Background: The activating NK receptor NKp80 triggers cytotoxicity by human NK cells via a cytoplasmic hemITAM sequence.
Results: A non-consensus hemITAM residue impairs the capacity of NKp80 to recruit Syk kinase and to trigger cytotoxicity.
Conclusion: Unlike typical hemITAM receptors, NKp80 does not efficiently recruit Syk kinase resulting in attenuated effector responses.
Significance: An attenuated cytotoxic responsiveness critically impacts on the immunomodulatory function of NKp80.
Keywords: Cell Signaling, Cell Surface Receptor, Cellular Immune Response, Immunology, Innate Immunity, Natural Killer (NK) Cell, Signaling, NK Receptor, Syk Kinase
Abstract
Cellular cytotoxicity is the hallmark of NK cells mediating both elimination of virus-infected or malignant cells, and modulation of immune responses. NK cytotoxicity is triggered upon ligation of various activating NK cell receptors. Among these is the C-type lectin-like receptor NKp80 which is encoded in the human Natural Killer Gene Complex (NKC) adjacent to its ligand, activation-induced C-type lectin (AICL). NKp80-AICL interaction promotes cytolysis of malignant myeloid cells, but also stimulates the mutual crosstalk between NK cells and monocytes.
While many activating NK cell receptors pair with ITAM-bearing adaptors, we recently reported that NKp80 signals via a hemITAM-like sequence in its cytoplasmic domain. Here we molecularly dissect the NKp80 hemITAM and demonstrate that two non-consensus amino acids, in particular arginine 6, critically impair both hemITAM phosphorylation and Syk recruitment. Impaired Syk recruitment results in a substantial attenuation of cytotoxic responses upon NKp80 ligation. Reconstituting the hemITAM consensus or Syk overexpression resulted in robust NKp80-mediated responsiveness. Collectively, our data provide a molecular rationale for the restrained activation potential of NKp80 and illustrate how subtle alterations in signaling motifs determine subsequent cellular responses. They also suggest that non-consensus alterations in the NKp80 hemITAM, as commonly present among mammalian NKp80 sequences, may have evolved to dampen NKp80-mediated cytotoxic responses toward AICL-expressing cells.
Introduction
Cytotoxic lymphocytes of the mammalian immune system police other self-cells for signs of threats such as viral infection or malignant transformation. While they share the same effector mechanisms eventually resulting in the death of self-cells identified as dangerous, they are subdivided by their molecular threat-recognizing sensors into CD8 αβ T cells, γδ T cells, and Natural Killer (NK)2 cells, respectively. While the former use T cell receptors as dominating recognition and activation units, NK cells employ a vast array of different receptor systems to detect deviations of normal self-cellular surfaces, probably reflecting their functional versatility (1, 2). For example, the NKG2D receptor enables NK cells to recognize infected and malignant cells via interaction with a number of cell stress-induced MHC class I-like molecules resulting in cytolysis of the harmful cells (3, 4). NK cell cytotoxicity, however, is also deployed to govern immune responses, e.g. by cytolysis of immature Dendritic Cells or activated T cells (2, 5, 6).
Receptors activating cellular cytotoxicity of human NK cells include receptors of the immunoglobulin-like (Ig)-like superfamily, such as the Natural Cytotoxicity Receptors (NCRs) NKp30, NKp44, and NKp46, the IgG receptor CD16, and C-type lectin-like receptors such as NKG2D and NKp80 (1, 2, 4). Most of these receptors associate with adaptor proteins such as CD3ζ (e.g. NKp30, NKp46, CD16), DAP12 (e.g. NKp44), or DAP10 (e.g. NKG2D) that contain tyrosine-based signaling motifs relaying activating signals via recruiting kinases to their phosphotyrosines (1, 7, 8). These signaling motifs are mostly ITAMs, which are tandem tyrosine units recruiting the tyrosine kinases Syk or ZAP-70 (1, 7–9). DAP10 contains a phosphatidylinositol-3 kinase (PI3K)-recruiting YXXM motif (1, 7–9).
Recently, we provided the first insights into signaling by the C-type lectin-like receptor (CTLR) NKp80 (10). NKp80 is expressed by virtually all human NK cells, and subsets of effector memory CD8 αβ T cells and γδ T cells, and stimulates their cytotoxic effector responses (11–13). NKp80 is also found on cytotoxic lymphocytes of primates, but is absent from rodents (14, 15). In humans, NKp80 ligates activation-induced C-type lectin (AICL) and thereby promotes cytolysis of malignant myeloid cells and mutual activation of NK cells and monocytes under inflammatory conditions (13). Notably, NKp80 and AICL are encoded just 7 kb apart in a tail-to-tail orientation in the human Natural Killer Gene Complex (NKC) by the genes KLRF1 and CLEC2B, respectively (13, 16).
In contrast to other activating NK receptors, NKp80 does not associate with the abovementioned adaptor proteins, but utilizes a tyrosine-containing sequence motif at the membrane-distal amino terminus of its cytoplasmic domain for signal initiation (10). A similar sequence motif was previously reported for some myeloid-specific CTLR such as Dectin-1 or CLEC-2 (17, 18). Since this motif resembles a single tyrosine unit of an ITAM, it has been termed hemITAM (17, 18). We previously reported that mutation of tyrosine 7 of the hemITAM completely abolishes the capacity of NKp80 to trigger cellular cytotoxicity (10). The corresponding tyrosine in hemITAMs of myeloid-specific CTLR has been shown, upon phosphorylation, to recruit Syk for signal transduction (17, 18). Our previously reported data strongly suggested critical involvement of Syk for NKp80 signal transduction, however, a direct physical interaction of Syk with tyrosine-phosphorylated NKp80 remained to be addressed (10).
Hence, we set out to scrutinize the hemITAM-like sequence of NKp80 for its capacity to recruit Syk and to activate NK cell cytotoxicity to obtain a better understanding of the signaling mechanisms and function of this unique human NK cell receptor.
EXPERIMENTAL PROCEDURES
Reagents
NKp80 mAb 5D12 was previously described (13) and used for flow cytometry as an AlexaFluor647-conjugate. Monoclonal Ab specific for pY (P-Tyr-100, Cell Signaling), β-actin (AC-15, peroxidase conjugate, Sigma), CD107a (APC conjugate, BD Pharmingen) were purchased, as well as sheep polyclonal Ab specific for NKp80 (R&D Systems), and Syk (Cell Signaling). Inhibitors piceatannol and PP2 were from Calbiochem and Sigma, respectively.
Cells and Cellular Transduction
Human peripheral blood lymphocytes (PBL) were isolated from healthy donors by density gradient centrifugation and subsequent depletion of plastic-adherent cells. For expansion of NK cells, PBL were cultured for 12 to 17 days on irradiated RPMI8866 feeder cells in RPMI1640 with 10% FCS and 100 units of IL-2/ml (Roche, Penzberg, Germany). Thereafter, about 70–80% of expanded cells represented CD3−CD56+ NK cells as determined by flow cytometry, and subsequently were used for immunoprecipitation.
NK-92MI cells (19) were maintained in IMDM with 10% FCS and 10% horse serum. The open reading frame of NKp80 with a carboxyl-terminal FLAG tag was cloned into pMXsIP (kindly provided by Toshio Kitamura, University of Tokyo) (20). NKp80 mutants were generated using pMXsIP-NKp80 as a template and the QuikChange site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions. Wild-type Syk and kinase-defective (KD) Syk constructs were cloned into pczCFG5IEGZ (kind gift of Ingolf Berberich, University of Würzburg, Germany). Retroviruses were generated using phoenix-ampho cells (Nolan Laboratory, Stanford University) for transduction of NK-92MI cells using standard protocols. NK-92MI cells with ectopic expression of both NKp80 and Syk were generated by subsequent transduction of pMXsIP-NKp80-transduced NK92MI cells with pczCFG5IEGZ-Syk constructs. NK-92MI cells stably expressing NKp80 were selected with 5 μg/ml puromycin (for pMXsIP), and for stable co-expression of Syk additionally with 0.3 mg/ml zeocin (for pczCFG5IEGZ). NKp80 surface expression was monitored with mAb 5D12 in flow cytometry. Syk expression was assessed by GFP co-expression in flow cytometry or by immunoblotting.
Cytotoxicity Assay
NKp80 variants expressed by NK-92MI transductants were tested for their capacity to trigger NK cytotoxicity in redirect cytolysis assays with antibody-loaded FcγR+ P815 cells as stimulators. P815 cells were labeled with 51Cr (Perkin Elmer) for 2 h at 37 °C. After washing, 51Cr-labeled P815 cells were co-cultured for 4 h with NK-92MI transductants in the presence of 0.5 μg/ml mAb 5D12 at different effector (E) to target (T) ratios. Subsequently, supernatants were mixed with OptiPhase Supermix scintillation mixture (PerkinElmer, Waltham) in an IsoPlate-96 and measured with a MicroBeta2 plate counter (PerkinElmer). Spontaneous release of target cells alone was less than 15% of the maximum release taken from target cells lysed in 1% Triton X-100. Percentage of lysis was calculated as follows: 100 × (experimental release-spontaneous release)/(maximum release-spontaneous release). Experiments were performed in triplicates.
Flow Cytometry and Degranulation Assays
NK cells were incubated with Alexa647-conjugated mAb 5D12 or the appropriate mouse isotype control in FACS buffer (PBS supplemented with 2 mm EDTA, 2% FCS, and 0.02% NaN3). After washing with FACS buffer, flow cytometric data were acquired using a FACSCanto II (BD Biosciences). Data analysis was done using CellQuest and FloJo software (BD Biosciences). For assessment of degranulation triggered by NKp80, 5D12, or IgG1 control were coated into 96-well flat-bottom cell culture plates (0.5 μg per well). After washing, 105 NK-92MI cells were added to each well in 0.1 ml medium containing anti-CD107a-APC (BD Pharmingen). After incubation for 2 h at 37 °C, cells were washed and analyzed by flow cytometry as described above.
Inhibitor Treatment, Immunoprecipitation, and Immunoblotting
After re-warming to 37 °C for 1 min, NK-92MI cells were treated with 2 mm pervanadate for the indicated times, and subsequently lysed by addition of lysis buffer (20 mm Tris/HCl, pH 7.5, 1.1% Nonidet P-40, 140 mm NaCl, 5 mm EDTA, 5 mm iodoacetamide, 10 mm Na4P2O7, 10 mm NaF, 1 mm Na3VO4, Complete protease inhibitor mixture (Roche)). For inhibition of Src- or Syk-family kinases, cells were pre-incubated for 20 min at room temperature with PP2 or piceatannol, respectively, before adding pervanadate (for 2 min) and subsequent cell lysis. Supernatants of cell lysates were added to 20 μl of protein A/G-Sepharose beads (Pierce) pre-coated with 10 μg 5D12 for immunoprecipitation. Following incubation at 4 °C for 2 h, beads were repeatedly washed, incubated with PNGaseF (New England Biolabs) following the manufacturer's instructions and, after addition of SDS-PAGE sample buffer, boiled for 5 min. After SDS-PAGE and blotting, PVDF membranes were probed with relevant antibodies and developed using enhanced chemiluminescence (Pierce).
RESULTS
Impaired Syk Recruitment by the NKp80 HemITAM in NK Cells
Recently, we reported that the immunoreceptor NKp80 triggers cytotoxicity by human NK cells via an atypical hemITAM and provided several lines of evidence for involvement of Syk kinase in the signaling pathway (10). HemITAMs of myeloid C-type lectin-like receptors such as Dectin-1 or CLEC-2 are known to directly recruit and to physically interact with Syk kinase upon phosphorylation (18, 21). Accordingly, Syk could be precipitated from cell lysates using synthetic peptides comprising the phosphorylated hemITAM of NKp80 (10). However, while these data indicated a direct interaction of Syk with phosphorylated NKp80, initial attempts to detect Syk in immunoprecipitates of NKp80 from lysates of NKp80-transduced NK-92MI cells (NK-92MI-NKp80) failed, raising the possibility that Syk may not or only inefficiently associate with phosphorylated NKp80 in the context of NK cells. These ambiguous results prompted us to investigate the NKp80 hemITAM in more detail.
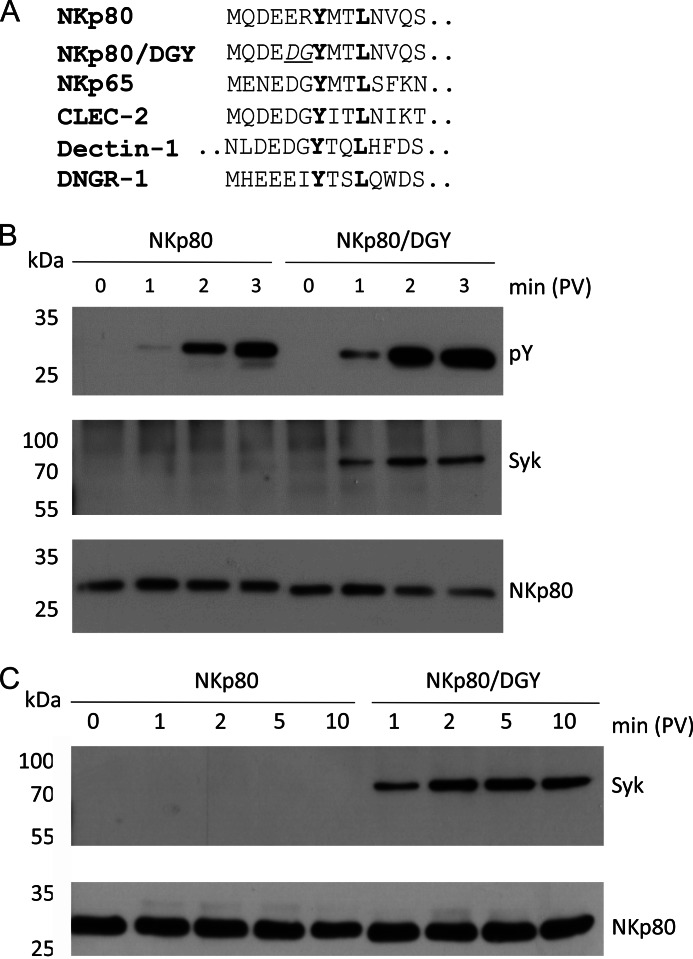
The hemITAM of NKp80 can be considered anomalous, because the two amino acids preceding the tyrosine of the hemITAM in NKp80 (ERYXXL) deviate from the hemITAM consensus (DGYXXL) deduced from the Syk-recruiting hemITAMs of Dectin-1 and CLEC-2 (Fig. 1A and (22)). Previously, we had shown that a mutant of NKp80 with these two amino acids substituted by the consensus amino acids (here: mutant DGY; Fig. 1A) is phosphorylated faster and at a higher stoichiometry than NKp80, and appeared more potent in triggering NK effector responses (10). To compare wild-type NKp80 and NKp80/DGY for Syk recruitment, NK-92MI cells stably transduced with NKp80 or NKp80/DGY were treated with pervanadate to achieve optimal phosphorylation of the hemITAM as a prerequisite for Syk recruitment. Subsequently, NKp80 precipitates of cellular lysates were probed for tyrosine phosphorylation and Syk co-immunoprecipitation. As previously reported, (10) we consistently observed a delayed and less pronounced tyrosine phosphorylation of NKp80 as compared with NKp80/DGY (Fig. 1B). Most notably, while we routinely and unambiguously detected Syk in the precipitates of NKp80/DGY, Syk remained undetectable in NKp80 precipitates (Fig. 1, B and C). These data clearly demonstrated that glutamate 5 and/or arginine 6 preceding the tyrosine of the hemITAM of NKp80 cause attenuated NKp80 phosphorylation and impaired Syk recruitment in NK-92MI cells.
FIGURE 1.
Syk recruitment by NKp80 is hampered by alterations of the consensus hemITAM sequence. A, hemITAM-containing amino terminal cytoplasmic sequences of the human C-type lectin-like receptors NKp80 (gene: KLRF1), NKp65 (KLRF2), CLEC-2 (CLEC1B), Dectin-1 (CLEC7A), and DNGR-1 (CLEC9A), respectively, and of the NKp80 mutant NKp80/DGY with a reconstituted hemITAM consensus. The first 14 amino acids (aa) are shown (Dectin-1: aa 9 to 22). B and C, NKp80 was immunoprecipitated from lysates of NK-92MI-NKp80 or NK-92MI-NKp80/DGY cells, respectively, after various times of pervanadate treatment using anti-NKp80 mAb 5D12. Tyrosine-phosphorylated NKp80 (pY) and co-immunoprecipitated Syk were detected by immunoblotting after removal of N-linked carbohydrates and reducing SDS-PAGE. Precipitated amounts of NKp80 were monitored for control with a polyclonal anti-NKp80 reagent. Comparable expression of NKp80 and NKp80/DGY by NK-92MI cells is shown in Fig. 5A.
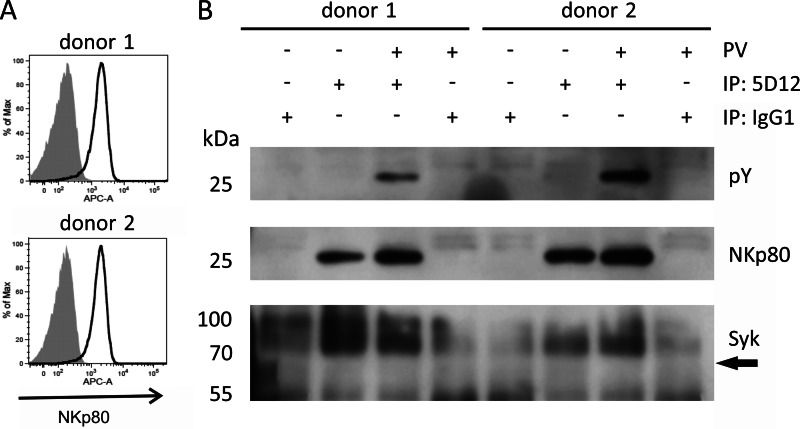
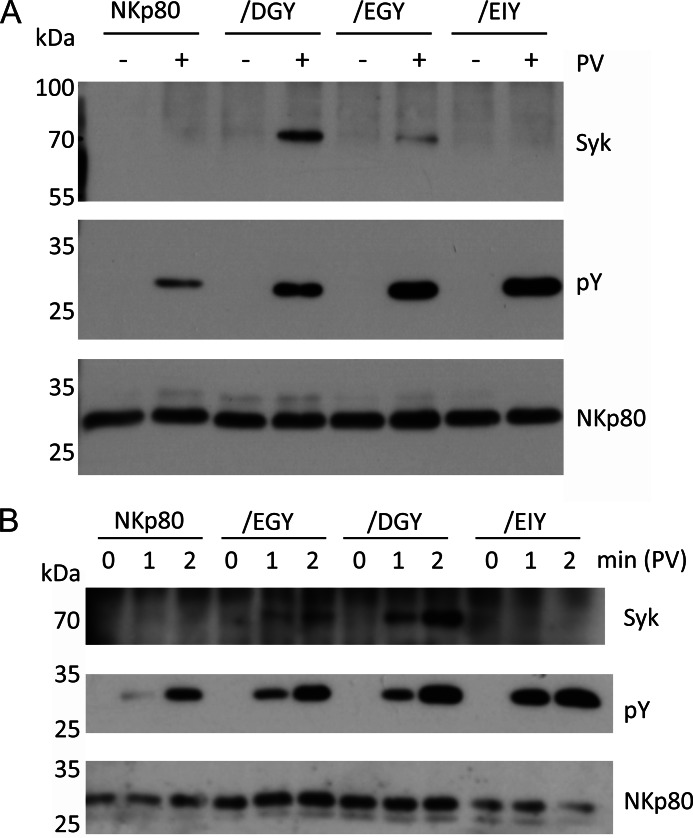
To assess whether these findings gained with an immortalized NK cell line also apply to primary NK cells, we studied the latter for NKp80 phosphorylation and Syk recruitment. Hence, NKp80 was precipitated from lysates of pervanadate-treated NK cells obtained from healthy donors and expanded in vitro in co-cultures with RPMI8866 cells. In line with the results from NK-92MI cells, tyrosine phosphorylation of NKp80 was readily detected for pervanadate-treated, but not for untreated NK cells (Fig. 2). Also, there was no Syk detectable in NKp80 precipitates, even though the experiment was scaled up to achieve maximum sensitivity and Syk was detectable in NK cell lysates (Fig. 6A). With the caveat that this experiment did not allow for a positive control, the results indicate that similarly to NK-92MI cells, recruitment of Syk by phosphorylated NKp80 is impaired in primary human NK cells.
FIGURE 2.
NKp80 of human NK cells is phosphorylated, but fails to recruit Syk. A, NKp80 surface expression by NK cells expanded in co-cultures with RPMI8866 cells from PBL of two healthy donors. Expanded NK cells (gate: CD3−CD56+) were stained with mAb NKp80 (solid lines) or an isotype control (filled) and analyzed by flow cytometry. B, NKp80 from lysates of pervanadate (PV)-treated or untreated human NK cells was immunoprecipitated with anti-NKp80 mAb 5D12 and precipitates were analyzed for NKp80 (polyclonal anti-NKp80 reagent), tyrosine phosphorylation (pY), or presence of Syk by immunoblotting after removal of N-linked carbohydrates. Arrow indicates anticipated position of Syk. Immunoprecipitations (IP) with an isotype control IgG1 is shown for control.
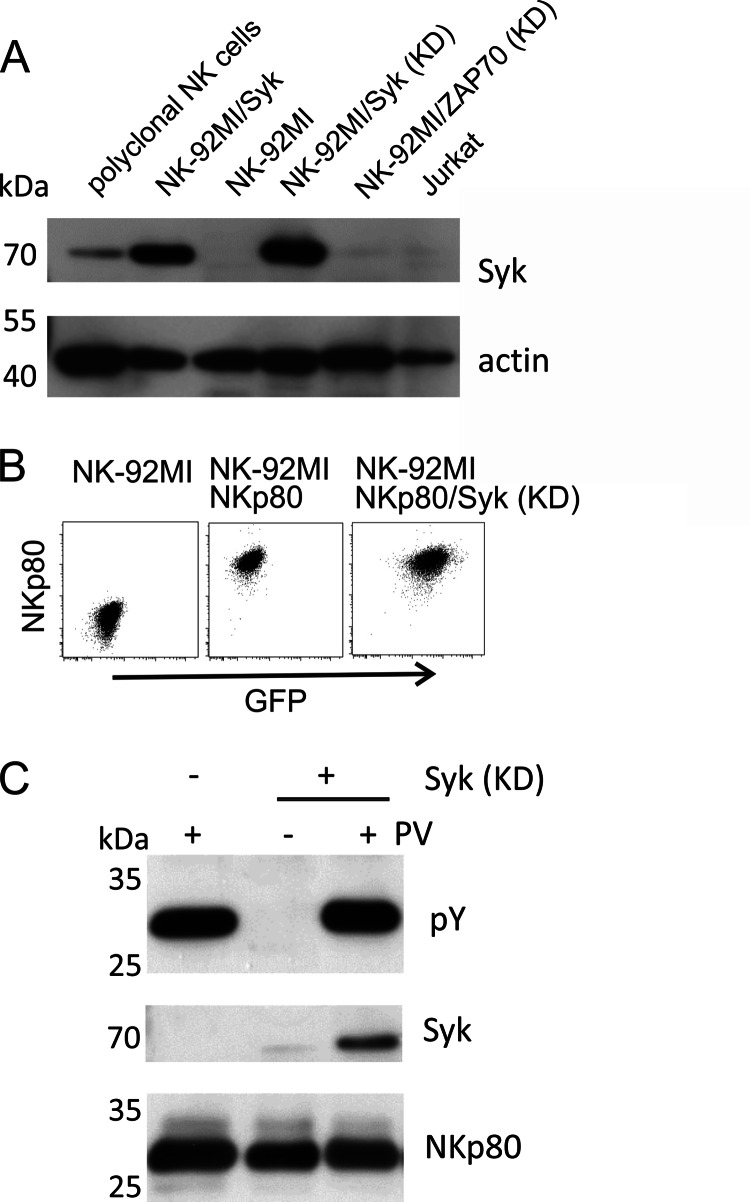
FIGURE 6.
Syk association with NKp80 is detectable upon Syk overexpression. A, immunoblots of lysates of expanded, polyclonal NK cells, Jurkat cells, and NK-92MI transduced with wildtype or kinase-deficient (KD) mutants of Syk or ZAP70 (B) FACS plots of NK-92MI cells ectopically expressing NKp80 or NKp80 in conjunction with Syk (KD). Syk expression is indicated by co-expressed GFP. C, NKp80 was precipitated from lysates of untreated or pervanadate-treated NK-92MI cells ectopically expressing NKp80 alone or in conjunction with Syk KD using mAb 5D12. Precipitates were assayed for tyrosine phosphorylation and co-precipitated Syk by immunoblotting. NKp80 precipitation was monitored with a polyclonal anti-NKp80 reagent.
Arginine 6 Hampers hemITAM Phosphorylation
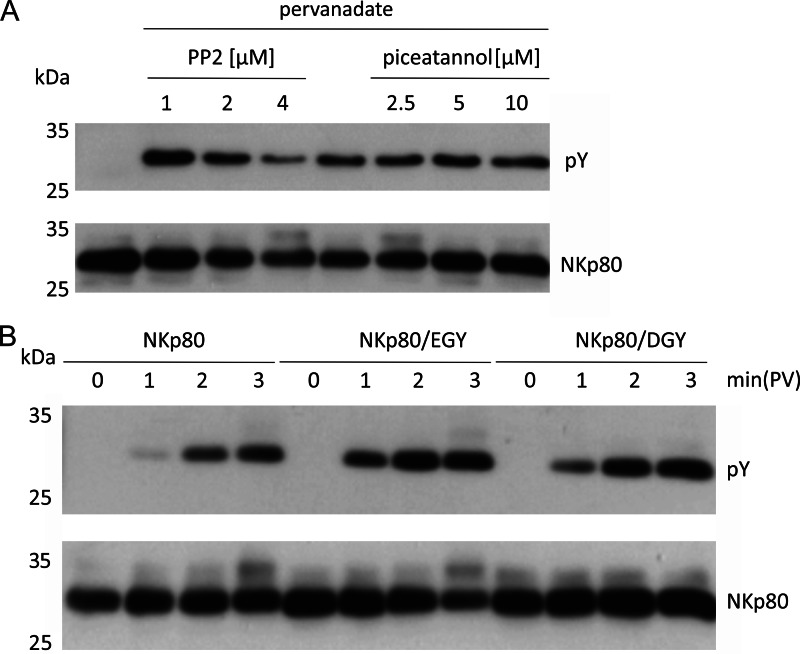
Most immunoreceptors are phosphorylated by Src-family kinases upon ligand engagement, but also an involvement of Syk in the phosphorylation of a hemITAM-bearing immunoreceptor (i.e. CLEC-2) was recently shown (23, 24). To assess involvement of Src-family kinases versus Syk kinases in the phosphorylation of NKp80, we treated NK-92MI-NKp80 cells with inhibitors for Src-family kinases (i.e. PP2) or Syk (i.e. piceatannol), respectively, followed by a short pervanadate treatment and subsequently assayed for tyrosine phosphorylation of precipitated NKp80. While piceatannol treatment had no effect on NKp80 phosphorylation, treatment with increasing concentrations of PP2 was paralleled by a decrease in NKp80 phosphorylation suggesting that NKp80 is phosphorylated by Src kinases (Fig. 3A). It is known that substrate specificity of Src kinases is determined by acidic and hydrophobic amino acids preceding the tyrosine to be phosphorylated (25). Hence, we assumed that arginine 6 of NKp80 may cause the delayed and less efficient tyrosine phosphorylation observed. To directly address this presumption, NKp80 with a single arginine to glycine mutation at position 6 (NKp80/EGY) was stably expressed in NK-92MI cells and tested together with NKp80 and NKp80/DGY for tyrosine phosphorylation. In accord with the presumption, NKp80/EGY was phosphorylated faster than NKp80, but comparable to NKp80/DGY (Figs. 3B and 4B) demonstrating that arginine 6 of NKp80 hampers NKp80 phosphorylation.
FIGURE 3.
Arginine 6 of the NKp80 hemITAM impedes NKp80 phosphorylation by Src kinases. A, NKp80 phosphorylation is Src-family kinase-dependent. NK-92MI-NKp80 cells were pretreated for 20 min with various concentrations of the Src-family kinase inhibitor PP2, or the Syk kinase inhibitor piceatannol, respectively. Before cell lysis, cells were pervanadate-treated (2 min) and precipitated NKp80 assayed for tyrosine phosphorylation. B, impaired tyrosine phosphorylation of NKp80 can be reversed by substituting arginine for glycine at position 6. NK-92MI-NKp80, NK-92MI-NKp80/EGY, or NK-92MI-NKp80/DGY transductants were treated for various times with pervanadate before cell lysis, NKp80 precipitation, and assessment of NKp80 tyrosine phosphorylation. A and B, NKp80 was precipitated from lysates using mAb 5D12 and assayed for tyrosine phosphorylation by immunoblotting. Precipitated amounts of NKp80 were monitored with a polyclonal anti-NKp80 reagent. Comparable expression of NKp80 and NKp80 mutants by NK-92MI cells is shown in Fig. 5A.
FIGURE 4.
Glutamate 5 and arginine 6 of the NKp80 hemITAM jointly contribute to impaired Syk recruitment by NKp80. A and B, substitution of both arginine 6 and glutamate 5 to the hemITAM consensus amino acids glycine 6 and aspartate 5 is strictly required for efficient Syk recruitment by NKp80. NK-92MI cells transduced with NKp80, NKp80/EGY, NKp80/EIY, or NKp80/DGY, respectively, were treated (A) for 5 min or (B) for various times with pervanadate before cell lysis, or left untreated. NKp80 was precipitated from lysates using mAb 5D12 and precipitates were assayed for tyrosine phosphorylation and co-precipitated Syk by immunoblotting. Precipitated amounts of NKp80 were monitored with a polyclonal anti-NKp80 reagent.
Glutamate 5 and Arginine 6 Both Contribute to Impaired Syk Recruitment by NKp80
Based on these results, it was possible that hampered tyrosine phosphorylation due to arginine 6 may be causative for the impaired Syk recruitment of NKp80. In fact, Syk co-immunoprecipitated with NKp80/EGY, though clearly less efficiently than with NKp80/DGY (Fig. 4, A and B). This was unexpected as these mutants only differ at position 5 by the rather similar acidic amino acids glutamate and aspartate. Further informative insights were obtained from the analysis of the NKp80 mutant EIY. Here, we substituted arginine 6 by isoleucine corresponding to the hemITAM sequence of DNGR-1 (Fig. 1A), a C-type lectin-like receptor expressed on a subset of dendritic cells and specifically detecting exposed actin filaments of necrotic cells (26–28). While NKp80/EIY was phosphorylated as efficiently as NKp80/DGY, there was clearly no Syk detectable in NKp80/EIY precipitates (Fig. 4).
Collectively, these results show the following: (i) arginine 6 (replacing the consensus glycine) immediately preceding the tyrosine of the NKp80 hemITAM hampers efficient tyrosine phosphorylation and Syk recruitment; (ii) isoleucine 6 does not affect tyrosine phosphorylation, but impairs Syk recruitment; (iii) Syk recruitment is not only affected by alterations at position 6, but also by a rather minor amino acid substitution at position 5, (iv) Syk recruitment is impaired independently of efficient phosphorylation of tyrosine 7.
Attenuated Cytotoxic Capacity of NKp80 Is Due to HemITAM Modifications Impairing Syk Recruitment
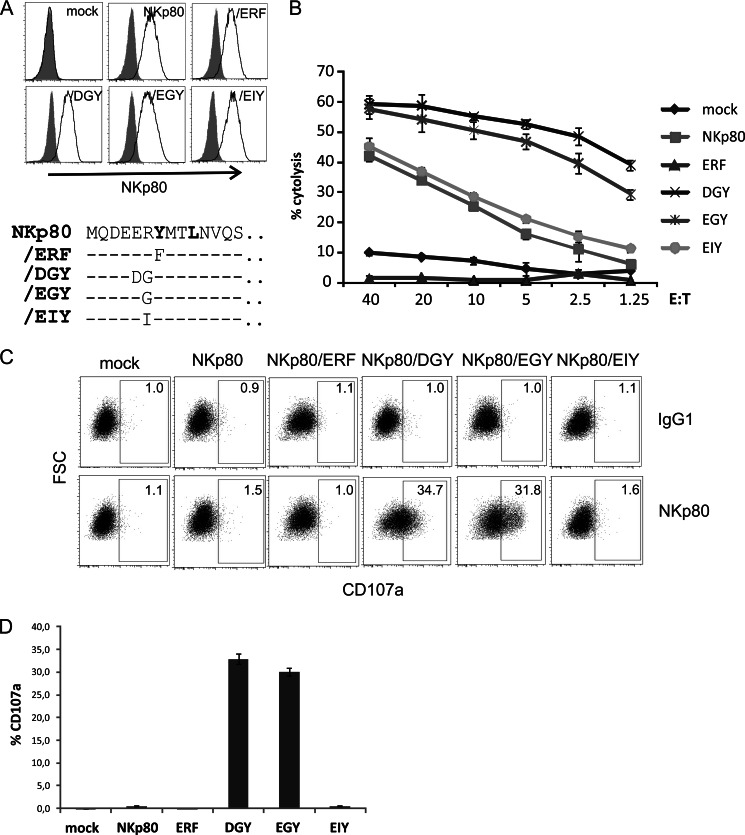
Next, we assessed functional implications of the biochemical findings described above. To address the triggering capacity of the NKp80 mutants, the respective NK-92MI transductants, which express these mutants at comparable levels at the cell surface (Fig. 5A), were subjected to cytotoxicity and degranulation assays. First, the capacity to trigger NK-mediated cytolysis was tested in a redirected cytotoxicity assay with Fc-receptor bearing P815 cells loaded with anti-NKp80 mAb 5D12. NKp80/DGY was substantially more potent in activating cytotoxicity than wild-type NKp80, whereas mutation of tyrosine 7 to phenylalanine (NKp80/ERF) fully abrogated NKp80 function (Fig. 5B). Of note, while the activating capacity of NKp80/EGY paralleled that of NKp80/DGY, the activating capacity of NKp80/EIY was comparable to that of wild-type NKp80 (Fig. 5B). This showed that the potency to trigger cytotoxic responses correlated with the capacity to recruit Syk, but not with the kinetics of tyrosine phosphorylation. In a next set of experiments, degranulation of NK-92MI transductants following NKp80 crosslinking by plate-bound mAb 5D12 was analyzed. Significant degranulation was only observed for NKp80/DGY and NKp80/EGY, but not with NKp80, NKp80/EIY, or NKp80/ERF, respectively (Fig. 5, C and D). Again, these functional data revealed a clear-cut difference in triggering capacity between NKp80 mutants able to recruit Syk (i.e. NKp80/DGY and/EGY), and NKp80 or NKp80 variants where Syk recruitment is impaired.
FIGURE 5.
Substitution of glycine 6 in the consensus hemITAM attenuates NKp80-mediated cytotoxicity. A, comparable surface expression of NKp80 mutants by transduced NK-92MI cells. NK-92MI cells stably transduced with NKp80 or NKp80 mutants/ERF,/DGY,/EGY, or/EIY, respectively, were stained with mAb 5D12 (solid lines) or an isotype control (filled) and analyzed by flow cytometry. Lower panel: Relevant aminoteminal sequences of NKp80 mutants are shown (aa 1 to 14) with dashes indicating amino acids identical to NKp80 sequence. B, attenuated NKp80-mediated cytotoxicity is associated with arginine 6 or isoleucine 6 replacing glycine 6 of the hemITAM consensus. NK-92MI-NKp80, NK-92MI-NKp80/EGY, NK-92MI-NKp80/ERF, NK-92MI-NKp80/EIY, NK-92MI-NKp80/DGY, or mock-transduced NK-92MI cells were co-cultivated for 4 h with 51Cr-labeled P815 cells in the presence of anti-NKp80 mAb 5D12 in a redirect cytolysis assay to determine cytotoxic potency of the NKp80 mutants. C and D, degranulation of NK-92MI transductants is strictly dependent on glycine 6 in the hemITAM. NK-92MI transductants were incubated for 2 h in 96-well plates coated with anti-NKp80 mAb 5D12 or an isotype control (IgG1) and subsequently assessed for cell surface CD107a exposure by flow cytometry. C, representative dot plots of 5D12- (lower panels) or IgG1 control-stimulated (upper panels) and (D) means of triplicates with standard deviations of one representative experiment out of three experiments.
Enforced Expression of Syk Promotes Syk Recruitment by NKp80 and Enhances NKp80-mediated Effector Responses
Next, we raised the question whether failure to detect Syk recruitment by NKp80 in cellular lysates may be due to insurmountable structural constraints excluding interaction of Syk with NKp80 or due to a lower affinity of NKp80 for Syk and, hence, more pronounced competition of Syk with other cellular proteins for NKp80 binding. The latter was suggested by our earlier findings of Syk binding to synthetic NKp80 phosphopeptides (10) and by the fact, that NK-92MI cells only contain rather low levels of Syk (Fig. 6A).
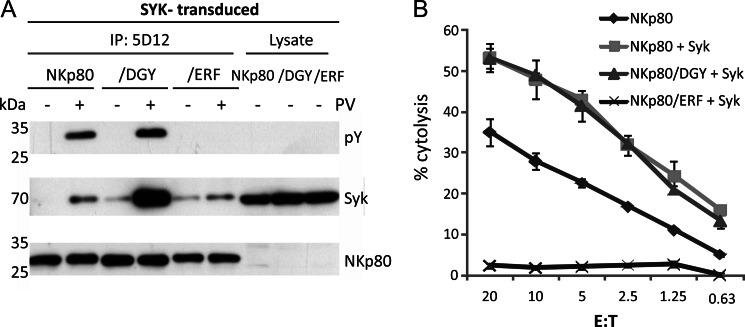
In case Syk unfavorably competes with other cellular proteins for association with phosphorylated NKp80, enforced expression of Syk may promote Syk association with NKp80. To address this presumption, we made use of the previously reported NK-92MI transductant expressing both NKp80 and a kinase-deficient form of Syk (KD-Syk) (10). These transductants express high amounts of KD-Syk that inhibits NKp80-mediated signaling (Fig. 6, A and B) (10). In contrast to NKp80 precipitates from NK-92MI-NKp80 cells, NKp80 precipitates from NK-92MI/NKp80 cells with KD-Syk contained Syk in detectable amounts (Fig. 6C). These findings prompted us to establish NK-92MI-NKp80 cells with ectopic overexpression of wild-type Syk to assess functional consequences thereof. Immunoprecipitations revealed that Syk associated with NKp80 in these cells upon pervanadate treatment (Fig. 7A). Next, NK-92MI cells ectopically expressing wild-type Syk together with either NKp80, NKp80/DGY, or NKp80/ERF, respectively, were assayed in redirected lysis assays for the functional potency. Remarkably, cytotoxic responses of NK-92MI cells transduced with NKp80 plus Syk were substantially stronger than responses of NK-92MI-NKp80 cells, and comparable to that of NKp80/DGY plus Syk (Fig. 7B). Superior reactivity was not due to Syk overexpression per se as transductants expressing both NKp80/ERF plus Syk remained functionally silent. Altogether, these data show that NKp80 can recruit Syk when abundantly present resulting in a substantially increased potential to trigger NK cytotoxicity.
FIGURE 7.
Attenuated NKp80-mediated cytotoxicity can be enhanced by overexpression of Syk. NK-92MI-NKp80, NK-92MI-NKp80/DGY, and NK-92MI-NKp80/ERF stably expressing high levels of wild type Syk were generated by transduction and assayed (A) for Syk co-immunoprecipitation with NKp80 and (B) for NKp80-mediated cytotoxicity in a redirected cytolysis assay. A, Syk-transduced NK-92MI-NKp80, NK-92MI-NKp80/DGY, and NK-92MI-NKp80/ERF cells were treated with pervanadate (2 min) and subsequently lysed. NKp80 was precipitated with anti-NKp80 mAb 5D12 and precipitates assessed for NKp80 tyrosine phosphorylation and co-precipitated Syk. NKp80 precipitation was monitored with a polyclonal anti-NKp80 reagent. For control, lysates were directly assessed for Syk. Note that amounts of NKp80 in lysates were below detection threshold (B) NK-92MI-NKp80, NK-92MI-NKp80-Syk, NK-92MI-NKp80/DGY-Syk, and NK-92MI-NKp80/ERF-Syk were co-cultivated with 51Cr-labeled P815 cells in the presence of anti-NKp80 mAb 5D12 and % cytolysis determined after 4 h of co-culture.
DISCUSSION
NKp80 uniquely differs from other human activating NK receptors by engaging a likewise C-type lectin-like glycoprotein (i.e. AICL) which is encoded in tight genetic linkage, and by transducing activating signals by a hemITAM-like sequence in its cytoplasmic domain. Considering the variety of activating receptors expressed by NK cells these distinct properties of NKp80 point to a peculiar function of NKp80 in NK cell biology. Unraveling the signaling pathway of NKp80, and, in particular, molecular dissection of the hemITAM which is central to NKp80 signaling, is expected to provide important clues for NKp80 function.
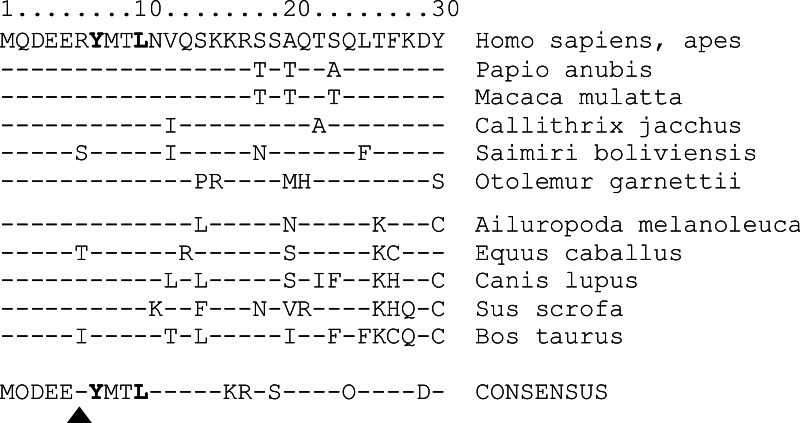
Here we show that amino acids glutamate 5 and arginine 6 preceding the hemITAM tyrosine 7 strongly affect the signaling capacity of NKp80 and, hence, determine quality and strength of the resulting effector responses. While both glutamate 5 and arginine 6 impair Syk recruitment with a critical impact on the initiation of downstream signaling pathways, arginine 6 also impedes phosphorylation of tyrosine 7, and thus likely the signaling strength. Watson and colleagues showed in a series of elegant experiments that signaling of CLEC-2 via Syk requires that the two SH2-binding domains of Syk dimerize two neighboring CLEC-2 molecules via binding to their phosphorylated hemITAMs provided either by a CLEC-2 homodimer or two adjacent CLEC-2 homodimers (22, 29). This intermolecular binding mode of Syk to hemITAMs obviously is different to its intramolecular binding mode to ITAMs and may impose additional structural restraints on Syk binding by hemITAM amino acids determining homodimerization and/or conformation (29). Hence, amino acids at hemITAM position 6 may either directly affect Syk binding or indirectly through conformational effects. Replacement of glutamate 5 and arginine 6 in NKp80 by aspartate 5 and glycine 6 preceding the hemITAM tyrosine 7 in the consensus hemITAM of the myeloid receptors CLEC-2 and Dectin-1, enabled efficient Syk recruitment, autonomous degranulation, and strong cytotoxic effector responses. Hence, it is tempting to speculate that the suboptimal hemITAM of NKp80 specifically evolved to dampen NKp80-mediated effector responses to avert otherwise detrimental effects toward AICL-expressing cells. This idea is supported by comparative analyses of mammalian NKp80 sequences. Expression of NKp80 has so far been documented for lymphocytes of primates (14, 15) and NKp80-like sequences are present in genomes of various mammals, including horse, dog, and cow, but not in rodents. Intriguingly, alignments of these mammalian NKp80 sequences reveal that the first 10 amino terminal amino acids comprising the hemITAM are highly conserved (Fig. 8), except for position 6 where arginine occasionally is replaced by isoleucine, threonine, or serine, respectively, but not by glycine. Isoleucine similarly to arginine impairs Syk recruitment by NKp80 (Fig. 4), further lending support to the idea that attenuation of the triggering potency of NKp80 specifically evolved to dampen effector responses upon AICL ligation. Unlike other activating NK cell receptors, such as NKG2D or NCRs that mediate recognition and elimination of dangerous infected or malignant cells (2, 4), immunomodulatory functions of NKp80 may require a more restrained activation potential.
FIGURE 8.
Conservation of the anomalous hemITAM in mammalian NKp80. Sequence alignment of the first 30 amino terminal amino acids of NKp80 in various mammalian species as deduced from the respective GenBankTM entries. Dashes indicate same amino acids as in upper sequence (humans, apes). Triangle denotes position 6 critically affecting Syk recruitment. Bottom line (CONSENSUS) indicates amino acids shared by all listed NKp80 sequences, while dashes indicate variable positions.
DNGR-1 (CLEC9A), a myeloid CTLR that recently has been shown to bind actin filaments of necrotic cells, contains a hemITAM with an isoleucine at position 6 similarly to NKp80/EIY (Figs. 1A and 5A) (26–28, 30, 31). While we did not detect Syk recruitment by NKp80/EIY, Syk recruitment by DNGR-1 has been reported (30, 31). Of note, the underlying evidence for direct physical interaction has primarly been derived from peptide pulldown experiments (30, 31). Our data illustrate that results from peptide pull-down experiments have to be interpreted with caution and emphasize the need to confirm physical interaction of signaling proteins with intact receptors in the appropriate cellular context. Hence, reassessment of Syk recruitment by DNGR-1 in immunoprecipitation experiments may be desirable, in particular, because functional consequences of DNGR-1 ligation reportedly differ from ligation of Dectin-1, e.g. with regard to DC activation and phagocytosis (18).
The myeloid hemITAM receptors CLEC-2 and Dectin-1 were shown to also mediate phagocytosis (32–34). Interestingly, Dectin-1-mediated phagocytosis in macrophages was shown to be Syk-independent, while it was strictly depending on the presence of the hemITAM tyrosine and the preceding triacidic DED motif (32–34). These data imply that the hemITAM serves as a docking site for additional signaling molecules apart from Syk. Our data also raise the possibility that Syk is not the major signaling component recruited by the anomalous hemITAM of NKp80: competition for binding to the NKp80 hemITAM between Syk and at least one other (unknown) signaling component in the cellular context of NK cells is indicated by our studies with NK-92MI cells overexpressing Syk. Together with data from our previous peptide pulldown experiments, where we did not observe a differential binding of Syk to phosphopeptides corresponding to the hemITAM of NKp80 versus the hemITAM of NKp80-DGY, this suggests that NK cells express a signaling protein efficiently competing with Syk for NKp80 binding in NK cells. Studies are ongoing to identify this protein that will importantly contribute to a better understanding of NKp80 function and hemITAM signaling capacities.
We recently provided the first report of NKp65, an activating receptor expressed by NK-92 cells that represents the closest relative to NKp80. NKp65 is encoded in close proximity to NKp80 in the NKC, and like NKp80, opposite to its ligand, i.e. KACL (35). The amino terminus of NKp65 matches the hemITAM consensus including aspartate and glycine at positions 5 and 6, respectively (Fig. 1A), and therefore may be expected to recruit Syk more efficiently than NKp80. Functional data using NK-92 cells as effectors also suggest that NKp65 is capable to trigger robust effector responses (35). However, as conventional NK cells do not express significant amounts of NKp65, the physiological significance of NKp65 as opposed to NKp80 remains unclear.
Taken together, we here show that the restrained potential of NKp80 to activate cytotoxicity can be ascribed to a single amino acid deviating from the hemITAM consensus and impairing Syk recruitment. We propose that this anomalous hemITAM of NKp80 may have specifically evolved to dampen NKp80-mediated cytotoxic effector responses in the context of the immunomodulatory function of NKp80.
Acknowledgments
We thank Christina Rohe for excellent technical assistance and Kevin Dennehy for suggestions.
This work was supported by a grant of the German Research Council (DFG; STE 828/6-1) (to A. S.).
- NK
- Natural Killer
- hemITAM
- hemi-immunoreceptor tyrosine-based activation motif
- NKC
- Natural Killer Gene complex
- AICL
- activation-induced C-type lectin
- NCR
- natural cytotoxicity receptor
- CTLR
- C-type lectin-like receptor
- KD
- kinase-defective
- E
- effector
- T
- target.
REFERENCES
- 1. Lanier L. L. (2008) Up on the tightrope: natural killer cell activation and inhibition. Nat. Immunol. 9, 495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. (2008) Functions of natural killer cells. Nat. Immunol. 9, 503–510 [DOI] [PubMed] [Google Scholar]
- 3. Bauer S., Groh V., Wu J., Steinle A., Phillips J. H., Lanier L. L., Spies T. (1999) Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285, 727–729 [DOI] [PubMed] [Google Scholar]
- 4. Waldhauer I., Steinle A. (2008) NK cells and cancer immunosurveillance. Oncogene 27, 5932–5943 [DOI] [PubMed] [Google Scholar]
- 5. Cerboni C., Zingoni A., Cippitelli M., Piccoli M., Frati L., Santoni A. (2007) Antigen-activated human T lymphocytes express cell-surface NKG2D ligands via an ATM/ATR-dependent mechanism and become susceptible to autologous NK- cell lysis. Blood 110, 606–615 [DOI] [PubMed] [Google Scholar]
- 6. Cooper M. A., Fehniger T. A., Fuchs A., Colonna M., Caligiuri M. A. (2004) NK cell and DC interactions. Trends Immunol. 25, 47–52 [DOI] [PubMed] [Google Scholar]
- 7. Bryceson Y. T., March M. E., Ljunggren H. G., Long E. O. (2006) Activation, coactivation, and costimulation of resting human natural killer cells. Immunol. Rev. 214, 73–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bryceson Y. T., Long E. O. (2008) Line of attack: NK cell specificity and integration of signals. Curr. Opin. Immunol. 20, 344–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bryceson Y. T., Chiang S. C., Darmanin S., Fauriat C., Schlums H., Theorell J., Wood S. M. (2011) Molecular mechanisms of natural killer cell activation. J. Innate. Immun. 3, 216–226 [DOI] [PubMed] [Google Scholar]
- 10. Dennehy K. M., Klimosch S. N., Steinle A. (2011) Cutting edge: NKp80 uses an atypical hemi-ITAM to trigger NK cytotoxicity. J. Immunol. 186, 657–661 [DOI] [PubMed] [Google Scholar]
- 11. Kuttruff S., Koch S., Kelp A., Pawelec G., Rammensee H. G., Steinle A. (2009) NKp80 defines and stimulates a reactive subset of CD8 T cells. Blood 113, 358–369 [DOI] [PubMed] [Google Scholar]
- 12. Vitale M., Falco M., Castriconi R., Parolini S., Zambello R., Semenzato G., Biassoni R., Bottino C., Moretta L., Moretta A. (2001) Identification of NKp80, a novel triggering molecule expressed by human NK cells. Eur. J. Immunol. 31, 233–242 [DOI] [PubMed] [Google Scholar]
- 13. Welte S., Kuttruff S., Waldhauer I., Steinle A. (2006) Mutual activation of natural killer cells and monocytes mediated by NKp80-AICL interaction. Nat. Immunol. 7, 1334–1342 [DOI] [PubMed] [Google Scholar]
- 14. Biassoni R., Fogli M., Cantoni C., Costa P., Conte R., Koopman G., Cafaro A., Ensoli B., Moretta A., Moretta L., De Maria A. (2005) Molecular and functional characterization of NKG2D, NKp80, and NKG2C triggering NK cell receptors in rhesus and cynomolgus macaques: monitoring of NK cell function during simian HIV infection. J. Immunol. 174, 5695–5705 [DOI] [PubMed] [Google Scholar]
- 15. Mavilio D., Benjamin J., Kim D., Lombardo G., Daucher M., Kinter A., Nies-Kraske E., Marcenaro E., Moretta A., Fauci A. S. (2005) Identification of NKG2A and NKp80 as specific natural killer cell markers in rhesus and pigtailed monkeys. Blood 106, 1718–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vogler I., Steinle A. (2011) Vis-a-vis in the NKC: genetically linked natural killer cell receptor/ligand pairs in the natural killer gene complex (NKC). J. Innate. Immun. 3, 227–235 [DOI] [PubMed] [Google Scholar]
- 17. Kerrigan A. M., Brown G. D. (2010) Syk-coupled C-type lectin receptors that mediate cellular activation via single tyrosine based activation motifs. Immunol. Rev. 234, 335–352 [DOI] [PubMed] [Google Scholar]
- 18. Sancho D., Reis e Sousa C. (2012) Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu. Rev. Immunol. 30, 491–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tam Y. K., Maki G., Miyagawa B., Hennemann B., Tonn T., Klingemann H. G. (1999) Characterization of genetically altered, interleukin 2-independent natural killer cell lines suitable for adoptive cellular immunotherapy. Hum. Gene Ther. 10, 1359–1373 [DOI] [PubMed] [Google Scholar]
- 20. Kitamura T. (1998) New experimental approaches in retrovirus-mediated expression screening. Int. J. Hematol. 67, 351–359 [DOI] [PubMed] [Google Scholar]
- 21. Kerrigan A. M., Brown G. D. (2011) Syk-coupled C-type lectins in immunity. Trends Immunol. 32, 151–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fuller G. L., Williams J. A., Tomlinson M. G., Eble J. A., Hanna S. L., Pöhlmann S., Suzuki-Inoue K., Ozaki Y., Watson S. P., Pearce A. C. (2007) The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YXXL-dependent signaling cascade. J. Biol. Chem. 282, 12397–12409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Séverin S., Pollitt A. Y., Navarro-Nuñez L., Nash C. A., Mourão-Sá D., Eble J. A., Senis Y. A., Watson S. P. (2011) Syk-dependent phosphorylation of CLEC-2: a novel mechanism of hem-immunoreceptor tyrosine-based activation motif signaling. J. Biol. Chem. 286, 4107–4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith-Garvin J. E., Koretzky G. A., Jordan M. S. (2009) T cell activation. Annu. Rev. Immunol. 27, 591–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Songyang Z., Carraway K. L., 3rd, Eck M. J., Harrison S. C., Feldman R. A., Mohammadi M., Schlessinger J., Hubbard S. R., Smith D. P., Eng C., and (1995) Catalytic specificity of protein-tyrosine kinases is critical for selective signalling. Nature 373, 536–539 [DOI] [PubMed] [Google Scholar]
- 26. Ahrens S., Zelenay S., Sancho D., Hanč P., Kjoer S., Feest C., Fletcher G., Durkin C., Postigo A., Skehel M., Batista F., Thompson B., Way M., Reis e Sousa C., Schulz O. (2012) F-actin is an evolutionarily conserved damage-associated molecular pattern recognized by DNGR-1, a receptor for dead cells. Immunity 36, 635–645 [DOI] [PubMed] [Google Scholar]
- 27. Poulin L. F., Reyal Y., Uronen-Hansson H., Schraml B. U., Sancho D., Murphy K. M., Håkansson U. K., Moita L. F., Agace W. W., Bonnet D., Reis e Sousa C. (2012) DNGR-1 is a specific and universal marker of mouse and human Batf3-dependent dendritic cells in lymphoid and nonlymphoid tissues. Blood 119, 6052–6062 [DOI] [PubMed] [Google Scholar]
- 28. Zhang J. G., Czabotar P. E., Policheni A. N., Caminschi I., Wan S. S., Kitsoulis S., Tullett K. M., Robin A. Y., Brammananth R., van Delft M. F., Lu J., O'Reilly L. A., Josefsson E. C., Kile B. T., Chin W. J., Mintern J. D., Olshina M. A., Wong W., Baum J., Wright M. D., Huang D. C., Mohandas N., Coppel R. L., Colman P. M., Nicola N. A., Shortman K., Lahoud M. H. (2012) The dendritic cell receptor Clec9A binds damaged cells via exposed actin filaments. Immunity 36, 646–657 [DOI] [PubMed] [Google Scholar]
- 29. Hughes C. E., Pollitt A. Y., Mori J., Eble J. A., Tomlinson M. G., Hartwig J. H., O'Callaghan C. A., Fütterer K., Watson S. P. (2010) CLEC-2 activates Syk through dimerization. Blood 115, 2947–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huysamen C., Willment J. A., Dennehy K. M., Brown G. D. (2008) CLEC9A is a novel activation C-type lectin-like receptor expressed on BDCA3+ dendritic cells and a subset of monocytes. J. Biol. Chem. 283, 16693–16701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sancho D., Joffre O. P., Keller A. M., Rogers N. C., Martínez D., Hernanz-Falcón P., Rosewell I., Reis e Sousa C. (2009) Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature 458, 899–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Herre J., Marshall A. S., Caron E., Edwards A. D., Williams D. L., Schweighoffer E., Tybulewicz V., Reis e Sousa C., Gordon S., Brown G. D. (2004) Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood 104, 4038–4045 [DOI] [PubMed] [Google Scholar]
- 33. Kerrigan A. M., Dennehy K. M., Mourão-Sá D., Faro-Trindade I., Willment J. A., Taylor P. R., Eble J. A., Reis e Sousa C., Brown G. D. (2009) CLEC-2 is a phagocytic activation receptor expressed on murine peripheral blood neutrophils. J. Immunol. 182, 4150–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Underhill D. M., Rossnagle E., Lowell C. A., Simmons R. M. (2005) Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 106, 2543–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spreu J., Kuttruff S., Stejfova V., Dennehy K. M., Schittek B., Steinle A. (2010) Interaction of C-type lectin-like receptors NKp65 and KACL facilitates dedicated immune recognition of human keratinocytes. Proc. Natl. Acad. Sci. U.S.A. 107, 5100–5105 [DOI] [PMC free article] [PubMed] [Google Scholar]