Background: mGluR7 is a presynaptic autoreceptor in the CNS, which is regulated by receptor phosphorylation.
Results: Protein phosphatase 1 (PP1) binds to mGluR7, promotes Ser-862 dephosphorylation, and increases receptor surface expression.
Conclusion: PP1 regulates mGluR7 trafficking and function.
Significance: Because mGluR7 is associated with memory function and neuropsychiatric disorders, understanding underlying regulatory mechanisms is important.
Keywords: G Protein Coupled Receptors (GPCR), Glutamate Receptor Metabotropic, Protein Phosphorylation, Receptor Endocytosis, Serine Threonine Protein Phosphatase, Synapses
Abstract
The metabotropic glutamate receptor type 7 (mGluR7) is the predominant group III mGluR in the presynaptic active zone, where it serves as an autoreceptor to inhibit neurotransmitter release. Our previous studies show that PKC phosphorylation of mGluR7 on Ser-862 is a key mechanism controlling constitutive and activity-dependent surface expression of mGluR7 by regulating a competitive interaction of calmodulin and protein interacting with C kinase (PICK1). As receptor phosphorylation and dephosphorylation are tightly coordinated through the precise action of protein kinases and phosphatases, dephosphorylation by phosphatases is likely to play an active role in governing the activity-dependent or agonist-induced changes in mGluR7 receptor surface expression. In the present study, we find that the serine/threonine protein phosphatase 1 (PP1) has a crucial role in the constitutive and agonist-induced dephosphorylation of Ser-862 on mGluR7. Treatment of neurons with PP1 inhibitors leads to a robust increase in Ser-862 phosphorylation and increased surface expression of mGluR7. In addition, Ser-862 phosphorylation of both mGluR7a and mGluR7b is a target of PP1. Interestingly, agonist-induced dephosphorylation of mGluR7 is regulated by PP1, whereas NMDA-mediated activity-induced dephosphorylation is not, illustrating there are multiple signaling pathways that affect receptor phosphorylation and trafficking. Importantly, PP1γ1 regulates agonist-dependent Ser-862 dephosphorylation and surface expression of mGluR7.
Introduction
The metabotropic glutamate receptors (mGluRs)3 are seven-transmembrane domain receptors that are linked via G proteins to intracellular signaling cascades. Eight different family members are subdivided into three groups based on sequence homology, pharmacological properties, and second messenger coupling (1). Among the mGluR family, mGluR7 belongs to the group III mGluRs, which are coupled to inhibitory G proteins. Group III mGluRs are often presynaptic (2, 3), and mGluR7 is highly expressed at the presynaptic active zone throughout the CNS and acts as an auto-regulatory receptor. Because of its low affinity for glutamate, mGluR7 has been proposed to be only activated under conditions of sustained synaptic activity and play a role in feedback inhibition to limit further release of glutamate at excitatory synapses (4, 5).
Like many GPCRs, mGluR7 undergoes constitutive and agonist-dependent endocytosis. The agonist-induced rapid endocytosis of mGluR7 underlies a presynaptic form of long term depression and a rapid switch to long term potentiation at naïve mossy fiber-interneuron synapses (6, 7). The tight regulation of mGluR7 endocytosis is thought to be regulated by several molecules interacting with the region surrounding amino acid Ser-862 as well as the PDZ binding domain of the short mGluR7 cytoplasmic tail (8, 9). Previously, we showed that both phosphorylation of Ser-862 and binding of PICK1 to the PDZ ligand located in the mGluR7 cytoplasmic tail are critical for regulating constitutive and agonist-induced mGluR7 trafficking. PICK1 recruits PKCα and facilitates PKCα-induced mGluR7 Ser-862 phosphorylation, which promotes stabilization of mGluR7 surface expression. By contrast, dephosphorylation destabilizes surface expression and induces internalization of mGluR7. In addition, phosphorylation of Ser-862 disrupts calmodulin binding, which normally competes with PICK1-PKCα for binding to mGluR7. The dephosphorylation triggered by neuronal activation or agonist treatment induces internalization of mGluR7. Thus, an interplay between PKC phosphorylation and CaM/PICK1 binding regulates receptor trafficking (10).
Receptor phosphorylation and dephosphorylation are tightly regulated processes mediated by the precise action of protein kinases and phosphatases. Similar to protein kinases, protein phosphatases actively participate in regulating synaptic changes and the activity-dependent alterations of synaptic transmission (11, 12). It is clear that PKC dramatically regulates mGluR7 trafficking, but the phosphatase(s) that target mGluR7 remain unknown. Therefore, we have focused on the role of serine/threonine protein phosphatases in the regulation of Ser-862 phosphorylation of mGluR7 and subsequent surface expression of mGluR7 at synapses. In this study, we show that the serine/threonine protein phosphatase 1 (PP1) has a crucial role in the constitutive and agonist-induced dephosphorylation of Ser-862 on mGluR7. Treatment of primary neurons with okadaic acid or calyculin A led to a dramatic increase of Ser-862 phosphorylation, indicating that PP1 has a specific effect on dephosphorylation of mGluR7. Treatment of mGluR7 with an agonist, l-AP4 or NMDA, induced a profound decrease in Ser-862 phosphorylation of mGluR7. Agonist-induced dephosphorylation of mGluR7 was blocked by okadaic acid, whereas NMDA-mediated activity-induced dephosphorylation was not, suggesting a specific role of PP1 in agonist-induced acute changes in mGluR7 surface expression. Interestingly, Ser-862 phosphorylation of both mGluR7a and mGluR7b was markedly enhanced by the inhibition of PP1. In addition, surface expression of mGluR7 was enhanced and internalization of mGluR7 was inhibited by okadaic acid or co-expression of a dominant negative form of PP1γ1. Taken together, these data show that PP1 has a dynamic role in the regulation of Ser-862 phosphorylation and surface expression of mGluR7.
EXPERIMENTAL PROCEDURES
Cells and Antibodies
HEK cells were maintained in DMEM containing 10% fetal bovine serum and 1% l-glutamine. Primary hippocampal or cortical neurons were harvested from embryonic day 18 Sprague-Dawley rat embryos and maintained in Neurobasal medium (Invitrogen) with l-glutamine and B-27 supplement (Invitrogen). The rabbit mGluR7 Ser-862 phosphorylation-specific antibody was described previously (10). An mGluR7b-specific antibody was generated by immunizing rabbits with a synthetic peptide Ac-CIPPVRKSVQKSVTWYTIPPTV-OH corresponding to amino acids 901–922 of the mGluR7b C terminus as described previously (3, 13). The following antibodies are purchased from commercial sources: mGluR7a, PP1γ1 catalytic subunit, PP2A catalytic subunit, and calcineurin antibody (Millipore); phospho-p70S6K (Cell Signaling Technology); nuclear factor of activated T cells (NFAT) c1 (Thermo Scientific); c-Myc (9E10), rabbit anti-FLAG, and α-tubulin antibody (Sigma).
Western Blotting and Immunoprecipitation
Rat brain or cells were solubilized in lysis buffer containing 50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 2 mm EDTA, 1% Triton X-100, and protease inhibitor mixture (Roche Applied Science) for 30 min on ice. The lysates were cleared by centrifugation at 20,000 × g for 15 min at 4 °C. The supernatants were mixed with 6× Laemmli buffer, resolved by SDS-PAGE, transferred to PVDF membrane, and analyzed by immunoblotting using the indicated antibodies. For immunoprecipitation, precleared supernatants were incubated with antibody-bound protein A or G beads (Sigma) for 4 h at 4 °C and washed four times with lysis buffer. Immunoprecipitates were subjected to Western blotting.
Surface Receptor Biotinylation Assay
Cell surface biotinylation was performed as described previously (14, 15). Briefly, primary cultured cortical neurons (days in vitro 14) were treated with 50 nm okadaic acid or dimethyl sulfoxide for 45 min at 37 °C, and washed three times with ice-cold PBS containing 1 mm MgCl2 and 0.1 mm CaCl2 (PBS++). Neurons were incubated with 1 mg/ml EZ-Link Sulfo-NHS-SS-biotin (Thermo) in PBS++ for 20 min at 4 °C with gentle shaking. Excess non-reactive biotinylation reagent was quenched by washing four times with 50 mm glycine in PBS++. Neurons were solubilized in lysis buffer containing 50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 2 mm EDTA, 1% Triton X-100, protease inhibitor mixture (Roche Applied Science) for 30 min on ice. The insoluble pellet was removed by centrifugation at 20,000 × g for 15 min at 4 °C. The remaining supernatant was then incubated with 30 μl of streptavidin-agarose beads (Pierce) for 3 h at 4 °C. After washing the beads four times with lysis buffer, the bound proteins were analyzed by Western blotting.
Receptor Internalization Assay
The receptor internalization assay has been described elsewhere in detail (16, 17). Briefly, primary hippocampal neurons (days in vitro 12–14) grown on glass coverslips were transfected with mGluR7 tagged with an N-terminal c-Myc epitope. Neurons were incubated with anti-Myc antibody for 10 min at room temperature to label surface-expressed receptors, rinsed, and returned to conditioned medium for 45 min at 37 °C in the absence or presence of 50 nm okadaic acid. The neurons were then washed, fixed with 4% paraformaldehyde/4% sucrose in PBS for 20 min, and blocked with 10% normal goat serum for 30 min. Surface receptors were visualized by staining with Alexa Fluor 568-conjugated secondary antibody (red). The neurons were then washed, permeabilized with 0.2% Triton X-100 for 5 min, and blocked with 10% normal goat serum for 1 h, and internalized receptors were visualized by staining with Alexa Fluor 488-conjugated secondary antibody (green). To detect FLAG expression in Fig. 5, rabbit anti-FLAG antibody (1:500) was incubated after blocking with normal goat serum, followed by co-staining with Alexa Fluor 648-conjugated secondary antibody. The neurons were mounted with ProLong Antifade kit (Invitrogen) and imaged with a 40× objective using a Zeiss LSM 510 or 710 confocal microscope. Maximum projection images were obtained from serial optical sections at 0.36-μm intervals. The amount of internalization was quantified by measuring the integrated intensity of green and red signals using MetaMorph software (version 7.0, Universal Imaging Corp.).
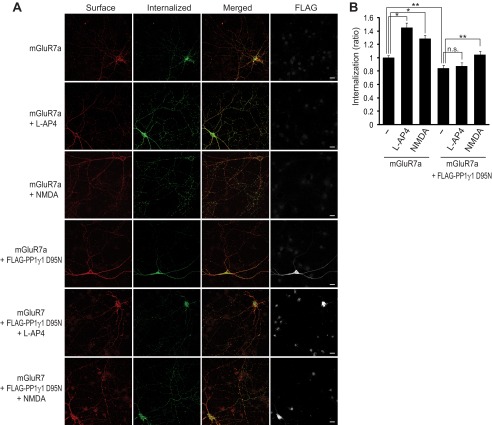
FIGURE 5.
Agonist-induced internalization of mGluR7a is inhibited by PP1γ1 D95N. A, hippocampal neurons were transiently transfected with FLAG-tagged PP1γ1 D95N and/or Myc-tagged mGluR7a. Surface-expressed receptors were labeled with anti-Myc antibody and returned to conditioned medium containing 400 μm l-AP4 or 50 μm NMDA for 15 min at 37 °C. The neurons were stained and images acquired as described under “Experimental Procedures.” Scale bar, 20 μm. B, a summary histogram quantifying the internalized receptors from A is shown as the ratio of the internalized fraction compared with total (surface + internalized) fraction. Data represent means ± S.E. *, p < 0.01; **, p < 0.05 (n > 25 neurons from three independent experiments). n.s. indicates p > 0.05.
RESULTS
Ser/Thr Protein Phosphatase 1 Regulates Ser-862 Phosphorylation of mGluR7
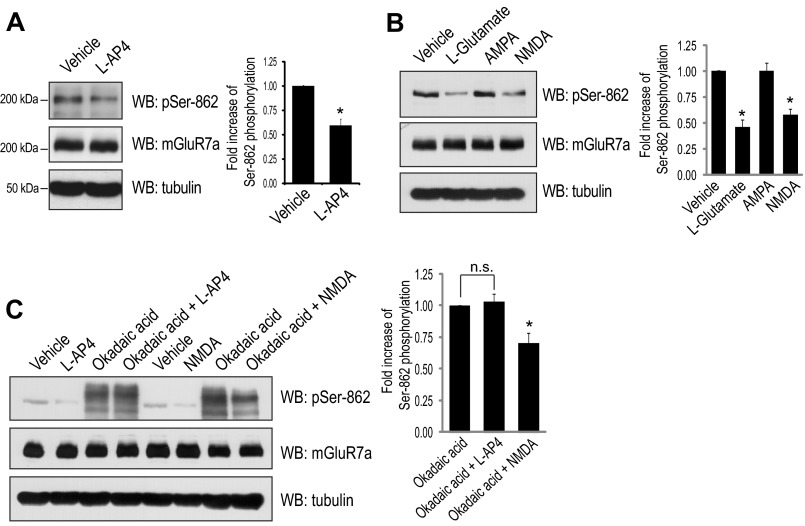
To evaluate the effect of protein phosphatase activity on Ser-862 phosphorylation of mGluR7, we first utilized several inhibitors of serine/threonine PP activity. Primary rat cortical neurons were treated with okadaic acid for 45 min, and Ser-862 phosphorylation of mGluR7 was detected by Western blot using a Ser-862 phosphorylation state-specific antibody that was previously characterized (10). Ser-862 phosphorylation of mGluR7 was dramatically increased by the treatment of neurons with 10–500 nm okadaic acid, whereas treatment with a lower concentration of okadaic acid (<5 nm) did not lead to any changes of Ser-862 phosphorylation (Fig. 1, A–C). Okadaic acid is a potent inhibitor of both PP1 and PP2A, but 100-fold more potent for PP2A than for PP1 (IC50 value is 10–270 and 0.2–2 nm for PP1 and PP2A, respectively) (11, 18, 19). Therefore, the inability of low dose okadaic acid to enhance mGluR7 phosphorylation suggests that Ser-862 of mGluR7 is a target of PP1 rather than PP2A. To confirm that PP1 regulates Ser-862 phosphorylation, we utilized several other PP inhibitors, including FK506, calyculin A, and fostriecin. FK506 is a protein phosphatase 2B-specific inhibitor (20). Calyculin A is a more potent inhibitor of PP1 and PP2A than okadaic acid and has a similar potency for PP1 and PP2A (IC50 = 1–14) (11, 18). Fostriecin is a potent and selective inhibitor of PP2A and PP4 and has no inhibitory activity against PP1 (21, 22). Both FK506 and fostriecin did not alter mGluR7 Ser-862 phosphorylation; however, there was a dramatic increase in mGluR7 phosphorylation with the treatment of calyculin A (Fig. 1, D and E). In addition, we observed increased phosphorylation of NFAT, which is a target molecule of calcineurin (protein phosphatase 2B) (23, 24), following the treatment of FK506 (Fig. 1F). The phosphorylation of p70S6K (at Thr-389), a downstream molecule of the mTOR signaling pathway (25–27), increased upon treatment with fostriecin, whereas mGluR7 Ser-862 phosphorylation was not altered (Fig. 1F). Therefore, PP2A and protein phosphatase 2B are not involved in regulating the phosphorylation of mGluR7. Time course experiments revealed that phosphorylation of Ser-862 begins to increase after a 15-min treatment with 50 nm okadaic acid (Fig. 1G). For this reason, neurons were treated with 50 nm okadaic acid for 45 min throughout this study. Together, these data demonstrate that Ser-862 of mGluR7 is dephosphorylated specifically by PP1.
FIGURE 1.
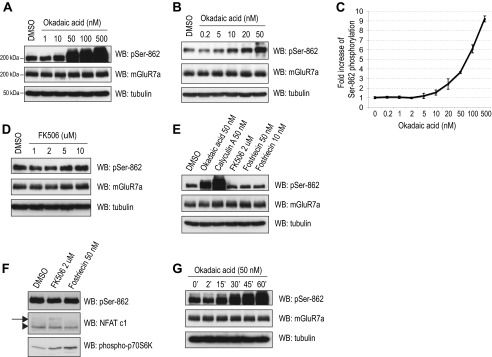
PP1 activity regulates phosphorylation of mGluR7 Ser-862. A and B, primary rat cortical neurons (days in vitro 12–14) were incubated with the indicated concentrations of okadaic acid at 37 °C for 45 min. Ser-862 phosphorylation of mGluR7, total expression of mGluR7, and expression of tubulin were evaluated by Western blot (WB) analysis. C, quantitation of the immunoblots from A and B was determined by measuring the band intensity of the pSer-862 blots compared with the intensity of total mGluR7a blots using NIH ImageJ software. Graphs represent means ± S.E. (n = 2–4). D, primary rat cortical neurons were incubated with the indicated concentrations FK506 at 37 °C for 45 min. E, primary rat cortical neurons were incubated with the indicated concentration PP inhibitors at 37 °C for 45 min. F, under the same experimental conditions as E, the phosphorylation of NFAT c1 and p70S6K at Thr-389 were examined. The arrow indicates phosphorylated NFAT c1, and the arrowhead indicates dephosphorylated NFAT c1. Representative blots are presented from two independent experiments. G, primary rat cortical neurons were incubated with 50 nm okadaic acid at 37 °C for various times. DMSO, dimethyl sulfoxide.
PP1 Regulates Agonist-induced Dephosphorylation of mGluR7
We recently found that mGluR7 undergoes endocytosis following treatment with an agonist l-AP4 (6, 10) and the reduced phosphorylation of mGluR7 Ser-862 leads to the internalization of mGluR7 (10). However, the direct effect of agonist treatment on Ser-862 phosphorylation has not been determined. To examine agonist-induced alterations in Ser-862 phosphorylation, l-AP4 (400 μm) was applied to primary rat cortical neurons for 15 min. To control the extracellular environment, conditioned culture medium was replaced with artificial cerebrospinal fluid without Mg2+ 30 min prior to l-AP4 treatment. We observed a 40% reduction of Ser-862 phosphorylation following treatment of primary neurons with l-AP4 (Fig. 2A). Treatment of neurons with high concentration of l-glutamate (1 mm) or NMDA (50 μm) also led to a dramatic reduction of Ser-862 phosphorylation (∼50%), whereas addition of AMPA (100 μm) had no effect on mGluR7 phosphorylation (Fig. 2B). To examine the PP1 dependence in agonist-induced mGluR7 dephosphorylation, okadaic acid was applied for 45 min prior to and during l-AP4 treatment (15 min). Okadaic acid completely blocked the decrease of Ser-862 phosphorylation by l-AP4, whereas dephosphorylation induced by NMDA was not completely blocked by okadaic acid (Fig. 2C). These data indicate that NMDA-mediated dephosphorylation mGluR7 is regulated by a mechanism that is distinct from that of l-AP4 and that NMDA-mediated dephosphorylation mGluR7 is not mediated by PP1.
FIGURE 2.
Agonist-induced dephosphorylation of mGluR7 is blocked by PP1 inhibition. A, l-AP4, an mGluR7 agonist, decreases Ser-862 phosphorylation of mGluR7. Primary rat cortical neurons were incubated with l-AP4 (400 μm) for 15 min. Ser-862 phosphorylation of mGluR7 was evaluated by Western blot (WB) analysis, and quantitation of the immunoblots is shown. Graphs represent means ± S.E. *, p < 0.05 (n = 4). B, high concentrations of l-glutamate decrease Ser-862 phosphorylation of mGluR7. Primary rat cortical neurons were treated with 1 mm l-glutamate, 100 μm AMPA, or 50 μm NMDA for 10 min. Ser-862 phosphorylation of mGluR7 was evaluated by Western blot analysis and quantitated. Graphs represent means ± S.E. *, p < 0.05 (n = 3). C, okadaic acid blocks l-AP4-induced dephosphorylation of mGluR7, whereas it does not block NMDA-induced dephosphorylation of mGluR7. Primary rat cortical neurons were incubated with okadaic acid (50 nm) for 45 min, and then l-AP4 or NMDA was applied for an additional 15 or 10 min, respectively. Western blot analysis was performed using the indicated antibodies. The relative okadaic acid-induced Ser-862 phosphorylation levels affected by an application of l-AP4 or NMDA were presented by quantitation of the Ser-862 band intensity using NIH ImageJ software. Graphs represent means ± S.E. *, p < 0.05; n.s. indicates p > 0.05 (n = 3).
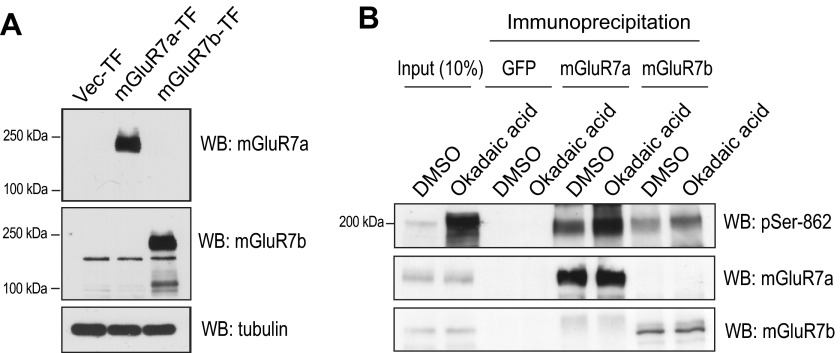
To determine whether Ser-862 on mGluR7a and/or mGluR7b is a target of PP1 in primary neurons, we examined isoform-specific mGluR7 Ser-862 dephosphorylation. An mGluR7a-specific antibody is commercially available, and we successfully generated an mGluR7b-specific antibody using a peptide corresponding to mGluR7b amino acids 901–922 (3). This antibody specifically recognized mGluR7b as confirmed in immunoblots of mGluR7a- or mGluR7b-expressing HEK cells (Fig. 3A). Primary cortical neurons were treated with okadaic acid, and the lysates were immunoprecipitated using mGluR7a- or mGluR7b-specific antibodies and probed using the phospho-Ser-862-specific antibody. Surprisingly, we found that okadaic acid treatment dramatically enhanced phosphorylation of Ser-862 on both mGluR7a and mGluR7b (Fig. 3B), indicating that both mGluR7a Ser-862 and mGluR7b Ser-862 are dephosphorylated by PP1.
FIGURE 3.
Ser-862 phosphorylation of both mGluR7a and mGluR7b is regulated by PP1γ1. A, generation of mGluR7b-specific antibody is shown. HEK cells were transfected with vector (Vec-TF), mGluR7a (mGluR7a-TF), or mGluR7b (mGluR7b-TF). Two days after transfection, cells were solubilized and immunoblotted with mGluR7a or mGluR7b antibody, respectively. B, primary cortical neurons were treated with dimethyl sulfoxide (DMSO) or okadaic acid (50 nm) for 45 min. Neurons were washed three times and solubilized using 1% Triton X-100 lysis buffer and immunoprecipitated with mGluR7a or mGluR7b antibody. Immunoprecipitates were resolved by SDS-PAGE and immunoblotted using the indicated antibodies. WB, Western blot.
Endocytosis of mGluR7 Is Reduced by the Inhibition of PP1
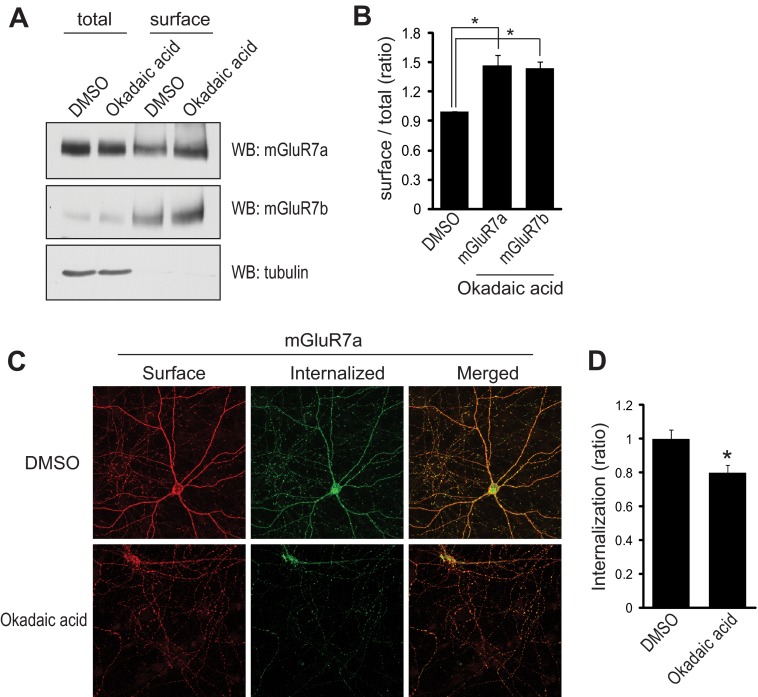
We have previously reported that PKCα increases Ser-862 phosphorylation of mGluR7 and stabilizes the receptor on the neuronal surface (10). As Ser-862 phosphorylation is markedly enhanced by the inhibition of PP1, we examined whether PP1 regulates mGluR7 expression by altering mGluR7 internalization. To analyze surface-expressed endogenous mGluR7a and mGluR7b, primary rat cortical neurons were subjected to cell surface biotinylation. Primary neurons were treated with okadaic acid or vehicle for 45 min and membrane-impermeable sulfo-NHS-SS-biotin was added to the cells for 20 min at 4 °C. Surface-expressed receptors were isolated using streptavidin-agarose beads. Treatment with okadaic acid increased surface expression of both mGluR7a and mGluR7b by ∼40% as compared with the cells treated with dimethyl sulfoxide (Fig. 4, A and B). We next examined whether the stabilization of mGluR7 on the neuronal surface is caused by the reduced internalization. To monitor the internalization of mGluR7 by okadaic acid, we transfected N-terminal Myc-tagged mGluR7a in primary hippocampal neurons, labeled surface-expressed mGluR7a with c-Myc antibody, and then allowed internalization of receptors at 37 °C for 45 min. Surface receptors were visualized with Alexa Fluor 568-labeled secondary antibodies (red) before permeabilization, whereas the internalized receptors were visualized using Alexa Fluor 488-labeled secondary antibodies (green) after permeabilization. We observed a 20% decrease in mGluR7 internalization in cells treated with okadaic acid (Fig. 4, C and D). Taken together, our data demonstrate that increased Ser-862 phosphorylation by inhibition of PP1 reduces the internalization of mGluR7 and stabilizes mGluR7 on neuronal surface.
FIGURE 4.
Surface expression of mGluR7 is regulated by PP1. A, surface expression of mGluR7a and mGluR7b was analyzed using biotinylation. Primary cortical neurons were biotinylated at 4 °C to label cell surface proteins. The neurons were treated with dimethyl sulfoxide (DMSO) or okadaic acid (50 nm) for 45 min. Surface-expressed receptors were isolated by streptavidin-agarose beads and immunoblotted using mGluR7a, mGluR7b, or tubulin antibody. Aliquots of the neuronal cell lysate were also analyzed to reveal expression of total mGluR7 or tubulin protein. B, relative surface expression of mGluR7a or mGluR7b was quantified from A by measuring the ratio of surface to total band intensities using NIH ImageJ software. Graphs represent means ± S.E. *, p < 0.05 (n = 3). C, hippocampal neurons were transiently transfected with Myc-tagged mGluR7a, and surface-expressed receptors were labeled with anti-Myc antibody. The neurons were returned to conditioned medium containing 50 nm okadaic acid at 37 °C for 45 min. The neurons were stained, and images were acquired as described under “Experimental Procedures.” D, a summary histogram quantifying the internalized receptors from C is shown as the ratio of the internalized fraction compared with total (surface + internalized) fraction. Data represent means ± S.E. *, p < 0.01 (n = 30 neurons from three independent experiments). WB, Western blot.
Agonist-induced Internalization of mGluR7a Is Inhibited by PP1γ1 D95N
We extended our study to include overexpression of the D95N mutant of PP1γ1, in which the catalytic domain is inactive (28) in primary neurons to address the different role of l-AP4 and NMDA in the internalization of mGluR7. We co-transfected PP1γ1 D95N with mGluR7a in primary hippocampal neurons and examined the internalization of mGluR7a. The internalization of mGluR7a was reduced by ∼20% in PP1γ1 D95N-transfected neurons, and the agonist l-AP4 did not have a significant effect on the internalization of mGluR7a in PP1γ1 D95N-transfected neurons, whereas NMDA partially rescued the internalization of mGluR7 (Fig. 5). Therefore, PP1γ1 D95N stabilizes mGluR7 on the neuronal surface by regulating mGluR7 Ser-862 phosphorylation.
DISCUSSION
The regulation of protein phosphorylation is a reversible process maintained by the balanced action of protein kinases and phosphatases that are crucial in directly modulating synaptic plasticity (29). As PKC-induced Ser-862 phosphorylation of mGluR7 is critical for a rapid change of mGluR7 trafficking and presynaptic plasticity, we have sought to identify the serine/threonine PP that dephosphorylates mGluR7 Ser-862 and opposes the role of PKCα. In the present study, among several serine/threonine PPs examined, we have identified PP1 as the key protein phosphatase regulating phosphorylation of Ser-862 on mGluR7. PP1 is involved in agonist-induced dephosphorylation of Ser-862 phosphorylation of mGluR7. Furthermore, PP1 regulates mGluR7 trafficking by modulating Ser-862 phosphorylation. Therefore, our data suggest PP1 is involved in reversing PKC-induced changes of mGluR7 trafficking and surface expression.
The time course of the okadaic acid effects on Ser-862 seems be slow compared with phosphorylation induced by kinases, which usually starts to increase within a few minutes. We have no clear explanation for this; however, one explanation might be the slow penetration of okadaic acid into the neurons, which has been reported in case of neutrophils (30). A second possibility is that the kinase activity on Ser-862 may be constitutively robust, such that the inhibitory effect of phosphatase activity may be reflected slowly.
We found that treatment with the mGluR7 agonists l-AP4 or glutamate lead to the dephosphorylation of mGluR7 in primary neurons. As l-AP4 induces mGluR7 internalization in neurons, this finding suggests that the surface expression of mGluR7 is tightly regulated by the Ser-862 phosphorylation status. To determine whether agonist-induced dephosphorylation and internalization of mGluR7 is dependent on the action of protein phosphatases, okadaic acid or the PP1γ1 D95N catalytic inactive mutant was utilized along with l-AP4 on primary cortical neurons. We found that l-AP4 did not induce dephosphorylation of Ser-862 and internalization of mGluR7 following the inhibition of PP1, demonstrating that l-AP4-induced dephosphorylation of Ser-862 is mediated by PP1. In sharp contrast, NMDA-induced dephosphorylation and internalization was not affected by inhibition of PP1. The NMDA-induced changes in Ser-862 phosphorylation status may be due to the activity of a distinct unidentified phosphatase, NMDA may directly inactivate kinases other than PKCα, or NMDA-induced changes may regulate different intracellular stores of mGluR7.
The effects of okadaic acid on the surface expression of mGluR7 are modest and the increase in mGluR7 Ser-862 phosphorylation by okadaic acid is not proportional to the amount of mGluR7 internalization. We believe that a mGluR7-interacting molecule such as calmodulin or PICK1 is also an important determinant for mGluR7 internalization. For example, PICK1 binding to phosphorylated mGluR7 is primarily modulated by calmodulin binding to mGluR7 and not by Ser-862 phosphorylation per se (10).
mGluR7 has two splice variants, mGluR7a and mGluR7b. There is an out-of-frame insertion before the last exon of the mGluR7 gene, resulting in alternative spliced isoforms, mGluR7a and mGluR7b. Therefore, 16 amino acids of mGluR7a and 23 amino acids of mGluR7b differ in their extreme C-terminal tails (31, 32). Previous studies showed that PP1γ1 directly interacts with mGluR7b, but not mGluR7a, as determined using GST pulldown assays or yeast two-hybrid system interactions (33–35). This binding is mediated primarily by the N-terminal domain of PP1γ1 and the C-terminal region of mGluR7b (KSVTW; amino acids 911–915). Trp-915 of mGluR7b is critical for this binding. The conserved docking motif interacting with the PP1 catalytic subunit is (R/K)-x(0,1)-(V/I)-x-F, which is found in many, but not all, proteins that bind to PP1γ1 (36–38). The proposed PP1γ1-binding motif is similar but not identical to this consensus motif in mGluR7b but is not conserved in the mGluR7a C-terminal region. Instead, the KSVT motif without Trp is conserved in the secondary intracellular loop both in mGluR7a and -b. Although PP1γ1, a catalytic subunit of PP, seems to bind to both mGluR7a and mGluR7b in co-immunoprecipitation experiments using HEK cells (data not shown), we were not able to identify a consensus interacting motif on mGluR7a. However, our findings that inhibition of PP1 increased phosphorylation of Ser-862 and surface expression of mGluR7a suggest that Ser-862 of mGluR7a as well as mGluR7b is a target of PP1. It is unlikely that mGluR7a and mGluR7b form a heterodimer and that PP1γ1 dephosphorylates mGluR7a in the heterodimer without directly binding to mGluR7a. Data against the heterodimer model comes from a previous report showing that mGluR7b displays are more restricted distribution in the brain than mGluR7a (39). In addition, mGluR7a co-immunoprecipitates with PP1γ1 in cells that do not express mGluR7b (data not shown). Rather than this heterodimeric model, we hypothesize that the binding of PP1γ1 to mGluR7a may be contributed by multiple domains, including both cytoplasmic and intracellular loops, or may be mediated indirectly by unidentified mGluR7-interacting molecules via transmembrane domains.
The serine/threonine PP family includes PP1, PP2A, protein phosphatase 2B (calcineurin), and PP4–7 in eukaryotic cells (11, 38). Among these phosphatases, the PP1 catalytic subunit is classified into PP1α, PP1β (also termed PP1δ), and PP1γ that are all present on distinct genes. PP1γ has two splice variants, PP1γ1 and PP1γ2. Although these subunits possess distinct tissue distribution, all of these are expressed in brain (11), and PP1γ1 is enriched in presynaptic boutons and dendrites (40). The catalytic subunit of PP1 is highly conserved and interacts with >50 regulatory subunits, which play inhibitory roles on PP1 activity (37) and anchor the phosphatase directly to a membrane receptor (12, 41). For instance, yotiao targets the catalytic subunit of PP1 or the regulatory subunit of protein kinase A to the NMDA receptor NR1, and PP1 inhibition by okadaic acid enhances peak NMDA-evoked currents (42). Thus, it is quite possible that an unidentified regulatory component may affect anchoring of phosphatases near mGluR7 at the presynaptic active zone. Further study of the spatiotemporal control of phosphatase interactions with mGluR7 in vivo will certainly be an important goal.
Acknowledgments
We thank J. D. Badger II for preparing primary neuron cultures, Dr. C. L. Smith (NINDS Light Imaging Facility) for assistance with confocal microscopy, and the NINDS Sequencing Facility.
This work was supported by the NINDS, National Institutes of Health, Intramural Research Program (to K. W. R.), the NCI, National Institutes of Health, Intramural Research Program (to P. A. R.), the new faculty research fund of the Ajou University School of Medicine, and the Basic Science Research Program (2011-0011694) and Medical Research Center Program (NRF-2012R1A5A048183) through the National Research Foundation of Korea.
- mGluR
- metabotropic glutamate receptor
- PP1
- serine/threonine protein phosphatase 1
- l-AP4
- l-(+)-2-amino-4-phosphonobutyric acid
- PICK1
- protein interacting with C kinase 1
- NFAT
- nuclear factor of activated T cells.
REFERENCES
- 1. Conn P. J., Pin J. P. (1997) Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 37, 205–237 [DOI] [PubMed] [Google Scholar]
- 2. Shigemoto R., Kulik A., Roberts J. D., Ohishi H., Nusser Z., Kaneko T., Somogyi P. (1996) Target-cell-specific concentration of a metabotropic glutamate receptor in the presynaptic active zone. Nature 381, 523–525 [DOI] [PubMed] [Google Scholar]
- 3. Shigemoto R., Kinoshita A., Wada E., Nomura S., Ohishi H., Takada M., Flor P. J., Neki A., Abe T., Nakanishi S., Mizuno N. (1997) Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J. Neurosci. 17, 7503–7522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. El Far O., Betz H. (2002) G-protein-coupled receptors for neurotransmitter amino acids: C-terminal tails, crowded signalosomes. Biochem. J. 365, 329–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fagni L., Ango F., Perroy J., Bockaert J. (2004) Identification and functional roles of metabotropic glutamate receptor-interacting proteins. Semin. Cell Dev. Biol. 15, 289–298 [DOI] [PubMed] [Google Scholar]
- 6. Pelkey K. A., Lavezzari G., Racca C., Roche K. W., McBain C. J. (2005) mGluR7 is a metaplastic switch controlling bidirectional plasticity of feedforward inhibition. Neuron 46, 89–102 [DOI] [PubMed] [Google Scholar]
- 7. Pelkey K. A., Yuan X., Lavezzari G., Roche K. W., McBain C. J. (2007) mGluR7 undergoes rapid internalization in response to activation by the allosteric agonist AMN082. Neuropharmacology 52, 108–117 [DOI] [PubMed] [Google Scholar]
- 8. Bockaert J., Perroy J., Bécamel C., Marin P., Fagni L. (2010) GPCR interacting proteins (GIPs) in the nervous system: Roles in physiology and pathologies. Annu. Rev. Pharmacol. Toxicol. 50, 89–109 [DOI] [PubMed] [Google Scholar]
- 9. Dev K. K., Nakajima Y., Kitano J., Braithwaite S. P., Henley J. M., Nakanishi S. (2000) PICK1 interacts with and regulates PKC phosphorylation of mGLUR7. J. Neurosci. 20, 7252–7257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suh Y. H., Pelkey K. A., Lavezzari G., Roche P. A., Huganir R. L., McBain C. J., Roche K. W. (2008) Corequirement of PICK1 binding and PKC phosphorylation for stable surface expression of the metabotropic glutamate receptor mGluR7. Neuron 58, 736–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herzig S., Neumann J. (2000) Effects of serine/threonine protein phosphatases on ion channels in excitable membranes. Physiol. Rev. 80, 173–210 [DOI] [PubMed] [Google Scholar]
- 12. Winder D. G., Sweatt J. D. (2001) Roles of serine/threonine phosphatases in hippocampal synaptic plasticity. Nat. Rev. Neurosci. 2, 461–474 [DOI] [PubMed] [Google Scholar]
- 13. Pelkey K. A., Topolnik L., Yuan X. Q., Lacaille J. C., McBain C. J. (2008) State-dependent cAMP sensitivity of presynaptic function underlies metaplasticity in a hippocampal feedforward inhibitory circuit. Neuron 60, 980–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shi Y., Suh Y. H., Milstein A. D., Isozaki K., Schmid S. M., Roche K. W., Nicoll R. A. (2010) Functional comparison of the effects of TARPs and cornichons on AMPA receptor trafficking and gating. Proc. Natl. Acad. Sci. U.S.A. 107, 16315–16319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suh Y. H., Terashima A., Petralia R. S., Wenthold R. J., Isaac J. T., Roche K. W., Roche P. A. (2010) A neuronal role for SNAP-23 in postsynaptic glutamate receptor trafficking. Nat. Neurosci. 13, 338–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lavezzari G., Roche K. W. (2007) Constitutive endocytosis of the metabotropic glutamate receptor mGluR7 is clathrin-independent. Neuropharmacology 52, 100–107 [DOI] [PubMed] [Google Scholar]
- 17. Lee J. H., Lee J., Choi K. Y., Hepp R., Lee J. Y., Lim M. K., Chatani-Hinze M., Roche P. A., Kim D. G., Ahn Y. S., Kim C. H., Roche K. W. (2008) Calmodulin dynamically regulates the trafficking of the metabotropic glutamate receptor mGluR5. Proc. Natl. Acad. Sci. U.S.A. 105, 12575–12580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ishihara H., Martin B. L., Brautigan D. L., Karaki H., Ozaki H., Kato Y., Fusetani N., Watabe S., Hashimoto K., Uemura D. (1989) Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem. Biophys. Res. Commun. 159, 871–877 [DOI] [PubMed] [Google Scholar]
- 19. Koss D. J., Hindley K. P., Riedel G., Platt B. (2007) Modulation of hippocampal calcium signalling and plasticity by serine/threonine protein phosphatases. J. Neurochem. 102, 1009–1023 [DOI] [PubMed] [Google Scholar]
- 20. Liu J., Farmer J. D., Jr., Lane W. S., Friedman J., Weissman I., Schreiber S. L. (1991) Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66, 807–815 [DOI] [PubMed] [Google Scholar]
- 21. Boger D. L., Ichikawa S., Zhong W. (2001) Total synthesis of fostriecin (CI-920). J. Am. Chem. Soc. 123, 4161–4167 [DOI] [PubMed] [Google Scholar]
- 22. Walsh A. H., Cheng A., Honkanen R. E. (1997) Fostriecin, an antitumor antibiotic with inhibitory activity against serine/threonine protein phosphatases types 1 (PP1) and 2A (PP2A), is highly selective for PP2A. FEBS Lett. 416, 230–234 [DOI] [PubMed] [Google Scholar]
- 23. Schwartz N., Schohl A., Ruthazer E. S. (2009) Neural activity regulates synaptic properties and dendritic structure in vivo through calcineurin/NFAT signaling. Neuron 62, 655–669 [DOI] [PubMed] [Google Scholar]
- 24. Shibasaki F., Hallin U., Uchino H. (2002) Calcineurin as a multifunctional regulator. J. Biochem. 131, 1–15 [DOI] [PubMed] [Google Scholar]
- 25. Millward T. A., Zolnierowicz S., Hemmings B. A. (1999) Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem. Sci. 24, 186–191 [DOI] [PubMed] [Google Scholar]
- 26. Peterson R. T., Desai B. N., Hardwick J. S., Schreiber S. L. (1999) Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycinassociated protein. Proc. Natl. Acad. Sci. U.S.A. 96, 4438–4442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petritsch C., Beug H., Balmain A., Oft M. (2000) TGF-β inhibits p70 S6 kinase via protein phosphatase 2A to induce G1 arrest. Genes Dev. 14, 3093–3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang J., Zhang Z., Brew K., Lee E. Y. (1996) Mutational analysis of the catalytic subunit of muscle protein phosphatase-1. Biochemistry 35, 6276–6282 [DOI] [PubMed] [Google Scholar]
- 29. Lee H. K. (2006) Synaptic plasticity and phosphorylation. Pharmacol. Ther. 112, 810–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kreienbühl P., Keller H., Niggli V. (1992) Protein phosphatase inhibitors okadaic acid and calyculin A alter cell shape and F-actin distribution and inhibit stimulus-dependent increases in cytoskeletal actin of human neutrophils. Blood 80, 2911–2919 [PubMed] [Google Scholar]
- 31. Corti C., Restituito S., Rimland J. M., Brabet I., Corsi M., Pin J. P., Ferraguti F. (1998) Cloning and characterization of alternative mRNA forms for the rat metabotropic glutamate receptors mGluR7 and mGluR8. Eur. J. Neurosci. 10, 3629–3641 [DOI] [PubMed] [Google Scholar]
- 32. Flor P. J., Van Der Putten H., Rüegg D., Lukic S., Leonhardt T., Bence M., Sansig G., Knöpfel T., Kuhn R. (1997) A novel splice variant of a metabotropic glutamate receptor, human mGluR7b. Neuropharmacology 36, 153–159 [DOI] [PubMed] [Google Scholar]
- 33. Croci C., Sticht H., Brandstätter J. H., Enz R. (2003) Group I metabotropic glutamate receptors bind to protein phosphatase 1C. Mapping and modeling of interacting sequences. J. Biol. Chem. 278, 50682–50690 [DOI] [PubMed] [Google Scholar]
- 34. Enz R. (2002) The metabotropic glutamate receptor mGluR7b binds to the catalytic γ-subunit of protein phosphatase 1. J. Neurochem. 81, 1130–1140 [DOI] [PubMed] [Google Scholar]
- 35. Enz R., Croci C. (2003) Different binding motifs in metabotropic glutamate receptor type 7b for filamin A, protein phosphatase 1C, protein interacting with protein kinase C (PICK) 1 and syntenin allow the formation of multimeric protein complexes. Biochem. J. 372, 183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Egloff M. P., Johnson D. F., Moorhead G., Cohen P. T., Cohen P., Barford D. (1997) Structural basis for the recognition of regulatory subunits by the catalytic subunit of protein phosphatase 1. EMBO J. 16, 1876–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Munton R. P., Vizi S., Mansuy I. M. (2004) The role of protein phosphatase-1 in the modulation of synaptic and structural plasticity. FEBS Lett. 567, 121–128 [DOI] [PubMed] [Google Scholar]
- 38. Shi Y. (2009) Serine/threonine phosphatases: mechanism through structure. Cell 139, 468–484 [DOI] [PubMed] [Google Scholar]
- 39. Kinoshita A., Shigemoto R., Ohishi H., van der Putten H., Mizuno N. (1998) Immunohistochemical localization of metabotropic glutamate receptors, mGluR7a and mGluR7b, in the central nervous system of the adult rat and mouse: a light and electron microscopic study. J. Comp. Neurol. 393, 332–352 [PubMed] [Google Scholar]
- 40. Strack S., Kini S., Ebner F. F., Wadzinski B. E., Colbran R. J. (1999) Differential cellular and subcellular localization of protein phosphatase 1 isoforms in brain. J. Comp. Neurol. 413, 373–384 [PubMed] [Google Scholar]
- 41. Cohen P. T. (2002) Protein phosphatase 1–targeted in many directions. J. Cell Sci. 115, 241–256 [DOI] [PubMed] [Google Scholar]
- 42. Westphal R. S., Tavalin S. J., Lin J. W., Alto N. M., Fraser I. D., Langeberg L. K., Sheng M., Scott J. D. (1999) Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science 285, 93–96 [DOI] [PubMed] [Google Scholar]