Background: The non-stop decay (NSD) mechanism has remained undetermined in mammalian cells.
Results: Degradation of unstable non-stop mRNA requires Hbs1, Dom34, and the exosome-Ski complex in mammalian cells.
Conclusion: The NSD mechanism exists in mammalian cells and involves a member of the eRF3 family of G proteins, Hbs1.
Significance: Our work provides a foundation for dissecting the detailed molecular basis of the human NSD mechanism.
Keywords: Exosomes, mRNA Decay, Ribosomes, RNA Turnover, Translation Release Factors, Hbs1, eRF3, Non-stop Decay
Abstract
In yeast, aberrant mRNAs lacking in-frame termination codons are recognized and degraded by the non-stop decay (NSD) pathway. The recognition of non-stop mRNAs involves a member of the eRF3 family of GTP-binding proteins, Ski7. Ski7 is thought to bind the ribosome stalled at the 3′-end of the mRNA poly(A) tail and recruit the exosome to degrade the aberrant message. However, Ski7 is not found in mammalian cells, and even the presence of the NSD mechanism itself has remained enigmatic. Here, we show that unstable non-stop mRNA is degraded in a translation-dependent manner in mammalian cells. The decay requires another eRF3 family member (Hbs1), its binding partner Dom34, and components of the exosome-Ski complex (Ski2/Mtr4 and Dis3). Hbs1-Dom34 binds to form a complex with the exosome-Ski complex. Also, the elimination of aberrant proteins produced from non-stop transcripts requires the RING finger protein listerin. These findings demonstrate that the NSD mechanism exists in mammalian cells and involves Hbs1, Dom34, and the exosome-Ski complex.
Introduction
mRNA decay is intimately linked to and regulated by translation. In recent years, it has become clear that members of the eRF3 (eukaryotic translation release factor 3) family of GTP-binding proteins act as signal transducers that couple translation to mRNA decay and play pivotal roles in the regulation of gene expression and mRNA quality control in eukaryotes. Members of this family are structurally characterized by a C-terminal elongation factor 1α-like GTP-binding domain and an N-terminal unique domain (1–4). The C-terminal domain is highly conserved among the members in eukaryotes, whereas the N-terminal domain is less conserved and unique to each family member. The most well studied member, eRF3, is a class II release factor, which brings the class I factor eRF1 into the ribosomal A site containing a termination codon (UAA, UGA, or UAG) and accelerates the peptidyl hydrolase activity of the ribosome (5–7). Involvement of eRF3 in the regulation of mRNA decay was first documented for nonsense-mediated mRNA decay (8), which eliminates mRNA with a premature termination codon (9). After nonsense-containing mRNA is transported to the cytoplasm, the translation termination complex eRF1-eRF3 binds to the ribosome that has arrived at the premature termination codon. The premature termination codon is thought to be recognized as aberrant either by inefficient translation termination caused by the faux UTR (10) or by downstream decay-accelerating elements, including the exon-exon junction complex (11–14). Depending on the species, distinct and/or unified mechanisms are proposed for nonsense-mediated mRNA decay (15–18).
eRF3 is also involved in the regulation of decay of normal mRNA. eRF3 forms a complex with a cytoplasmic poly(A)-binding protein and regulates the deadenylation of general mRNA in both yeast and mammals (19–21). We have proposed that the translation termination complex eRF1-eRF3 binds to poly(A)-binding protein competitively with the deadenylases, and release of the termination complex after translation ends triggers the association of the deadenylases with poly(A)-bound poly(A)-binding protein and accelerates deadenylation of the mRNA (21).
In contrast to the mRNAs with termination codons, mRNAs that lack in-frame termination codons are recognized and degraded by a mechanism called non-stop decay (NSD)3 (22–24). This system involves another eRF3 family member, Ski7, and the auxiliary Ski complex composed of the DExH RNA helicase Ski2, the tricopeptide repeat protein Ski3, and the WD repeat protein Ski8 (25). NSD also requires the exosome, which consists of nine core proteins, including Rrp40 and the catalytic protein Rrp44 (26). Ski7 associates with the cytoplasmic form of the exosome and the Ski complex through its N-terminal domain (3). Because termination codons are missing in the message, the ribosome continues to translate the 3′-UTR and even the poly(A) tail. In a proposed model, Ski7 binds to the ribosome stalled at the 3′-end of the mRNA and recruits the exosome to trigger fast 3′-to-5′ exonucleolytic degradation of the message. Thus, NSD is translation-dependent (23), but in contrast to normal decay, NSD is not dependent on deadenylation (22). Also, NSD does not require nonsense-mediated mRNA decay factors, including Upf1 (23). The components involved in the yeast NSD mechanism are conserved in mammals except for the key regulator Ski7.
In contrast, mRNAs with a structural propensity to cause ribosome stalling are degraded by no-go decay (27–29). In this mechanism, Hbs1 (the closest relative of eRF3) in complex with the eRF1 homolog Dom34 functions as a regulator (4). As is the case for eRF1-eRF3, the Hbs1-Dom34 complex binds to the A site of the ribosome that is stalled during translation elongation in a codon-nonspecific manner and triggers endonucleolytic cleavage by an unknown nuclease to eliminate the aberrant message (27, 30–33). Recent findings demonstrated that Hbs1-Dom34 also functions as a codon-nonspecific translation termination factor to release peptidyl-tRNA and to accelerate recycling of the stalled ribosome (34, 35).
These mRNA decay mechanisms are believed to be conserved among eukaryotes with the exception of NSD. Ski7 is found only in a subset of saccharomycete yeast cells but not in mammalian cells, and even the presence of the NSD mechanism itself has remained largely enigmatic. In contrast, there are reports supporting the presence of NSD in mammalian cells. First, Frischmeyer et al. (23) have shown that a non-stop β-glucuronidase reporter mRNA was low at steady state in HeLa cells. Second, α-globin non-stop mRNA was reported to be unstable in MEL and HeLa cells (24). In contrast, there are published data refuting the existence of NSD in mammals. A study using a GFP-based reporter in HeLa cells showed that the steady-state amount of non-stop mRNA was not significantly reduced relative to the wild-type message, and no significant difference was observed between the stability of the wild-type and non-stop mRNAs (36). Moreover, even in clinical cases, inconsistent results have been reported. In Diamond-Blackfan anemia, mutant RPS19 (ribosomal protein S19) mRNA lacking a stop codon was low compared with wild-type mRNA from a healthy donor (37). Similar results were reported for other clinical cases (38). In contrast, in a mitochondrial neurogastrointestinal encephalopathy patient, mutant (non-stop) TYMP mRNA was stable and coexisted with wild-type mRNA at similar levels (39).
Here, we examined the existence of NSD in mammals in detail based on the following five criteria: (i) stability of non-stop mRNA, (ii) dependence on translation, (iii) requirement for eRF3 family GTP-binding protein, (iv) requirement for the exosome-Ski complex, and (v) complex formation between the eRF3 family G protein and the exosome. We demonstrate that NSD actually exists in mammalian cells and involves Hbs1-Dom34 in complex with the exosome-Ski complex.
EXPERIMENTAL PROCEDURES
Plasmids
pFBG control (with the in-frame stop codon in the β-globin 3′-UTR mutated) was constructed by inverse PCR using pFLAG-CMV5/TO-β-globin (21) and the primer pair NH0193/NH0194. pFBG non-stop was generated by inverse PCR using pFBG control and NH0195/NH0004. To construct p5FBG control and p5FBG non-stop, the β-globin gene was amplified by PCR using either pFBG control or pFBG non-stop and the primer pair NH0047/NH0048 and inserted into the HindIII and EcoRV sites of pCMV-5×FLAG (40) to yield pCMV-5×FLAG-β-globin control or pCMV-5×FLAG-β-globin non-stop, respectively. The 5×FLAG-tagged β-globin gene was then amplified by PCR using either pCMV-5×FLAG-β-globin control or pCMV-5×FLAG-β-globin non-stop and the primer pair NH0618/NH0048 and inserted into the EcoRV and PstI sites of pFLAG-CMV5/TO (21). pIRE-FBG non-stop was generated by inverse PCR using pFBG non-stop and the primer pair NH0670/NH0671. pT7-TR, which expresses the T7-tagged tetracycline receptor, was generated by inverse PCR using pcDNA6/TR (Invitrogen) and the primer pair NH0383/NH0389. To construct pFLAG-Hbs1, cDNA encoding Hbs1 was amplified by PCR using petmRFS (2) and the primer pair NH0273/NH0414 and inserted into the EcoRI and XhoI sites of pCMV-FLAG (21). To construct pFLAG-Dom34, cDNA encoding Dom34 was amplified by RT-PCR using cDNA reverse-transcribed from HeLa cell total RNA as a template and the primer pair NH0275/NH0276 and inserted into the EcoRI site of pCMV-FLAG. To construct p5×Myc-Ski2, cDNA encoding Ski2 was amplified by PCR using the primer pair NH0237/NH0238 and the BC015758 clone (Open Biosystems) as a template and inserted into the EcoRI and XhoI sites of pCMV-5×Myc (41). To construct p5×Myc-Dis3, cDNA encoding Dis3 was amplified by PCR using the primer pair NH0089/NH0090 and the KIAA1008 clone as a template and inserted into the SalI and BglII sites of pCMV-5×Myc. Either pEGFP-C1 (Clontech) or p5×FLAG-EGFP (41) was used as a transfection/loading control. Primer sequences are listed in supplemental Table 1.
Cell Culture and DNA/RNA Transfection
HeLa cells were cultured in Dulbecco's modified Eagle's medium (Nissui) supplemented with 5% fetal bovine serum. DNA/RNA transfection was performed using Lipofectamine 2000 (Invitrogen) or polyethylenimine Max (Polysciences, Inc.) according to the manufacturers' instructions.
Northern Analysis and Real-time PCR
HeLa cells were transfected with the specified plasmids and siRNAs using Lipofectamine 2000. Total RNA was isolated and analyzed by Northern blotting as described previously (21, 41). In the transcriptional pulse-chase analysis, HeLa cells were transfected with pT7-TR, p5×FLAG-EGFP, either pFBG control or pFBG non-stop, and the specified siRNAs. 48 h after transfection, the cells were treated with 10 ng/ml tetracycline for 2.5 h to induce transcription, washed twice with phosphate-buffered saline to remove tetracycline completely, and harvested at the specified times after transcriptional shutoff. Total RNA was isolated and analyzed by Northern blotting. The levels of 5FBG, FBG, and 5×FLAG-EGFP mRNAs were quantitated from the Northern blot using Image Gauge Version 4.23 (Fujifilm). Real-time PCR analysis was performed using the StepOne real-time PCR system with Power SYBR Green PCR Master Mix (Applied Biosystems). Listerin and Mtr4 mRNAs were amplified using the primer pairs NH0619/NH0620 and NH0730/NH0731, respectively. GAPDH mRNA was amplified as described previously (41). Primer and siRNA sequences are listed in supplemental Tables 1 and 2, respectively.
Immunoprecipitation Assay and Western Blotting
For the immunoprecipitation assay, cells were lysed in buffer A (20 mm Tris-HCl (pH 7.5), 50 mm NaCl, 2.5 mm EDTA, 0.5% Nonidet P-40, 1 mm DTT, 0.1 mm PMSF, 2 μg/ml aprotinin, 2 μg/ml leupeptin, and 1 μg/ml RNase A (Sigma)) at 4 °C for 30 min. The mixture was centrifuged at 15,000 × g for 20 min, and the supernatant was subsequently incubated with anti-FLAG-agarose (Sigma) at 4 °C for 1 h. The resin was then washed three times with buffer A. Bound protein was eluted with SDS-PAGE sample buffer and analyzed by Western blotting. For analysis of the total cell lysate by Western blotting, cells were harvested and then boiled with SDS-PAGE sample buffer. Antibodies for Western blotting were as follows: anti-FLAG (Sigma), anti-Myc (Roche Applied Science), anti-GST (Cell Signaling), anti-Hbs1 (raised against His-tagged Hbs1), anti-Dom34 (raised against His-tagged Dom34), anti-GAPDH (Millipore or raised against His-tagged GAPDH), anti-Ski2 (Proteintech), and anti-Dis3 (Santa Cruz Biotechnology).
RESULTS
Both Non-stop mRNA and Protein Are Expressed at Lower Levels in Mammalian Cells
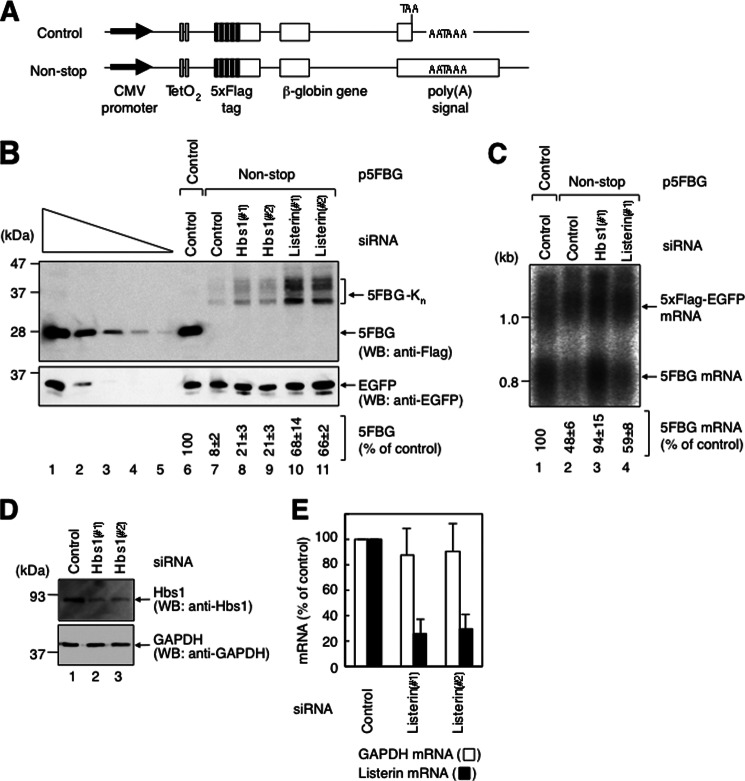
To analyze the expression of the gene that lacks a termination codon, we constructed N-terminally FLAG-tagged β-globin reporter genes: FLAG-β-globin control (FBG control) and FLAG-β-globin non-stop (FBG non-stop). The FBG control reporter was generated by mutating two in-frame termination codons in the 3′-UTR of the FLAG-β-globin reporter (21). The FBG non-stop reporter was generated by removing the bona fide termination codon of FBG control. Furthermore, we constructed 5×FLAG-tagged β-globin reporter genes: 5×FLAG-β-globin control (5FBG control) and 5×FLAG-β-globin non-stop (5FBG non-stop) (Fig. 1A) because FLAG-β-globin proteins were not detectable by Western blotting using anti-FLAG antibody.
FIGURE 1.
Both non-stop mRNA and protein are expressed at lower levels in HeLa cells. A, diagram of each of the two 5×FLAG-β-globin constructs. Arrows indicate the CMV promoter. Open boxes indicate the coding frame. Black boxes indicate the FLAG tag. Gray boxes indicate the tetracycline operator (TetO2). B, listerin functions in the reduced expression of non-stop protein. HeLa cells were transfected with pEGFP-C1; either p5FBG control (lanes 1–6) or p5FBG non-stop (lanes 7–11); and control luciferase siRNA (lanes 6 and 7), Hbs1 siRNA-1 (Hbs1(#1); lane 8), Hbs1 siRNA-2 (Hbs1(#2); lane 9), listerin siRNA-1 (Listerin(#1); lane 10), or listerin siRNA-2 (Listerin(#2); lane 11). Total cell lysate was analyzed by Western blotting (WB) using anti-FLAG (upper panel) or anti-EGFP (lower panel) antibody. Lanes 1–5, in which 3-fold dilutions of the lysate were analyzed, show that the condition is semiquantitative. Numbers immediately below each lane represent the level of 5×FLAG-β-globin protein normalized to the level of EGFP protein, with the level calculated from lane 1 defined as 100%. C, Hbs1 functions in the reduced expression of non-stop mRNA. HeLa cells were transfected with p5×FLAG-EGFP; either p5FBG control (lane 1) or p5FBG non-stop (lanes 2–4); and control luciferase siRNA (lanes 1 and 2), Hbs1 siRNA-1 (lane 3), or listerin siRNA-1 (lane 4). Numbers immediately below each lane represent the level of 5FBG mRNA normalized to the level of 5×FLAG-EGFP mRNA. D, down-regulation of Hbs1 expression. HeLa cells were transfected with control luciferase siRNA (lane 1), Hbs1 siRNA-1 (lane 2), or Hbs1 siRNA-2 (lane 3). Total cell lysate was analyzed by Western blotting using anti-Hbs1 (upper panel) or anti-GAPDH (lower panel) antibody. E, down-regulation of listerin expression. HeLa cells were transfected with control luciferase siRNA, listerin siRNA-1, or listerin siRNA-2. Total RNA isolated from the cells was analyzed by real-time PCR. The levels of listerin and GAPDH mRNAs were quantitated. Results in B–E are representative of three independently performed experiments.
We first examined the amount of mRNA and protein produced from the reporter genes at steady state. HeLa cells were cotransfected with either p5FBG control or p5FBG non-stop and a reference plasmid expressing EGFP as a transfection/loading control. Western blot analysis of the total cell lysate showed that 5FBG non-stop protein was decreased to 8 ± 2% of the control (Fig. 1B, compare lanes 6 and 7). 5FBG non-stop migrated on the SDS-polyacrylamide gel at 30∼40 kDa (Fig. 1B, lane 7) because polylysine is translated from the poly(A) tail of the mRNA to cause ribosome stalling on the poly(A) tail (42). In contrast, Northern blot analysis of total cellular RNA showed that the steady-state level of 5FBG non-stop mRNA was decreased to 48 ± 6% of the control (Fig. 1C, lane 2). Thus, 5FBG non-stop protein and mRNA were both expressed at a lower level.
It has been shown that non-stop mRNAs are degraded via a Ski7-dependent pathway in yeast (23, 22, 43). In mammals, Hbs1 is most closely related to Ski7 and is established as a regulator of no-go decay. Also, a previous study demonstrated that a single gene referred to as HBS1/SKI7 from Lachancea kluyveri can complement both ski7Δ and hbs1Δ mutants of Saccharomyces cerevisiae (44). This led us to speculate that human Hbs1 might be involved in the decay of non-stop mRNA. Thus, we adopted a siRNA-mediated knockdown strategy to assess the effect of Hbs1 knockdown on the expression of non-stop mRNA and protein. Western blot analysis showed that both Hbs1 siRNA-1 and Hbs1 siRNA-2 efficiently reduced cellular Hbs1 protein (Fig. 1D). The down-regulation of Hbs1 expression increased the level of 5FBG non-stop mRNA (Fig. 1C, compare lanes 2 and 3) and protein (Fig. 1B, compare lanes 7–9). We conclude that Hbs1 functions in the reduction of non-stop mRNA in mammalian cells. Hbs1 in mammals is suggested to play the same role in non-stop mRNA decay as Ski7 in yeast.
Knockdown of Hbs1 increased the amount of 5FBG non-stop mRNA to a level comparable to 5FBG control mRNA (94 ± 15%) (Fig. 1C, compare lanes 1 and 3) but the level of 5FBG non-stop protein to 21 ± 3% of that of 5FBG control protein (Fig. 1B, compare lanes 6, 8, and 9). Based on previous studies, the difference seemed to be caused by selective degradation of 5FBG non-stop protein and/or translational repression of 5FBG non-stop (36, 42, 45, 46). It has been shown that Ltn1-mediated ubiquitination of non-stop proteins results in their degradation in yeast (45, 46). Ltn1 is conserved among eukaryotes, and its mammalian ortholog is known as listerin (47). Therefore, we next assessed whether the mechanism of non-stop protein degradation by the E3 ubiquitin ligase is functional in mammalian cells. We examined the effect of siRNA-mediated knockdown of listerin on the reduced expression of non-stop mRNA and protein. Real-time PCR analysis showed that listerin siRNA-1 and listerin siRNA-2 down-regulated listerin mRNA expression to 26 ± 12 and 30 ± 12% of the control level, respectively (Fig. 1E). The down-regulation increased the level of 5FBG non-stop protein to 68 ± 14 and 66 ± 2% of that of 5FBG control protein, respectively (Fig. 1B, compare lanes 7, 10, and 11) but had little effect on 5FBG non-stop mRNA (Fig. 1C, lanes 2 and 4). These results indicate that the expression of non-stop mRNA is reduced not only at the mRNA level but also at the protein level, and the latter is mostly mediated by the listerin E3 ubiquitin ligase.
Decay of Unstable Non-stop mRNA Requires Translation
To gain a better understanding of the decay pathways of non-stop mRNA in mammalian cells, we next analyzed the decay kinetics. For this purpose, we used the FBG reporter instead of the 5FBG reporter because FBG mRNA migrated faster than 5FBG on agarose gel and was separated clearly from 5×FLAG-EGFP mRNA (transfection/loading control). The steady-state level of FBG non-stop mRNA was decreased to 51 ± 11% of FBG control mRNA (Fig. 2A, lane 2), which is similar to the result using 5FBG reporter constructs (Fig. 1C, lane 2).
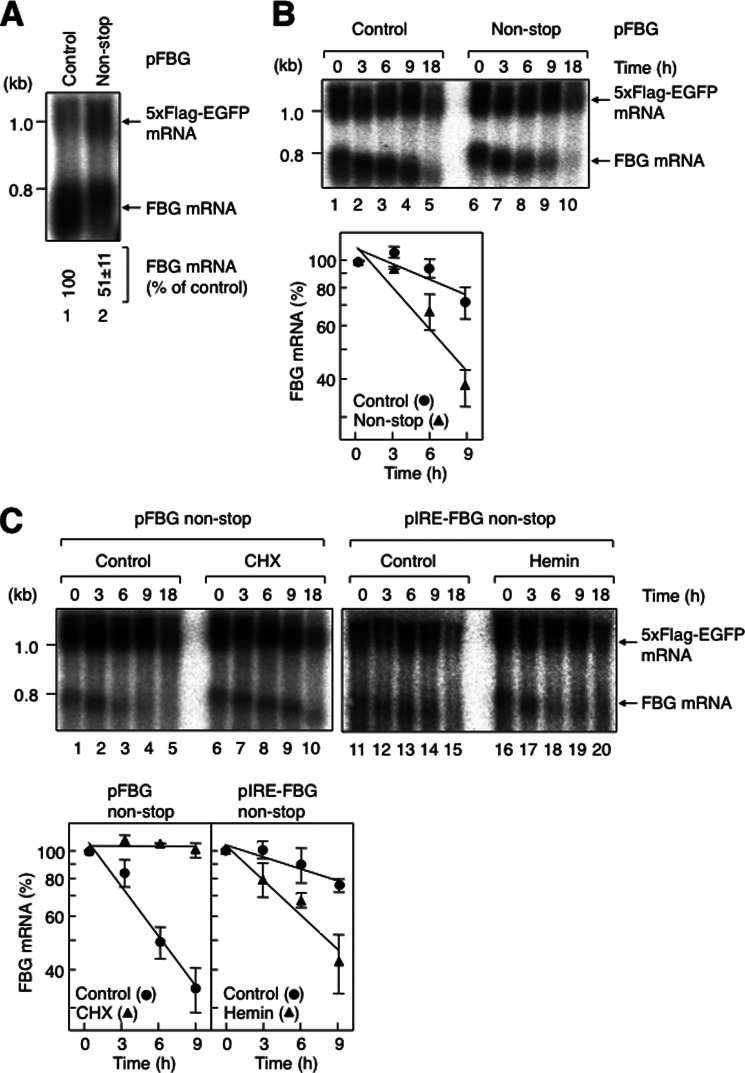
FIGURE 2.
Non-stop mRNA is unstable, and its decay requires translation in HeLa cells. A, steady-state levels of β-globin non-stop mRNAs. HeLa cells were transfected with p5×FLAG-EGFP and either pFBG control (lane 1) or pFBG non-stop (lane 2). Numbers immediately below each lane represent the level of FBG mRNA normalized to the level of 5×FLAG-EGFP mRNA. B, degradation of β-globin non-stop mRNAs. HeLa cells were cotransfected with p5×FLAG-EGFP, pT7-TR, and either pFBG control (upper panel, lanes 1–5) or pFBG non-stop (lanes 6–10). 1 day later, FBG mRNA expression was induced by tetracycline for 2.5 h, and the cells were harvested at the specified times after stopping FLAG-β-globin mRNA transcription. The levels of wild-type FBG mRNAs, which were normalized to the levels of 5×FLAG-EGFP mRNAs, were quantitated with the levels of the mRNA from the 0-h time point defined as 100% (lower panel). C, translation is required for the fast degradation of β-globin non-stop mRNA. HeLa cells were cotransfected with p5×FLAG-EGFP, pT7-TR, and either pFBG non-stop (upper panel, lanes 1–10) or pIRE-FBG non-stop (lanes 11–20). The transcriptional pulse-chase analysis was performed as described for B, except that the cells were treated with 100 μg/ml cycloheximide (CHX; upper panel, lanes 6–10) or 50 μm hemin (lanes 16–20). The levels of FBG mRNAs were quantitated as described for B (lower panel). Error bars represent S.D. for three independent experiments.
To determine whether the reduced expression of non-stop mRNA was due to increased decay of the mRNA, the decay kinetics of the non-stop mRNA was monitored using a tetracycline regulatory transcriptional pulse-chase approach as described previously (21, 41). HeLa cells were transfected with a transcriptional repressor plasmid expressing the tetracycline receptor, a reference plasmid expressing 5×FLAG-EGFP, and either the FBG control (Fig. 2B, lanes 1–5) or FBG non-stop (lanes 6–10) reporter plasmid. The half-lives of FBG control and FBG non-stop mRNAs were 17.5 ± 4.8 and 6.1 ± 0.8 h, respectively (Fig. 2B, lower panel). We conclude that non-stop mRNA is degraded fast in HeLa cells.
Non-stop mRNA decay is characterized by its dependence on translation in yeast (23, 43). To confirm that the decay observed for non-stop mRNA in mammalian cells meets this criteria, we first analyzed the effect of cycloheximide on the mRNA decay. Cycloheximide treatment greatly increased the half-life of FBG non-stop mRNA from 5.7 ± 0.9 h to >18 h (Fig. 2C, compare lanes 1–5 and 6–10). These results are consistent with previous reports showing that a translation inhibitor increases the level of non-stop mRNA at steady state (23, 37). To further confirm the translation dependence, we constructed an FBG non-stop reporter bearing an IRE within its 5′-UTR (48, 49) and analyzed the mRNA decay of IRE-FBG non-stop mRNA. When hemin was added as an iron source to inactivate iron regulatory protein and permit translation, the half-life of IRE-FBG non-stop mRNA was decreased from 22.6 ± 5.9 to 7.5 ± 1.4 h (Fig. 2C, compare lanes 11–15 and 16–20). We conclude that translation is required for the fast decay of non-stop mRNA.
Non-stop mRNA Decay Requires Hbs1 and Dom34
As described above, Hbs1 is involved in the reduction of non-stop mRNA in mammalian cells (Fig. 1C). To determine whether Hbs1 functions in the fast degradation of non-stop mRNA, we applied a knockdown approach to the transcriptional pulse-chase analysis. HeLa cells were transfected with either Hbs1 siRNA-1 (Fig. 3B, lane 11-20) or control siRNA (lanes 1–10) and a transcriptional repressor plasmid expressing the tetracycline receptor, a reference plasmid expressing 5×FLAG-EGFP, and either the FBG control (Fig. 3B, lanes 1–5 and 11–15) or FBG non-stop (lanes 6–10 and 16–20) reporter plasmid. Down-regulation of Hbs1 protein expression was confirmed by Western blotting using anti-Hbs1 antibody and, as a control, anti-GAPDH antibody (Fig. 3A, compare lanes 1 and 2). This down-regulation increased the half-life of FBG non-stop mRNA from 5.3 ± 0.9 to 12.9 ± 2.6 h (Fig. 3B, compare lanes 6–10 and 16–20). The half-life of FBG non-stop mRNA was close to that of FBG control mRNA (Fig. 3, B, compare lanes 11–15 and 16–20; and C). The increased half-life of FBG non-stop mRNA was obtained with a second siRNA (Hbs1 siRNA-2) whose target sequence was different from that of Hbs1 siRNA-1 (Fig. 3D). We conclude that Hbs1 functions in the decay of non-stop mRNA.
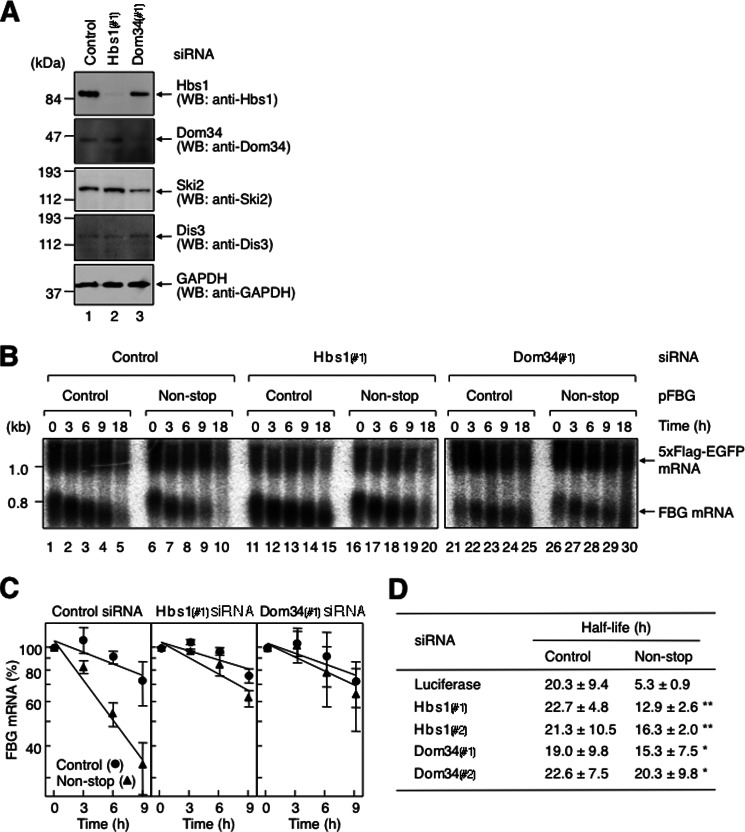
FIGURE 3.
Non-stop mRNA decay requires Hbs1 and Dom34. A, down-regulation of Hbs1 and Dom34 expression. HeLa cells were transfected with control luciferase siRNA (lane 1), Hbs1 siRNA-1 (Hbs1(#1); lane 2), or Dom34 siRNA-1 (Dom34(#1); lane 3). Total cell lysate was analyzed by Western blotting (WB) using the indicated antibodies. B, down-regulation of either Hbs1 or Dom34 expression inhibits fast degradation of non-stop mRNA. HeLa cells were transfected with p5×FLAG-EGFP; pT7-TR; either pFBG control (lanes 1–5, 11–15, and 21–25) or pFBG non-stop (lanes 6–10, 16–20, and 26–30); and control luciferase siRNA (lanes 1–10), Hbs1 siRNA-1 (lanes 11–20), or Dom34 siRNA-1 (lanes 21–30). The transcriptional pulse-chase analysis was performed as described in the legend to Fig. 2, except that the siRNAs were cotransfected. C, the levels of FBG mRNAs were quantitated as described in the legend to Fig. 2. D, the half-lives of FBG mRNAs were calculated. The asterisks denote the levels of statistical significance (Student's t test) between luciferase siRNA and either Hbs1 or Dom34 siRNA: *, p < 0.05; **, p < 0.01.
Previous studies demonstrated that Hbs1 binds Dom34 directly to form a complex (4), and the Hbs1-Dom34 complex functions in no-go decay (27, 31). These findings prompted us to investigate whether Dom34 is involved in the fast degradation of non-stop mRNA. Knockdown of Dom34 with Dom34 siRNA-1 in HeLa cells was performed as described above for Hbs1 (Fig. 3A, compare lanes 1 and 3). The down-regulation of Dom34 expression increased the half-life of FBG non-stop mRNA from 5.3 ± 0.9 h to >18 h (Fig. 3B, compare lanes 6–10 and 26–30). The half-life of FBG non-stop mRNA was equivalent to that of FBG control mRNA (Fig. 3, B, compare lanes 21–25 and 26–30; and C). The increased half-life of FBG non-stop mRNA was also obtained with a second siRNA (Dom34 siRNA-2) whose target sequence was different from that of Dom34 siRNA-1 (Fig. 3D). Thus, the down-regulation inhibits fast degradation of non-stop mRNA. However, it should be noted that when Dom34 was depleted, the expression levels of Hbs1 and Ski2 were decreased to 40∼60% of the control (Fig. 3A). The decreased levels of these proteins could partially contribute to inhibit the fast degradation because Dom34 depletion completely inhibited the fast decay provided that 40∼60% of Hbs1 and Ski2 were still remained. Taken together, these results indicate that both Hbs1 and Dom34 function in the decay of non-stop mRNA.
Non-stop mRNA Decay Requires the Exosome-Ski Complex
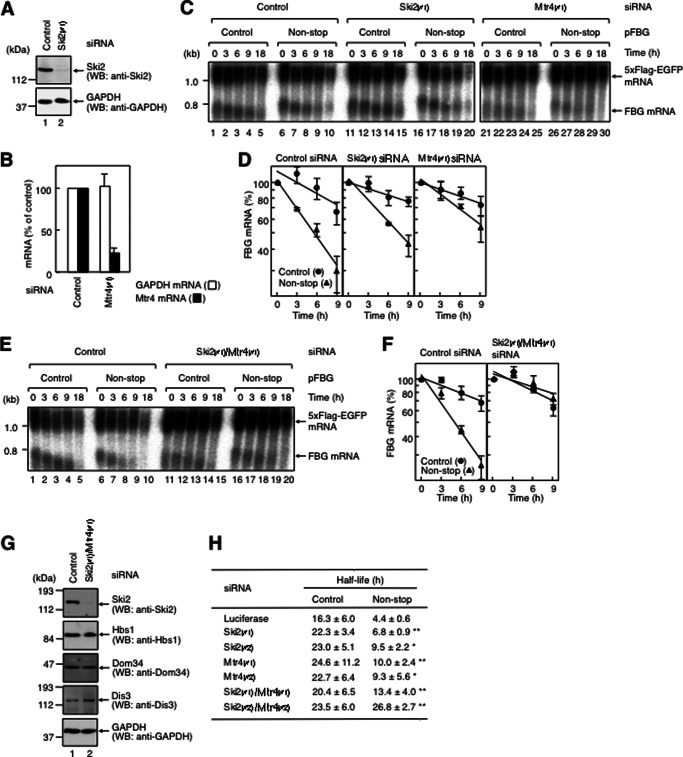
In yeast, non-stop mRNAs are degraded mainly in a 3′-to-5′ direction by the exosome (23, 22). Thus, we next aimed to determine whether non-stop mRNAs in mammalian cells are degraded by the exosome complex. We examined the decay of non-stop mRNA upon Ski2 down-regulation for the following two reasons. First, exosome-mediated 3′-to-5′ degradation of general mRNA requires a Ski complex consisting of the helicase Ski2, Ski3, and Ski8 (25, 50). Second, in yeast, the Ski complex binds Ski7, to which Hbs1 is most closely related in mammals (3, 51). Because two different yeast Ski2 homologs, Ski2 and Mtr4, are present in the human genome, we performed siRNA-mediated knockdown of both Ski2 and Mtr4 in HeLa cells.
HeLa cells were transfected with either Ski2 siRNA-1 or Mtr4 siRNA-1 as described above for Hbs1. Down-regulation of Ski2 and Mtr4 was confirmed by Western blotting and real-time PCR, respectively (Fig. 4, A and B). Ski2 and Mtr4 down-regulation increased the half-life of FBG non-stop mRNA from 4.4 ± 0.5 to 6.8 ± 0.9 and 10.0 ± 2.4 h, respectively (Fig. 4, C, compare lanes 6–10, 16–20, and 26–30; and D). The result shows that siRNA-mediated knockdown of either Ski2 or Mtr4 partially increased the half-lives of FBG non-stop mRNA. Therefore, HeLa cells were transfected with both Ski2 siRNA-1 and Mtr4 siRNA-1 as described above. The simultaneous Ski2/Mtr4 down-regulation increased the half-life of FBG non-stop mRNA from 4.4 ± 0.5 to 13.4 ± 4.0 h (Fig. 4, E, compare lanes 6–10 and 16–20; and F). The half-life of FBG non-stop mRNA was equivalent to that of FBG control mRNA upon Ski2/Mtr4 down-regulation (Fig. 4, E, compare lanes 11–15 and 16–20; and F). The increased half-life of FBG non-stop mRNA was also observed with Ski2 siRNA-2 and Mtr4 siRNA-2 (Fig. 4H). The expression levels of Hbs1, Dom34, and the exo/endonuclease Dis3 (see below) were not affected by Ski2/Mtr4 down-regulation (Fig. 4G). These results suggest that Ski2 and Mtr4 function redundantly in the fast decay of non-stop mRNA.
FIGURE 4.
Non-stop mRNA decay requires the exosome-Ski complex. A, down-regulation of Ski2 expression. HeLa cells were transfected with either control luciferase siRNA (lane 1) or Ski2 siRNA-1 (Ski2(#1); lane 2). Total cell lysate was analyzed by Western blotting (WB) using the indicated antibodies. B, down-regulation of Mtr4 expression. HeLa cells were transfected with control luciferase siRNA or Mtr4 siRNA-1 (Mtr4(#1)). Total RNA isolated from the cells was analyzed by real-time PCR. The levels of Mtr4 and GAPDH mRNAs were quantitated. Results are representative of three independently performed experiments. C, down-regulation of either Ski2 or Mtr4 expression partially inhibits fast degradation of non-stop mRNA. HeLa cells were transfected with p5×FLAG-EGFP; pT7-TR; either pFBG control (lanes 1–5, 11–15, and 21–25) or pFBG non-stop (lanes 6–10, 16–20, and 26–30); and control luciferase siRNA (lanes 1–10), Ski2 siRNA-1 (lanes 11–20), or Mtr4 siRNA-1 (lanes 21–30). The transcriptional pulse-chase analysis was performed as described in the legend to Fig. 3. D, the levels of FBG mRNAs were quantitated as described in the legend to Fig. 2. E, down-regulation of Ski2/Mtr4 expression inhibits fast degradation of non-stop mRNA. HeLa cells were transfected with p5×FLAG-EGFP, pT7-TR, either pFBG control (lanes 1–5 and 11–15) or pFBG non-stop (lanes 6–10 and 16–20), and either control luciferase siRNA (lanes 1–10) or Ski2 siRNA-1/Mtr4 siRNA-1 (lanes 11–20). The transcriptional pulse-chase analysis was performed as described in the legend to Fig. 2, except that the siRNAs were cotransfected. F, the levels of FBG mRNAs were quantitated as described in the legend to Fig. 2. G, down-regulation of Ski2/Mtr4 expression. HeLa cells were transfected with control luciferase siRNA (lane 1) or Ski2 siRNA-1/Mtr4 siRNA-1 (lane 2). Total cell lysate was analyzed by Western blotting using the indicated antibodies. H, the half-lives of FBG mRNAs were calculated. The asterisks denote the levels of statistical significance (Student's t test) between luciferase siRNA and either Ski2 or Mtr4 siRNA: *, p < 0.05; **, p < 0.01.
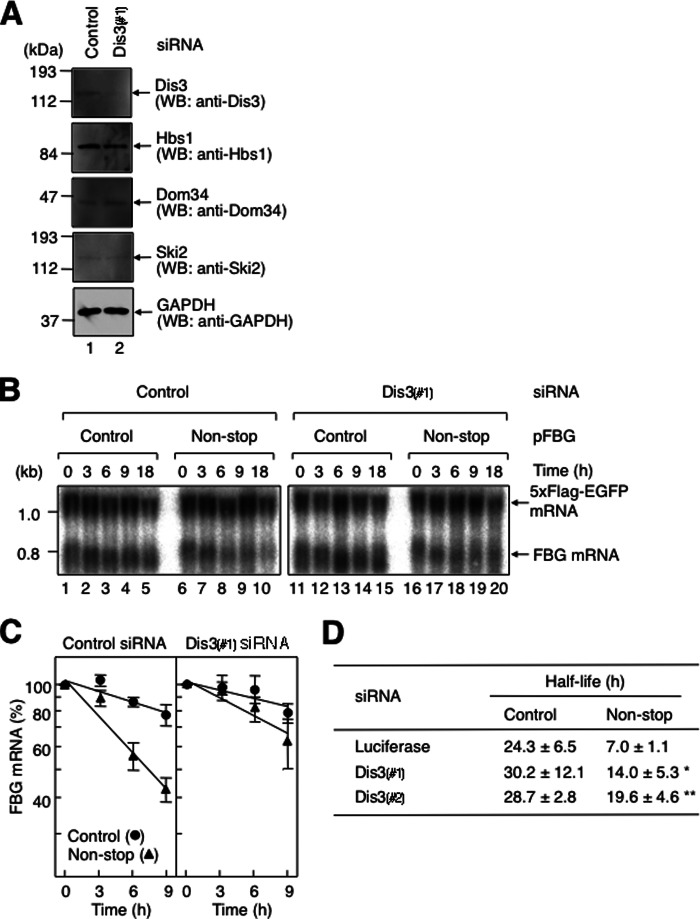
In yeast, non-stop mRNA is degraded by Rrp44, which has both exonuclease and endonuclease activities conferred by the PIN domain in the N terminus and the RNase II/R domain in the C terminus (26, 52, 53). Human Dis3 is similar to yeast Rrp44 not only in terms of sequence conservation but also in possessing the two distinct ribonucleolytic activities. Although Dis3 is localized mainly in the nucleus, a significant amount of Dis3 is detected in the cytoplasm. Moreover, human Dis3 can complement a yeast Rrp44 disruption (54). To elucidate whether the 3′-to-5′ exonuclease in the exosome complex promotes the degradation of non-stop mRNA in mammalian cells, we next examined the decay of non-stop mRNA by knocking down Dis3. HeLa cells were transfected with Dis3 siRNA-1 as described above for Hbs1. Down-regulation of Dis3 was confirmed by Western blotting using anti-Dis3 antibody and, as a control, anti-GAPDH antibody (Fig. 5A). Under this condition, the expression levels of Hbs1, Dom34, and Ski2 were not affected (Fig. 5A). The Dis3 down-regulation increased the half-life of FBG non-stop mRNA from 7.0 ± 1.1 to 14.0 ± 5.3 h (Fig. 5B, compare lanes 6–10 and 16–20; and C and D). The increased half-life of FBG non-stop mRNA was observed with a second siRNA (Dis3 siRNA-2) whose target sequence was different from that of Dis3 siRNA-1 (Fig. 5D). These results indicate that 3′-to-5′ exonuclease in the exosome complex promotes the degradation of non-stop mRNA. Notably, Dis3 is suggested to function predominantly in the decay of non-stop mRNA.
FIGURE 5.
Non-stop mRNA decay requires Dis3. A, down-regulation of Dis3 expression. HeLa cells were transfected with either control luciferase siRNA (lane 1) or Dis3 siRNA-1 (Dis3(#1); lane 2). Total cell lysate was analyzed by Western blotting (WB) using the indicated antibodies. B, down-regulation of Dis3 expression inhibits fast degradation of non-stop mRNA. HeLa cells were transfected with p5×FLAG-EGFP, pT7-TR, either pFBG control (lanes 1–5 and 11–15) or pFBG non-stop (lanes 6–10 and 16–20), and either control luciferase siRNA (lanes 1–10) or Dis3 siRNA-1 (lanes 11–20). The transcriptional pulse-chase analysis was performed as described in the legend to Fig. 2. C, the levels of FBG mRNAs were quantitated as described in the legend to Fig. 2. D, the half-lives of FBG mRNAs were calculated. The asterisks denote the levels of statistical significance (Student's t test) between luciferase and Dis3 siRNAs: *, p < 0.05;**, p < 0.01.
Hbs1-Dom34 Binds to Form a Complex with the Exosome
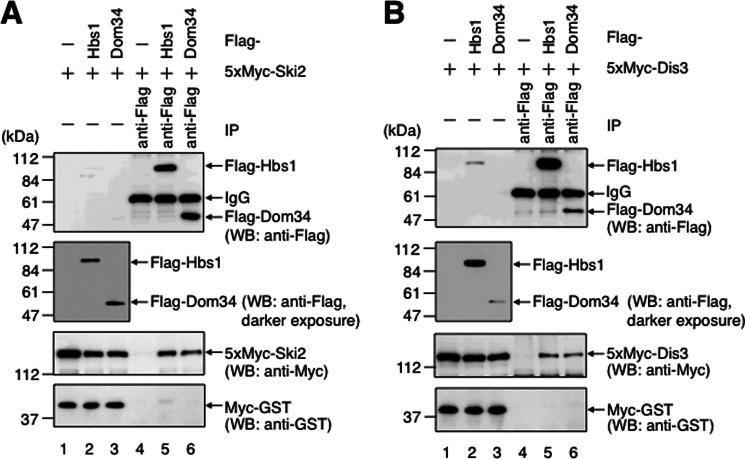
Ski7 recruits the exosome to non-stop mRNA in yeast (22, 23). If, as in the case of yeast Ski7, Hbs1 functions in the fast degradation of non-stop mRNA in mammalian cells, Hbs1 should interact with the exosome complex. To test this hypothesis, we assessed the interaction between Hbs1 and the exosome complex by conducting co-immunoprecipitation experiments using HeLa cells. HeLa cells were transfected with p5×Myc-Ski2, pMyc-GST, and either pFLAG-Hbs1 or pFLAG, and the cell extract was immunoprecipitated using anti-FLAG antibody. Western blot analysis showed that FLAG-Hbs1 co-purified with 5×Myc-Ski2 (Fig. 6A, lane 5). When the same experiment was performed with p5×Myc-Dis3 instead of p5×Myc-Ski2, FLAG-Hbs1 also co-purified with 5×Myc-Dis3 (Fig. 6B, lane 5). Additionally, Myc-GST was not detectably co-purified with FLAG-Hbs1, indicating that 5×Myc-Ski2 and 5×Myc-Dis3 were co-purified specifically (Fig. 6, A and B, lower panel). The interactions seem not to be mediated by RNA because all of these experiments were performed in the presence of RNase A. From these results, we conclude that Hbs1 interacts with the exosome-Ski complex.
FIGURE 6.
Hbs1 and Dom34 interact with the exosome-Ski complex. HeLa cells were transfected with either p5×Myc-Ski2 (A) or p5×Myc-Dis3 (B) and also pFLAG (lanes 1 and 4), pFLAG-Hbs1 (lanes 2 and 5), or pFLAG-Dom34 (lanes 3 and 6). The cell extracts were subjected to an immunoprecipitation (IP) assay using anti-FLAG antibody. Immunoprecipitation was performed in the presence of RNase A. The immunoprecipitates (lanes 4–6) and inputs (lanes 1–3; 10% of the amount immunoprecipitated) were analyzed by Western blotting (WB) using the indicated antibodies. The results are representative of three independently performed experiments.
As described previously, Hbs1 and Dom34 bind directly, and the formation of the Hbs1-Dom34 complex results in a significant conformational change in Dom34, which increases the affinity of the complex for the A site of the ribosome (32, 55, 56). Thus, in the no-go decay mechanism, Hbs1 and Dom34 are thought to form a complex at the A site of the stalled ribosome. These results led us to speculate that Hbs1 in complex with Dom34 recruits the exosome to the ribosome stalled on non-stop mRNA. To investigate this possibility, we analyzed the interaction between Dom34 and the exosome by conducting co-immunoprecipitation experiments. HeLa cells were cotransfected with pFLAG-Dom34, pMyc-GST, and either p5×Myc-Ski2 (Fig. 6A) or p5×Myc-Dis3 (Fig. 6B), and the cell extract was immunoprecipitated using anti-FLAG antibody. Western blot analysis showed that both 5×Myc-Ski2 and 5×Myc-Dis3, but not Myc-GST, were co-purified with FLAG-Dom34 (Fig. 6, A and B, lane 6). We conclude that Dom34, as well as Hbs1, interacts with the exosome complex. These results suggest that the exosome is recruited to the Hbs1-Dom34 complex, which is fitted into the stalled ribosome on non-stop mRNA.
DISCUSSION
The presence of a quality control mechanism for aberrant mRNAs lacking in-frame termination codons remained enigmatic in mammalian cells. One of the primary reasons for this is the absence of a Ski7 protein in mammalian cells. Also, it was reported that the decay rate of non-stop mRNA is not different from that of wild-type mRNA (36, 39). In this study, we have demonstrated that a quality control mechanism for aberrant non-stop mRNA actually exists in mammalian cells based on the following five criteria. (i) Non-stop mRNA is unstable and degraded quickly (Fig. 2B); (ii) the decay is dependent on translation (Fig. 2C); (iii) the decay requires an eRF3 family GTP-binding protein (Fig. 3); (iv) the decay requires the exosome-Ski complex (Figs. 4 and 5); and (v) the decay-regulating eRF3 family G protein forms a complex with the exosome (Fig. 6).
Thus, as in yeast, an eRF3-like GTP-binding protein, the exosome, and a Ski complex are all involved in the decay of non-stop mRNA in mammalian cells. This makes it possible to surmise that the Hbs1-Dom34 complex binds to the stalled ribosome at the 3′-end of mRNA and recruits the exosome-Ski complex to degrade the aberrant massage. A major difference is that Hbs1 is used instead of Ski7 in mammalian cells.
Recent findings have demonstrated that Hbs1-Dom34 acts as a ribosome-recycling factor, which dissociates the ribosome into the subunits to release the stalled ribosome from the message (33–35, 57, 58). The findings are reasonable because mRNA with a stalled ribosome is not a good substrate for the 3′-to-5′ exonucleolytic degradation by the exosome. The findings, taken together with our results presented here, suggest that Hbs1-Dom34 binds to the stalled ribosome at the 3′-end of non-stop mRNA and induces both dissociation of the ribosome into the subunits for its recycling and simultaneous recruitment of the exosome-Ski complex for degrading the aberrant message. Very recently, Inada and co-workers (59) also showed that Hbs1 is involved in NSD in the yeast S. cerevisiae. However, as described above, saccharomycete yeast cells have a unique member of the eRF3 G protein family, Ski7, which has already been established as a regulator of NSD in yeast. Thus, it is reasonable to assume that in contrast to other organisms, including mammals, saccharomycete yeast cells have evolved a specialized mechanism for NSD, in which two eRF3 family members are involved: the yeast Hbs1 inherited a ribosome-recycling function of the ancestral Hbs1, and Ski7 inherited an exosome-recruiting function.
Also, we have presented data suggesting that the product of non-stop mRNA is subjected to protein quality control and that listerin, the mammalian ortholog of yeast Ltn1, functions as the E3 ubiquitin ligase. More studies are needed to evaluate in detail the mechanisms of the two quality control systems developed for non-stop mRNA and protein.
Acknowledgments
We are grateful to O. Jean-Jean and H. Philippe for providing Hbs1 cDNA.
This work was supported by Grant-in-aid for Scientific Research on Innovative Areas “RNA Regulation” 20112005 from the Ministry of Education, Culture, Sports, Science and Technology of Japan and Grant-in-aid for Scientific Research (B) 21370080 from the Japan Society for the Promotion of Science (to S. H.).

This article contains supplemental Tables 1 and 2.
- NSD
- non-stop decay
- FBG
- FLAG-β-globin
- IRE
- iron-responsive element
- EGFP
- enhanced GFP.
REFERENCES
- 1. Hoshino S., Imai M., Mizutani M., Kikuchi Y., Hanaoka F., Ui M., Katada T. (1998) Molecular cloning of a novel member of the eukaryotic polypeptide chain-releasing factors (eRF). Its identification as eRF3 interacting with eRF1. J. Biol. Chem. 273, 22254–22259 [DOI] [PubMed] [Google Scholar]
- 2. Wallrapp C., Verrier S. B., Zhouravleva G., Philippe H., Philippe M., Gress T. M., Jean-Jean O. (1998) The product of the mammalian orthologue of the Saccharomyces cerevisiae HBS1 gene is phylogenetically related to eukaryotic release factor 3 (eRF3) but does not carry eRF3-like activity. FEBS Lett. 440, 387–392 [DOI] [PubMed] [Google Scholar]
- 3. Araki Y., Takahashi S., Kobayashi T., Kajiho H., Hoshino S., Katada T. (2001) Ski7p G protein interacts with the exosome and the Ski complex for 3′-to-5′ mRNA decay in yeast. EMBO J. 20, 4684–4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carr-Schmid A., Pfund C., Craig E. A., Kinzy T. G. (2002) Novel G-protein complex whose requirement is linked to the translational status of the cell. Mol. Cell. Biol. 22, 2564–2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frolova L., Le Goff X., Rasmussen H. H., Cheperegin S., Drugeon G., Kress M., Arman I., Haenni A. L., Celis J. E., Philippe M. (1994) A highly conserved eukaryotic protein family possessing properties of polypeptide chain release factor. Nature 372, 701–703 [DOI] [PubMed] [Google Scholar]
- 6. Stansfield I., Jones K. M., Kushnirov V. V., Dagkesamanskaya A. R., Poznyakovski A. I., Paushkin S. V., Nierras C. R., Cox B. S., Ter-Avanesyan M. D., Tuite M. F. (1995) The products of the SUP45 (eRF1) and SUP35 genes interact to mediate translation termination in Saccharomyces cerevisiae. EMBO J. 14, 4365–4373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhouravleva G., Frolova L., Le Goff X., Le Guellec R., Inge-Vechtomov S., Kisselev L., Philippe M. (1995) Termination of translation in eukaryotes is governed by two interacting polypeptide chain release factors, eRF1 and eRF3. EMBO J. 14, 4065–4072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maquat L. E. (2004) Nonsense-mediated mRNA decay: splicing, translation and mRNP dynamics. Nat. Rev. Mol. Cell Biol. 5, 89–99 [DOI] [PubMed] [Google Scholar]
- 9. Czaplinski K., Ruiz-Echevarria M. J., Paushkin S. V., Han X., Weng Y., Perlick H. A., Dietz H. C., Ter-Avanesyan M. D., Peltz S. W. (1998) The surveillance complex interacts with the translation release factors to enhance termination and degrade aberrant mRNAs. Genes Dev. 12, 1665–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Amrani N., Ganesan R., Kervestin S., Mangus D. A., Ghosh S., Jacobson A. (2004) A faux 3′-UTR promotes aberrant termination and triggers nonsense-mediated mRNA decay. Nature 432, 112–118 [DOI] [PubMed] [Google Scholar]
- 11. Nagy E., Maquat L. E. (1998) A rule for termination-codon position within intron-containing genes: when nonsense affects RNA abundance. Trends Biochem. Sci. 23, 198–199 [DOI] [PubMed] [Google Scholar]
- 12. Lykke-Andersen J., Shu M. D., Steitz J. A. (2001) Communication of the position of exon-exon junctions to the mRNA surveillance machinery by the protein RNPS1. Science 293, 1836–1839 [DOI] [PubMed] [Google Scholar]
- 13. Kim V. N., Kataoka N., Dreyfuss G. (2001) Role of the nonsense-mediated decay factor hUpf3 in the splicing-dependent exon-exon junction complex. Science 293, 1832–1836 [DOI] [PubMed] [Google Scholar]
- 14. Le Hir H., Gatfield D., Izaurralde E., Moore M. J. (2001) The exon-exon junction complex provides a binding platform for factors involved in mRNA export and nonsense-mediated mRNA decay. EMBO J. 20, 4987–4997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kashima I., Yamashita A., Izumi N., Kataoka N., Morishita R., Hoshino S., Ohno M., Dreyfuss G., Ohno S. (2006) Binding of a novel SMG-1-Upf1-eRF1-eRF3 complex (SURF) to the exon junction complex triggers Upf1 phosphorylation and nonsense-mediated mRNA decay. Genes Dev. 20, 355–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huntzinger E., Kashima I., Fauser M., Saulière J., Izaurralde E. (2008) SMG6 is the catalytic endonuclease that cleaves mRNAs containing nonsense codons in metazoan. RNA 14, 2609–2617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eberle A. B., Lykke-Andersen S., Mühlemann O., Jensen T. H. (2009) SMG6 promotes endonucleolytic cleavage of nonsense mRNA in human cells. Nat. Struct. Mol. Biol. 16, 49–55 [DOI] [PubMed] [Google Scholar]
- 18. Muhlrad D., Parker R. (1994) Premature translational termination triggers mRNA decapping. Nature 370, 578–581 [DOI] [PubMed] [Google Scholar]
- 19. Hoshino S., Imai M., Kobayashi T., Uchida N., Katada T. (1999) The eukaryotic polypeptide chain releasing factor (eRF3/GSPT) carrying the translation termination signal to the 3′-poly(A) tail of mRNA. Direct association of eRF3/GSPT with polyadenylate-binding protein. J. Biol. Chem. 274, 16677–16680 [DOI] [PubMed] [Google Scholar]
- 20. Hosoda N., Kobayashi T., Uchida N., Funakoshi Y., Kikuchi Y., Hoshino S., Katada T. (2003) Translation termination factor eRF3 mediates mRNA decay through the regulation of deadenylation. J. Biol. Chem. 278, 38287–38291 [DOI] [PubMed] [Google Scholar]
- 21. Funakoshi Y., Doi Y., Hosoda N., Uchida N., Osawa M., Shimada I., Tsujimoto M., Suzuki T., Katada T., Hoshino S. (2007) Mechanism of mRNA deadenylation: evidence for a molecular interplay between translation termination factor eRF3 and mRNA deadenylases. Genes Dev. 21, 3135–3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Hoof A., Frischmeyer P. A., Dietz H. C., Parker R. (2002) Exosome-mediated recognition and degradation of mRNAs lacking a termination codon. Science 295, 2262–2264 [DOI] [PubMed] [Google Scholar]
- 23. Frischmeyer P. A., van Hoof A., O'Donnell K., Guerrerio A. L., Parker R., Dietz H. C. (2002) An mRNA surveillance mechanism that eliminates transcripts lacking termination codons. Science 295, 2258–2261 [DOI] [PubMed] [Google Scholar]
- 24. Kong J., Liebhaber S. A. (2007) A cell type-restricted mRNA surveillance pathway triggered by ribosome extension into the 3′ untranslated region. Nat. Struct. Mol. Biol. 14, 670–676 [DOI] [PubMed] [Google Scholar]
- 25. Brown J. T., Bai X., Johnson A. W. (2000) The yeast antiviral proteins Ski2p, Ski3p, and Ski8p exist as a complex in vivo. RNA 6, 449–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lebreton A., Tomecki R., Dziembowski A., Séraphin B. (2008) Endonucleolytic RNA cleavage by a eukaryotic exosome. Nature 456, 993–996 [DOI] [PubMed] [Google Scholar]
- 27. Doma M. K., Parker R. (2006) Endonucleolytic cleavage of eukaryotic mRNAs with stalls in translation elongation. Nature 440, 561–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harigaya Y., Parker R. (2010) No-go decay: a quality control mechanism for RNA in translation. Wiley Interdiscip. Rev. RNA 1, 132–141 [DOI] [PubMed] [Google Scholar]
- 29. Onouchi H., Nagami Y., Haraguchi Y., Nakamoto M., Nishimura Y., Sakurai R., Nagao N., Kawasaki D., Kadokura Y., Naito S. (2005) Nascent peptide-mediated translation elongation arrest coupled with mRNA degradation in the CGS1 gene of Arabidopsis. Genes Dev. 19, 1799–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee H. H., Kim Y.-S., Kim K. H., Heo I., Kim S. K., Kim O., Kim H. K., Yoon J. Y., Kim H. S., Kim D. J., Lee S. J., Yoon H. J., Kim S. J., Lee B. G., Song H. K., Kim V. N., Park C. M., Suh S. W. (2007) Structural and functional insights into Dom34, a key component of no-go mRNA decay. Mol. Cell 27, 938–950 [DOI] [PubMed] [Google Scholar]
- 31. Passos D. O., Doma M. K., Shoemaker C. J., Muhlrad D., Green R., Weissman J., Hollien J., Parker R. (2009) Analysis of Dom34 and its function in no-go decay. Mol. Biol. Cell 20, 3025–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van den Elzen A. M. G., Henri J., Lazar N., Gas M. E., Durand D., Lacroute F., Nicaise M., van Tilbeurgh H., Séraphin B., Graille M. (2010) Dissection of Dom34-Hbs1 reveals independent functions in two RNA quality control pathways. Nat. Struct. Mol. Biol. 17, 1446–1452 [DOI] [PubMed] [Google Scholar]
- 33. Becker T., Armache J.-P., Jarasch A., Anger A. M., Villa E., Sieber H., Motaal B. A., Mielke T., Berninghausen O., Beckmann R. (2011) Structure of the no-go mRNA decay complex Dom34-Hbs1 bound to a stalled 80S ribosome. Nat. Struct. Mol. Biol. 18, 715–720 [DOI] [PubMed] [Google Scholar]
- 34. Shoemaker C. J., Eyler D. E., Green R. (2010) Dom34:Hbs1 promotes subunit dissociation and peptidyl-tRNA drop-off to initiate no-go decay. Science 330, 369–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pisareva V. P., Skabkin M. A., Hellen C. U. T., Pestova T. V., Pisarev A. V. (2011) Dissociation by Pelota, Hbs1 and ABCE1 of mammalian vacant 80S ribosomes and stalled elongation complexes. EMBO J. 30, 1804–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Akimitsu N., Tanaka J., Pelletier J. (2007) Translation of nonSTOP mRNA is repressed post-initiation in mammalian cells. EMBO J. 26, 2327–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chatr-Aryamontri A., Angelini M., Garelli E., Tchernia G., Ramenghi U., Dianzani I., Loreni F. (2004) Nonsense-mediated and nonstop decay of ribosomal protein S19 mRNA in Diamond-Blackfan anemia. Hum. Mutat. 24, 526–533 [DOI] [PubMed] [Google Scholar]
- 38. Klauer A. A., van Hoof A. (2012) Degradation of mRNAs that lack a stop codon: a decade of nonstop progress. Wiley Interdiscip. Rev. RNA 3, 649–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Torres-Torronteras J., Rodriguez-Palmero A., Pinós T., Accarino A., Andreu A. L., Pintos-Morell G., Martíí R. (2011) A novel nonstop mutation in TYMP does not induce nonstop mRNA decay in a MNGIE patient with severe neuropathy. Hum. Mutat. 32, E2061–E2068 [DOI] [PubMed] [Google Scholar]
- 40. Ruan L., Osawa M., Hosoda N., Imai S., Machiyama A., Katada T., Hoshino S., Shimada I. (2010) Quantitative characterization of Tob interactions provides the thermodynamic basis for translation termination-coupled deadenylase regulation. J. Biol. Chem. 285, 27624–27631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hosoda N., Funakoshi Y., Hirasawa M., Yamagishi R., Asano Y., Miyagawa R., Ogami K., Tsujimoto M., Hoshino S. (2011) Anti-proliferative protein Tob negatively regulates CPEB3 target by recruiting Caf1 deadenylase. EMBO J. 30, 1311–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ito-Harashima S., Kuroha K., Tatematsu T., Inada T. (2007) Translation of the poly(A) tail plays crucial roles in nonstop mRNA surveillance via translation repression and protein destabilization by proteasome in yeast. Genes Dev. 21, 519–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Inada T., Aiba H. (2005) Translation of aberrant mRNAs lacking a termination codon or with a shortened 3′-UTR is repressed after initiation in yeast. EMBO J. 24, 1584–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van Hoof A. (2005) Conserved functions of yeast genes support the duplication, degeneration and complementation model for gene duplication. Genetics 171, 1455–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wilson M. A., Meaux S., van Hoof A. (2007) A genomic screen in yeast reveals novel aspects of nonstop mRNA metabolism. Genetics 177, 773–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bengtson M. H., Joazeiro C. A. P. (2010) Role of a ribosome-associated E3 ubiquitin ligase in protein quality control. Nature 467, 470–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chu J., Hong N. A., Masuda C. A., Jenkins B. V., Nelms K. A., Goodnow C. C., Glynne R. J., Wu H., Masliah E., Joazeiro C. A. P., Kay S. A. (2009) A mouse forward genetics screen identifies LISTERIN as an E3 ubiquitin ligase involved in neurodegeneration. Proc. Natl. Acad. Sci. U.S.A. 106, 2097–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hentze M. W., Caughman S. W., Casey J. L., Koeller D. M., Rouault T. A., Harford J. B., Klausner R. D. (1988) A model for the structure and functions of iron-responsive elements. Gene 72, 201–208 [DOI] [PubMed] [Google Scholar]
- 49. Thermann R., Neu-Yilik G., Deters A., Frede U., Wehr K., Hagemeier C., Hentze M. W., Kulozik A. E. (1998) Binary specification of nonsense codons by splicing and cytoplasmic translation. EMBO J. 17, 3484–3494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Anderson J. S., Parker R. (1998) The 3′ to 5′ degradation of yeast mRNAs is a general mechanism for mRNA turnover that requires the SKI2 DEVH box protein and 3′ to 5′ exonucleases of the exosome complex. EMBO J. 17, 1497–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang L., Lewis M. S., Johnson A. W. (2005) Domain interactions within the Ski2/3/8 complex and between the Ski complex and Ski7p. RNA 11, 1291–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schaeffer D., Tsanova B., Barbas A., Reis F. P., Dastidar E. G., Sanchez-Rotunno M., Arraiano C. M., van Hoof A. (2009) The exosome contains domains with specific endoribonuclease, exoribonuclease and cytoplasmic mRNA decay activities. Nat. Struct. Mol. Biol. 16, 56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schaeffer D., van Hoof A. (2011) Different nuclease requirements for exosome-mediated degradation of normal and nonstop mRNAs. Proc. Natl. Acad. Sci. U.S.A. 108, 2366–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tomecki R., Kristiansen M. S., Lykke-Andersen S., Chlebowski A., Larsen K. M., Szczesny R. J., Drazkowska K., Pastula A., Andersen J. S., Stepien P. P., Dziembowski A., Jensen T. H. (2010) The human core exosome interacts with differentially localized processive RNases: hDIS3 and hDIS3L. EMBO J. 29, 2342–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kobayashi K., Kikuno I., Kuroha K., Saito K., Ito K., Ishitani R., Inada T., Nureki O. (2010) Structural basis for mRNA surveillance by archaeal Pelota and GTP-bound EF1α complex. Proc. Natl. Acad. Sci. U.S.A. 107, 17575–17579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen L., Muhlrad D., Hauryliuk V., Cheng Z., Lim M. K., Shyp V., Parker R., Song H. (2010) Structure of the Dom34-Hbs1 complex and implications for no-go decay. Nat. Struct. Mol. Biol. 17, 1233–1240 [DOI] [PubMed] [Google Scholar]
- 57. Shoemaker C. J., Green R. (2011) Kinetic analysis reveals the ordered coupling of translation termination and ribosome recycling in yeast. Proc. Natl. Acad. Sci. U.S.A. 108, E1392–E1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Becker T., Franckenberg S., Wickles S., Shoemaker C. J., Anger A. M., Armache J.-P., Sieber H., Ungewickell C., Berninghausen O., Daberkow I., Karcher A., Thomm M., Hopfner K. P., Green R., Beckmann R. (2012) Structural basis of highly conserved ribosome recycling in eukaryotes and archaea. Nature 482, 501–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tsuboi T., Kuroha K., Kudo K., Makino S., Inoue E., Kashima I., Inada T. (2012) Dom34:Hbs1 plays a general role in quality-control systems by dissociation of a stalled ribosome at the 3′ end of aberrant mRNA. Mol. Cell 46, 518–529 [DOI] [PubMed] [Google Scholar]