Background: The Hog1 stress-activated protein kinase (SAPK) regulates mRNA biogenesis in response to osmostress.
Results: The nuclear pore complex is regulated upon osmostress by the Hog1 SAPK.
Conclusion: mRNA export is controlled by the HOG signaling pathway.
Significance: Efficient mRNA biogenesis of stress-responsive genes requires of the coordination between synthesis and mRNA export.
Keywords: Gene Expression, MAP Kinases (MAPKs), p38 MAPK, Signal Transduction, Stress Response, mRNA Export, Yeast
Abstract
The control of mRNA biogenesis is exerted at several steps. In response to extracellular stimuli, stress-activated protein kinases (SAPK) modulate gene expression to maximize cell survival. In yeast, the Hog1 SAPK plays a key role in reprogramming the gene expression pattern required for cell survival upon osmostress by acting during transcriptional initiation and elongation. Here, we genetically show that an intact nuclear pore complex is important for cell survival and maximal expression of stress-responsive genes. The Hog1 SAPK associates with nuclear pore complex components and directly phosphorylates the Nup1, Nup2, and Nup60 components of the inner nuclear basket. Mutation of those factors resulted in a deficient export of stress-responsive genes upon stress. Association of Nup1, Nup2, and Nup60 to stress-responsive promoters occurs upon stress depending on Hog1 activity. Accordingly, STL1 gene territory is maintained at the nuclear periphery upon osmostress in a Hog1-dependent manner. Cells containing non-phosphorylatable mutants in Nup1 or Nup2 display reduced expression of stress-responsive genes. Together, proper mRNA biogenesis of stress-responsive genes requires of the coordinate action of synthesis and export machineries by the Hog1 SAPK.
Introduction
In response to stress, cells regulate gene expression to maximize cell survival. Cellular signaling cascades regulate specific transcriptional activities that convert extracellular information into modulation of gene expression (1, 2). Activation of the yeast HOG8 (high osmolarity glycerol) signaling pathway in response to osmostress results in a transient and acute regulation of the transcriptional program of the cell (3). The central core of the HOG pathway consists of a tier of kinases highly conserved among eukaryotic cells that results in the phosphorylation and nuclear accumulation of the Hog1 stress-activated protein kinase (SAPK) when extracellular stimuli is sensed (4–6). Transcription profiling studies together with binding analyses of Hog1-dependent transcription factors have shown that Hog1 is a major regulator of the osmostress-regulated transcription that involves a complex and highly specific network of genes (7–15).
The Hog1 SAPK regulates gene expression at multiple levels (3). The SAPK directly phosphorylates several unrelated transcription factors such as Sko1, Hot1, Smp1, or Rtg1/Rtg3 (16–21). Moreover, in response to stress, the kinase associates with several target loci through physical interaction with specific transcription factors (22, 23). Once stably associated with chromatin, Hog1 has diverse functions in the stimulation of gene expression. Recruitment of Hog1 to promoters is essential to coordinate chromatin remodeling and assembly of the transcriptional machinery upon stress (19, 23–26). Moreover, the SAPK physically interacts with elongating RNA polymerase II and cross-links to osmostress-transcribed regions allowing for efficient histone eviction during transcriptional elongation (27–29). In addition, Hog1 has also been involved in the control of mRNA stability as well as in the translation of stress-responsive genes (13, 14, 30, 31).
The nuclear pore complex (NPC) mediates nucleocytoplasmic transport of macromolecules in all eukaryotes (for review, see Refs. 32–34). NPCs have largely symmetrical, doughnut-shape structures that lie within a pore connecting the inner and outer layers of the nuclear envelope. In the yeast Saccharomyces cerevisiae the NPC is 50 MDa in size and consists of multiple copies of ∼30 different proteins called nucleoporins (Nups) positioned in a flexible structure (34–37). Not all nucleoporins are equally accessible, and they have completely different roles depending on their location at the scaffold, the nuclear basket, or the cytoplasmic filaments (for review, see Refs. 34 and 38).
Besides trafficking of macromolecules, the nuclear pore has been proposed to exert a direct role in gene expression through physical interactions of Nups with the nuclear genome; indeed, recent work in yeast has revealed that some genes can undergo dynamic recruitment to the periphery upon transcriptional activation (for review, see Refs. 39–42). Localization of individual genes within the nucleus can be dynamically controlled, and many inducible yeast genes such as the GAL genes rapidly relocalize from the nucleoplasm to the nuclear periphery after activation by their association with Nups (43–47). Other examples of nuclear pore components interacting with active genes during the initial steps of transcription are described for INO1 (48–50), RAP1/GCR1/GCR2 genes (51), or α-factor-induced genes (52). Although many studies pointed out that recruitment to the nuclear periphery appears to have a functional role in promoting transcriptional activation, gene anchoring may not be a general requirement for gene expression (53, 54). For instance, it was reported that repressive environment at the nuclear periphery establishes a negative feedback loop that enables the GAL locus to respond rapidly to changes in environmental conditions (55).
In this study we show that nuclear pore integrity is essential for maximal gene expression and adaptation to osmostress. The Hog1 SAPK interacts with and phosphorylates specific components of the NPC to facilitate mRNA export upon osmostress. Therefore, the Hog1 SAPK regulates several steps in mRNA biogenesis from initiation to mRNA export, which might define a dedicated path for optimal expression of osmostress-responsive genes.
EXPERIMENTAL PROCEDURES
Yeast Strains
S. cerevisiae strain BY4741 (MATa his3-Δ1 leu2-Δ0 met15-Δ0 ura3-Δ0) and its derivatives (nup42, nup100, nup53, nup157, seh1, nup188, nup170, nup133, nup120, nup84, nup2, nup60, pom34, pom152, nup59, nup1, and gle2) as well as TAP-tagged (Nup42, Nup100, Nup53, Nup157, Seh1, Nup188, Nup170, Nup133, Nup120, Nup84, Nup2, Nup60, Pom34, Pom152, Nup59, Nup1, Gle2, Nic96, Nup116, Nup49, Nup159, Nup57, Nup85, Nup192, Nup145, Gle1, and Nup82) and GFP-tagged (Nup1, Nup2, and Nup60) strains were obtained from the EROSCARF collection. For phospho-mass spectrometry, NUP2-TAP::HIS3 HOG1::kanMX4 was obtained. For localization studies, strains were HOT1-GFP::HIS3 with NUP2::kanMX4, NUP60 ::kanMX4, and NUP188::kanMX4 (YAG200, YAG201, and YAG202), MSN2-GFP::HIS3 with NUP2::kanMX4, NUP60::kanMX4, and NUP188::kanMX4 (YAG203, YAG204, and YAG205) and SKO1-GFP::HIS3 with NUP2::kanMX4, NUP60 ::kanMX4, and NUP188::kanMX4 (YAG206, YAG207, and YAG208). Tagging strains obtained in this study were YSR43 (NUP1-6HA::HIS3), YSR44 (NUP1-6HA::HIS3 HOG1::kanMX4), YSR218 (NUP60-6HA::HIS3), YSR221 (NUP60-HA::HIS3 HOG1::kanMX4), YSR38 (NUP2-6HA::HIS3), YSR47 (NUP2-6HA::HIS3 HOG1 ::kanMX4), and YAG209 (CSE2-6HA::KanMX4). Tagging of genomic ORFs with HA epitope was done with a PCR-based strategy. Strains used for FACS cytometry analysis were YSR237 (NUP2::kanMX4 STL1-qVenus::LEU2 pRS413), YSR238 (NUP2::kanMX4 STL1-qVenus::LEU2 pRS413-Nup2), YSR239 (NUP2::kanMX4 STL1-qVenus::LEU2 pRS413-Nup2T361A), YSR325 (NUP1::kanMX4 STL1-qVenus::LEU2 pRS413), YSR240 (NUP1::kanMX4 STL1-qVenus::LEU2 pRS413-Nup1), and YSR273 (NUP1::kanMX4 STL1-qVenus::LEU2 pRS413-Nup1S11A/T159A/S161A).
Plasmids
The plasmids used in this study were pGEX4T-Hog1 and pGEX4T-Pbs2EE (PBS2 with S514E and T518E mutations), which are described in Bilsland-Marchesan et al. (56). NUP1 (PSR19), NUP1S11AT159AS161A (PSR45), NUP2 (PSR3), NUP2T361A (PSR44), and NUP60 (PSR21) were cloned into pGEX4T. RPN2 was cloned into pGEX6P.1 (pAG36). NUP1 (pSR60), NUP1S11A/T159A/S161A (pSR77), NUP2 (pSR57), and NUP2T361A (pSR66) were cloned into pRS413. STL1-qVenus construct is described in Pelet et al. (57). pRS416 HOG-GFP is described in Ferrigno et al. (6).
Northern Blot Analysis
Yeast strains were grown to mid-log phase in rich medium at an absorbance at 660 nm of 0.6–0.9 and then subjected to osmostress (0.4 or 1 m NaCl) for the length of time indicated in Figs. 2 and 3. Total RNA and expression of specific genes were probed using radiolabeled PCR fragments containing the entire ORF of STL1 (1.7 kbp), CTT1 (1.7 kbp), GRE2 (1.0 kbp), and the non-coding exon of RDN18 (1.8 kbp). Signals were quantified with phosphorimaging (Fujifilm BAS-5000), and autoradiographs were obtained on Kodak Biomax XAR film (Sigma).
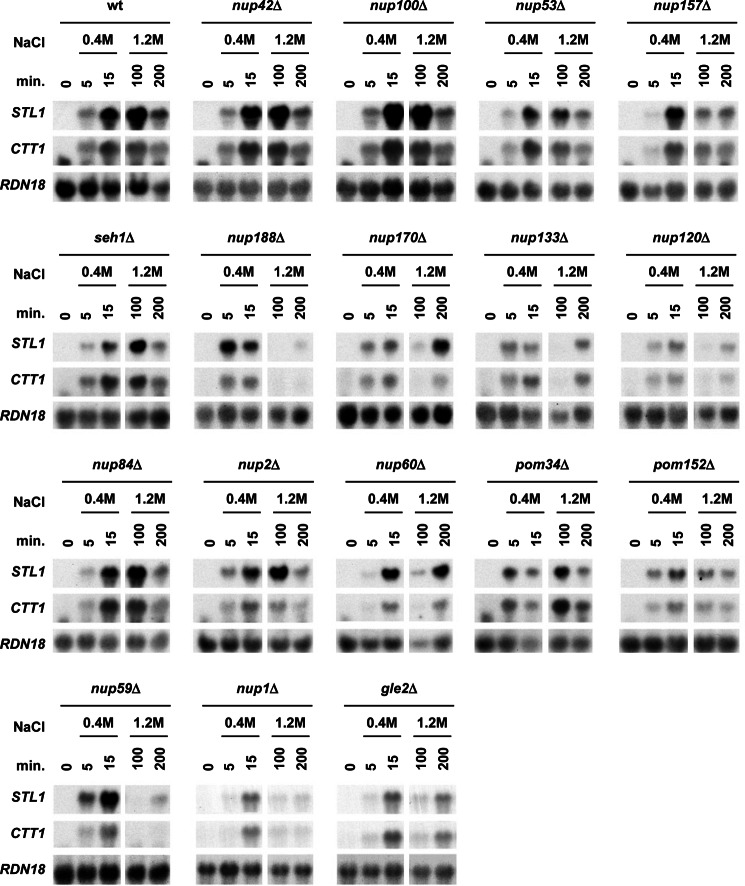
FIGURE 2.
NPC is essential for gene expression upon osmostress. WT and the indicated viable NPC mutant strains were grown to mid-log phase in rich medium and subjected to 0.4 m NaCl or 1.2 m NaCl osmostress for the indicated lengths of time. Total RNA was extracted and blotted against STL1 and CTT1 and RDN18 as the loading control.
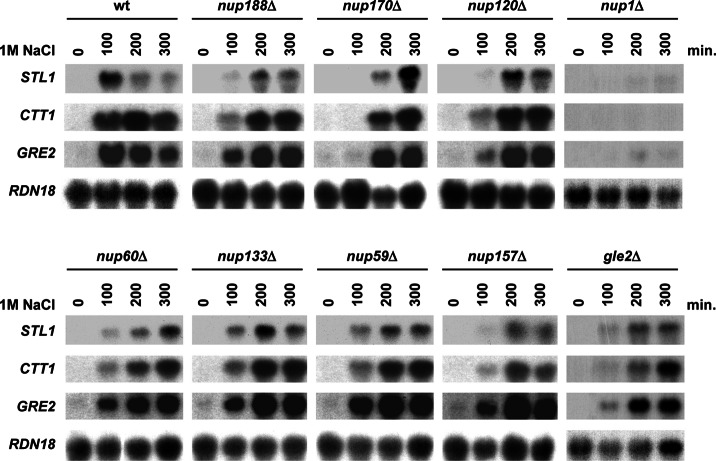
FIGURE 3.
NPC is essential for gene expression upon osmostress. WT and the indicated strains were grown to mid-log phase in rich medium and then subjected to osmostress (1 m NaCl) for the indicated length of time. Total RNA was extracted and assayed by Northern blot for transcript levels of STL1, CTT1, and GRE2 and RDN18 as the loading control.
GST Pulldown Experiments
Cells expression expressing GST or GST-Hog1 and specific TAP-tagged proteins grown in mid-log phase were treated with 0.4 m NaCl for 10 min and then collected by brief centrifugation at 4 °C. Pellets were harvested with glass beads in the FastPrep-24 (Qbiogene) in lysis buffer A (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 15 mm EDTA, 15 mm EGTA, 2 mm DTT, 0.1% Triton X-100, 1 mm PMSF, 1 mm benzamidine, 2 mg/ml leupeptin, 2 mg/ml pepstatin, 25 mm β-glycerophosphate, 1 mm sodium pyrophosphate, 10 mm sodium fluoride, 100 mm sodium orthovanadate), and lysates were clarified by centrifugation and quantified by the Bradford assay (Bio-Rad). As a control, 25 mg of whole-cell extract was blotted with anti-GST antibody to check the expression levels of the tagged proteins. Alternatively, 2 mg of cleared supernatant was incubated with 50 μl of glutathione-Sepharose beads (GE Healthcare) overnight at 4 °C. Beads were washed extensively with buffer A, resuspended in loading buffer, and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. PAP antibody (Sigma) was used to detect the TAP-tagged proteins.
Kinase Assay
The GST fusion proteins encoding NUPs, Hog1, and Pbs2EE were expressed in Escherichia coli DH5α and purified using glutathione-Sepharose beads (GE Healthcare) in STET buffer (10 mm Tris-HCl, pH 8.0, 100 mm NaCl, 1 mm EDTA, pH 8.0, 5% Triton X-100, 2 mm DTT, 1 mm phenylmethylsulfonyl fluoride, 1 mm benzamidine, 2 mg/ml leupeptin, 2 g/ml pepstatin). A 1-μg sample of Hog1 was activated with 0.5 μg of Pbs2EE in the presence of kinase buffer (50 mm Tris-HCl pH 7.5, 10 mm MgCl2, 2 mm DTT) and 50 mm ATP. After 20 min at 30 °C, eluted NUP protein was added to the previous mixture together with [γ-32P]ATP (0.1mCi/ml) and incubated for 15 min at 30 °C. The reaction was terminated by the addition of SDS-loading buffer. Labeled proteins were resolved by SDS/PAGE, stained, dried, and detected by autoradiography.
TAP Purification
27 TAP tagged strains were grown to mid-log exponential phase in 250 ml of YPD. Protein extracts were extracted in buffer A as above and incubated with Prot A-Sepharose beads (Sigma) for 2 h at 4 °C. Beads were washed four times with Buffer A and four times with kinase buffer. Kinase assay was performed with Pbs2EE-activated GST-Hog1 as above. The reaction was stopped by the addition of SDS-loading buffer. Labeled proteins were resolved by SDS/PAGE, transferred for Western blotting, and detected by autoradiography.
Phospho-mass Spectrometry
NUP2-TAP-tagged wild type and hog1Δ strains were grown in 1000 ml of YPD to A660 2. Unstressed and stressed (0.4 m NaCl, 5 min) cultures were harvested, and pellets were frozen on dry ice. The pellet was resuspended in 8 ml of lysis buffer supplied with protease and phosphatase inhibitors, and standard glass bead lysis was performed. Lysates were cleared by spinning 45 min at 4750 rpm at 4 °C. 0.5-ml IgG beads were added, and the sample was incubated for 120 min at 4 °C. IgG beads were subsequently washed 3 times in lysis buffer supplied with 0.5 m NaCl followed by 3 washes with 1× Tris/DTT. Purified TAP-tagged NUP2 was eluted with 3.5 m MgCl2 (1st elution 0.5 ml, 2nd elution 1 ml, and 3rd elution 0.5 ml). The eluate was immediately diluted by adding 3 ml Tris/DTT and concentrated in a Centricon column to 0.5 ml, rediluted with 3.5 ml Tris/DTT, and concentrated to 0.5 ml. The concentrated eluate was precipitated by adding 2 ml of ice-cold acetone, incubated overnight at −20 °C, and pelleted by 14.000 rpm centrifugation for 10 min. Pellet was air-dried and stored at −80 °C. Phosphopeptide enrichment was performed according to Zhou et al. (58). No fractionation step was included. 50–100 μg of purified protein was trypsinized and subjected to phosphopeptide enrichment. Phospho-enriched samples were analyzed in a LTQ-Orbitrap, and results were processed with MaxQuant (59). In the case of double-phosphorylated peptides, only intensities of the respective single phosphorylation events that could be unambiguously mapped were analyzed in this study.
In Situ Hybridization of Yeast Cells
Localization of STL1 transcripts was performed following the detailed protocol from the Singer laboratory (64). 5′Cy3-end-labeled 60-bp oligonucleotides were designed covering the ORF region of STL1 gene. Wild type, mex67-5, nup2Δ, and nup60Δ cells were grown to A660 0.5. To inactivate Mex67, the thermosensitive mutant mex67-5 was shifted to 37 °C for 20 min. NaCl was added to a final concentration of 0.4 m and incubated for 20 min. After salt treatment, the cultures were fixed with formaldehyde, and each strain was used to detect STL1 RNA specifically. Pictures were taken using a Leica DM6000B fluorescence microscope with a 63× PL APO objective.
Chromatin Immunoprecipitation
Chromatin immunoprecipitation was performed as described previously (25). Yeast cultures were grown to early log phase (A660 0.6–1.0) before aliquots of the culture were exposed to osmostress (0.4 or 1.2 m NaCl) for the length of time specified in legends. For cross-linking, yeast cells were treated with 1% formaldehyde for 20 min at room temperature. Antibody used in this study was monoclonal anti-HA 12CA5. Quantitative PCR analysis of stress genes and constitutively expressed genes used the following primers with locations indicated by the distance from the respective ATG initiation codon: STL1 (−372/−112 and +1000/+1280), CTT1 (−432/−302 and +736/+836), and TEL (telomeric region on the right arm of chromosome VI). Experiments were done on three independent chromatin preparations, and quantitative PCR analysis was done in real time using an Applied Biosystems 7700 sequence detector. Immunoprecipitation efficiency was calculated in triplicate by dividing the amount of PCR product in the immunoprecipitated sample by that in the TEL sequence control. The binding data are presented as -fold induction with respect to the non-treated condition.
Statistical Mapping of STL1 Gene Territory
In vivo localization of STL1 locus was performed as previously described (60). Briefly, a mCherry-Nop1 Nup49GFP TetRGFP strain was used to introduce an array of TetR DNA binding sites downstream of the STL1 locus by homologous recombination. Agar patches of rich media with or without 0.4 m NaCl were used to image cells in cover slides sealed with VaLaP (1/3 Vaseline®, 1/3 lanoline, 1/3 paraffin). Confocal microscopy was limited to 20 min after mounting and using a confocal microscope with an incubator for live cell experiments (The Cube and The Box; Life Imaging Service) at 30 °C and performed with an Andor Revolution Nipkow-disk confocal system installed on a Olympus IX-81 featuring a Yokogawa CSU22 confocal spinning disk unit and a cooled Andor EMCCD camera (DU 888). The system was controlled using the mode “Revolution FAST” of Andor Revolution IQ software (Andor Technology). Images were acquired using an Olympus 100× objective (Plan APO, 1.4 NA, oil immersion). Single laser lines used for excitation were diode-pumped solid state lasers (DPSSL) exciting GFP fluorescence at 488 nm (50 milliwatts, Coherent) and mCherry fluorescence at 561 nm (50 milliwatts, Cobolt JiveTM); a Semrock bi-bandpass emission filter (Em01-R488/568–15) allowed collection of green and red fluorescence. Pixel size was 65 nm. For three-dimensional analysis, Z-stacks of 41 images with a 250-nm Z-step were used. Exposure time was 200 ms. Image analysis was done with MATLAB by aligning nuclei using the axis between nucleus and nucleolus three-dimensional centroids. Between 2000 and 3000 cells were analyzed (wild type control, n = 3047 cells; wild type NaCl, n = 2762 cells; hog1Δ control, n = 2882 cell; hog1Δ NaCl, n = 2195). The statistical analyses (p value) presented in the figure correspond to a Kolmogorov-Smirnov test testing for equal distribution.
Flow Cytometry
Saturated overnight cultures grown in synthetic medium were diluted and grown for 24 h at log phase (below A660 0.4). Cells were stressed by mixing 200 μl of culture with 100 μl of stress solution (SD medium + NaCl). Protein translation was stopped after 45 min by the addition of cycloheximide (0.1 mg/ml). Cells were briefly sonicated, and fluorescence was measured by flow cytometry (FACSCalibur, BD Biosciences).
RESULTS
Nuclear Pore Integrity Is Essential for Growth and Gene Expression upon Osmostress
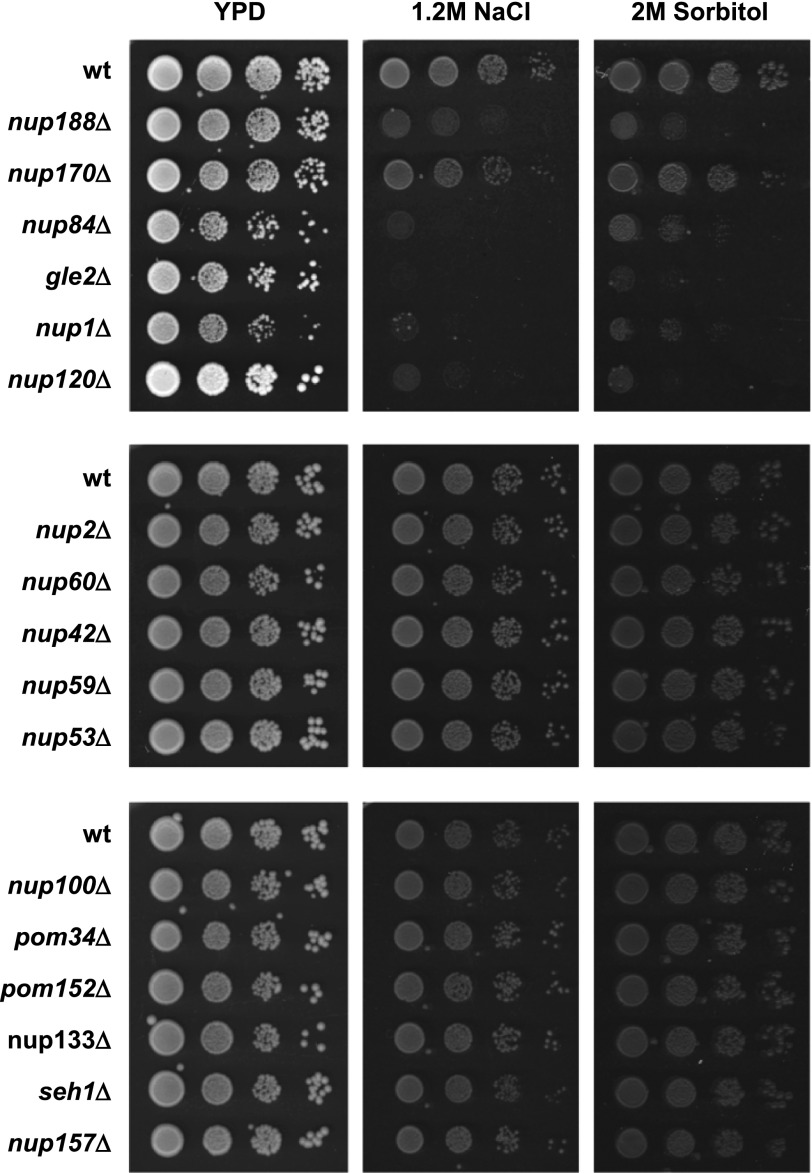
We performed an exhaustive genome-wide genetic screening searching for mutations that render cells sensitive at high osmolarity to systematically identify novel activities required for full expression of the gene program required for cell survival upon osmostress. This screen identified several transcriptional-related complexes such as the Rpd3 histone deacetylase complex, SAGA, Mediator, the Ubp3 ubiquitin protease, and components of the TEC as well as the RSC complex that are important for gene expression in response to osmostress (24–26, 61). In addition, we also found that mutations in three genes encoding components of the NPC yielded cells osmosensitive (NUP120, NUP170, and NUP188). Then, we systematically extended our screen and monitored cell growth in the presence of high osmolarity in all viable null mutant yeast strains deficient in components of the NPC (17 in total). We assessed the sensitivity of all those strains in 1.2 m NaCl and 2 m sorbitol and found that several mutants in components of the NPC complex (nup188Δ, nup170Δ, nup84Δ, gle2Δ, nup1Δ, and nup120Δ) were sensitive to osmostress (Fig. 1). The mutations included nucleoporins acting as adaptors, coat, cytoplasmic filaments, and components of the nuclear basket.
FIGURE 1.
NPC is essential for growth upon osmostress. Mutations on specific NPC components render cells osmosensitive. Wild type (wt) and the indicated mutant strains were grown to mid-log exponential phase and spotted, making serial dilutions in YPD plates without or with 1.2 m NaCl or 2 m sorbitol. Growth at 30 °C was scored after 3 days.
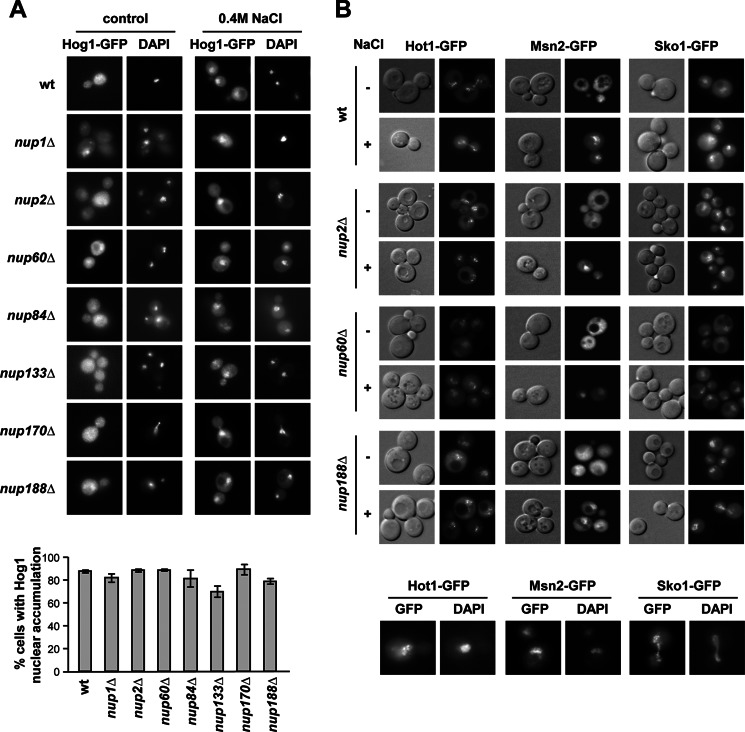
We then also assessed systematically whether mutations in components of the NPC resulted in reduced osmostress gene expression. We found that expression of osmoresponsive genes such as STL1, CTT1, and GRE2 was significantly affected or delayed under severe osmostress in nup188Δ, nup170Δ, gle2Δ, nup120Δ, nup133Δ, nup59Δ, nup157Δ, nup1Δ, and nup60Δ mutant strains (Figs. 2 and 3). Of note, localization of Hog1 upon stress was not altered in any of the mutant strains tested (Fig. 4A). Again, all those mutations included components present in diverse domains of the NPC. The expression of STL1, CTT1, and GRE2 is driven by different transcription factors (i.e. Hot1, Msn2/Msn4, and Sko1, respectively) and thus indicate a general defect on stress-responsive mRNA accumulation rather than a defect associated with a given transcription factor. Of note, localization of Sko1, Msn2, or Hot1 (fused to GFP) in all nucleoporin mutants analyzed was similar to wild type (Fig. 4B). Taken together, our data suggest that a subset of components of the NPC complex, which includes elements in different regions of the NPC, is important for maximal gene expression and cell growth in response to osmostress.
FIGURE 4.
Localization of Hog1 or transcription factors Hot1, Msn2, and Sko1 is not altered in NPC mutant strains. A, Hog1 localization was not affected in NPC mutant strains. WT and the indicated NPC mutant strains expressing Hog1-GFP were grown in SD medium to mid-log phase and subjected to osmostress (0.4 m NaCl, 5 min). DAPI staining to reveal nuclei and epifluorescence pictures were taken without (basal conditions) and with stress. The percentage of cells with Hog1 nuclear accumulation upon stress is shown in the lower panel; data are the mean and S.D. of three independent experiments. B, Hot1, Msn2, and Sko1 localization is not affected in NPC mutant strains. WT and the indicated NPC mutant strains expressing Hot1-GFP, Mns2-GFP, and Sko1-GFP were grown in SD medium to mid-log phase and subjected to osmostress (0.4 m NaCl, 5 min). Nomarsky and epifluorescence pictures were taken without (−) and with stress (+). DAPI staining to reveal nuclei in the wild type strain upon stress is shown in the lower panel.
The Hog1 SAPK Associates with the NPC Complex
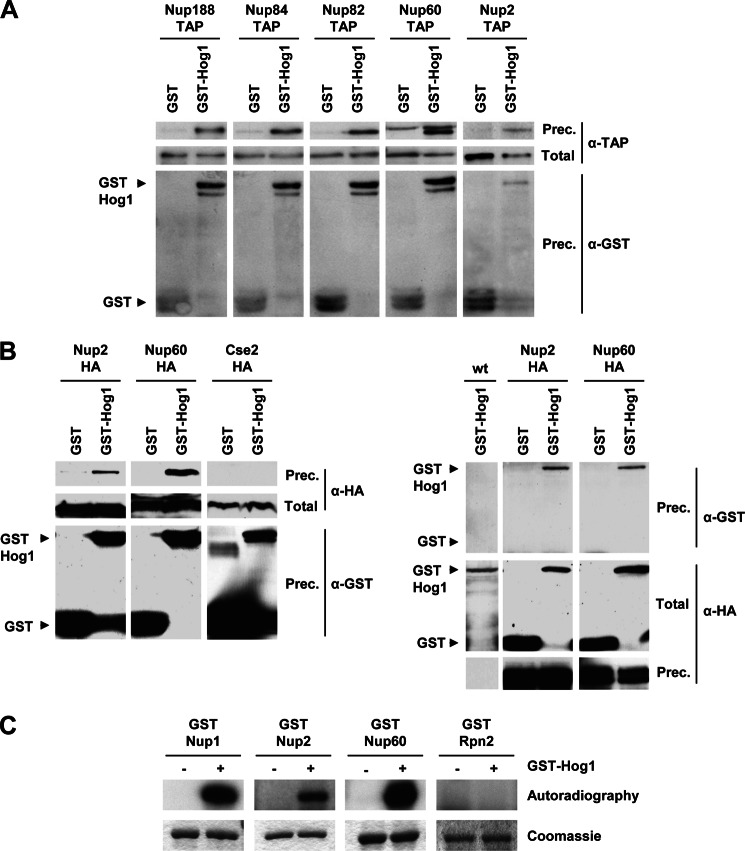
Hog1 interacts with a number of substrates to control gene expression. The possibility that Hog1 and the NPC complex physically interact was addressed by performing GST pulldown experiments in extracts from osmotically stressed cells expressing GST-Hog1 and a selected group of TAP-tagged nucleoporins (Nup2, Nup188, Nup84, Nup82, and Nup60). In all cases, GST-Hog1 but not the GST control, co-precipitated the TAP-tagged NPC components (Fig. 5A). Similar results were obtained when association of GST-Hog1 to HA-tagged Nup2 or Nup60 was assessed in reciprocal co-precipitations (Fig. 5B). Thus, the pulldown experiments indicated that Hog1 physically associates with the NPC complex, which provides biochemical evidence for the relationship between NPC and the SAPK.
FIGURE 5.
Hog1 co-precipitates with the NPC and phosphorylates some of its components. A, in vivo binding of Hog1 and the NPC is shown. TAP-tagged Nup188, Nup84, Nup82, Nup60, or Nup2 strains expressing GST or GST-Hog1 were grown to mid-log phase and subjected to brief osmotic shock (0.4 m NaCl, 10 min). GST proteins were pulled down by glutathione-Sepharose 4B, and the presence of TAP-tagged proteins was probed by immunoblotting using anti-TAP (PAP; Sigma) (upper panel). Total extract represents <20% of total input protein (middle panel). Amounts of precipitated (prec.) GST proteins were detected using anti-GST (lower panel). B, GST-Hog1 and Nup2-HA or Nup60-HA reciprocal immunoprecipitations are shown. Nup2-HA and Nup60-HA co-immunoprecipitates with GST-Hog1 in a GST pulldown assay (left panels). Cse2-HA was added as negative control. Correspondingly, Hog1 co-immunoprecipitates with Nup2-HA or Nup60-HA in a HA pulldown assay (right panels). C, in vitro phosphorylation of Nup2, Nup60, and Nup1 by Hog1 is shown. Recombinant GST-fused proteins from E. coli were phosphorylated in vitro by Hog1. A non-relevant protein (GST-tagged Rpn2) was added as a control to exclude possible nonspecific phosphorylation of Nups by Hog1. Phosphorylated proteins were resolved by SDS-PAGE and detected by autoradiography (upper panel). Total protein levels were detected by staining with Coomassie Brilliant Blue (lower panel).
Components in the Nuclear Basket of the NPC Are Phosphorylated by Hog1
Then we used an in vitro phosphorylation assay to assess whether Hog1 directly phosphorylates any component of the NPC. Initially, we purified 27 TAP-tag nucleoporins from the yeast TAP-tag collection (Open Biosystems) and subjected them to an in vitro kinase assay using E. coli-purified Hog1 that was activated in the presence of a constitutive MAPKK allele (Pbs2EE) (18). 3 of 27 of the purified TAP-nucleoporins from yeast, Nup1, Nup2, and Nup60 were phosphorylated by the Hog1 SAPK. To validate the results from the TAP assay, we fused Nup1, Nup2, and Nup60 to GST expressed and purified from E. coli. All three proteins, but not the Rpn2 negative control, were phosphorylated when they were incubated with active Hog1, indicating that these Nups are direct substrates for the Hog1 SAPK (Fig. 5C). Of note, all three target nucleoporins are components of the nuclear basket.
Export of Stress-responsive mRNAs Is Altered upon Osmostress in nup2 and nup60 Mutants
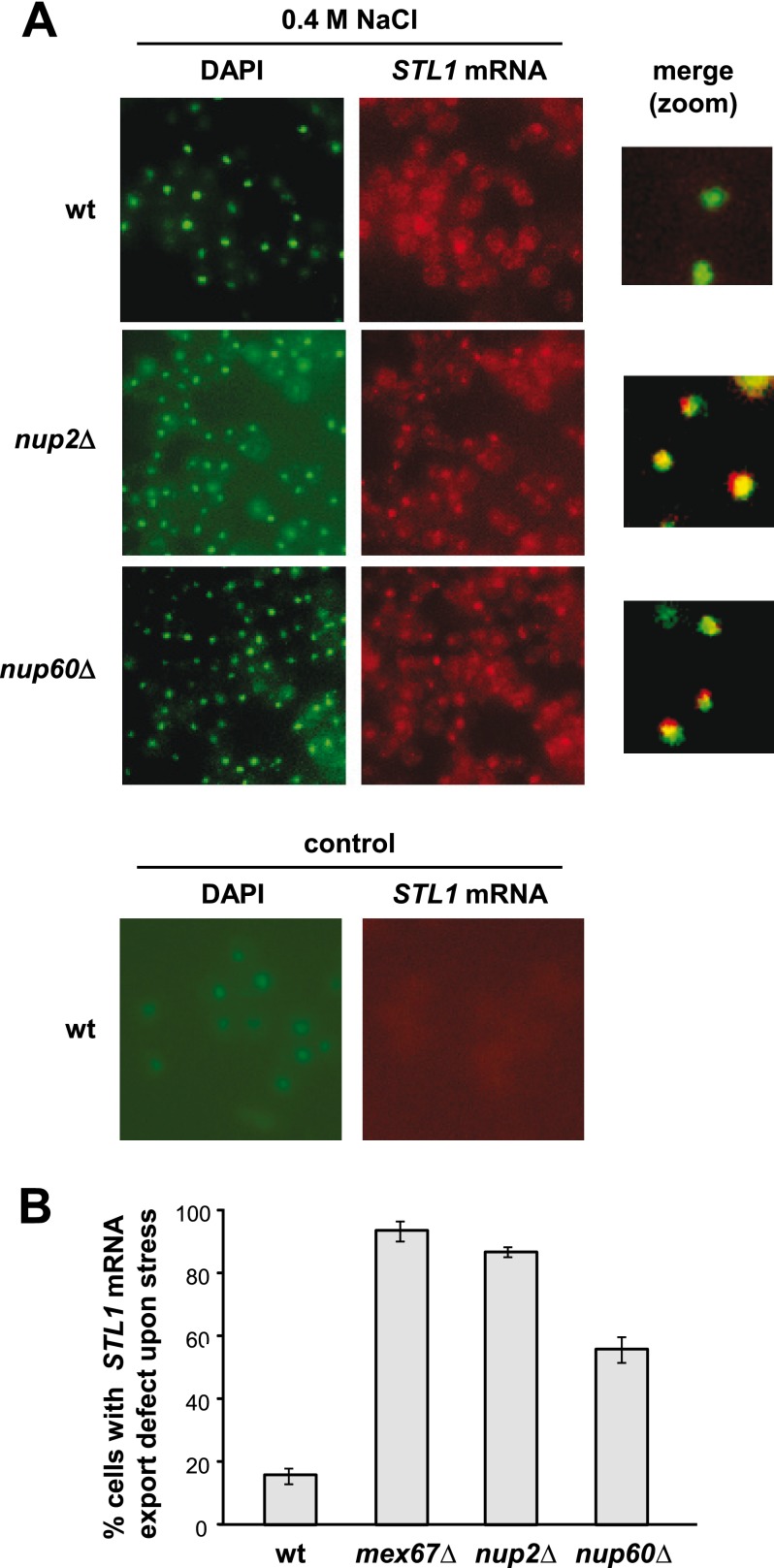
Nucleoporins present in the nuclear basket are important for mRNA export (62, 63). Whereas nup1 mutant cells show a clear defect in poly(A)+ export, mutation of nup2 and nup60 only showed a mild poly(A)+ export defect in most cells (64). Of note, a subset of cells displayed a more severe defect, suggesting that not all mRNAs were equally dependent on a specific nucleoporin for export (64). To determine whether Nup2 and Nup60 are responsible for stress-responsive mRNA export upon osmostress, in situ hybridization of specific STL1 mRNA was determined. Wild type, mex67-5, nup2Δ, and nup60Δ cells were grown in YPD and subjected to osmostress (0.4 m NaCl) for 20 min. Localization of STL1 mRNA by in situ hybridization of yeast cells was performed using a Cy3-end-labeled STL1 following standard methods (see “Experimental Procedures”). STL1 is a highly induced mRNA that is not produced in the absence of stress. As shown in Fig. 6, in contrast to wild type, export of STL1 mRNA was clearly altered in nup2 and nup60 mutant strains in response to osmostress. A temperature-sensitive allele of MEX67 (mex67-5) was used as a control of impaired total mRNA export. Thus, export of the STL1 stress-responsive mRNA is altered in nup2 and nup60 mutants.
FIGURE 6.
nup2 and nup60 mutants display defects in STL1 mRNA export upon osmostress. A, the localization of STL1 RNA upon stress (0.4 m NaCl; upper panels) and in basal conditions (lower panel) was assessed by in situ hybridization using Cy3-labbeled probes against STL1. DNA was stained with DAPI. A merged zoom image is shown in the right panels. B, the percentage of cells with impaired export of STL1 mRNA in response to osmostress is shown. A temperature-sensitive allele of MEX67 (mex67-5) was used as a positive control of impaired total mRNA export. Data are the mean and S.D. of three independent experiments.
Nuclear Basket Proteins Nup1, Nup2, and Nup60 Associate with Stress-responsive Genes
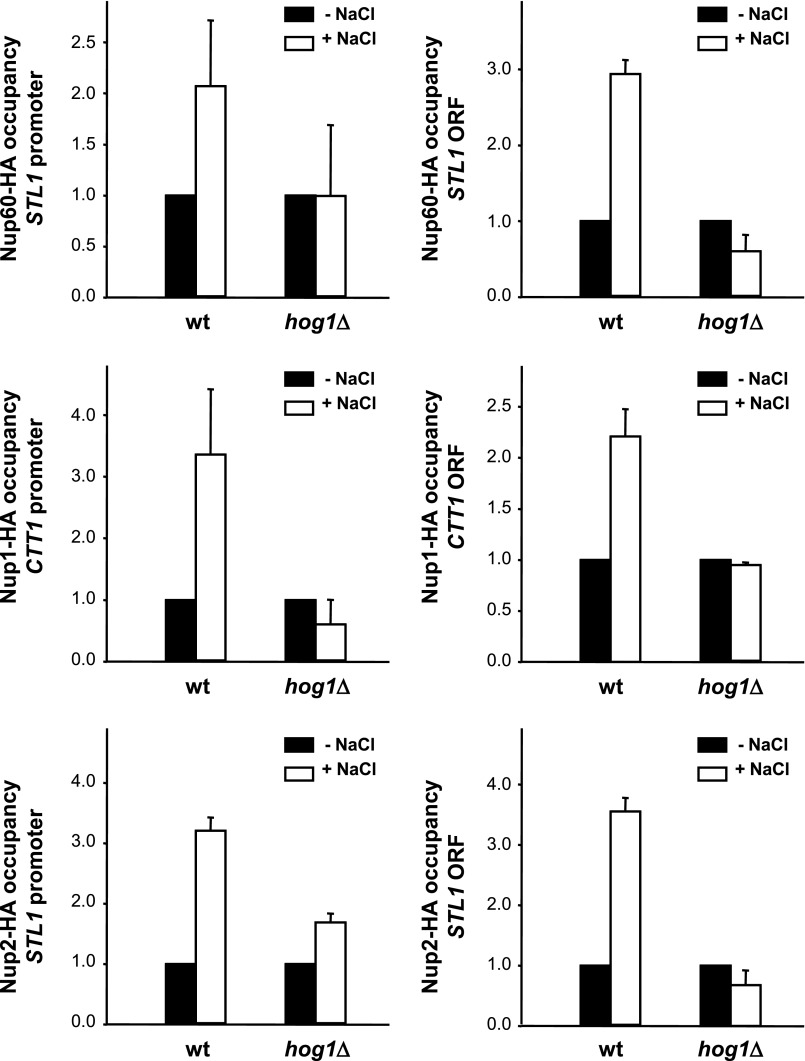
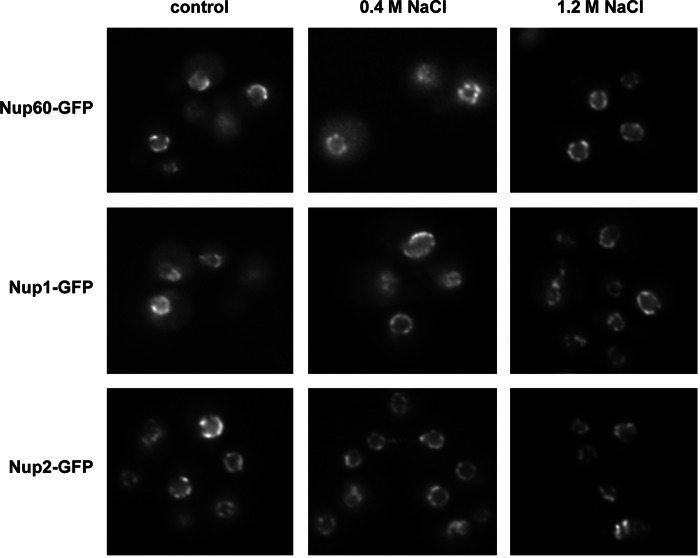
Hog1 associates with stress-responsive gene promoters and throughout the entire transcribed region of target genes in response to stress (22, 28, 29). Because we found that Hog1 interacted with the NPC, we asked whether components of the NPC were also recruited to osmoresponsive genes upon stress. We used chromatin immunoprecipitation to assess the binding of Nup60, Nup1, and Nup2 at promoters and ORFs of osmoresponsive genes (such as STL1 and CTT1) before and after the addition of NaCl. Chromatin from wild type and hog1 cells, both expressing integrated HA-tagged Nups, was immunoprecipitated and quantified by real-time PCR. As shown in Fig. 7, Nups associated specifically with osmoresponsive promoters and ORFs in response to osmostress. In contrast, no association of Nups was observed in a hog1 strain, indicating that recruitment of NPC components to chromatin in response to osmostress is dependent on Hog1. Of note, no significant changes in Nup1, Nup2, or Nup60 (fused to GFP) localization were observed upon osmostress (Fig. 8). Taken together, Hog1 is required for NPC association to stress-responsive genes upon osmostress.
FIGURE 7.
Nuclear basket proteins bind to stress-dependent genes through Hog1 upon osmostress. WT and hog1 strains expressing HA-tagged Nup60, Nup1, and Nup2 were grown to mid-log phase and subjected (open bars) to osmostress (0.4 m NaCl, 10 min) or not (filled bars). Proteins were immunoprecipitated using anti-HA monoclonal antibody, and binding to the promoter (left panels) and ORF (right panels) regions of STL1 and CTT1 loci was analyzed. The real-time PCR results are shown as -fold induction of treated relative to non-treated (time 0) samples normalized to a telomere internal control. Data are the mean and S.D. of three independent experiments. The statistical significance of the difference was assessed by a paired Student's t test of acceptance of equality at (p value < 0.05) comparing the Nup binding of a wild type versus hog1 upon stress.
FIGURE 8.
NPC localization is not affected upon osmostress. Yeast strains expressing Nup60-GFP, Nup1-GFP, or Nup2-GFP were grown in SD medium to mid-log phase and subjected to osmostress (0.4 m NaCl or 1.2 m NaCl, 5 min). Epifluorescence pictures were taken without (basal conditions) and with stress.
Hog1 Is Required to Maintain STL1 at the Nuclear Periphery
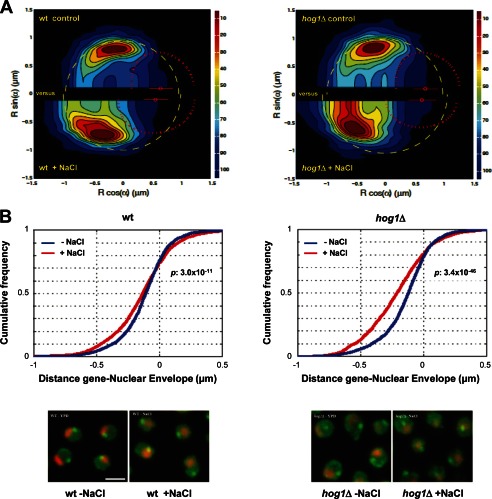
The association of NUPS at stress-loci prompted us to analyze the location of the STL1 gene within the nucleus by high resolution statistical mapping using confocal microscopy. This technique permits analysis of the spatial location of a given locus within subnuclear domains (60). We assessed STL1 location in wild type and hog1 cells before and after osmostress (0.4 m NaCl for a period of 5–20 min). Live confocal images were gathered and statistically analyzed, and radial distances of STL1 loci to nuclear envelope were calculated (see “Experimental Procedures”). The STL1 probability density map (with continuous distribution) based on the analysis is shown in Fig. 9A. STL1 gene was localized near the nuclear envelope under non-stressed growth conditions in wild type and hog1 cells, about 20 kb from the Tel4R position (65). In a wild type strain, the distance of STL1 toward the edge of nucleolus or to the nucleolar center was only affected marginally upon osmostress (the radial distance relative to the nuclear envelope increased slightly; Fig. 9B). In contrast, in a hog1 mutant strain, the relative distance of STL1 to the nuclear envelope increased markedly. These results would suggest that Hog1 is required to maintain the peripheral location of the STL1 gene in response to osmostress. Correspondingly, Hog1 is required for the association of Nups to STL1 loci upon osmostress.
FIGURE 9.
Hog1 is required to maintain STL1 at the nuclear periphery. A, shown are STL1 probability density maps based on analysis of >2,000 nuclei grown in basal (control) and 0.4 m NaCl conditions (upper and lower halves of the map) in WT and hog1 strains (right and left panels). R indicates STL1 probability density map in cylindrical coordinates. Dashed yellow circle, “median” nuclear envelope; dashed red curve, “median” nucleolus; small red circle, “median” location of nucleolar centroid; red bar, range between the percentiles 10 and 90 of the nucleolus centroid position. Scale bars, 1 μm. B, STL1 distance relative to the nuclear envelope increases in a Hog1-dependent manner is shown. Radial distance of STL1 locus to nuclear envelope (cumulative frequencies) is shown in control (blue) versus 0.4 m NaCl (red) conditions in WT and hog1 strains (right and left panels). 0 is the nuclear envelop; a negative value indicates a gene position within the nucleus. See “Experimental Procedures” for details. Illustrations of STL1 localization (green dot) images representative of the quantification are shown. Scale bars, 2 μm. Shown is peripheral localization of STL1 compared with nuclear periphery in wt-YPD, wt-NaCl, and hog1-YPD and without any preferential nucleolar localization in hog1-NaCl.
Phosphorylation of Nup1 and Nup2 by Hog1 Is Important for Maximal Stress-responsive mRNA Biogenesis
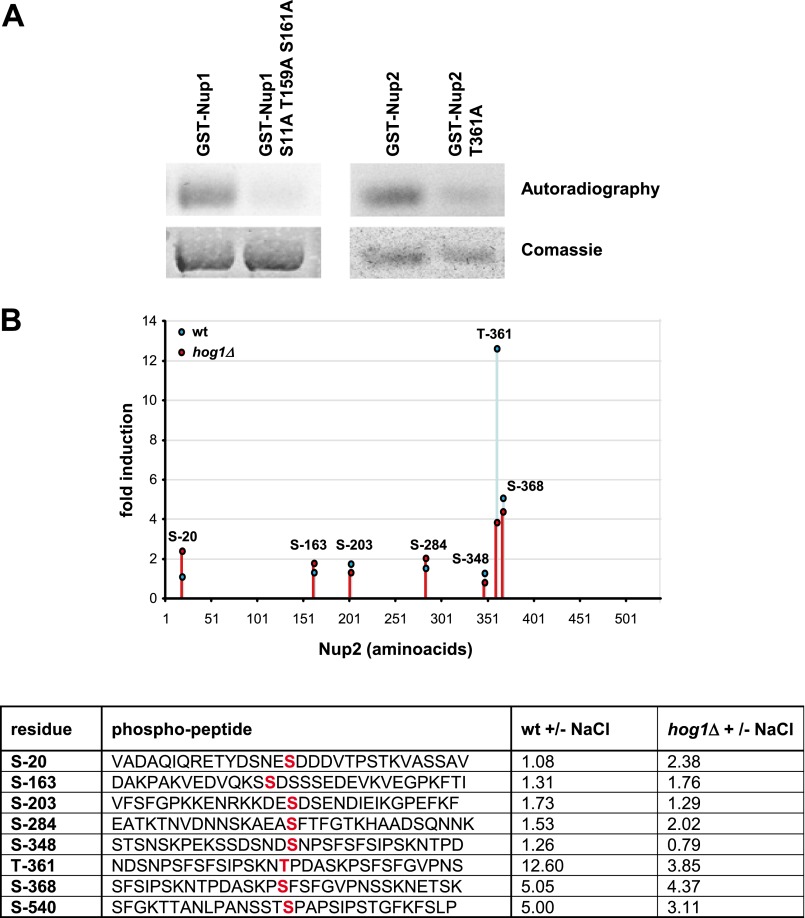
Because several basket nucleoporins were important for mRNA biogenesis and they were also phosphorylated by Hog1, we assessed the relevance of NUP phosphorylation in mRNA biogenesis and export. To identify the phosphorylation sites in the Nup1, Nup2, and Nup60 for the SAPK, several fragments of these proteins were expressed and purified from E. coli and then incubated with activate Hog1 in presence of radioactive ATP. Nup1 contained 15 putative Ser-Pro/Trp-Pro sites, whereas Nup2 and Nup60 contained 7 and 9 putative Ser-Pro/Trp-Pro sites, respectively. After systematic mutation analyses we could not significantly reduce the phosphorylation of Nup60 by Hog1 in vitro. In contrast, fragment analyses and site-directed mutagenesis of Nup1 and Nup2 permitted the creation of a triple mutant to Ala in Nup1 (nup1S11A/T159A/S161A) and a single mutant in Nup2 (nup2T361A) that displayed a strong reduction in the phosphorylation status by Hog1 (Fig. 10A). It is worth noting that despite systematic mutagenic analyses of Nup1 and Nup2, we were never able to abolish completely the phosphorylation in vitro of these proteins. This suggests that alternative putative phosphorylation sites for Hog1 (either canonical or non canonical) might exist in these proteins.
FIGURE 10.
A, Nup1 and Nup2 are phosphorylated in vitro by Hog1. Recombinant GST-fused wild type and mutant proteins were purified from E. coli, and in vitro kinase assays were performed as in Fig. 2B. Phosphorylated proteins were resolved by SDS-PAGE and detected by autoradiography (upper panel). GST-tagged Nup proteins were detected by staining with Coomassie Brilliant Blue (lower panel). B, shown is -fold change in phosphorylation upon 0.4 m NaCl treatment (5 min) at the indicated residues in Nup2 in wild type (blue) and hog1 mutant (red) strains. Ratios have been calculated from absolute phosphorylation intensities of unstressed and osmostressed wild type and hog1 mutant samples. The table shows the Nup2 residues that have been found to be phosphorylated in this study (first column), the position of the phosphorylated residue (red) in the MS-detected phosphopeptide (second column), and ratios of wild type stressed/wild type unstressed and hog1 mutant stressed/hog1 mutant unstressed (third and fourth column, respectively).
We then assessed in vivo phosphorylation of Nup1 and Nup2 by MS phospho-proteomics. Nup1 was highly problematic for technical reasons, and many of the peptides were not identified in the MS. Nevertheless, we could detect phosphorylation of the Thr-159 and Ser-161 sites in vivo. In contrast, Nup2 could be extensively analyzed by MS. Phospho-proteomic analyses showed that Nup2 was phosphorylated at least in eight different sites under non-stress conditions. Remarkably, only phosphorylation of Thr-361 was induced (>10-fold) in response to stress. This phosphorylation was abolished in a hog1 strain (Fig. 10B). Therefore, the Nup2 is phosphorylated at Thr-361 in vitro and in vivo by Hog1.
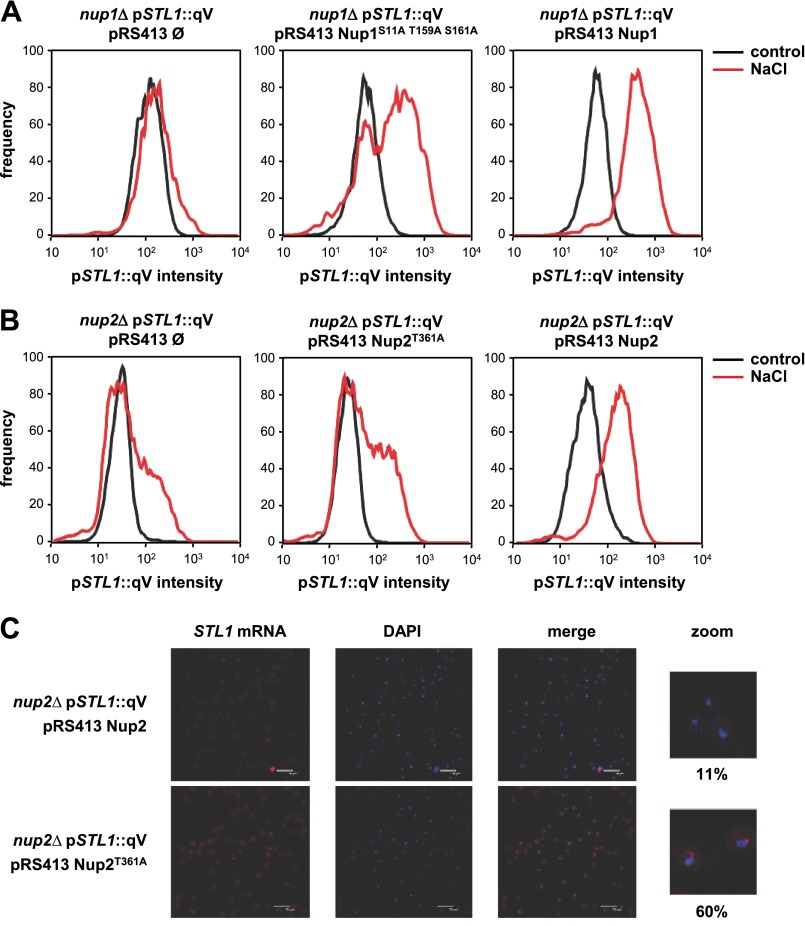
Next, we assessed the relevance of the phosphorylation of Nup1 and Nup2 mutations in gene expression. We analyzed gene expression in mutant strains carrying centromeric plasmids carrying the nup1S11A/T159A/S161A or nup2T361A mutant alleles. We took advantage of a sensitive method to quantify gene expression in single cells. Briefly, we fused the STL1 promoter to a GFPqv and followed gene expression by flow cytometry (57) in wild type or cells expressing the Nup1S11AT159AS161A and Nup2T361A mutant proteins in the absence or presence of stress. Albeit the maximal degree of expression was similar between the wild type and the Nup1S11AT159AS161A mutant, expression of STL1 was slower in the mutant when compared with wild type (Fig. 11A). In contrast, mutation of the Nup2T361A resulted in both a delay and a reduction in mRNA accumulation that was comparable with the null nup2 mutant (Fig. 11B). Therefore, mutation of the phosphorylation sites by the SAPK in Nup1 and Nup2 resulted in altered mRNA accumulation upon osmostress.
FIGURE 11.
Phosphorylation of Nup1 and Nup2 by Hog1 mediates proper stress-responsive mRNA biogenesis. nup1 (A) and nup2 (B) mutant strains harboring the quadruple-Venus (qV) fluorescence reporter driven by the STL1 promoter (pSTL1) and the indicated low copy number (pRS413) constructs were measured by flow cytometry upon osmostress (1 m NaCl, 70 min). C, shown is analysis of nuclear STL1 mRNA export in the nup2 mutant strain carrying the NUP2 wild type or the nup2T361A allele upon osmostress. The localization of STL1 RNA (left panel) was assessed by in situ hybridization using Cy3-labeled probes against STL1 in response to 0.4 m NaCl. DNA was stained with DAPI (middle panel). Merged images are shown (right panel). Scale bars, 1 μm (white line). Merged zoom images are shown with the percentage of impaired export of STL1 mRNA in response to osmostress.
Because the transcriptional defect was more exacerbated in the Nup2T361A mutant, we assessed STL1 mRNA export by in situ hybridization in nup2-deficient cells carrying a wild type allele of NUP2 or the nup2T361A mutant. Whereas the wild type strain only displayed 10% of cells with mRNA specks, >60% of the cells carrying the nup2T361A mutation presented defects in mRNA export (Fig. 11C). Overall, our results suggest that the phosphorylation of nuclear basket nucleoporins by Hog1 is important for proper mRNA export in response to osmostress.
DISCUSSION
Yeast cells respond to increases in external osmolarity by activating the stress-activated Hog1 SAPK. A major outcome of the activation of Hog1 is the regulation of gene expression, and it has been shown that Hog1 controls gene expression by acting in several steps of mRNA biogenesis (2, 3). Here, we provide evidence that mRNA export, an additional step in mRNA biogenesis, is also controlled by the SAPK.
The identification by genetic means of mutants in the NPC that were osmosensitive and displayed reduced expression of representative stress-responsive mRNAs prompted us to systematically assess which components of the NPC were important for stress adaptation and whether they were targeted by the SAPK. We found that specific mutations in nucleoporins localized in the adaptor, coat, cytoplasmic filaments, and nuclear basket resulted in defects in adaptation and mRNA accumulation upon stress. Mutations in NUPs did not lead to alteration on transcription factor localization. This suggests that integrity of the NPC is important for proper mRNA accumulation upon osmostress. Remarkably, only nuclear basket nucleoporins were directly phosphorylated by the Hog1 SAPK. Thus, this might suggest that several components of the NPC are important for proper stress-responsive mRNA accumulation/export, but only the components that remain at the nuclear side can be directly regulated by the SAPK. Correspondingly, active Hog1 accumulates into the nucleus in response to stress (6).
Nucleoporins have been involved in macromolecular trafficking, proteins, and mRNAs as well as in regulatory functions (33, 66). The phosphorylation of nucleoporins by cyclin-dependent kinases and MAPKs has been reported in yeast and mammals (67, 68). Here, we have shown that the recruitment of nucleoporins occurs at stress loci in response to Hog1 activation. In contrast, Hog1 protein transport is not altered by the deletion of those nucleoporins. Thus, the role of nucleoporin regulation upon osmostress seems to be related to mRNA export. Correspondingly, STL1 mRNA export is altered upon stress in cells deficient in nuclear basket nup2 and nup60 mutants. Furthermore, a point mutation in Nup2 (Nup2T361A) that reduces Hog1-dependent phosphorylation also results in defective STL1 export. Taken together, our data support that mRNA export is controlled by the phosphorylation of NUP components by the SAPK. The fact that null mutations of the NUPs showed a more dramatic effect on gene expression than the non-phosphorylatable mutants could be due to additional phosphorylation sites targeted by the SAPK. Alternatively, it is possible that phosphorylation of the NUPs serves to modulate the efficiency of the transport that would be completely impaired in the null mutants.
Tethering genomic loci to the nuclear envelope has been postulated as a mechanism to optimize gene expression (62, 63, 69). However, the molecular mechanism and functional significance of peripheral localization of transcription-induced genes is poorly understood and whether it is the cause or consequence of transcription activation is still unclear (48, 55, 70). However, several studies have shown a direct link between NPC and gene localization (43, 45–47, 49, 51). Moreover, peripheral localization of certain loci has been linked to NPC phosphorylation. For instance, it has been reported that phosphorylation of Nup1 by Cdk1 alters INO1 and GAL1 nuclear localization (68). Here, we have shown that STL1 is located near the nuclear envelope under normal conditions possibly due to its close proximity to the Ter4R location. However, it is only in response to stress that NUPs associate to the STL1 loci, indicating the direct tethering of the loci to NPC upon stress. In a hog1 strain, NUPs do not associate to STL1, and the STL1 locus is not retained at the nuclear periphery. Taken together, our data suggest that association of NPC to stress-responsive loci might serve to optimally couple mRNA production and export. Alternatively, the association of NUPs to promoters might be relevant to stabilize transcriptional complexes at the loci of transcribed genes. Actually, the mutation of the phosphorylation sites for Hog1 in Nup1 and Nup2 seem not only to diminish gene expression but also delays mRNA production. A similar scenario is observed when chromatin remodeling at stress-loci is altered yielding a bimodal response similar to the observed here (57). We cannot formally exclude the presence of nucleoplasmic pool of phosphorylated Nups. In Drosophila, the presence of nucleoplasmic Nups away from the NPC, in the nucleoplasm, has been reported (71). Although we cannot directly address this point with STL1 due to its telomer proximity, it is worth mentioning that upon induction of a telomer proximal gene, HXK1, the distance to periphery decreased upon NPC association (46).
In response to stress, general transcription seems to be down-regulated in contrast to an increase of stress-responsive mRNAs (13–15). Thus, the data provided here also point toward the existence of a specialized pathway that would include the control of several steps in mRNA biogenesis. This would favor the production of stress-responsive mRNAs, whereas there is a major down-regulation of mRNA synthesis of general and housekeeping genes. The Hog1 SAPK has been involved in mRNA synthesis, the control of mRNA stability (13, 30), and translation (31). The data provided here suggest that the SAPK also controls the export of stress-responsive mRNAs via the phosphorylation of NUPS at the nuclear basket. Indeed, there are many examples reporting the importance of additional levels in the regulation of gene expression after its synthesis in the nucleus (72). All together, the data support a model by which a single signaling molecule is able to exert the control of several steps in mRNA biogenesis.
Acknowledgments
We are grateful to S. Obejas and A. Fernandez for technical assistance and L. Subirana for experimental support.
This work was supported by MINECO (Spanish government) Grant BFU2012-33503, the Consolider Ingenio 2010 program (Grant CSD2007-0015), the Fundación Marcelino Botín (to F. P.), and Grant BFU2011-26722 (to E. d. N.).
- HOG
- high osmolarity glycerol
- NPC
- nuclear pore complex
- YPD
- yeast extract/peptone/dextrose
- qV
- quadruple-Venus
- TAP
- tandem affinity purification.
REFERENCES
- 1. de Nadal E., Ammerer G., Posas F. (2011) Controlling gene expression in response to stress. Nat. Rev. Genet. 12, 833–845 [DOI] [PubMed] [Google Scholar]
- 2. Weake V. M., Workman J. L. (2010) Inducible gene expression. Diverse regulatory mechanisms. Nat. Rev. Genet. 11, 426–437 [DOI] [PubMed] [Google Scholar]
- 3. de Nadal E., Posas F. (2010) Multilayered control of gene expression by stress-activated protein kinases. EMBO J. 29, 4–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Macia J., Regot S., Peeters T., Conde N., Solé R., Posas F. (2009) Dynamic signaling in the Hog1 MAPK pathway relies on high basal signal transduction. Sci. Signal. 2, ra13. [DOI] [PubMed] [Google Scholar]
- 5. Saito H., Posas F. (2012) Response to hyperosmotic stress. Genetics 192, 289–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferrigno P., Posas F., Koepp D., Saito H., Silver P. A. (1998) Regulated nucleo/cytoplasmic exchange of HOG1 MAPK requires the importin β homologs NMD5 and XPO1. EMBO J. 17, 5606–5614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gasch A. P., Spellman P. T., Kao C. M., Carmel-Harel O., Eisen M. B., Storz G., Botstein D., Brown P. O. (2000) Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11, 4241–4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Posas F., Chambers J. R., Heyman J. A., Hoeffler J. P., de Nadal E., Ariño J. (2000) The transcriptional response of yeast to saline stress. J. Biol. Chem. 275, 17249–17255 [DOI] [PubMed] [Google Scholar]
- 9. Rep M., Krantz M., Thevelein J. M., Hohmann S. (2000) The transcriptional response of Saccharomyces cerevisiae to osmotic shock. Hot1p and Msn2p/Msn4p are required for the induction of subsets of high osmolarity glycerol pathway-dependent genes. J. Biol. Chem. 275, 8290–8300 [DOI] [PubMed] [Google Scholar]
- 10. Yale J., Bohnert H. J. (2001) Transcript expression in Saccharomyces cerevisiae at high salinity. J. Biol. Chem. 276, 15996–16007 [DOI] [PubMed] [Google Scholar]
- 11. Ni L., Bruce C., Hart C., Leigh-Bell J., Gelperin D., Umansky L., Gerstein M. B., Snyder M. (2009) Dynamic and complex transcription factor binding during an inducible response in yeast. Genes Dev. 23, 1351–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Capaldi A. P., Kaplan T., Liu Y., Habib N., Regev A., Friedman N., O'Shea E. K. (2008) Structure and function of a transcriptional network activated by the MAPK Hog1. Nat. Genet. 40, 1300–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miller C., Schwalb B., Maier K., Schulz D., Dümcke S., Zacher B., Mayer A., Sydow J., Marcinowski L., Dölken L., Martin D. E., Tresch A., Cramer P. (2011) Dynamic transcriptome analysis measures rates of mRNA synthesis and decay in yeast. Mol. Syst. Biol. 7, 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nadal-Ribelles M., Conde N., Flores O., González-Vallinas J., Eyras E., Orozco M., de Nadal E., Posas F. (2012) Hog1 bypasses stress-mediated down-regulation of transcription by RNA polymerase II redistribution and chromatin remodeling. Genome Biol. 13, R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cook K. E., O'Shea E. K. (2012) Hog1 controls global reallocation of RNA Pol II upon osmotic shock in Saccharomyces cerevisiae. G3 2, 1129–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Proft M., Serrano R. (1999) Repressors and upstream repressing sequences of the stress-regulated ENA1 gene in Saccharomyces cerevisiae. bZIP protein Sko1p confers HOG-dependent osmotic regulation. Mol. Cell. Biol. 19, 537–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rep M., Reiser V., Gartner U., Thevelein J. M., Hohmann S., Ammerer G., Ruis H. (1999) Osmotic stress-induced gene expression in Saccharomyces cerevisiae requires Msn1p and the novel nuclear factor Hot1p. Mol. Cell. Biol. 19, 5474–5485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Proft M., Pascual-Ahuir A., de Nadal E., Ariño J., Serrano R., Posas F. (2001) Regulation of the Sko1 transcriptional repressor by the Hog1 MAP kinase in response to osmotic stress. EMBO J. 20, 1123–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alepuz P. M., de Nadal E., Zapater M., Ammerer G., Posas F. (2003) Osmostress-induced transcription by Hot1 depends on a Hog1-mediated recruitment of the RNA Pol II. EMBO J. 22, 2433–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Nadal E., Casadomé L., Posas F. (2003) Targeting the MEF2-like transcription factor Smp1 by the stress-activated Hog1 mitogen-activated protein kinase. Mol. Cell. Biol. 23, 229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruiz-Roig C., Noriega N., Duch A., Posas F., de Nadal E. (2012) The Hog1 SAPK controls the Rtg1/Rtg3 transcriptional complex activity by multiple regulatory mechanisms. Mol. Biol. Cell 23, 4286–4296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alepuz P. M., Jovanovic A., Reiser V., Ammerer G. (2001) Stress-induced map kinase Hog1 is part of transcription activation complexes. Mol. Cell 7, 767–777 [DOI] [PubMed] [Google Scholar]
- 23. Proft M., Struhl K. (2002) Hog1 kinase converts the Sko1-Cyc8-Tup1 repressor complex into an activator that recruits SAGA and SWI/SNF in response to osmotic stress. Mol. Cell 9, 1307–1317 [DOI] [PubMed] [Google Scholar]
- 24. De Nadal E., Zapater M., Alepuz P. M., Sumoy L., Mas G., Posas F. (2004) The MAPK Hog1 recruits Rpd3 histone deacetylase to activate osmoresponsive genes. Nature 427, 370–374 [DOI] [PubMed] [Google Scholar]
- 25. Zapater M., Sohrmann M., Peter M., Posas F., de Nadal E. (2007) Selective requirement for SAGA in Hog1-mediated gene expression depending on the severity of the external osmostress conditions. Mol. Cell. Biol. 27, 3900–3910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Solé C., Nadal-Ribelles M., Kraft C., Peter M., Posas F., de Nadal E. (2011) Control of Ubp3 ubiquitin protease activity by the Hog1 SAPK modulates transcription upon osmostress. EMBO J. 30, 3274–3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pascual-Ahuir A., Struhl K., Proft M. (2006) Genome-wide location analysis of the stress-activated MAP kinase Hog1 in yeast. Methods 40, 272–278 [DOI] [PubMed] [Google Scholar]
- 28. Pokholok D. K., Zeitlinger J., Hannett N. M., Reynolds D. B., Young R. A. (2006) Activated signal transduction kinases frequently occupy target genes. Science 313, 533–536 [DOI] [PubMed] [Google Scholar]
- 29. Proft M., Mas G., de Nadal E., Vendrell A., Noriega N., Struhl K., Posas F. (2006) The stress-activated Hog1 kinase is a selective transcriptional elongation factor for genes responding to osmotic stress. Mol. Cell 23, 241–250 [DOI] [PubMed] [Google Scholar]
- 30. Romero-Santacreu L., Moreno J., Pérez-Ortín J. E., Alepuz P. (2009) Specific and global regulation of mRNA stability during osmotic stress in Saccharomyces cerevisiae. RNA 15, 1110–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Warringer J., Hult M., Regot S., Posas F., Sunnerhagen P. (2010) The HOG pathway dictates the short-term translational response after hyperosmotic shock. Mol. Biol. Cell 21, 3080–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tran E. J., Wente S. R. (2006) Dynamic nuclear pore complexes. Life on the edge. Cell 125, 1041–1053 [DOI] [PubMed] [Google Scholar]
- 33. Lim R. Y., Ullman K. S., Fahrenkrog B. (2008) Biology and biophysics of the nuclear pore complex and its components. Int. Rev. Cell Mol. Biol. 267, 299–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hoelz A., Debler E. W., Blobel G. (2011) The structure of the nuclear pore complex. Annu. Rev. Biochem. 80, 613–643 [DOI] [PubMed] [Google Scholar]
- 35. Rout M. P., Aitchison J. D., Suprapto A., Hjertaas K., Zhao Y., Chait B. T. (2000) The yeast nuclear pore complex. composition, architecture, and transport mechanism. J. Cell Biol. 148, 635–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kampmann M., Blobel G. (2009) Three-dimensional structure and flexibility of a membrane-coating module of the nuclear pore complex. Nat. Struct. Mol. Biol. 16, 782–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aitchison J. D., Rout M. P. (2012) The yeast nuclear pore complex and transport through it. Genetics 190, 855–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. D'Angelo M. A., Hetzer M. W. (2008) Structure, dynamics, and function of nuclear pore complexes. Trends Cell Biol. 18, 456–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brown C. R., Silver P. A. (2007) Transcriptional regulation at the nuclear pore complex. Curr. Opin. Genet. Dev. 17, 100–106 [DOI] [PubMed] [Google Scholar]
- 40. Taddei A. (2007) Active genes at the nuclear pore complex. Curr. Opin. Cell Biol. 19, 305–310 [DOI] [PubMed] [Google Scholar]
- 41. Ahmed S., Brickner J. H. (2007) Regulation and epigenetic control of transcription at the nuclear periphery. Trends Genet. 23, 396–402 [DOI] [PubMed] [Google Scholar]
- 42. Akhtar A., Gasser S. M. (2007) The nuclear envelope and transcriptional control. Nat. Rev. Genet. 8, 507–517 [DOI] [PubMed] [Google Scholar]
- 43. Casolari J. M., Brown C. R., Komili S., West J., Hieronymus H., Silver P. A. (2004) Genome-wide localization of the nuclear transport machinery couples transcriptional status and nuclear organization. Cell 117, 427–439 [DOI] [PubMed] [Google Scholar]
- 44. Cabal G. G., Genovesio A., Rodriguez-Navarro S., Zimmer C., Gadal O., Lesne A., Buc H., Feuerbach-Fournier F., Olivo-Marin J. C., Hurt E. C., Nehrbass U. (2006) SAGA interacting factors confine sub-diffusion of transcribed genes to the nuclear envelope. Nature 441, 770–773 [DOI] [PubMed] [Google Scholar]
- 45. Schmid M., Arib G., Laemmli C., Nishikawa J., Durussel T., Laemmli U. K. (2006) Nup-PI. The nucleopore-promoter interaction of genes in yeast. Mol. Cell 21, 379–391 [DOI] [PubMed] [Google Scholar]
- 46. Taddei A., Van Houwe G., Hediger F., Kalck V., Cubizolles F., Schober H., Gasser S. M. (2006) Nuclear pore association confers optimal expression levels for an inducible yeast gene. Nature 441, 774–778 [DOI] [PubMed] [Google Scholar]
- 47. Luthra R., Kerr S. C., Harreman M. T., Apponi L. H., Fasken M. B., Ramineni S., Chaurasia S., Valentini S. R., Corbett A. H. (2007) Actively transcribed GAL genes can be physically linked to the nuclear pore by the SAGA chromatin modifying complex. J. Biol. Chem. 282, 3042–3049 [DOI] [PubMed] [Google Scholar]
- 48. Brickner D. G., Cajigas I., Fondufe-Mittendorf Y., Ahmed S., Lee P. C., Widom J., Brickner J. H. (2007) H2A.Z-mediated localization of genes at the nuclear periphery confers epigenetic memory of previous transcriptional state. PLoS Biol. 5, e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brickner J. H., Walter P. (2004) Gene recruitment of the activated INO1 locus to the nuclear membrane. PLoS. Biol. 2, e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ahmed S., Brickner D. G., Light W. H., Cajigas I., McDonough M., Froyshteter A. B., Volpe T., Brickner J. H. (2010) DNA zip codes control an ancient mechanism for gene targeting to the nuclear periphery. Nat. Cell Biol. 12, 111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Menon B. B., Sarma N. J., Pasula S., Deminoff S. J., Willis K. A., Barbara K. E., Andrews B., Santangelo G. M. (2005) Reverse recruitment. The Nup84 nuclear pore subcomplex mediates Rap1/Gcr1/Gcr2 transcriptional activation. Proc. Natl. Acad. Sci. U.S.A. 102, 5749–5754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Casolari J. M., Brown C. R., Drubin D. A., Rando O. J., Silver P. A. (2005) Developmentally induced changes in transcriptional program alter spatial organization across chromosomes. Genes Dev. 19, 1188–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dieppois G., Iglesias N., Stutz F. (2006) Cotranscriptional recruitment to the mRNA export receptor Mex67p contributes to nuclear pore anchoring of activated genes. Mol. Cell. Biol. 26, 7858–7870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Abruzzi K. C., Belostotsky D. A., Chekanova J. A., Dower K., Rosbash M. (2006) 3′-End formation signals modulate the association of genes with the nuclear periphery as well as mRNP dot formation. EMBO J. 25, 4253–4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Green E. M., Jiang Y., Joyner R., Weis K. (2012) A negative feedback loop at the nuclear periphery regulates GAL gene expression. Mol. Biol. Cell 23, 1367–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bilsland-Marchesan E., Ariño J., Saito H., Sunnerhagen P., Posas F. (2000) Rck2 kinase is a substrate for the osmotic stress-activated mitogen-activated protein kinase Hog1. Mol. Cell. Biol. 20, 3887–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pelet S., Rudolf F., Nadal-Ribelles M., de Nadal E., Posas F., Peter M. (2011) Transient activation of the HOG MAPK pathway regulates bimodal gene expression. Science 332, 732–735 [DOI] [PubMed] [Google Scholar]
- 58. Zhou H., Ye M., Dong J., Corradini E., Cristobal A., Heck A. J., Zou H., Mohammed S. (2013) Robust phosphoproteome enrichment using monodisperse microsphere-based immobilized titanium (IV) ion affinity chromatography. Nat. Protoc. 8, 461–480 [DOI] [PubMed] [Google Scholar]
- 59. Cox J., Mann M. (2008) MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 [DOI] [PubMed] [Google Scholar]
- 60. Berger A. B., Cabal G. G., Fabre E., Duong T., Buc H., Nehrbass U., Olivo-Marin J. C., Gadal O., Zimmer C. (2008) High-resolution statistical mapping reveals gene territories in live yeast. Nat. Methods 5, 1031–1037 [DOI] [PubMed] [Google Scholar]
- 61. Mas G., de Nadal E., Dechant R., Rodríguez de la Concepción ML, Logie C., Jimeno-González S., Chávez S., Ammerer G., Posas F. (2009) Recruitment of a chromatin remodelling complex by the Hog1 MAP kinase to stress genes. EMBO J. 28, 326–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Liang Y., Hetzer M. W. (2011) Functional interactions between nucleoporins and chromatin. Curr. Opin. Cell Biol. 23, 65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mekhail K., Moazed D. (2010) The nuclear envelope in genome organization, expression, and stability. Nat. Rev. Mol. Cell Biol. 11, 317–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Powrie E. A., Zenklusen D., Singer R. H. (2011) A nucleoporin, Nup60p, affects the nuclear and cytoplasmic localization of ASH1 mRNA in S. cerevisiae. RNA 17, 134–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Therizols P., Duong T., Dujon B., Zimmer C., Fabre E. (2010) Chromosome arm length and nuclear constraints determine the dynamic relationship of yeast subtelomeres. Proc. Natl. Acad. Sci. U.S.A. 107, 2025–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Köhler A., Hurt E. (2010) Gene regulation by nucleoporins and links to cancer. Mol. Cell 38, 6–15 [DOI] [PubMed] [Google Scholar]
- 67. Kosako H., Yamaguchi N., Aranami C., Ushiyama M., Kose S., Imamoto N., Taniguchi H., Nishida E., Hattori S. (2009) Phosphoproteomics reveals new ERK MAP kinase targets and links ERK to nucleoporin-mediated nuclear transport. Nat. Struct. Mol. Biol. 16, 1026–1035 [DOI] [PubMed] [Google Scholar]
- 68. Brickner D. G., Brickner J. H. (2010) Cdk phosphorylation of a nucleoporin controls localization of active genes through the cell cycle. Mol. Biol. Cell 21, 3421–3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Capelson M., Hetzer M. W. (2009) The role of nuclear pores in gene regulation, development and disease. EMBO Rep. 10, 697–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Light W. H., Brickner D. G., Brand V. R., Brickner J. H. (2010) Interaction of a DNA zip code with the nuclear pore complex promotes H2A.Z incorporation and INO1 transcriptional memory. Mol. Cell 40, 112–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kalverda B., Pickersgill H., Shloma V. V., Fornerod M. (2010) Nucleoporins directly stimulate expression of developmental and cell-cycle genes inside the nucleoplasm. Cell 140, 360–371 [DOI] [PubMed] [Google Scholar]
- 72. Choder M. (2011) mRNA imprinting. Additional level in the regulation of gene expression. Cell Logist. 1, 37–40 [DOI] [PMC free article] [PubMed] [Google Scholar]