Background: Sharp-1 inhibits skeletal muscle differentiation.
Results: SUMO modification impacts of Sharp-1-mediated inhibition of myogenesis.
Conclusion: Sumoylation acts as a signal for recruitment of the chromatin modifier G9a.
Significance: These studies link sumoylation of a transcription factor with changes in chromatin structure and differentiation of skeletal muscle precursor cells.
Keywords: Chromatin Modification, Differentiation, Epigenetics, Helix-Loop-Helix Transcription Factors, Histone Methylation, Myogenesis, Skeletal Muscle, Sumoylation, Transcription Factors
Abstract
Sumoylation is an important post-translational modification that alters the activity of many transcription factors. However, the mechanisms that link sumoylation to alterations in chromatin structure, which culminate in tissue specific gene expression, are not fully understood. In this study, we demonstrate that SUMO modification of the transcription factor Sharp-1 is required for its full transcriptional repression activity and function as an inhibitor of skeletal muscle differentiation. Sharp-1 is modified by sumoylation at two conserved lysine residues 240 and 255. Mutation of these SUMO acceptor sites in Sharp-1 does not impact its subcellular localization but attenuates its ability to act as a transcriptional repressor and inhibit myogenic differentiation. Consistently, co-expression of the SUMO protease SENP1 with wild type Sharp-1 abrogates Sharp-1-dependent inhibition of myogenesis. Interestingly, sumoylation acts as a signal for recruitment of the co-repressor G9a. Thus, enrichment of G9a, and histone H3 lysine 9 dimethylation (H3K9me2), a signature of G9a activity, is dramatically reduced at muscle promoters in cells expressing sumoylation-defective Sharp-1. Our findings demonstrate how sumoylation of Sharp-1 exerts an impact on chromatin structure and transcriptional repression of muscle gene expression through recruitment of G9a.
Introduction
Small ubiquitin-like modifier (SUMO)3 is one of the best characterized members of ubiquitin-like proteins involved in regulation of transcription factors (1–3). There are four SUMO isoforms in mammals (SUMO-1, SUMO-2, SUMO-3, and SUMO-4). Similar to ubiquitin, SUMO is also ligated to lysine residues in target proteins, and the target lysine usually embedded within a canonical consensus ΨKXE, where Ψ is a hydrophobic amino acid, K is the acceptor lysine for covalent conjugation of SUMO, X is any amino acid, and E is glutamic acid. SUMO is covalently attached to substrates through the activities of an enzyme cascade similar to the ubiquitination cycle: SUMO is activated by the E1 activation enzyme and transferred to the sole E2 enzyme Ubc9, which then conjugates to the substrate by a specific E3 ligase. Proteins from the PIAS (protein inhibitor of activated STAT) family, RanBP2 (Ran-binding protein 2), and Pc2 (Polycomb 2) have been identified as SUMO E3 ligases (3–5). Sumoylation is a highly dynamic and reversible modification with substrates undergoing rapid conjugation and deconjugation. The removal of SUMO is catalyzed by SUMO-specific isopeptidases of SENP (sentrin-specific protease) family (2, 6). Despite the similarity between the sumoylation and ubiquitination pathway, the functional consequences of these two modifications are quite different. Unlike ubiquitination which primarily facilitates the target protein for degradation, sumoylation has diverse effects including regulation of protein-protein interactions, subcellular localization, protein stability, and alteration of transcriptional activity of substrate proteins. Transcription factors are the largest group of target proteins whose functions are modified by sumoylation, and in most reported studies, sumoylation poses a negative effect on the activities of transcription factors (7–9).
Sharp-1 is a basic helix-loop-helix-Orange domain containing transcriptional repressor that is expressed in many cell types during embryonic development as well as in adult tissues (10–14). Sharp-1 binds to class B E-box sites CACGTG with high affinity to repress transcription of target genes (15, 16). Unlike related Hey and Hes sub-family members, which recruit the co-repressor TLE (transducin-like enhancer of split)/Groucho through a WRPW motif, Sharp-1 associates with distinct co-repressors, including histone deacetylase 1 (HDAC1), SirT1, and the histone methyltransferase G9a (12, 17).
The myogenic regulatory factor MyoD plays a central role in differentiation of skeletal muscle precursor cells. MyoD heterodimerizes with ubiquitously expressed E proteins and binds to E-box sequences (CANNTG) present in promoters of muscle genes to turn on their expression. Sharp-1 is expressed at high levels in precursor cells, and its levels decline during differentiation. Both gain of function and loss of function studies have shown that Sharp-1 impairs myogenic differentiation through antagonism of MyoD (10, 17). The mechanisms underlying Sharp-1-dependent inhibition of differentiation include dimerization with MyoD and E proteins. In addition, we have recently shown that Sharp-1 interacts with G9a, that mediates repressive histone H3 lysine 9 dimethylation (H3K9me2) marks and recruits it to MyoD target promoters. Consistent with the recruitment of G9a, enrichment in H3K9me2 is apparent at MyoD target promoters in Sharp-1-overexpressing cells. Moreover, MyoD methylation at lysine 104 (Lys-104) is also enhanced by G9a (10, 18). Thus, inhibition of G9a expression or activity partially rescues Sharp-1-dependent repression of myogenesis (17). Although these studies have implicated G9a as a mediator of Sharp-1-dependent inhibition of myogenesis, the molecular mechanisms and signals that regulate its recruitment by Sharp-1 are unclear.
In this study, we provide evidence that SUMO modification of Sharp-1 serves as a platform for recruitment of the co-repressor G9a and its ability to inhibit myogenesis. We demonstrate that Sharp-1 is sumoylated at two highly conserved lysine residues Lys-240 and Lys-255, which is further enhanced by PIAS3 and PIASxα. Mutation of these lysine acceptor sites in Sharp-1 (Sharp-1 2KR) abolishes sumoylation without any impact on its subcellular localization. However, in contrast to wild type Sharp-1, which inhibits MyoD transcriptional activity and myogenic differentiation, the sumoylation-defective mutant Sharp-1 2KR is significantly less efficient at blocking MyoD function and myogenesis. Interestingly, unlike wild type Sharp-1, Sharp-1 2KR exhibits a markedly reduced association with G9a and is insensitive to inhibitors of G9a activity. Taken together, these studies reveal a key role for SUMO modification of Sharp-1 in the recruitment of G9a and inhibition of myogenic differentiation.
EXPERIMENTAL PROCEDURES
Cell Culture and Differentiation Assays
C2C12 cells were cultured and maintained in growth medium consisting of DMEM with 20% FBS (Hyclone). HEK293 and C3H10T1/2 (10T1/2) cells were maintained in DMEM supplemented with 10% FBS (Invitrogen), and COS-7 cells in DMEM with 10% calf serum (Hyclone).
C2C12 cells were co-transfected with a 9:1 ratio of an expression vector for pCS2, Sharp-1, or Sharp-1 2KR and pBabe (which confers resistance to puromycin). To test the role of sumoylation, SENP1 was transfected as indicated. 48 h after transfection, cells were selected in medium containing 2 μg/ml puromycin for 2 days. Selected cells were differentiated in medium consisting of DMEM plus 2% horse serum (Hyclone). For myogenic conversion assays, 10T1/2 cells were transfected with equivalent levels of MyoD alone or with Sharp-1 or Sharp-1 2KR. Undifferentiated (day 0) and differentiated cells (day 6) were harvested for analysis of protein lysates by Western blot and fixed for immunofluorescence assays. To quantify differentiation, myogenic index was calculated as the ratio of nuclei in MHC+ myotubes/total nuclei across four different microscopic fields. 700–1000 nuclei were counted.
Immunofluorescence Assays
For differentiation assays, cells were washed with PBS and fixed in ice-cold 4% paraformaldehyde. After permeabilization, cells were incubated with anti-MHC (MY32) (Sigma) antibody and detected with secondary antibody coupled with Alexa Fluor (Molecular Probes). Slides were mounted in Vectashield mounting medium (Vector Laboratories) containing DAPI to stain nuclei. Images were captured using a Nikon Eclipse TE 2000-U fluorescence microscope using MetaMorph software (version 7.0r3). COS-7 cells were used to examine subcellular localization of Myc-tagged Sharp-1 and Sharp-1 2KR. Cells were seeded at a density of 1 × 104 cells/well in six-well plates. 24 h later, cells were transfected with Myc-Sharp-1 and Myc-Sharp-1 2KR, or additionally co-transfected with SUMO-1. 48 h after transfection, cells were fixed, incubated with mouse anti-Myc antibody, and detected with Texas Red-coupled secondary antibody. Cells were visualized on a Zeiss LSM 510 META confocal laser-scanning microscope.
Plasmids and Mutagenesis
FLAG-mPIAS1, FLAG-mPIAS3 FLAG-mPIASxα, FLAG-mPIASy, SUMO-1, SENP1 were kindly provided by Martin Lee (19). pCS2-Myc-Sharp-1 has been described (17). To mutate potential sumoylation residues from lysine to arginine in Sharp-1, the QuikChangeTM site-directed mutagenesis kit (Stratagene) was used. The primers for generating pCS2-Myc-Sharp-1 K240R were as follows: 5′-CGC GCG GCC GTC CGA CAG GAG CCA CCC-3′ and 5′-GGG TGG CTC CTG TCG GAC GGC CGC GCG-3′; primers for Sharp-1 2KR were as follows: 5′-CCC AAG AGG CCG CGA CTG GAG GCG CGC-3′ and 5′-GCG CGC CTC CAG TCG CGG CCT CTT GGG-3′. The cDNA was sequenced entirely to confirm the presence of directed mutations.
Immunoprecipitation and Western Blotting
To detect sumoylation, expression vectors were transfected using LipofectamineTM 2000 as described (20). Cells were collected 48 h after transfection and lysed in presence of 20 mm N-ethylmaleimide (Sigma) using an ice-cold lysis buffer (50 mm Tris-HCl, pH 8.0, 50 mm NaCl, 1 mm EDTA, 0.1% Triton X-100, 0.5 mm PMSF, and protease inhibitors (Roche Diagnostics). Lysates were analyzed by Western blotting using the following antibodies: anti-SUMO-1, anti-Myc, anti-FLAG, and anti-β-actin. For co-immunoprecipitation assays, Myc-tagged Sharp-1 and Sharp-1 2KR were transfected into C2C12 cells. Lysates were immunoprecipitated using Myc-agarose beads and analyzed for association with endogenous MyoD by Western blotting using anti-MyoD (Santa Cruz Biotechnology) or endogenous G9a using anti-G9a antibody (Cell Signaling).
ChIP Assays
C2C12 cells were transfected with vector alone, Sharp-1 or Sharp-1 2KR, and SENP-1. ChIP assays were performed using a kit (Upstate) with 2 μg of H3K9me2 antibody (Millipore), 10 μl of G9a antibody (Abcam), and 2 μg anti-Sharp-1 antibody (Santa Cruz Biotechnology). DNA was amplified with primers specific to myogenin promoter as described previously (17).
Luciferase Assays
HEK293T cells were transfected with 9E-TK-luc reporter along with Sharp-1 or Sharp-1 2KR. 10T1/2 cells were transfected with pMyogLuc promoter reporter, MyoD, Sharp-1, and Sharp-1 2KR as indicated in the figures together with 5 ng of Renilla luciferase. 24 h after transfection, UNC0638 (Sigma) was added for 24 h, after which cells were lysed and assayed using the Dual-Luciferase reporter assay system (Promega). Each transfection was performed in triplicate and repeated at least twice. Values were reported as means with S.D. (shown as error bars).
Statistical Analysis
Error bars indicate mean ± S.D. Statistical analysis was performed using Student's t test, and p values < 0.05 were considered statistically significant.
RESULTS
Sharp-1 Is SUMO-modified
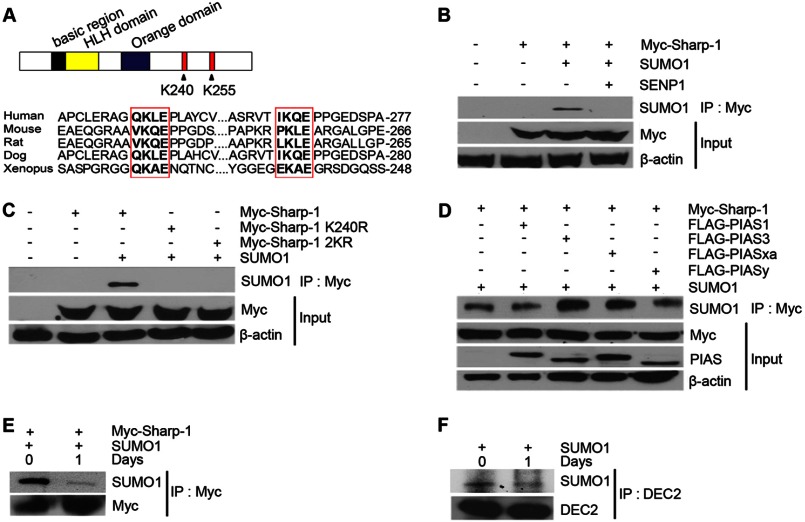
We have recently reported that Sharp-1 interacts with the co-repressor G9a to inhibit differentiation of skeletal muscle precursor cells (17). The interaction mapped to amino acid residues 173–265 in Sharp-1. Further examination of this region revealed two highly conserved lysine residues that perfectly matched the consensus sumoylation motif: lysine 240 in the sumoylation motif VKQE; and Lys-255 in the sequence PKLE (Fig. 1A). To determine whether Sharp-1 undergoes sumoylation, we transiently co-transfected HEK293 cells with Myc-Sharp-1 and SUMO-1. Cells were lysed in the presence of N-ethylmaleimide, an inhibitor of SUMO hydrolases, and lysates were immunoprecipitated with Myc-agarose beads followed by Western blotting with anti-SUMO-1 antibody. Interestingly, in the presence of SUMO-1, Sharp-1 appeared to be sumoylated (Fig. 1B). Furthermore, in presence of co-transfected SENP1, which is able to remove SUMO conjugates from target proteins, Sharp-1 sumoylation was almost abolished. These results confirmed that Sharp-1 is SUMO-conjugated in cells.
FIGURE 1.
Sharp-1 is SUMO-modified. A, the domain structure Sharp-1 is shown with the basic, helix-loop-helix (HLH), and the Orange domains (upper panel). Potential sumoylation sites at Lys-240 and Lys-255 are indicated. Alignment of the two SUMO consensus motifs in Sharp-1 cDNA from human, mouse, rat, dog, and Xenopus showed high conservation across species. B, cells were co-transfected with constructs encoding Myc-Sharp-1, SUMO-1, and SENP1 as indicated. Lysates were subjected to immunoprecipitation with Myc-agarose beads followed by immunoblotting with anti-SUMO-1 antibody. Anti-Myc antibody was used to detect expression of Sharp-1. β-Actin served as a loading control. C, cells were co-transfected with Myc-Sharp-1, Sharp-1 K240R, and Sharp-1 2KR along with SUMO-1. Lysates were immunoprecipitated with Myc-agarose beads followed by Western blotting with anti-SUMO-1 antibody. D, FLAG-tagged PIAS1, PIAS3, PIASxα, and PIASy were co-transfected with Myc-Sharp-1 and SUMO-1. Cell lysates were immunoprecipitated with Myc-agarose beads, and immunoprecipitates were subjected to Western blotting with anti-SUMO-1 antibody. Sharp-1 and PIAS proteins were detected with anti-Myc and anti-FLAG antibodies, respectively. E, C2C12 cells were co-transfected with Myc-Sharp-1 and SUMO-1. Cells were harvested as undifferentiated cells (day 0) and 1 day after differentiation (day 1), immunoprecipitated with Myc-agarose beads followed by Western blotting with anti-SUMO-1 and anti-Myc antibodies. F, C2C12 cells were transfected with SUMO-1. Endogenous Sharp-1 was immunoprecipitated from day 0 and day 1 lysates using anti-DEC2 (Sharp-1) antibody followed by Western blotting with anti-SUMO-1 antibody.
To validate that Lys-240 and Lys-255 are bona fide sumoylation sites, we generated point mutants changing the putative target lysine residues to arginine with site-directed mutagenesis. These mutants as well as wild type Sharp-1 were co-expressed with SUMO-1 in cells. Immunoprecipitation and Western blotting analysis revealed that mutation of Lys-240 alone (K240R), or both Lys-240 and Lys-255 (2KR) to arginine abrogated sumoylation even in the presence of SUMO-1, indicating that Lys-240 is the major site for SUMO modification of Sharp-1 (Fig. 1C).
Most sumoylation reactions are enhanced by specific SUMO E3 ligases, of which the PIAS family proteins have been well characterized (5). To determine whether PIAS proteins regulate Sharp-1 sumoylation, we co-transfected cells with Myc-Sharp-1, SUMO-1 and FLAG-PIAS1, PIAS3, PIASxα, and PIASy. Cell lysates were immunoprecipitated with Myc-agarose beads, followed by Western blotting with anti-SUMO-1 antibody. Overexpression of PIAS3 and PIASxα enhanced sumoylation of Sharp-1, whereas the presence of PIAS1 and PIASy had a minimal impact, suggesting that PIAS3 and PIASxα act as E3 SUMO ligases for Sharp-1 (Fig. 1D). To examine whether sumoylation of Sharp-1 is physiologically relevant in myogenesis, we first sought to determine whether Sharp-1 is SUMO conjugated in muscle cells. C2C12 myoblasts were co-transfected with Myc-Sharp-1 and SUMO-1. Cell lysates from undifferentiated cells and 24 h after induction of differentiation were immunoprecipitated with Myc-agarose beads followed by Western blotting with anti-SUMO-1 antibody. Sumoylation of Sharp-1 was detected in undifferentiated C2C12 cells and was reduced upon differentiation (Fig. 1E). To further validate this finding, endogenous Sharp-1 was immunoprecipitated from undifferentiated and differentiated C2C12 cells. Consistent with the previous results, endogenous Sharp-1 was SUMO-conjugated to higher levels in undifferentiated cells compared with differentiated cells (Fig. 1F).
Subcellular Localization of Sharp-1 Is Not Altered by SUMO Modification
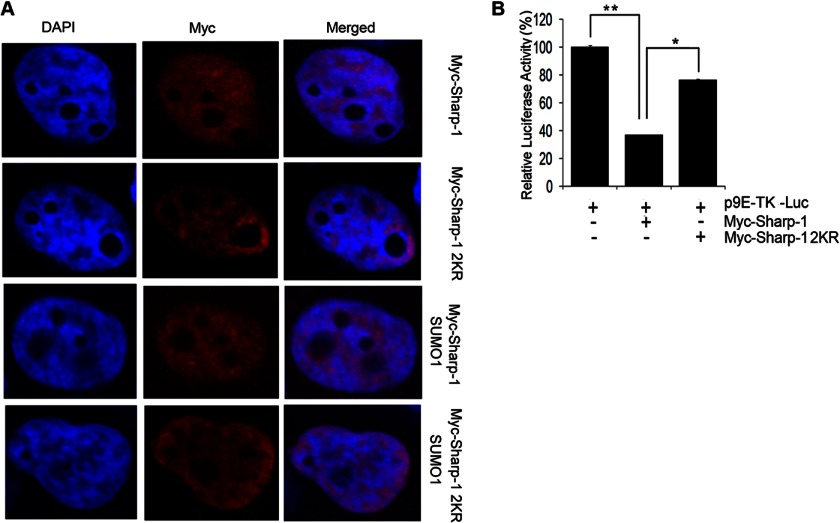
Because sumoylation has been shown to affect subcellular distribution of a number of target proteins, we analyzed localization of Sharp-1 and Sharp-1 2KR. Cells were transfected with Myc-Sharp-1 or Myc-Sharp-1 2KR in the absence and presence of SUMO-1 and visualized by confocal microscopy. Both proteins showed almost identical patterns of localization (Fig. 2A), suggesting that the nuclear localization of Sharp-1 is independent of its sumoylation status. To test the impact of sumoylation in Sharp-1-mediated transcriptional repression, cells were transfected with 9E-TK-Luc, a reporter harboring Sharp-1 binding sites (21). Consistent with previous reports (17), overexpression of wild type Sharp-1 significantly repressed reporter activity. In contrast, Sharp-1 2KR was considerably less potent in mediating transcriptional repression of the reporter (Fig. 2B).
FIGURE 2.
Sumoylation impacts Sharp-1-mediated transcriptional repression but not its subcellular localization. A, COS-7 cells were transfected with Sharp-1 and Sharp-1 2KR alone or together with SUMO-1. 48 h later, cells were fixed and stained with anti-Myc antibody. DAPI was used to stain and visualize nuclei. B, 293T cells were transfected with 9E-TK-Luc reporter (100 ng) along with Sharp-1 (50 ng) or Sharp-1 2KR (50 ng) as indicated. 48 h later, cells were harvested, and luciferase activity was measured. Error bars indicate mean ± S.D. *, p < 0.05; **, p < 0.01.
Sumoylation of Sharp-1 Is Required to Inhibit Myogenic Differentiation
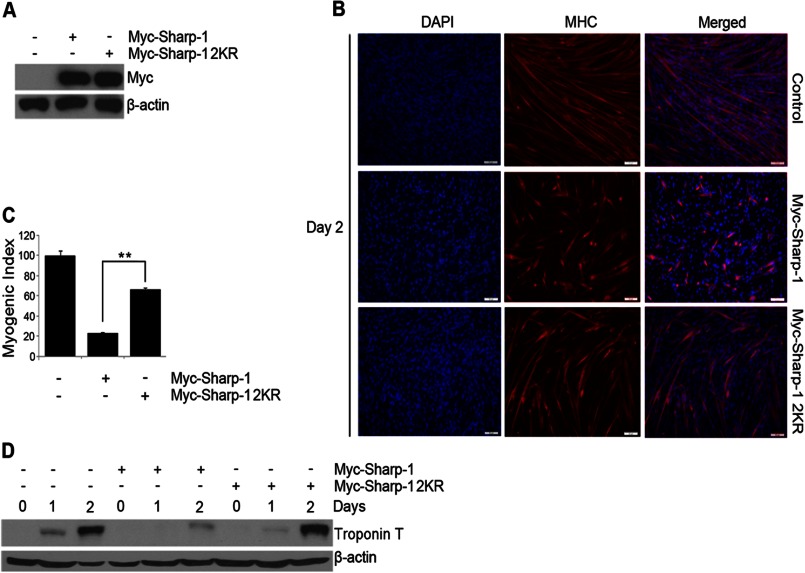
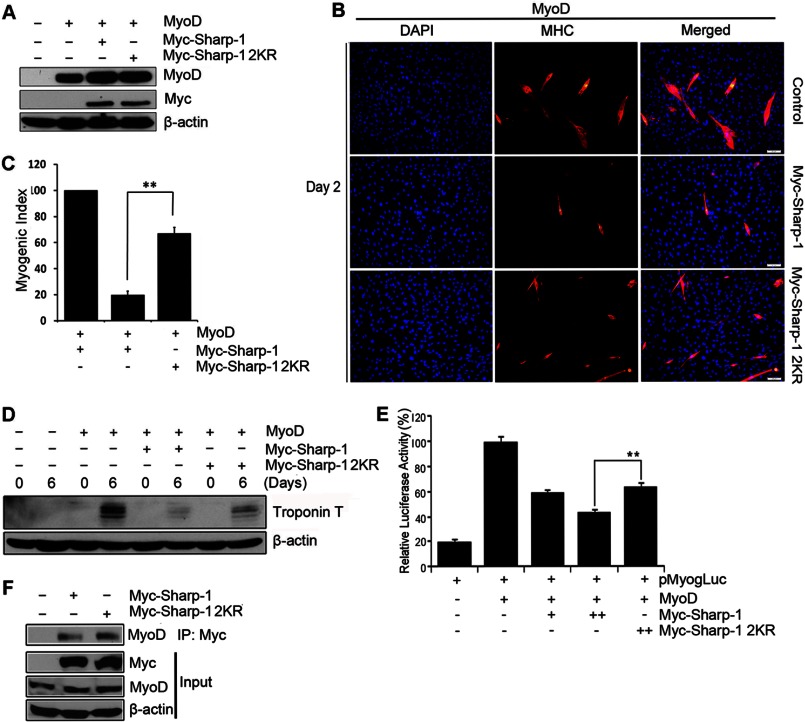
We have previously found that Sharp-1 inhibits the differentiation of skeletal muscle precursor cells (10, 17). To investigate the biological relevance of Sharp-1 sumoylation, we tested the possibility that sumoylation is involved in this process. C2C12 cells were co-transfected with Myc-tagged Sharp-1, Sharp-1 2KR together with the puromycin resistance vector pBabe. Both Sharp-1 and Sharp-1 2KR were expressed at similar levels (Fig. 3A). After selection, cells were analyzed for their ability to differentiate relative to control vector expressing cells. Consistent with our previous reports (10, 17), overexpression of Sharp-1 resulted in significant inhibition in myogenic differentiation as evidenced by a reduced number of terminally differentiated cells expressing MHC compared with vector-transfected cells (Fig. 3, B and C). Interestingly, in contrast to wild type Sharp-1, the SUMO-defective mutant Sharp-1 2KR was not as efficient in inhibiting differentiation (Fig. 3, B and C). To confirm this finding, the expression of troponin T, a differentiation marker was examined. Consistently, troponin T levels were reduced in Sharp-1-expressing cells, whereas Sharp-1 2KR-expressing cells did not show a significant change in the expression of this marker at late stages of differentiation (Fig. 3D).
FIGURE 3.
Mutation of sumoylation sites abrogates the ability of Sharp-1 to suppress myogenesis. A, C2C12 cells were co-transfected with Sharp-1 or Sharp-1 2KR along with a puromycin resistance vector. Empty vector (pCS2) was transfected in control cells. Sharp-1 and Sharp-1 2KR expression was determined by Western blotting with anti-Myc antibody. B and C, MHC staining (B) and myogenic index (C) was assessed in Sharp-1- and 2KR-expressing cells compared with control cells at day 2 of differentiation. D, cell lysates at days 0, 1, and 2 of differentiation from control, Sharp-1, and Sharp-1 2KR cells were analyzed by Western blotting using troponin T antibody. Error bars indicate mean ± S.D. **, p < 0.01.
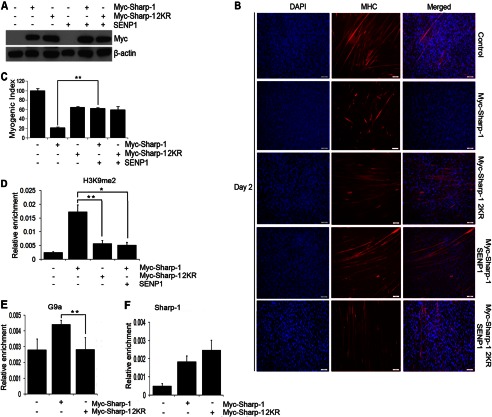
To validate that Sharp-1-mediated muscle differentiation inhibition is SUMO-dependent, we investigated whether desumoylation of Sharp-1 resulted in a phenotype similar to Sharp-1 2KR-expressing cells. The SUMO protease SENP1 was co-transfected with equivalent levels of Sharp-1 and Sharp-1 2KR in C2C12 cells (Fig. 4A). Consistent with a role for sumoylation, inhibition of myogenesis by Sharp-1 was partially reversed with expression of SENP1 (Fig. 4, B and C). On the other hand, SENP1 had no impact on Sharp-1 2KR. We then investigated the molecular mechanisms underlying the differential impact on differentiation between Sharp-1 and Sharp-1 2KR. We have recently demonstrated that Sharp-1 recruits G9a, and correspondingly H3K9me2, is enhanced in Sharp-1-overexpressing cells (17). We therefore analyzed H3K9me2 repression marks in cells expressing Sharp-1 and Sharp-1 2KR. Consistent with our recent report, H3K9me2 was enriched in C2C12 cells expressing Sharp-1 compared with control cells. Interestingly, however, no enrichment was observed in cells expressing equivalent levels of Sharp-1 2KR and Sharp-1 co-transfected with SENP1 (Fig. 4D). Corresponding with enrichment of H3K9me2, G9a occupancy was higher in Sharp-1-overexpressing cells relative to Sharp-1 2KR cells. However, no significant differences were apparent in Sharp-1 recruitment between the two cell lines (Fig. 4, E and F). Together, these results demonstrate that sumoylation of Sharp-1 is important for its ability to inhibit myogenic differentiation and for enrichment of G9a-dependent H3K9me2 marks at the myogenin promoter. These results also suggest that sumoylation may be important for recruitment of G9a.
FIGURE 4.
SENP1 rescues Sharp-1-mediated suppression of myogenesis. A, C2C12 cells were transfected with vector alone (control), Myc-Sharp-1, or Sharp-1 2KR individually or together with SENP1. Lysates were immunoblotted with anti-Myc antibody. B and C, after selection, differentiation was analyzed at day 2 with anti-MHC antibody. Nuclei were stained with DAPI (B). The myogenic index was determined. D, ChIP assays were performed to determine H3K9me2 enrichment at the myogenin promoter in cells expressing vector, Sharp-1, Sharp-1 2KR, and Sharp-1 along with SENP1. E and F, ChIP assay was performed to determine occupancy of G9a (E) and Sharp-1 (F) at the myogenin promoter in cells expressing vector alone, Sharp-1, and Sharp-1 2KR. Error bars indicate mean ± S.D.
Sharp-1 Sumoylation Is Essential for Suppression of MyoD Transcriptional Activity and Function
Because our previous studies have shown that Sharp-1 inhibits MyoD function (17), we examined the impact of Sharp-1 sumoylation specifically on MyoD transcriptional activity and MyoD-dependent myogenic conversion. 10T1/2 cells were transfected with MyoD alone, or with equivalent amount of Sharp-1 and Sharp-1 2KR (Fig. 5A). MyoD-dependent myogenic conversion was inhibited in cells expressing MyoD and Sharp-1. However, Sharp-1 2KR-expressing cells exhibited a higher percentage of MHC+ cells (Fig. 5B) and increased myogenic index (Fig. 5C), indicating that Sharp-1 2KR was less potent in the repression of MyoD function. Consistent with the effect on myogenic differentiation, troponin T expression was reduced to a greater extent in Sharp-1-overexpressing cells relative to control cells, but not in cells expressing Sharp-1 2KR (Fig. 5D). We then tested the impact of Sharp-1 sumoylation on MyoD transcriptional activity, which is inhibited by Sharp-1 (10, 18). 10T1/2 cells were transfected with the myogenin promoter reporter pMyog-Luc (22) along with MyoD alone or together with Sharp-1 or Sharp-1 2KR. Cell extracts were analyzed for luciferase activity. As reported previously, Sharp-1 significantly repressed the myogenin promoter, whereas the 2KR mutant was less effective (Fig. 5E). To examine whether reduced transcriptional repression by Sharp-1 2KR may be a consequence of impaired association with MyoD, we tested interaction of wild type and sumoylation defective Sharp-1 with endogenous MyoD. C2C12 were transfected with equivalent levels of Myc-tagged Sharp-1 and Sharp-1 2KR. Lysates were immunoprecipitated and examined for association with MyoD (Fig. 5F). Consistent with our previous reports, Sharp-1 interacted with MyoD (10). No significant difference was apparent in the ability of Sharp-1 2KR to interact with MyoD indicating that sumoylation is not involved in the association of Sharp-1 and MyoD.
FIGURE 5.
SUMO-modified Sharp-1 inhibits MyoD transcriptional activity and function. A, 10T1/2 cells transfected with MyoD, Myc-Sharp-1, and Myc-Sharp-1 2KR were analyzed for expression of MyoD and Sharp-1 by Western blotting. B–D, myogenic conversion assays were performed in cells transfected with MyoD, MyoD, and Sharp-1, or MyoD and Sharp-1 2KR. 6 days later, differentiated cells were stained with anti-MHC antibody (red). Nuclei were stained with DAPI (B). Differentiation was quantified by plotting myogenic index (C). Troponin T expression was assessed by Western blotting (D). E, 10T1/2 cells were transfected with pMyog-Luc promoter (100 ng) together with MyoD (50 ng), Sharp-1 (25 and 50 ng), or Sharp-1 2KR (50 ng) as indicated. 48 h later, cells were harvested and assayed for luciferase activity. Error bars indicate mean ± S.D. F, C2C12 cells were transfected with Myc-tagged Sharp-1 and Sharp-1 2KR. 24 h later, lysates were immunoprecipitated, and interaction of Sharp-1 and Sharp-1 2KR with endogenous MyoD was analyzed by Western blotting with anti-MyoD antibody. Lysates (input) were immunoblotted with anti-Myc and anti-MyoD antibodies to detect Sharp-1and MyoD expression.
SUMO Modification of Sharp-1 Is Essential for Its Interaction with G9a
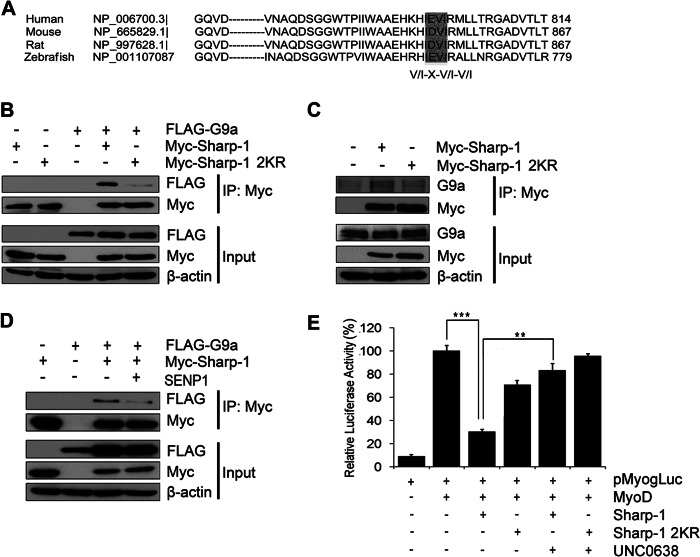
We have recently demonstrated that Sharp-1 interacts with G9a through a region spanning the sumoylation motifs (17). Several reports suggest that protein-protein interactions are SUMO modification-dependent. A SUMO binding motif (SBM) consensus sequence (V/I)X(V/I)(V/I) present in many proteins is known to be important for their recruitment by SUMO-modified transcription factors (23). To investigate the mechanisms that render non-SUMO modified Sharp-1 a less efficient suppressor of myogenesis, we examined the cDNA sequence of G9a. Interestingly, we identified a SBM consensus ID/EVI, which is conserved in G9a from various species (Fig. 6A). Moreover, G9a SBM is within its ankyrin (ANK) repeat sequence, which is essential for G9a interaction with Sharp-1 (17). To examine whether SUMO modification of Sharp-1 is essential for its interaction with G9a, co-immunoprecipitation assays were performed. 293 cells were co-transfected with FLAG-G9a, Myc-Sharp-1, and Myc-Sharp-1 2KR. Consistent with previous studies (17), immunoprecipitation of Sharp-1 revealed its association with G9a (Fig. 6B). Interestingly, the association of Sharp-1 2KR with G9a was greatly reduced. To validate these findings in muscle cells, we examined the interaction of Sharp-1 and Sharp-1 2KR with endogenous G9a in C2C12 cells. Similar to the impact seen in 293 cells, Sharp-1 2KR exhibited reduced interaction with endogenous G9a in C2C12 cells compared with wild type Sharp-1 (Fig. 6C). To corroborate the findings that sumoylation regulates the interaction between G9a and Sharp-1, we examined the impact of SENP1 on the association of Sharp-1 with G9a. Consistent with the reduced interaction of G9a with Sharp-1 2KR, expression of SENP1 reduced association of wild type Sharp-1 and G9a (Fig. 6D). We then tested whether inhibition of G9a impacts SUMO-dependent transcriptional repression mediated by Sharp-1. To address this, the myogenin promoter reporter pMyog-Luc was transfected in 10T1/2 cells along with MyoD, Sharp-1, and Sharp-1 2KR. 24 h later, 0.25 μm UNC0638, a pharmacological inhibitor of G9a methyltransferase activity, was added. In presence of UNC0638, transcriptional repression mediated by Sharp-1 was reversed. However, Sharp-1 2KR-mediated repression was not significantly altered (Fig. 6E). Taken together, these results demonstrate that recruitment of G9a is sumoylation-dependent and impacts the ability of Sharp-1 to mediate transcriptional repression of MyoD transcriptional activity and myogenic differentiation.
FIGURE 6.
SUMO modification of Sharp-1 is critical for interaction with G9a. A, alignment of G9a cDNA sequence from human, mouse, rat, and zebrafish revealed a conserved SBM (highlighted). B, cells transfected with FLAG-G9a, Myc-Sharp-1, or Myc-Sharp-1 2KR were immunoprecipitated with Myc-agarose beads and immunoblotted with anti-FLAG and anti-Myc antibodies. Input (lysates) were analyzed for G9a and Sharp-1 expression. C, C2C12 cells were transfected with Myc-Sharp-1 or Myc-Sharp-1 2KR and immunoprecipitated with Myc-agarose beads, and endogenous G9a interaction was analyzed by immunoblotting with anti-G9a antibody. Lysates (input) were immunoblotted with anti-G9a and anti-Myc antibodies to detect G9a and Sharp-1 expression. D, cells were transfected with Myc-Sharp-1, FLAG-G9a, alone or together with SENP1. Lysates were immunoprecipitated and immunoblotted with anti-FLAG and anti-Myc antibodies. E, 10T1/2 cells were transfected with pMyog-Luc promoter (100 ng) together with MyoD (50 ng), Sharp-1 (50 ng), and Sharp-1 2KR (50 ng) as indicated. 24 h after transfection, UNC0638 was added to cells expressing Sharp-1 and Sharp-1 2KR. 24 h later, cells were harvested and analyzed for luciferase activity. Error bars indicate mean ± S.D.
DISCUSSION
In this study, we report that Sharp-1 undergoes SUMO-1-dependent sumoylation at conserved lysine residues Lys-240 and Lys-255. The E3 SUMO ligases PIAS3 and PIASxα enhance Sharp-1 sumoylation. Mutation of the SUMO acceptor lysine residues does not impact the subcellular localization of Sharp-1; however, it does attenuate its transcriptional repression capacity, abrogate interaction with the chromatin modifier G9a, and impacts its function as a repressor of myogenic differentiation.
Similar to other members of the basic helix-loop-helix-Orange subfamily, Sharp-1 is a potent transcriptional repressor (10, 12, 17). However, the mechanisms underlying transcriptional regulation by Sharp-1 are unclear. Our previous studies linked the transcriptional repression activity of Sharp-1 to its role as an inhibitor of cellular differentiation along the skeletal muscle as well as the adipocytic lineage (10, 17, 24). The repression of myogenic differentiation by Sharp-1 relies on inhibition of MyoD transcriptional activity. This is achieved, in part, through dimerization with MyoD and E-proteins. In addition, Sharp-1 recruits G9a through a C-terminal region spanning amino acid residues 173–265. In support of this, disruption of G9a interaction results in reduced transcriptional repression of MyoD by Sharp-1. Moreover, overexpression of Sharp-1 results in enrichment of H3K9me2 marks that are mediated by G9a at MyoD target promoters. Conversely, loss of G9a expression or activity attenuates the ability of Sharp-1 to repress myogenic differentiation with a concomitant reduction of H3K9me2 occupancy. Collectively, these data demonstrate that G9a mediates transcriptional repression and inhibition of myogenesis by Sharp-1.
Sumoylation plays an important role in the regulation of transcription factor activity and function, which includes an impact on protein stability, cellular localization, DNA-binding, and protein-protein interactions. An emerging theme among these is the role of sumoylation in transcriptional repression by facilitating assembly of complexes that regulate chromatin accessibility and gene expression. Consistent with this notion, SUMO modification at Lys-240 and Lys-255 in Sharp-1 is essential for its full transcriptional repression activity. This is evidenced by the attenuated ability of the SUMO-defective mutant Sharp-1 2KR in mediating repression of MyoD transcriptional activity and MyoD-dependent myogenic conversion of fibroblast cells compared with wild type Sharp-1. Similarly, co-expression of SENP1 with wild type Sharp-1 mimics the phenotype of Sharp-1 2KR cells. Interestingly, sumoylation of Sharp-1 at Lys-240 and Lys-255 creates an interface for recruitment of G9a and possibly assembly of other chromatin modifiers/remodeling complexes. Thus, loss of Sharp-1 sumoylation correlates with reduced G9a-dependent H3K9me2 marks and repression of muscle promoters. These findings are in line with other reports that have demonstrated a link between SUMO modification of transcription factors and transcriptional repression via recruitment of co-repressors. Several studies have linked sumoylation with HDACs. For instance, SBM containing HDAC6 and HDAC2 are recruited by sumoylated p300 and Elk-1, respectively, to target promoters to repress transcription (25, 26). Similarly, sumoylation of Stra13, a basic helix-loop-helix factor related to Sharp-1, facilitates interaction with HDAC1 (20). In addition to G9a, a previous study has demonstrated that Sharp-1 interacts with HDAC1 and Sirt1 (12). However, these interactions map to a different region in Sharp-1 that include amino acids 265–410 for association with HDAC1, and the basic helix-loop-helix domain for Sirt1. Thus, the recruitment of HDAC1 and Sirt1 is not likely to be dependent on sumoylation of Sharp-1.
Although many studies have shown the role of sumoylation in regulation of transcription factor activity, the role of sumoylation in control of cellular differentiation is largely unclear. During myogenic differentiation, the overall levels of SUMO-modified proteins decline (27). While a few transcription factors such as the homeoprotein Msx1 and myocyte enhancer factor (MEF2) are known to be sumoylated, the role of SUMO modification of these transcription factors in regulation of muscle gene expression remains to be clarified. For instance, Msx1 is SUMO-modified and was recently shown to repress myogenesis by recruiting G9a (28, 29). However, the functional relevance of SUMO modification of Msx1 in recruitment of G9a or in the control of myogenesis has not been reported. Similarly, the MEF2 transcription factors, which play a key role during myogenesis by activating muscle-specific genes, are also known to be sumoylated. MEF2A is modified by SUMO-1, and MEF2C and MEF2D are modified by SUMO-2 and SUMO-3 (30, 31). Sumoylation of MEF2 proteins represses their transcriptional activity, but whether it impacts their function in myogenesis is unclear. A recent study demonstrated that Pax7 is SUMO-modified, and sumoylation at Lys-85 is important for Pax7-mediated repression of myogenesis and transactivation of selective target genes (32). However, the mechanisms by which sumoylation of Pax7 regulates its transcriptional activity have not been reported. In this regard, our findings identify a novel regulatory axis that links sumoylation to the control of skeletal muscle differentiation via regulation of G9a recruitment by Sharp-1.
Acknowledgments
We thank M. B. H. Lee, G. Pavlath, M. Rossner, and M. Walsh for various plasmid constructs.
This work was supported by the Singapore Ministry of Health National Medical Research Council Exploratory/Development grant (to R. T.).
- SUMO
- small ubiquitin-like modifier
- HDAC1
- histone deacetylase 1
- SBM
- SUMO binding motif
- MEF2
- myocyte enhancer factor 2.
REFERENCES
- 1. Müller S., Ledl A., Schmidt D. (2004) SUMO: a regulator of gene expression and genome integrity. Oncogene 23, 1998–2008 [DOI] [PubMed] [Google Scholar]
- 2. Gill G. (2003) Post-translational modification by the small ubiquitin-related modifier SUMO has big effects on transcription factor activity. Curr. Opin. Genet. Dev. 13, 108–113 [DOI] [PubMed] [Google Scholar]
- 3. Melchior F. (2000) SUMO–nonclassical ubiquitin. Annu. Rev. Cell Dev. Biol. 16, 591–626 [DOI] [PubMed] [Google Scholar]
- 4. Geiss-Friedlander R., Melchior F. (2007) Concepts in sumoylation: a decade on. Nat. Rev. Mol. Cell Biol. 8, 947–956 [DOI] [PubMed] [Google Scholar]
- 5. Kotaja N., Karvonen U., Jänne O. A., Palvimo J. J. (2002) PIAS proteins modulate transcription factors by functioning as SUMO-1 ligases. Mol. Cell. Biol. 22, 5222–5234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malakhov M. P., Mattern M. R., Malakhova O. A., Drinker M., Weeks S. D., Butt T. R. (2004) SUMO fusions and SUMO-specific protease for efficient expression and purification of proteins. J. Struct. Funct. Genomics 5, 75–86 [DOI] [PubMed] [Google Scholar]
- 7. Gill G. (2005) Something about SUMO inhibits transcription. Curr. Opin. Genet. Dev. 15, 536–541 [DOI] [PubMed] [Google Scholar]
- 8. Girdwood D. W., Tatham M. H., Hay R. T. (2004) SUMO and transcriptional regulation. Semin. Cell Dev. Biol. 15, 201–210 [DOI] [PubMed] [Google Scholar]
- 9. Johnson E. S. (2004) Protein modification by SUMO. Annu. Rev. Biochem. 73, 355–382 [DOI] [PubMed] [Google Scholar]
- 10. Azmi S., Ozog A., Taneja R. (2004) Sharp-1/DEC2 inhibits skeletal muscle differentiation through repression of myogenic transcription factors. J. Biol. Chem. 279, 52643–52652 [DOI] [PubMed] [Google Scholar]
- 11. Azmi S., Taneja R. (2002) Embryonic expression of mSharp-1/mDEC2, which encodes a basic helix-loop-helix transcription factor. Mech. Dev. 114, 181–185 [DOI] [PubMed] [Google Scholar]
- 12. Fujimoto K., Hamaguchi H., Hashiba T., Nakamura T., Kawamoto T., Sato F., Noshiro M., Bhawal U. K., Suardita K., Kato Y. (2007) Transcriptional repression by the basic helix-loop-helix protein Dec2: multiple mechanisms through E-box elements. Int. J. Mol. Med. 19, 925–932 [PubMed] [Google Scholar]
- 13. Fujimoto K., Shen M., Noshiro M., Matsubara K., Shingu S., Honda K., Yoshida E., Suardita K., Matsuda Y., Kato Y. (2001) Molecular cloning and characterization of DEC2, a new member of basic helix-loop-helix proteins. Biochem. Biophys. Res. Commun. 280, 164–171 [DOI] [PubMed] [Google Scholar]
- 14. Li Y., Zhang H., Xie M., Hu M., Ge S., Yang D., Wan Y., Yan B. (2002) Abundant expression of Dec1/stra13/sharp2 in colon carcinoma: its antagonizing role in serum deprivation-induced apoptosis and selective inhibition of procaspase activation. Biochem. J. 367, 413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Azmi S., Sun H., Ozog A., Taneja R. (2003) mSharp-1/DEC2, a basic helix-loop-helix protein functions as a transcriptional repressor of E box activity and Stra13 expression. J. Biol. Chem. 278, 20098–20109 [DOI] [PubMed] [Google Scholar]
- 16. Sun H., Ghaffari S., Taneja R. (2007) bHLH-Orange Transcription Factors in Development and Cancer. Transl. Oncogenomics 2, 107–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ling B. M., Gopinadhan S., Kok W. K., Shankar S. R., Gopal P., Bharathy N., Wang Y., Taneja R. (2012) G9a mediates Sharp-1-dependent inhibition of skeletal muscle differentiation. Mol. Biol. Cell 23, 4778–4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ling B. M., Bharathy N., Chung T. K., Kok W. K., Li S., Tan Y. H., Rao V. K., Gopinadhan S., Sartorelli V., Walsh M. J., Taneja R. (2012) Lysine methyltransferase G9a methylates the transcription factor MyoD and regulates skeletal muscle differentiation. Proc. Natl. Acad. Sci. U.S.A. 109, 841–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee M. B., Lebedeva L. A., Suzawa M., Wadekar S. A., Desclozeaux M., Ingraham H. A. (2005) The DEAD-box protein DP103 (Ddx20 or Gemin-3) represses orphan nuclear receptor activity via SUMO modification. Mol. Cell. Biol. 25, 1879–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Y., Rao V. K., Kok W. K., Roy D. N., Sethi S., Ling B. M., Lee M. B., Taneja R. (2012) SUMO modification of Stra13 is required for repression of cyclin D1 expression and cellular growth arrest. PloS One 7, e43137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rossner M. J., Oster H., Wichert S. P., Reinecke L., Wehr M. C., Reinecke J., Eichele G., Taneja R., Nave K. A. (2008) Disturbed clockwork resetting in Sharp-1 and Sharp-2 single and double mutant mice. PloS One 3, e2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Friday B. B., Horsley V., Pavlath G. K. (2000) Calcineurin activity is required for the initiation of skeletal muscle differentiation. J. Cell Biol. 149, 657–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Song J., Durrin L. K., Wilkinson T. A., Krontiris T. G., Chen Y. (2004) Identification of a SUMO-binding motif that recognizes SUMO-modified proteins. Proc. Natl. Acad. Sci. U.S.A. 101, 14373–14378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gulbagci N. T., Li L., Ling B., Gopinadhan S., Walsh M., Rossner M., Nave K. A., Taneja R. (2009) SHARP1/DEC2 inhibits adipogenic differentiation by regulating the activity of C/EBP. EMBO Rep. 10, 79–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Girdwood D., Bumpass D., Vaughan O. A., Thain A., Anderson L. A., Snowden A. W., Garcia-Wilson E., Perkins N. D., Hay R. T. (2003) P300 transcriptional repression is mediated by SUMO modification. Mol. Cell. 11, 1043–1054 [DOI] [PubMed] [Google Scholar]
- 26. Yang S. H., Sharrocks A. D. (2004) SUMO promotes HDAC-mediated transcriptional repression. Mol. Cell 13, 611–617 [DOI] [PubMed] [Google Scholar]
- 27. Riquelme C., Barthel K. K., Qin X. F., Liu X. (2006) Ubc9 expression is essential for myotube formation in C2C12. Exp. Cell Res. 312, 2132–2141 [DOI] [PubMed] [Google Scholar]
- 28. Gupta V., Bei M. (2006) Modification of Msx1 by SUMO-1. Biochem. Biophys. Res. Commun. 345, 74–77 [DOI] [PubMed] [Google Scholar]
- 29. Wang J., Abate-Shen C. (2012) The MSX1 homeoprotein recruits G9a methyltransferase to repressed target genes in myoblast cells. PloS One 7, e37647. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30. Grégoire S., Yang X. J. (2005) Association with class IIa histone deacetylases upregulates the sumoylation of MEF2 transcription factors. Mol. Cell. Biol. 25, 2273–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Riquelme C., Barthel K. K., Liu X. (2006) SUMO-1 modification of MEF2A regulates its transcriptional activity. J. Cell. Mol. Med. 10, 132–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luan Z., Liu Y., Stuhlmiller T. J., Marquez J., García-Castro M. I. (2013) SUMOylation of Pax7 is essential for neural crest and muscle development. Cell Mol Life Sci. 70, 1793–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]