Background: Deletion of the sphingolipid enzyme Isc1 in yeast makes cells sensitive to hydroxyurea.
Results: Phytoceramides are the main sphingolipid involved in yeast cell response to hydroxyurea toxicity.
Conclusion: C18:1-phytoceramides are identified as the specific ceramide that provide protection from hydroxyurea in a Cdc55-dependent manner.
Significance: This is the first specific sphingolipid species identified to play a role in the genotoxic response pathway.
Keywords: Cell Cycle, Phosphatase, Sphingolipid, Stress Response, Yeast, Hydroxyurea
Abstract
Recent studies showed that deletion of ISC1, the yeast homologue of the mammalian neutral sphingomyelinase, resulted in an increased sensitivity to hydroxyurea (HU). This raised an intriguing question as to whether sphingolipids are involved in pathways initiated by HU. In this study, we show that HU treatment led to a significant increase in Isc1 activity. Analysis of sphingolipid deletion mutants and pharmacological analysis pointed to a role for ceramide in mediating HU resistance. Lipid analysis revealed that HU induced increases in phytoceramides in WT cells but not in isc1Δ cells. To probe functions of specific ceramides, we developed an approach to supplement the medium with fatty acids. Oleate (C18:1) was the only fatty acid protecting isc1Δ cells from HU toxicity in a ceramide-dependent manner. Because phytoceramide activates protein phosphatases in yeast, we evaluated the role of CDC55, the regulatory subunit of ceramide-activated protein phosphatase PP2A. Overexpression of CDC55 overcame the sensitivity to HU in isc1Δ cells. However, addition of oleate did not protect the isc1Δ,cdc55Δ double mutant from HU toxicity. These results demonstrate that HU launches a lipid pathway mediated by a specific sphingolipid, C18:1-phytoceramide, produced by Isc1, which provides protection from HU by modulating Swe1 levels through the PP2A subunit Cdc55.
Introduction
Bioactive sphingolipids constitute an important group of regulatory molecules in the eukaryotic cell, and they have been implicated in a multitude of cellular functions ranging from regulation of cell growth to angiogenesis, inflammation, and responses to stress stimuli. The group of bioactive sphingolipids includes ceramide, sphingosine 1-phosphate, sphingosine, and ceramide 1-phosphate. Overall, ceramide has been appreciated as a transducer of stress stimuli; however, recent biochemical and analytical studies have revealed significant complexity and diversity in ceramide structure, and we have recently proposed that ceramides are actually a family of closely related molecules that may mediate distinct functions.
The yeast Saccharomyces cerevisiae has emerged as a powerful model in the study of sphingolipid metabolism and function. Indeed, most of the key enzymes of metabolism of bioactive sphingolipids were initially identified in yeast (1). Also, stress stimuli in yeast, such as heat stress, have been shown to regulate the acute production of bioactive sphingolipids, which in turn have been implicated in mediating many key responses such as cell cycle arrest, regulation of translation, and regulation of nutrient permeases (2, 3).
The sphingolipid enzyme Isc1, the yeast homologue of mammalian neutral sphingomyelinases, catalyzes the hydrolysis of complex sphingolipids, inositol phosphorylceramide (IPC),3 mannosylinositol phosphorylceramide (MIPC), and mannosyldiinositol phosphorylceramide (M(IP)2C), to dihydro- and phytoceramides (4–6). Deletion of the ISC1 gene has revealed that this enzyme is important for many biological processes, such as growth on nonfermentable carbon sources, gene expression during the diauxic shift (7–9), and the response to stress conditions such as high salt, heat stress, and oxidative stress (10–12).
In previous studies, we described a role for ISC1 in the genotoxic stress response to both the ribonucleotide reductase inhibitor HU and the alkylating agent methyl methanesulfonate (13). Isc1 has also been found to be involved in cellular morphogenesis under genotoxic stress (14). Deletion of the ISC1 gene conferred sensitivity to HU, mediated by the activation of a latent G2/M checkpoint. Mechanistically, the results implicated persistent phosphorylation on tyrosine 19 of the cyclin-dependent kinase Clb2-Cdc28, a known activator of the G2/M checkpoint (13, 15). In turn, this was traced to persistent activation of the Swe1 kinase responsible for phosphorylating Clb2-Cdc28. Either mutation of the phosphorylation site tyrosine 19 of Clb2-Cdc28 or the deletion of SWE1 was able to rescue the growth defect of isc1Δ on HU. These results indicated that Isc1 regulates phosphorylation of Clb2-Cdc28 and the G2/M checkpoint activation mediated by controlling Swe1 activity. This unexpected involvement of an enzyme of lipid metabolism in mediating the response to HU raised a number of important questions. Here, we address the question of whether Isc1 mediates response to HU action as a downstream effector. We also endeavor to determine the specific lipid mediator of Isc1 in response to HU and the downstream targets of the lipid mediator involved in Swe1 level regulation.
To address these questions, we employed biochemical and genetic approaches. The results demonstrated activation of Isc1 in response to HU. Genetic analysis suggested that phytoceramides were the likely mediators. Because yeast contain many species of ceramides, distinguished by the length of the fatty acyl group, α-hydroxylation of the fatty acid, and the hydroxylation of the sphingoid base, we employed a lipidomic approach. These results suggested more specifically a role for phytoceramide, but not dihydroceramides or α-hydroxyceramides, in the HU response. We next developed an approach to selectively enrich yeast cells in specific ceramides by providing selected fatty acid precursors. These results specifically supported a role for C18:1-phytoceramide in mediating the HU response. Our data define a highly specific pathway of sphingolipid metabolism in the response to HU and implicate Cdc55, the regulatory subunit of phosphatase PP2A, as the downstream target of C18:1-phytoceramide. The implications of these results for the delineation of ceramide-specific pathways are also discussed.
EXPERIMENTAL PROCEDURES
Strains and Media
Strains used in this study were as follows: JK9-3d (MATa trp1 leu2-3 his4 ura3 ade2 rme1), JK9-3d, isc1Δ (MATa trp1 leu2-3 his4 ura3 ade2 rme1 isc1::KanMX) from our laboratory (4), and JMY 1469 (MATa SWE1myc:HIS2 ade his2 leu2-3, 112 trp1-1 ura3Δns) a gift from D. Lew (Duke University). ISC1 was deleted in JMY 1469 strains to obtain JMY 1469,isc1Δ (13).
BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0), deletion library mutant strains were also used. The isogenic BY4741 deletion mutants used were as follows: isc1Δ, sur1Δ, sur2Δ, sur4Δ, csg2Δ, fen1Δ, scs7Δ, ipt1Δ, lag1Δ, lac1Δ, ypc1Δ, and ydc1Δ.
Deletion of CDC55 was done using the genomic DNA extracted from Y300-cdc55Δ (MATa ura3-1 his3-11,15 leu2-3,112 trp1-1 ade2-1 can1-100 cdc55::HIS3), a gift from Y. Wang (Florida State University) (16). PCR was performed using forward primer 5′-TAGCGCACAATGGCCGGGTTAATTAA-3′ and reverse primer 5′-GGGAAGATATGGGTAATTATAATGCG-3′ from Operon. The PCR product was used to transform BY4741 and BY4741-isc1Δ strains. Colonies growing on histidine minus (HIS−) agar plates were checked for the deletion of CDC55 by extracting DNA and using the same primers to perform PCR as follows: W303-1A (MATa can1-100 ade2-1 his3-11,15 leu2-3 112 trp1-1 ura3-1); lac1Δ,lag1Δ, same as W303-1A, but lac1::LEU2 lag1::TRP1, a gift from M. Jazwinski (University of Louisiana).
Standard yeast media, yeast extract, peptone, dextrose, adenine (YPDA), Synthetic Complete (SC) and SC-inositol, and SD URA− dropout media were used in this study. Minimal SD base with galactose and raffinose URA− dropout media was used to overexpress genes under GAL promotor, purchased from Clontech, catalog no. 630420. HU was purchased from US Biological and was employed at 7.5 and 10 mg/ml in liquid and solid SC and YPDA media, respectively. Fumonisin B1 was purchased from ENZO Life Sciences and was used at a concentration of 400 μm. Fatty acids were purchased from Sigma; myristate, palmitate, stearate, oleate, arachidate, and hexacosanate were dissolved in methanol then added to SC medium or SC URA− with or without agar, at a concentration of 0.003%.
Spot tests were performed as described previously (13); briefly, cells were grown to stationary phase overnight, then diluted to an A600 of 0.1, and then grown to an A600 of 0.8. The cultures were diluted to an A600 of 0.3 for the first spot on the left. 1:10 dilutions were done for the consecutive spots on the plates. Plates were incubated at 30 °C for 3 days.
Plasmids
pYES2 containing a galactose-inducible promoter was from Invitrogen. ISC1 ORF containing a FLAG tag was cloned into pYES2 using restriction enzymes KpnI and XbaI (4). ISC1-D163A and ISC1-K168A were generated in our laboratory (17). YPC1 and YDC1 plasmid construction was done as follows; BY4741 genomic DNA was prepared using yeast DNA extraction kit from thermo scientific catalog no. 78870.
The YPC1 and YDC1 genes were cloned using the following primers: YPC1, forward, 5′-CGTAAGCGTTAATGGAAAACAAT-3′, and reverse, 5′-CTTCGTGATTCGTCATTAAATAGAAC-3′; YDC1, forward, 5′-TCACCCTATTGTGGGATTGAA-3′, and reverse, 5′-TTCGGTCAATTGCACGTACA-3′.
PCR was carried out using the following programs: YPC1, 94 °C for 1 min, 49 °C for 1 min, 72 °C for 2 min, and 72 °C for 10 min; YDC1, 94 °C for 1 min, 56 °C for 1 min, 72 °C for 2 min, and 72 °C for 10 min, 30 cycles. The PCR product was then cloned using a PCR 2.1-TOPO vector from Invitrogen catalog no. K4560-40; TOP10 competent bacteria cells purchased from Invitrogen were transformed with the made plasmid. Bacterial colonies grown on plates containing 50 μg/ml carbenicillin plates were screened for positive clones containing the YPC1 and the YDC1 inserts by digesting plasmid recovered by a standard Qiagen miniprep using BamHI and XhoI restriction enzymes and BamHI and NotI, respectively. The positive clones were extracted from the gel and cloned into YEp24-PL (YEp24) (18).
Bacterial transformation was then performed, and positive clones were identified and verified if they contained the YEp24 containing YPC1 or YDC1 by restriction digestion with BamHI and XhoI or BamHI and NotI.
Enzymatic Activity Assay
The assay for Isc1 activity was conducted as described previously (4). Briefly, sphingomyelin labeled with 14C on the choline moiety was used as a substrate, and the hydrolysis of choline-methyl-14C-sphingomyelin was determined by liquid scintillation counting.
Western Blot Analysis
Western blots were performed as described previously (13). An anti-Myc tag was use to detect Swe1-Myc and also an anti-Swe1 from Santa Cruz Biotechnology catalog no. sc7171. Antibody against PSTAIRE (PSTAIRE domain of Cdc2 p34) from Santa Cruz Biotechnology, catalog no. sc-53, was used as a loading control.
Lipid Analyses by HPLC-MS/MS
Levels of dihydroceramide, phytosphingosine, phytosphingosine 1-phosphate, dihydrosphingosine, and dihydrosphingosine 1-phosphate were measured by the high performance liquid chromatography/mass spectrometry (LC-MS/MS) methodology as described previously (19). Analytical results of lipids were expressed as lipid level/total cell number
Lipid Determination
Cells were grown overnight and then resuspended in the morning to an absorbance of A600 of 0.15 in 50 ml of medium. Cells were grown to reach an A600 of 0.7 and then were either treated with HU or vehicle. Cells were harvested after 3 and 20 h of treatment with HU. Absorbance at A600 was measured; all the cultures were diluted to A600 of 0.7. Cells were then centrifuged at 3000 × g, and the pellets were resuspended in 1 ml of a lipid extraction solvent containing isopropyl alcohol (50%), diethyl ether (10%), pyridine (2%), ammonia (25%), and water (15%). Acid-washed glass beads (425–600 μm) were added to a 200-μl volume. Tubes were placed on a Destroyer vortex for five repeats of a 3-min on, 1-min off regiment. The cells were then transferred into 15-ml tubes by pouring, and the Eppendorf tubes were washed with 1 ml of extraction solvent and poured into 15-ml tubes, the tubes contained ∼2 ml of solvent, and cells and glass beads were sent to the Lipidomic Core at the Medical University of South Carolina for lipid analysis.
Thin Layer Chromatography (TLC)
Overnight cultures were diluted to an A600 of 0.1 and then grown to an A600 of 0.6 in SC medium. Cells were then centrifuged and washed twice with SC-inositol medium (YNB-inositol catalog no. 1533-050 from Sunrise Science Products) and inoculated into 20 ml of SC-inositol containing 4 μCi/ml [3H]myoinositol for labeling for 3 h with or without HU. Cell cultures were then centrifuged, and the pellets were used for sphingolipid extraction. Cells were washed twice with water and resuspended into 1 ml of water and transferred to glass tubes. 1 ml of solvent A, ethanol/ether/pyridine (1:0.33:0.067 v/v), was added to the tubes and then incubated at 57 °C for 30 min. The cells were centrifuged at 2000 rpm for 5 min; supernatant was transferred to new glass tube. 1 ml of solvent made of chloroform/methanol/water (16:16:5 v/v) was added, and an additional 1 ml of sodium hydroxide (0.2 n) in methanol was added. The mixture was incubated at 30 °C for 45 min and then 1.1 ml of 0.5% EDTA was added. 0.2 ml of acetic acid (1 n) was added to neutralize the mixture. 0.5 ml of chloroform was added to extract the nonacetylated lipids. The mixture was vortexed and centrifuged at 3000 rpm for 5 min. The lower phase on the tube was extracted and transferred to a new glass tube. The lipids were dried under nitrogen. The dried lipids were resuspended in 150 μl of solvent A. 50 liters were spotted on a TLC plate. The plate was developed in the first dimension in chloroform/methanol/ammonia (9:7:2 v/v). The plate was put in a cassette with a autoradiography film and incubated at −80 °C for 5 days.
Statistics
All data represent the median of at least three independent experiments unless otherwise stated on the figure legend. GraphPad Prism software was used in all illustrations.
RESULTS
HU Treatment Leads to an Increase of ISC1 Activity
To determine whether ISC1 is involved in a regulatory pathway downstream of HU, we investigated the effects of HU on Isc1 activity. An in vitro enzymatic assay was performed in WT cells overexpressing ISC1, and the results showed a net increase in Isc1 activity in response to HU starting at 90 min, and the activity remained elevated throughout the time course that lasted 20 h as shown (Fig. 1A). To investigate if the Isc1 enzymatic activity is required for its role in protecting cells from HU toxicity, catalytically inactive mutants of Isc1 were expressed in an isc1Δ strain transformed with a pYES2 vector containing wild type ISC1 and the two inactive mutants, ISC1-G163A and ISC1-K168A. Neither catalytically inactive mutant was able to protect cells from HU toxicity (Fig. 1B). In contrast, wild type ISC1 allowed cells to resume growth on HU. The level of IPC, MIPC, and M(IP)2C decreased significantly when WT cells were treated with HU after 3 h (Fig. 1C). This change in complex sphingolipid due to HU treatment corresponds to the increase of Isc1 activity after HU. These results indicate that HU treatment increases activity of Isc1 and that this activity is needed to protect cells from HU toxicity.
FIGURE 1.
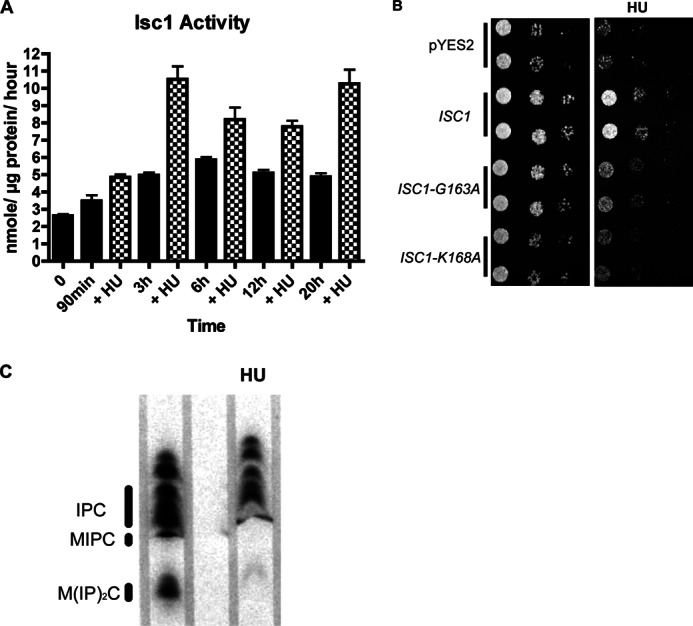
Activation of Isc1 in response to HU. A, effects of HU on Isc1 activity. Enzymatic activity of Isc1, expressed under a galactose promoter, was measured using sphingomyelin as a substrate (see “Experimental Procedures” for details) following HU at different time points as indicated. B, requirement for the enzymatic activity of Isc1 for protection of cells from HU toxicity. Cells deleted in the ISC1 gene were transformed with vector control pYES2, and plasmids containing ISC1, ISC1-K168A, and ISC1-G167A were expressed under a galactose promoter. A spot test was done on SC URA− plates containing galactose/raffinose with or without HU. Stationary cells were diluted to 0.1 and grown to A600 of 0.8. The culture were diluted to an A600 of 0.3 and spotted (5 × 104 cells), and a 1:10 serial dilution was done starting from the first spot on the left of A600 0.3. C, TLC analysis of complex sphingolipids (IPC, MIPC, and M(IP)2C). WT cells with and without HU were labeled with [3H]myoinositol for 3 h. The resulting labeled lipids were extracted and separated by TLC and developed for visualization by autoradiography. All experiments have been repeated three independent times.
Identifying the Lipid Species That Are Regulated by HU
Because HU activated Isc1, it became important to determine the candidate lipid mediators of Isc1 action. This is a particularly difficult issue with bioactive sphingolipids because these molecules are interconvertible in cells. For example, the production of ceramide by Isc1 may result in further metabolism to sphingoid bases and then to their phosphates (long chain base phosphates) by the action of ceramidases and sphingoid base kinases, respectively.
To pinpoint the functionally relevant class of sphingolipids, we adopted a combined genetic and pharmacological approach using deletion, inhibition, and overexpression of key enzymes involved in interconversion of bioactive sphingolipids. Analysis of the deletion mutants dpl1Δ, scs7Δ, sur1Δ, ipt1Δ, lag1Δ, lac1Δ, ysr3Δ, lcb3Δ, lcb4Δ, lcb5Δ, fen1Δ, sur2Δ, sur4Δ, csh1Δ, csg2Δ, ypc1Δ, and ydc1Δ (Fig. 2, A and B) revealed no effects for most of these genes except for dpl1Δ and sur4Δ, which were sensitive to HU. DPL1 is the dihydrosphingosine 1-phosphate/phytosphingosine 1-phosphate lyase gene that catalyzes the formation of fatty aldehydes and phosphoethanolamine in yeast (20, 21). The dpl1Δ sensitivity to HU suggests that a generation of fatty aldehydes and/or ethanolamine phosphate or accumulation of sphingoid base phosphates can affect cellular responses to HU. The Sur4 enzyme catalyzes the fatty acid elongation step and is implicated in the production of dihydroceramides and phytoceramides with longer chain fatty acids (longer than C16) (22), suggesting the possible importance of these particular ceramides in cell protection from HU. Furthermore, we found that double deletion of LAG1 and LAC1, the two highly homologous enzymes that codify for the acyl-CoA-ceramide synthase, also rendered cells more sensitive to HU (Fig. 2C). It is worthy of notice that the double mutant has a slow growth phenotype but was highly sensitive to HU treatment. Double deletion of LCB4 and LCB5, the two long chain base kinases that catalyze the production of the phosphorylated forms of phytosphingosine or dihydrosphingosine, showed no sensitivity to HU (Fig. 2D).
FIGURE 2.
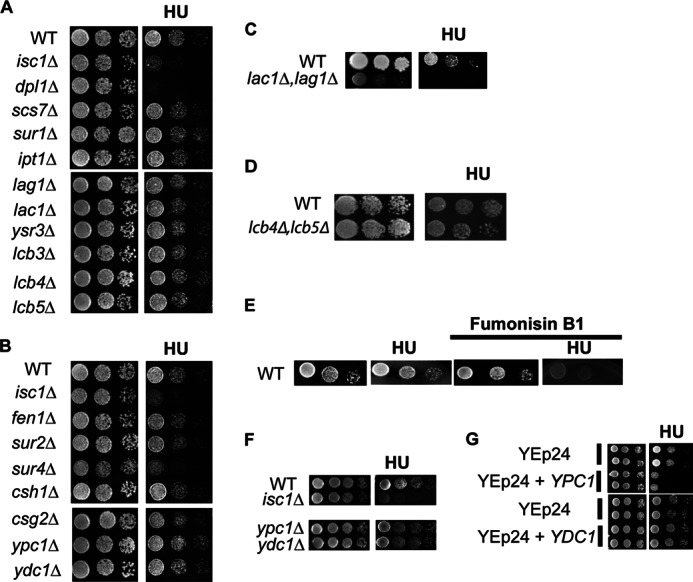
Sensitivity or resistant assessment to HU of yeast mutants carrying gene deletions in various sphingolipid enzymes. A and B, spot test assessment of HU sensitivity or resistance in sphingolipid deletion enzymes mutants. C, spot test of the double deleted strain lac1Δ,lag1Δ; D, double deleted strain lcb4Δ,lcb5Δ. E, effects of fumonisin B1 on resistance of WT cells to HU. The spot tests were done on YPDA plates containing fumonisin B1 with or without HU. F, effects of YPC1 and YDC1 deletion in the presence of HU. G, overexpression of YPC1 and YDC1 using a multicopy plasmid YEp24 on WT resistance to HU toxicity. The spot tests were done on SC URA− plates with or without 7.5 mg/ml HU. Plates in YPDA were treated with vehicle or with 10 mg/ml HU. All figures are representative experiments from three independent ones.
Excluding results with dpl1Δ, analysis of all the other enzymes (Isc1, Sur4, Lag1, Lac1, Ypc1, and Ydc1) that showed sensitivity or resistance to the drug points specifically at ceramide as the product involved in HU response and not sphingoid bases or more complex sphingolipid species. The results also rule out a specific role for α-hydroxyceramide because SCS7 deletion mutant showed no response to HU treatment.
To confirm that ceramides are important for protecting cells from HU toxicity, a pharmacological approach was additionally employed using fumonisin B1, an inhibitor of ceramide synthases. The results (Fig. 2E) showed that fumonisin B1-treated WT cells became sensitive to HU. Taken together, these results suggest that one or more ceramides play critical roles in cell survival in response to HU.
Interestingly, deletion of the two ceramidases YPC1 and YDC1 rendered cells more resistant to HU. This was observed during the initial screening of sphingolipid deletion mutants (Fig. 2B) and was confirmed with additional spot tests illustrated in Fig. 2F. These results clearly support a role for ceramide and not further downstream metabolites (such as sphingoid bases or their phosphates) in mediating the resistance to HU. These are also corroborated by the studies with fumonisin B1.
These results in turn suggested that YPC1 and/or YDC1 overexpression would make cells more sensitive because of the increased Ypc1 and/or Ydc1 activity and the consequent decrease of phytoceramide and/or dihydroceramide levels. When wild type cells overexpressed YPC1, they became more sensitive to HU (Fig. 2G). Thus, these results again support a role for ceramide and not further downstream products.
Very interestingly, and in contrast to the results with YPC1, overexpression of YDC1 had no effect on the sensitivity of wild type cells to HU (Fig. 2G). These results begin to distinguish among “ceramide” species in the HU response by providing evidence that phytoceramides (the preferred substrates of YPC1) and not dihydroceramides (the preferred substrates of YDC1) (23) are the candidate mediators of the action of Isc1 in response to HU. A map illustrating the yeast sphingolipid pathway indicates the responses to the sphingolipid deletion mutants (sensitivity or resistance) to HU (Fig. 3).
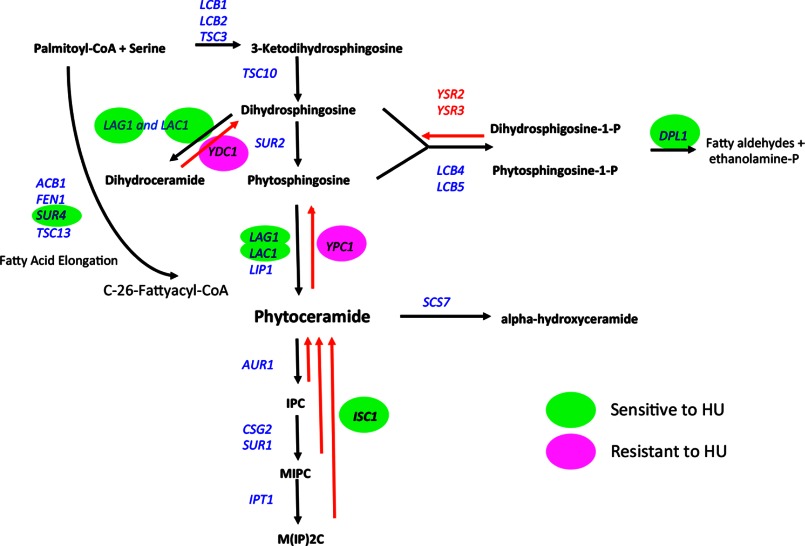
FIGURE 3.
Basic scheme of the sphingolipid pathway in S. cerevisiae with enzymes color-tagged for sensitivity or resistance to HU, green (sensitive), magenta (resistant), not colored (no sensitivity or resistance was observed or not tested); black arrows represent de novo pathway, red arrows represent the salvage pathway.
Based on the above results, it became important to determine whether a subset of ceramide molecular species was involved in the HU response. In yeast, ceramides can exist in at least 40 distinct molecular species based on the length of the fatty acyl chain, hydroxylation of the sphingoid base (phyto series), and hydroxylation of the fatty acyl group (α-hydroxy series) (24). Therefore, the sphingolipid response to HU was analyzed in the wild type and in isc1Δ cells, using LC-tandem mass spectrometry.
Several ceramide species showed some changes in the various conditions; therefore, we elected to focus on specific ceramides that would show the following patterns: 1) increases in response to HU, and 2) loss of this increase in isc1Δ. These criteria define the subset of ceramides that are directly produced in response to HU in an ISC1-dependent manner. To make sure that WT and isc1Δ cells would not lose viability after 20 h of HU treatment, a colony-forming unit assay was done. The results showed that 68% of WT cells were viable, and 57% of isc1Δ cells were viable (data not shown). Thus, there was only a modest 11% increase in cell death in isc1Δ, but this difference was not statistically significant.
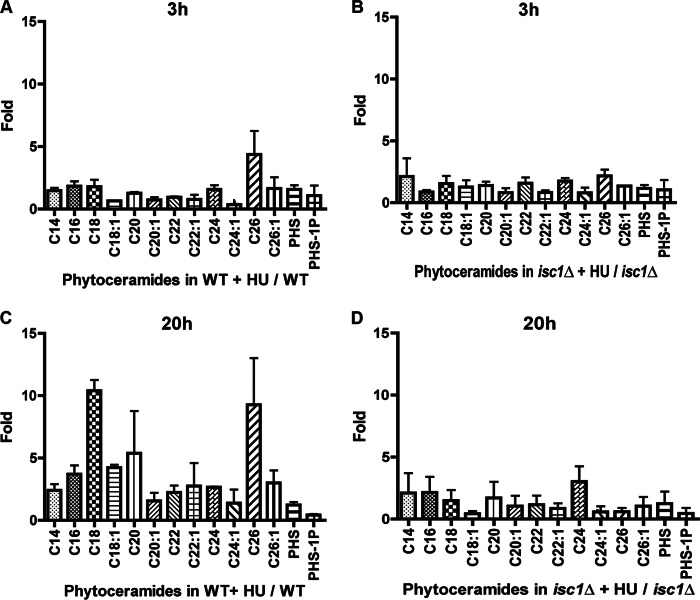
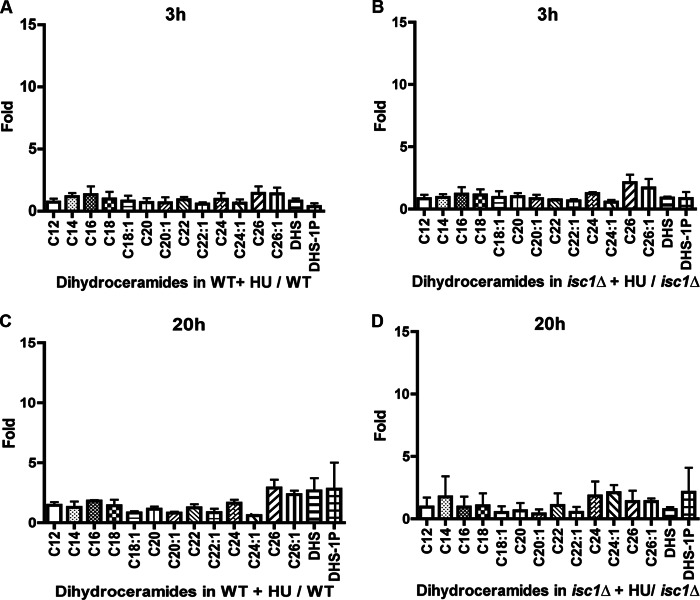
After 20 h of HU, many phytoceramide species increased in WT but failed to do so in isc1Δ cells. These changes were not seen after 3 h of HU treatment (Fig. 4, A and B). The most prominent increases were in C18-, C18:1-, C20-, and C26-phytoceramides after 20 h of treatment (Fig. 4, C and D). These results pointed toward the phytoceramide subspecies most likely involved in the protection against HU. In contrast, analysis of dihydroceramides (Fig. 5, A–D) did not reveal major changes in response to HU in their levels after 3 and 20 h in WT and isc1Δ cells. No changes were detected in the levels of α-hydroxyphytoceramides. In fact, deletion of SCS7, the α-hydroxylase that transforms phytoceramide into α-hydroxyceramide, did not show sensitivity to HU, indicating that these species do not play a major role in protecting cells from the genotoxic effect of HU (data not shown). Importantly, overexpression of YPC1 in WT cells abolished the increases in phytoceramides in response to HU, and more so the C16, C18, C18:1, C26, and C26:1 species (Fig. 6, A and B). These results therefore point to a subset of phytoceramides as candidate regulators of the HU response.
FIGURE 4.
Analysis of ceramide species following HU treatment in WT and isc1Δ cells by mass spectrometry. Phytoceramide levels were determined after 3 and 20 h of treatment with HU. To determine the fold changes of phytoceramide species due to HU, the values obtained from the treated samples were divided by the untreated ones. Fold changes in phytoceramides are represented in A for WT, in B for isc1Δ after 3 h, in C for WT, and in D, for isc1Δ after 20 h. All measurements represent the mean of two independent experiments.
FIGURE 5.
Dihydroceramide species after 3 h of HU in WT cells (A) compared with the dihydroceramide fold changes in isc1Δ (B). C and D are fold changes of dihydroceramides after 20 h in WT and isc1Δ, respectively. All measurements represent the mean of two independent experiments.
FIGURE 6.

Changes in phytoceramide levels upon overexpression of YPC1. A, fold changes in phytoceramides in WT cells transformed with vector control YEp24 compared with WT cells overexpressing YPC1 in YEp24 plasmid after 20 h HU treatment (B). All measurements represent the mean of three independent experiments.
Specific Role for C18:1-Phytoceramide in Mediating Protection from HU
Because the above results suggested that one or more phytoceramides may be specifically involved in the HU response, it became important to determine whether generating phytoceramides was sufficient to protect isc1Δ cells from the HU effects and if indeed a specific phytoceramide was needed. Given the very hydrophobic nature of long chain and very long chain ceramides, it was not possible to directly administer these lipids to yeast culture. Therefore, we sought a different approach to selectively enrich yeast in specific ceramides. To this end, specific fatty acid precursors, myristate (C14), palmitate (C16), stearate (C18), oleate (C18:1), arachidate (C20), and hexacosanoic acid (C26), were separately added to the medium to determine whether isc1Δ cell growth on HU can be rescued.
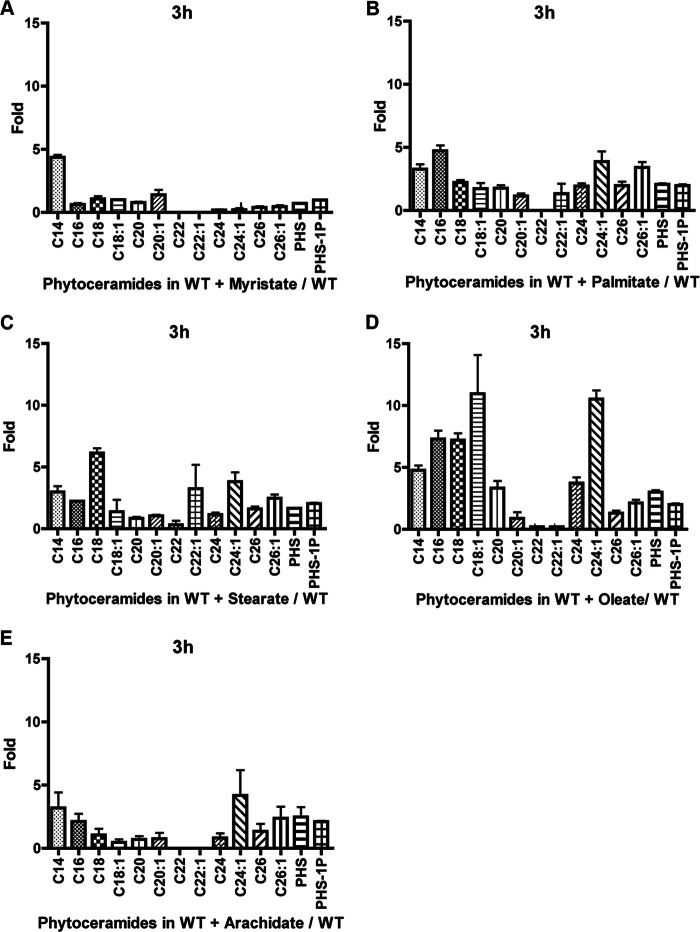
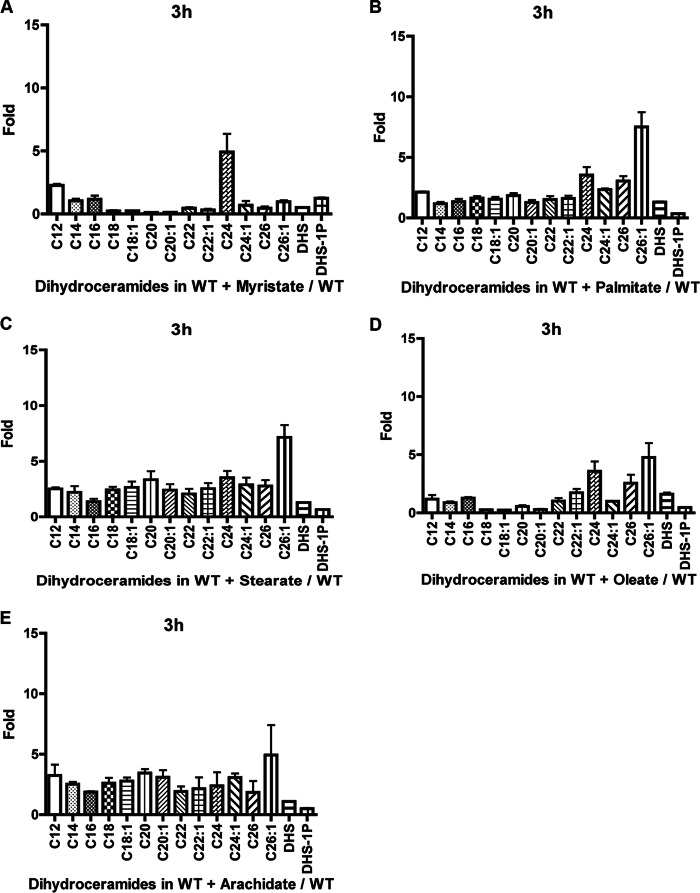
Initially, the effects of these fatty acids on ceramide composition was determined to establish whether addition of fatty acids could influence the increase in specific ceramides with the corresponding fatty acid. Therefore, the lipid levels were analyzed in WT cells growing on a medium supplemented individually with C14, C16, C18, C18:1, and C20 fatty acids. C26 was not developed further due to very poor solubility in culture medium. When myristate was added to the medium for 3 h, the levels of C14-phytoceramides increased significantly compared with all other species (Fig. 7A). Addition of palmitate resulted in increased levels of the phytoceramide species C14, C16, C24:1, and C26:1, with C16 being the highest (Fig. 7B). Stearate addition increased C18-phytoceramide but not C18:1-phytoceramide significantly compared with other species. However, other chain lengths such as C14, C16, C22:1, and C24:1 increased but not to the extent of the C18 increase (Fig. 7C). Conversely, oleate increased the levels of C14, C16, C18, C18:1, C24, and C24:1, with C18:1 registering the more significant increase (Fig. 7D). Arachidate increased the levels of C14- and phyto-C24:1-phytoceramide (Fig. 7E). No increase of C20 was detected. The poor response to arachidate in increasing the specific chain length was probably due to the poor solubility of this fatty acid when added to the medium. Dihydroceramide species seemed less responsive to the addition of fatty acids, as we noticed no specific chain length increases corresponding to the respective fatty acid added (Fig. 8, A–E). Taken together, these results show that besides arachidate, C14, C16, C18, and C18:1 fatty acids selectively increased the levels of their respective phytoceramides with some effects for some of the fatty acids on other ceramides. This then allowed us to explore the ability of these fatty acids to rescue the growth defect of isc1Δ in response to HU.
FIGURE 7.
Effect of addition of fatty acid on phytoceramide species levels increases after 3 h of HU treatment. Effect when adding myristate (A), palmitate (B), stearate (C), oleate (D), and arachidate (E). The values are represented as a fold change of treated with fatty acid compared with untreated. The results are the mean of three independent experiments.
FIGURE 8.
Effect of addition of fatty acids on dihydroceramide species levels increases in WT cells after 3 h of HU treatment. Effect when adding myristate (A), palmitate (B), stearate (C), oleate (D), and arachidate (E). The values are represented as a fold change of treated with fatty acid compared with untreated. The experiments are the mean of three independent experiments.
The results showed that oleate (C18:1) was the only fatty acid that when added to the medium rescued the growth of isc1Δ in the presence of HU (Fig. 9A). To verify that oleate did not interfere with HU toxicity in a nonspecific manner, we evaluated the KAR3 deletion strain known to be sensitive to HU (25); Kar3 is a microtubule motor protein that functions in mitosis and meiosis and has no known relationship to sphingolipids. Adding oleate to the medium containing HU had no effect on HU toxicity (data not shown).
FIGURE 9.
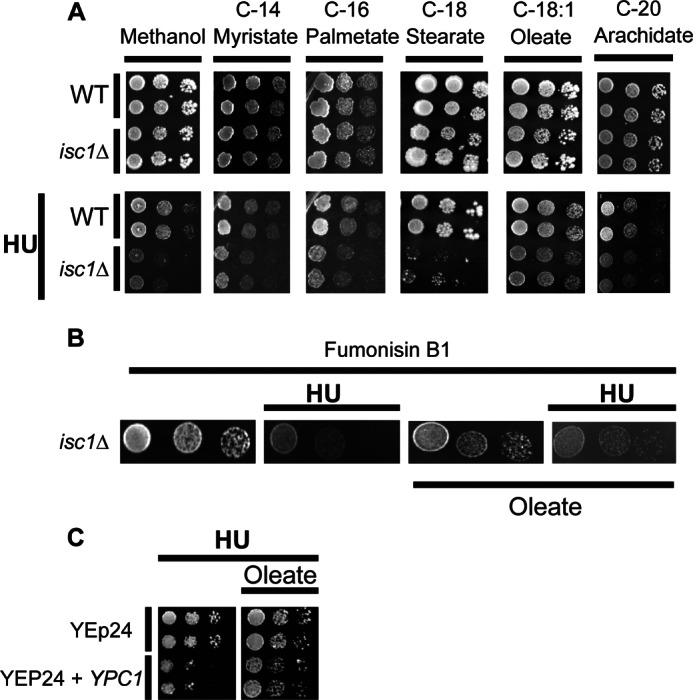
Evaluation of the ability of specific fatty acids to rescue cells from HU toxicity. A, spot tests were performed on WT and isc1Δ on SC medium supplemented with different chain length fatty acids (myristate, palmitate, stearate, oleate, and arachidate) and the same fatty acids with HU. This experiment was independently repeated three times. B, effects of fumonisin B1 on isc1Δ cells' HU sensitivity rescue by oleate. C, effect of oleate on the HU sensitivity of WT cells overexpressing the YPC1 gene. These experiments were repeated three times.
To determine whether the effects of oleate were due to incorporation into phytoceramide, isc1Δ cells were treated with fumonisin B1 to inhibit the synthesis of phytoceramide in response to oleate. The results showed that fumonisin B1 prevented the ability of oleate to reverse HU sensitivity (Fig. 9B), indicating that the protective effects of oleate were due to incorporation of the C18:1 fatty acids into phytoceramide. Moreover, oleate was also able to rescue the increased sensitivity to HU of cells overexpressing YPC1 (Fig. 9C).
Taken together, the above results pointed to C18:1 phytoceramide as the candidate. However, due to the overlap in effects of some of the fatty acids on ceramide species, we resorted to examining which lipids showed the most specific relationship to the protection from HU. Table 1 summarizes the increase of at least 3-fold registered in each chain length species of phytoceramides and dihydroceramides in WT and isc1Δ treated with HU and the increase due to the addition of oleate or other fatty acids. The only species that increased in WT with HU, failed to do so in isc1Δ with HU and increased when oleate was added but did not increase when other fatty acids were provided was C18:1-phytoceramide. Thus, taken together, these results show that phytoceramides are sufficient to mediate the protective effects of ISC1 in response to HU, and the results also suggest that C18:1-phytoceramide is the primary ceramide species involved in this process.
TABLE 1.
Comparison between the effect of oleate and other fatty acids on phytoceramide and dihydroceramide levels in WT and isc1Δstrains
The results show the changes in the levels of each phytoceramide and dihydroceramide species listed by chain length in four different conditions of growth as follows: 1) WT with HU; 2) isc1Δ with HU; 3) with addition of oleate, and 4) with addition of other fatty acids. + indicates an increase of at least three times the specific species. − indicates no increase or an increase of less than three times the level of the specific species. A total of four conditions were analyzed.
| Increased in WT with HU | Increased in isc1Δ with HU | Increased by oleate | Increased by other fatty acids | |
|---|---|---|---|---|
| Phytoceramides | ||||
| PHS | − | − | + | − |
| PHS 1-phosphate | − | − | − | − |
| C14 | − | − | + | + |
| C16 | + | − | + | + |
| C18 | + | − | + | + |
| C18:1 | + | − | + | − |
| C20 | + | − | + | + |
| C20:1 | − | − | − | − |
| C22 | − | − | − | − |
| C22:1 | − | − | − | + |
| C24 | − | + | + | + |
| C224:1 | − | − | + | + |
| C26 | + | − | − | + |
| C26:1 | − | − | − | − |
| Dihydroceramides | ||||
| DHS | − | − | − | − |
| DHS 1-phosphate | − | − | − | − |
| C12 | − | − | − | + |
| C14 | − | − | − | − |
| C16 | − | − | − | − |
| C18 | − | − | − | − |
| C18:1 | − | − | − | − |
| C20 | − | − | − | + |
| C20:1 | − | − | − | + |
| C22 | − | − | − | − |
| C22:1 | − | − | − | − |
| C24 | − | − | + | + |
| C24:1 | − | − | − | − |
| C26 | + | − | − | + |
| C26:1 | + | − | + | + |
Oleate Decreases Swe1 Kinase Levels in isc1Δ in the Presence of HU
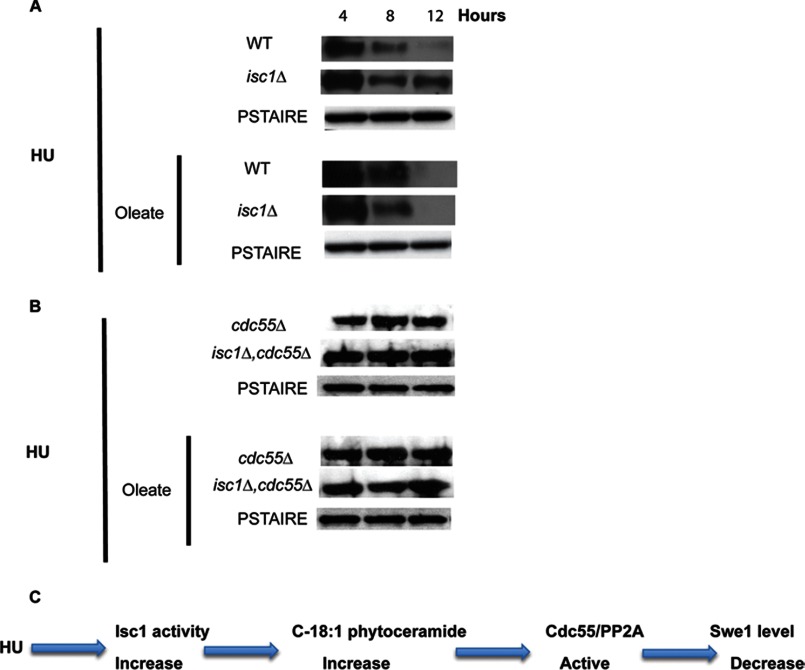
In a previous study, we showed that HU sensitivity of isc1Δ cells was due to Swe1 kinase stabilization, leading to increased phosphorylation of the cyclin kinase Clb2-Cdc28 resulting in a G2/M checkpoint activation. Those results revealed a necessary role for sphingolipids in regulating the Swe1/Cdc28 checkpoint. The above results with oleate allowed us to investigate whether phytoceramides are sufficient to regulate the stabilization of Swe1 levels. Degradation of Swe1 has been found to be regulated by the regulatory subunit of protein phosphatase 2A (PP2A), CDC55 (26). Deletion of CDC55 rendered cells sensitive to the drug (Fig. 10A), consistent with previous results (16). Interestingly overexpression of CDC55 was able to protect isc1Δ cells from the HU toxicity (Fig. 10B); thus, Cdc55 functions downstream of Isc1 in the pathway leading to HU. Importantly, addition of oleate failed to protect the single deletion cdc55Δ strain and the double deletion isc1Δ,cdc55Δ from HU toxicity (Fig. 10A, right panel) suggesting that oleate acts upstream of Cdc55.
FIGURE 10.
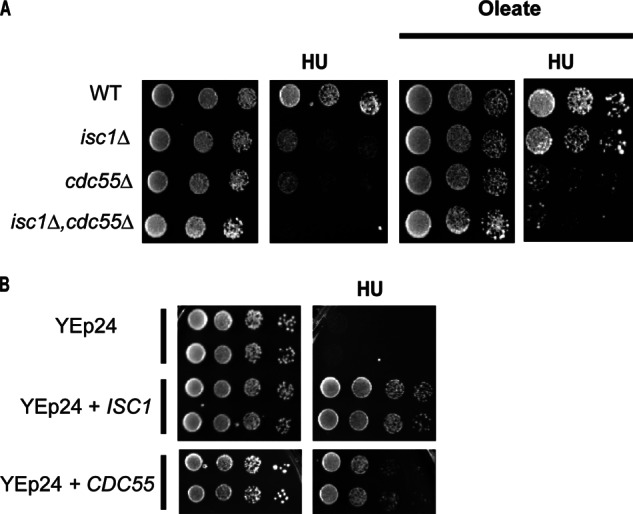
Role of Cdc55 in protecting isc1Δ from HU. A, deletion of CDC55 renders cells more sensitive to HU, and addition of oleate does not protect cdc55Δ from HU toxicity. Double deletion mutant isc1Δ,cdc55Δ is sensitive to HU in the presence and absence of oleate. B, overexpression of CDC55 protects isc1Δ cells from HU toxicity. These experiments were repeated three times.
Next, we evaluated the levels of Swe1 kinase in the presence of oleate and HU in isc1Δ and in isc1Δ,cdc55Δ double deletion strain. The results in Fig. 11A show that oleate was sufficient to induce loss of Swe1 in isc1Δ treated with HU, thus overcoming the defect observed in the isc1Δ cells. However, oleate had no effect on the stabilization levels of Swe1 in the double deletion isc1Δ,cdc55Δ strain (Fig. 11B). This indicated that Cdc55 deletion in the isc1Δ strain was sufficient to abrogate the protective effect of oleate.
FIGURE 11.
Effects of oleate on Swe1 levels in isc1Δ cells treated with HU. A, Western blot to monitor the levels of Swe1 protein was performed in WT and isc1Δ cells treated with HU ± oleate at 4, 8, and 12 h. Anti-Myc antibody was used to detect Swe1-myc. B, using anti-Swe1 antibody, the levels of the Swe1 protein were evaluated in the absence of CDC55 and in the double mutant isc1Δ,cdc55Δ treated with HU with or without oleate. PSTAIR antibody was used as a loading control. C, schematic description of our findings proposing the following events: when cells are treated with HU, Isc1 activity increases leading to an increase of phytoceramides, specifically C18:1. These ceramides activate Cdc55/PP2A phosphatase, which in turn induces the decrease of Swe1 levels leading to dephosphorylation of Clb2-Cdc28 leading to a G2/M cell cycle progression. Experiments in A and B were independently repeated three times.
DISCUSSION
The goal of this study was to define the regulation of bioactive sphingolipids by Isc1 in response to HU and to determine whether a specific sphingolipid species is required for cell protection against HU by regulating downstream target Swe1 kinase. The results initially demonstrated a requirement for enzymatic activity of Isc1 in protecting cells from HU, and the results subsequently pointed to phytoceramides as the main sphingolipids responsible for protecting cells from HU genotoxic effects. Further analysis highly implicated C18:1-phytoceramide as the main mediator of the Isc1 response.
The initial results clearly implicate Isc1 and its product phytoceramide in the HU genotoxic effect. Treating cells with HU induced an increase of Isc1 enzymatic activity, and the overexpressing inactive form, G167A and K168A, failed to protect the cells from the HU toxicity. Furthermore, HU treatment resulted in a significant increase in ceramides as shown by mass spectrometry. Also, we found that phytoceramides are the only sphingolipid species that increased with HU but failed to increase in ISC1-deleted cells.
To confirm the involvement of ceramides, we evaluated the sensitivity to HU of deletions of each nonessential sphingolipid gene. This analysis indicated that in addition to ISC1, deletion of SUR4, DPL1, and double deletion of LAG1 and LAC1 increased the sensitivity to HU. With the exception of DPL1, these genes are involved in the generation of ceramide by the de novo (Sur4, Lag1, and Lac1) or salvage pathway (Isc1). Furthermore, deletion of YPC1 or YDC1 provided protection from HU toxicity, thus ruling out a key role for sphingoid bases. Moreover, the overexpression of YPC1 but not YDC1 made the WT cells more sensitive to HU. In contrast, double deletion of LCB4 and LCB5, known to cause accumulation of LCBs, showed no sensitivity to HU, indicating that LCBs have no role in HU protection. In addition, no changes in LCB levels were detected when lipid levels were checked after HU treatment. Together, these results clearly implicate ceramides as the major players in regulating the sensitivity to HU. It is important to note that deletion of SUR2, which produces mainly phytoceramides, showed no sensitivity to HU. This result may suggest that phytoceramides have no role in protecting cells from HU. However, genetic, biochemical, and pharmacological methods indicated clearly that the main species are phytoceramides. It is therefore possible that the observation that sur2Δ is not sensitive could be due to the large accumulation of dihydroceramides and dihydrosphingosines in this mutant, probably making dihydroceramide's IPC that would be used as a substrate for activated Isc1 that may suffice to replace the need for phytoceramides and provide protection from HU toxicity.
At this point, we cannot explain the role of DPL1 in sensitivity to HU. Deletion of DPL1 induces accumulation of the phosphorylated LCB. Accumulation of phosphorylated LCB has been shown to cause slow growth and even a lethal phenotype in DPL1 and YSR2 double mutant (27). Thus, dpl1Δ is sensitive to HU either because of accumulation of phosphorylated LCB or because of decrease of long chain aldehyde and/or ethanolamine.
Sphingolipidomic analysis indicated that not one but several phytoceramide subspecies increased in WT after HU treatment. One challenging experimental goal was identifying the specific phytoceramide subspecies distinguished by the fatty acyl group length that specifically protects the cells from HU. Addition of different chain length fatty acids in the medium has been used previously to increase lipid species (28). Among the fatty acids we used, oleate was the only one that provides HU protection to isc1Δ cells and to WT cells overexpressing YPC1. Phytoceramide C18:1 was the main species to increase following oleate addition. Importantly, C18:1-phytoceramide was the only species that satisfied the following characteristics: 1) increased in WT after HU treatment; 2) did not increase in ISC1 deletion; 3) decreased with overexpression of YPC1; 4) increased when oleate was added to the medium, and 5) did not increase when other fatty acids were added to the medium. Thus, taken together, these results clearly implicate C18:1-phytoceramide as the most likely candidate. Our data suggest that both sphingolipid syntheses, through the de novo pathway and the hydrolytic pathway, are involved in the protection against HU. Here, we suggest that the salvage pathway plays a more important role; this is supported by our in vivo data, implicating a more efficient and quick response of the cells to DNA damage translated by the activation of one enzyme ISC1 to produce the lipid species C18:1-phytoceramide that would regulate the downstream target CDC55.
Mechanistically, the results place C18:1-phytoceramide downstream of Isc1 (overcomes defect of ISC1 deletion) and as a regulator upstream of Swe1 and CDC55. In fact, the levels of Swe1 in isc1Δ in the presence of HU were lowered to WT levels when oleate was added to the medium.
These findings raised important questions on how the Isc1/ceramide pathway mediates this response to HU; our results show that the downstream pathway involves protein phosphatases, and especially the CDC55 regulatory subunit of the ceramide-activated protein phosphatase PP2A. As suggested in a previous study (26), Swe1 levels are controlled by Cdc55, a regulatory subunit of the p subunit of ceramide-activated protein phosphatase, the eukaryotic class of serine/threonine protein phosphatases (29, 30). In yeast it has been shown that C2-ceramide, a cell-permeable analogue of ceramide, caused a dose-dependent inhibition of growth and that yeast cells exhibited a ceramide-activated phosphatase activity (29). A G1 arrest in response to ceramide was shown to be mediated by the regulatory subunit Cdc55 and by the Sit4 catalytic subunit, and deletion of CDC55 protected the cells from C2-ceramide sensitivity (31). Furthermore, CDC55 was also found to protect the amphiphysin-like mutant Rvs161 and that overexpression of CDC55 suppresses multiple growth defects in this mutant (32). In this study, overexpression of CDC55 rescued isc1Δ viability in HU, whereas the double mutant isc1Δ,cdc55Δ was not rescued from HU by oleate. Furthermore, deletion of CDC55 prevented oleate from correcting the levels of Swe1. Thus, these results implicate Cdc55 downstream of Isc1/C18:1-phytoceramides.
These current results implicate for the first time a bioactive lipid as a key mediator of DNA damage response in yeast. Although our previous study implicated Isc1 in HU response, those results, based on genetic analysis, could not determine whether Isc1 was a component of a signaling pathway initiated by HU/DNA damage or whether it affected those responses in an indirect manner. These current results show that Isc1 is a regulated component of those pathways. Thus, HU activates isc1 and increases ceramides. In turn, this regulates the response to HU, and it specifically mediates the response of SWE1 through the CDC55 phosphatase pathway (Fig. 11C). Although the activation mechanisms of Isc1 by HU remain unknown, we speculate based on preliminary data that it may be related to the regulation of Isc1 stability.
Another major implication of these studies pertains to defining a specific and distinct role for subclasses and specific species of ceramide. Emerging evidence is already pointing to the complexity of ceramide, both structurally and functionally. Thus, we have estimated that mammals may harbor at least 200–300 distinct ceramides that are distinguished by their fatty acyl chains, hydroxylations on different carbons, length of the sphingoid chain, double bonds, and the 1-head group. Importantly, these structurally distinct ceramides are products of a combinatorial pathway of synthesis and modification that allow creation of many more ceramides from a limited set of precursors. These considerations have led us to propose the “Many Ceramides” hypothesis whereby distinct ceramides are regulated by distinct pathways and participate in specific and distinct responses (24). Although there have been strong hints for these diverse functions in mammalian studies, the current results provide a solid genetic, biochemical and pharmacological, and mechanistic basis for defining a very specific pathway of C18:1-phytoceramide in the HU response. In mammalian studies, C18:1-ceramide, generated by human ceramide synthase 1, was found to be the candidate species that inhibited the activity of human telomerase reverse transcriptase via deacetylation of the Sp3 transcription factor in A549 human lung adenocarcinoma cells (33), although those studies did not rule out other downstream metabolites of ceramide. Other studies showed an increase of predominantly C16-ceramides and C24-ceramides after 3 and 24 h, respectively, after B-cell apoptotic stimulation with the B-cell receptor (34) with distinct roles proposed for each.
A distinct involvement for C18-ceramide in cell death induction was also described in head and neck squamous cell carcinoma. In these cells C18-ceramide production increased due to elevated ceramide synthase 1 (LASS1) enzymatic activity following gemcitabine/doxorubicin treatment (35).
In this study, we also provide a rather simple approach for probing lipid-specific functions using supplementation of fatty acids along with lipidomic analysis and specific pharmacological and genetic manipulations.
In conclusion, the results identified a specific sphingolipid species, C18:1-phytoceramides, as the lipid mediator for protection from genotoxic effects of HU. We also provided mechanistic evidence that phytoceramide C18:1 can regulate the Swe1 kinase levels in the isc1Δ cells in a Cdc55-dependent manner. The results in this study establish a strong platform for future studies to dissect the molecular interface between a sphingolipid species and a phosphatase. This study also reveals a new mechanism characterized by the activation of a sphingolipid pathway in a HU genotoxic dependent manner.
Acknowledgments
We are grateful Dr. Cungui Mao for revising the manuscript and to Lina Obeid, Chiara Luberto, and the Hannun group members for helpful discussions. We thank the Lipidomics Center at the Medical University of South Carolina for all lipid analyses performed in this study. Research at the Lipidomics Shared Resource, Hollings Cancer Center, Medical University of South Carolina, was supported in part by National Institutes of Health Grant P30 CA138313 and at the Lipidomics Core in the SC Lipidomics and Pathobiology COBRE, Department Biochemistry, Medical University of South Carolina, by National Institutes of Health Grant P20 RR017677.
This work was supported, in whole or in part, by National Institutes of Health Grants R01GM063265 and R01GM43825 (to Y. A. H.).
- IPC
- inositol phosphorylceramide
- HU
- hydroxyurea
- MIPC
- mannosylinositol phosphorylceramide
- M(IP)2C
- mannosyldiinositol phosphorylceramide
- LCB
- long chain base.
REFERENCES
- 1. Dickson R. C. (1998) Sphingolipid functions in Saccharomyces cerevisiae: comparison with mammals. Annu. Rev. Biochem. 67, 27–48 [DOI] [PubMed] [Google Scholar]
- 2. Jenkins G. M., Hannun Y. A. (2001) Role for de novo sphingoid base biosynthesis in the heat-induced transient cell cycle arrest of Saccharomyces cerevisiae. J. Biol. Chem. 276, 8574–8581 [DOI] [PubMed] [Google Scholar]
- 3. Cowart L. A., Hannun Y. A. (2007) Selective substrate supply in the regulation of yeast de novo sphingolipid synthesis. J. Biol. Chem. 282, 12330–12340 [DOI] [PubMed] [Google Scholar]
- 4. Sawai H., Okamoto Y., Luberto C., Mao C., Bielawska A., Domae N., Hannun Y. A. (2000) Identification of ISC1 (YER019w) as inositol phosphosphingolipid phospholipase C in Saccharomyces cerevisiae. J. Biol. Chem. 275, 39793–39798 [DOI] [PubMed] [Google Scholar]
- 5. Matmati N., Hannun Y. A. (2008) Thematic review series: sphingolipids. ISC1 (inositol phosphosphingolipid-phospholipase C), the yeast homologue of neutral sphingomyelinases. J. Lipid Res. 49, 922–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clarke C. J., Snook C. F., Tani M., Matmati N., Marchesini N., Hannun Y. A. (2006) The extended family of neutral sphingomyelinases. Biochemistry 45, 11247–11256 [DOI] [PubMed] [Google Scholar]
- 7. Sawai H., Hannun Y. A. (1999) Ceramide and sphingomyelinases in the regulation of stress responses. Chem. Phys. Lipids 102, 141–147 [DOI] [PubMed] [Google Scholar]
- 8. Vaena de Avalos S., Okamoto Y., Hannun Y. A. (2004) Activation and localization of inositol phosphosphingolipid phospholipase C, Isc1p, to the mitochondria during growth of Saccharomyces cerevisiae. J. Biol. Chem. 279, 11537–11545 [DOI] [PubMed] [Google Scholar]
- 9. Kitagaki H., Cowart L. A., Matmati N., Montefusco D., Gandy J., de Avalos S. V., Novgorodov S. A., Zheng J., Obeid L. M., Hannun Y. A. (2009) ISC1-dependent metabolic adaptation reveals an indispensable role for mitochondria in induction of nuclear genes during the diauxic shift in Saccharomyces cerevisiae. J. Biol. Chem. 284, 10818–10830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Almeida T., Marques M., Mojzita D., Amorim M. A., Silva R. D., Almeida B., Rodrigues P., Ludovico P., Hohmann S., Moradas-Ferreira P., Côrte-Real M., Costa V. (2008) Isc1p plays a key role in hydrogen peroxide resistance and chronological lifespan through modulation of iron levels and apoptosis. Mol. Biol. Cell 19, 865–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cowart L. A., Hannun Y. A. (2005) Using genomic and lipidomic strategies to investigate sphingolipid function in the yeast heat-stress response. Biochem. Soc. Trans. 33, 1166–1169 [DOI] [PubMed] [Google Scholar]
- 12. Betz C., Zajonc D., Moll M., Schweizer E. (2002) ISC1-encoded inositol phosphosphingolipid phospholipase C is involved in Na+/Li+ halotolerance of Saccharomyces cerevisiae. Eur. J. Biochem. 269, 4033–4039 [DOI] [PubMed] [Google Scholar]
- 13. Matmati N., Kitagaki H., Montefusco D., Mohanty B. K., Hannun Y. A. (2009) Hydroxyurea sensitivity reveals a role for ISC1 in the regulation of G2/M. J. Biol. Chem. 284, 8241–8246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tripathi K., Matmati N., Zheng W. J., Hannun Y. A., Mohanty B. K. (2011) Cellular morphogenesis under stress is influenced by the sphingolipid pathway gene ISC1 and DNA integrity checkpoint genes in Saccharomyces cerevisiae. Genetics 189, 533–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McMillan J. N., Longtine M. S., Sia R. A., Theesfeld C. L., Bardes E. S., Pringle J. R., Lew D. J. (1999) The morphogenesis checkpoint in Saccharomyces cerevisiae: cell cycle control of Swe1p degradation by Hsl1p and Hsl7p. Mol. Cell. Biol. 19, 6929–6939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu H., Wang Y. (2006) The function and regulation of budding yeast Swe1 in response to interrupted DNA synthesis. Mol. Biol. Cell 17, 2746–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Okamoto Y., Vaena de Avalos S., Hannun Y. A. (2003) Functional analysis of ISC1 by site-directed mutagenesis. Biochemistry 42, 7855–7862 [DOI] [PubMed] [Google Scholar]
- 18. Reid R. J., Kauh E. A., Bjornsti M. A. (1997) Camptothecin sensitivity is mediated by the pleiotropic drug resistance network in yeast. J. Biol. Chem. 272, 12091–12099 [DOI] [PubMed] [Google Scholar]
- 19. Bielawski J., Pierce J. S., Snider J., Rembiesa B., Szulc Z. M., Bielawska A. (2010) Sphingolipid analysis by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). Adv. Exp. Med. Biol. 688, 46–59 [DOI] [PubMed] [Google Scholar]
- 20. Saba J. D., Nara F., Bielawska A., Garrett S., Hannun Y. A. (1997) The BST1 gene of Saccharomyces cerevisiae is the sphingosine 1-phosphate lyase. J. Biol. Chem. 272, 26087–26090 [DOI] [PubMed] [Google Scholar]
- 21. Gottlieb D., Heideman W., Saba J. D. (1999) The DPL1 gene is involved in mediating the response to nutrient deprivation in Saccharomyces cerevisiae. Mol. Cell Biol. Res. Commun. 1, 66–71 [DOI] [PubMed] [Google Scholar]
- 22. Oh C. S., Toke D. A., Mandala S., Martin C. E. (1997) ELO2 and ELO3, homologues of the Saccharomyces cerevisiae ELO1 gene, function in fatty acid elongation and are required for sphingolipid formation. J. Biol. Chem. 272, 17376–17384 [DOI] [PubMed] [Google Scholar]
- 23. Mao C., Xu R., Bielawska A., Szulc Z. M., Obeid L. M. (2000) Cloning and characterization of a Saccharomyces cerevisiae alkaline ceramidase with specificity for dihydroceramide. J. Biol. Chem. 275, 31369–31378 [DOI] [PubMed] [Google Scholar]
- 24. Hannun Y. A., Obeid L. M. (2011) Many ceramides. J. Biol. Chem. 286, 27855–27862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu H., Jin F., Liang F., Tian X., Wang Y. (2011) The Cik1/Kar3 motor complex is required for the proper kinetochore-microtubule interaction after stressful DNA replication. Genetics 187, 397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang H., Jiang W., Gentry M., Hallberg R. L. (2000) Loss of a protein phosphatase 2A regulatory subunit (Cdc55p) elicits improper regulation of Swe1p degradation. Mol. Cell. Biol. 20, 8143–8156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim S., Fyrst H., Saba J. (2000) Accumulation of phosphorylated sphingoid long chain bases results in cell growth inhibition in Saccharomyces cerevisiae. Genetics 156, 1519–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Al-Feel W., DeMar J. C., Wakil S. J. (2003) A Saccharomyces cerevisiae mutant strain defective in acetyl-CoA carboxylase arrests at the G2/M phase of the cell cycle. Proc. Natl. Acad. Sci. U.S.A. 100, 3095–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fishbein J. D., Dobrowsky R. T., Bielawska A., Garrett S., Hannun Y. A. (1993) Ceramide-mediated growth inhibition and CAPP are conserved in Saccharomyces cerevisiae. J. Biol. Chem. 268, 9255–9261 [PubMed] [Google Scholar]
- 30. Dobrowsky R. T., Kamibayashi C., Mumby M. C., Hannun Y. A. (1993) Ceramide activates heterotrimeric protein phosphatase 2A. J. Biol. Chem. 268, 15523–15530 [PubMed] [Google Scholar]
- 31. Nickels J. T., Broach J. R. (1996) A ceramide-activated protein phosphatase mediates ceramide-induced G1 arrest of Saccharomyces cerevisiae. Genes Dev. 10, 382–394 [DOI] [PubMed] [Google Scholar]
- 32. McCourt P. C., Morgan J. M., Nickels J. T., Jr. (2009) Stress-induced ceramide-activated protein phosphatase can compensate for loss of amphiphysin-like activity in Saccharomyces cerevisiae and functions to reinitiate endocytosis. J. Biol. Chem. 284, 11930–11941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wooten-Blanks L. G., Song P., Senkal C. E., Ogretmen B. (2007) Mechanisms of ceramide-mediated repression of the human telomerase reverse transcriptase promoter via deacetylation of Sp3 by histone deacetylase 1. FASEB J. 21, 3386–3397 [DOI] [PubMed] [Google Scholar]
- 34. Kroesen B. J., Jacobs S., Pettus B. J., Sietsma H., Kok J. W., Hannun Y. A., de Leij L. F. (2003) BcR-induced apoptosis involves differential regulation of C16 and C24-ceramide formation and sphingolipid-dependent activation of the proteasome. J. Biol. Chem. 278, 14723–14731 [DOI] [PubMed] [Google Scholar]
- 35. Senkal C. E., Ponnusamy S., Rossi M. J., Bialewski J., Sinha D., Jiang J. C., Jazwinski S. M., Hannun Y. A., Ogretmen B. (2007) Role of human longevity assurance gene 1 and C18-ceramide in chemotherapy-induced cell death in human head and neck squamous cell carcinomas. Mol. Cancer Ther. 6, 712–722 [DOI] [PubMed] [Google Scholar]