Abstract
Objectives. We sought to analyze how early exposure to the 1918 influenza pandemic is associated with old-age mortality by cause of death.
Methods. We analyzed the National Health Interview Survey (n = 81 571; follow-up 1989–2006; 43 808 deaths) and used year and quarter of birth to assess timing of pandemic exposure. We used Cox proportional and Fine-Gray competing hazard models for all-cause and cause-specific mortality, respectively.
Results. Cohorts born during pandemic peaks had excess all-cause mortality attributed to increased noncancer mortality. We found evidence for a trade-off between noncancer and cancer causes: cohorts with high noncancer mortality had low cancer mortality, and vice versa.
Conclusions. Early disease exposure increases old-age mortality through noncancer causes, which include respiratory and cardiovascular diseases, and may trigger a trade-off in the risk of cancer and noncancer causes. Potential mechanisms include inflammation or apoptosis. The findings contribute to our understanding of the causes of death behind the early disease exposure–later mortality association. The cancer–noncancer trade-off is potentially important for understanding the mechanisms behind these associations.
Adverse early life conditions may have lasting effects on old-age health and mortality.1–8 Some even consider reductions in early life disease exposure to be a primary driver of historical mortality declines.9 Although the precise mechanisms linking early disease exposure to poor adult health remain unclear, numerous pathways have been postulated including those relating to fetal undernutrition and dysregulation of immune function.3,10,11
In animal models, experimental evidence suggests a negative causal effect of early disease exposure on later health.12–14 For humans, historical epidemics have been used to study the effects of early disease exposure on later health.1,2,4,15 These studies often find that those born around the time of an epidemic exhibit worse adult health and mortality than do neighboring cohorts.1,2,4 However, the causes of death contributing to the excess mortality are not known. Moreover, research on early exposure to the deadliest epidemic of the 20th century—the 1918 influenza pandemic—is mixed, showing increased cardiovascular disease prevalence and lower socioeconomic attainment,1,4 but no long-term mortality effects.15
We investigated whether US cohorts with early exposure to the 1918 pandemic experience differential mortality at old ages compared with neighboring cohorts. The 1918 pandemic, caused by the influenza A virus (subtype H1N1), arrived in the United States in 3 waves.16 During the first wave, which began in March 1918 and was completed by July 1918, incidence rates were high, but mortality was only slightly elevated. The second and the deadliest wave began in September 1918 and lasted until the end of the year. The third wave, with a mortality impact between those of the first 2 waves, occurred from January 1919 to March 1919. Approximately 30% of the US population was infected and about 0.5% of the population died because of the pandemic, mostly from pneumonia.16 Excess mortality had an unusual pattern as those aged 20 to 40 years were affected particularly strongly.16
The advantages of focusing on the 1918 pandemic are threefold. First, the pandemic arrived unexpectedly and lasted for only a short period, allowing treatment of the pandemic as a “natural experiment” wherein cohorts born months apart experienced different exposures but were otherwise compositionally similar in terms of other childhood characteristics and environmental conditions. Moreover, the exposed and nonexposed cohorts were born in a narrow enough time interval that timing of birth is not systematically linked to subsequent differences in the adult environment. Second, in contrast to older epidemics, existing data permit cause-of-death analyses. Third, although food shortages and disease tended to co-occur in historical populations, the 1918 pandemic allows focusing on disease because there were no generalized food shortages in the United States during the pandemic. Nutritional deprivation caused by disease, however, may function as a mediator.
We extended previous research in 3 important dimensions. First, although earlier studies have analyzed the relationship between early disease exposure and later-life mortality,2,15,17 it is not known what causes of death drive the association. We analyzed mortality by cause, which can enhance our understanding of potential mechanisms. Second, previous research on early disease exposure and later mortality has analyzed annual birth cohorts.2,5,15 We distinguished cohorts by year and quarter of birth, which provides a far more nuanced analysis of exposure timing. Third, previous work on the long-lasting effects of the pandemic has not accounted for the fact that the pandemic arrived in waves.1,4,15 Because of variation in the immediate mortality effects of each wave, there may be differences with respect to long-lasting effects. Our analysis accounted for exposure to each wave.
METHODS
We used data from the National Health Interview Survey (NHIS), an annual cross-sectional survey of the US noninstitutionalized population. We used the 1989–2004 surveys because we focused on US-born people and country of birth is not known before 1989, and death linkages are currently not available for surveys conducted after 2004. The 1989–2004 surveys are linked with the National Death Index through December 31, 2006, in the NHIS–Linked Mortality Files. These data allow for mortality analysis by year and quarter of birth. The mortality period assessed (1989–2006) falls under both the International Classification of Diseases, Ninth Revision (ICD-9; 1979–1998) and International Classification of Diseases, 10th Revision (ICD-10; 1999–2006) guidelines for US cause-of-death coding.18,19 We used a consistent set of 113 underlying cause-of-death recodes provided in the NHIS–Linked Mortality Files,20 with deaths occurring before 1999 recoded into comparable ICD-10 groupings by the National Center for Health Statistics. We analyzed all-cause mortality and mortality by 3 major cause-of-death categories: (1) cardiovascular diseases including heart disease, cerebrovascular diseases, and diseases of the circulatory system (ICD-10: I00–I78; hereafter: CVD); (2) malignant neoplasms excluding those of the trachea, bronchus, and lung (ICD-10: C00–C97 excluding C33–C34), and (3) all other causes, among which respiratory diseases is the largest category.
Birth Cohorts and Exposure Timing
We included the 1913–1924 birth cohorts. These included those born during the 2 years spanning the pandemic (1918–1919) and 5 cohorts born before (1913–1917) and after (1920–1924) the pandemic. The sample size was 81 571 persons with 43 808 deaths.
We grouped the cohorts into 5 categories according to exposure timing:
the 1913q1–1917q2 cohorts (“q” refers to quarter of year) were exposed after their first birthday;
the 1917q3–1918q1 cohorts were exposed during first year of life but not at birth or in gestation;
the 1918q2, 1918q4, and 1919q1 cohorts were exposed in the third trimester and at birth, as each of the cohorts was born during one of the pandemic waves;
the 1918q3 and 1919q2–q4 cohorts were each exposed early in gestation (first or second trimester) but not at birth; and
the 1920q1–1924q4 cohorts were not directly exposed.
These 5 categories are not an exhaustive description of the exposure experience. For example, in group 3, the 1919q1 cohort was exposed not only late in gestation and at birth (to the third wave), but also in the second trimester (to the second wave). Likewise, in group 4, the 1918q3 cohort had second-trimester exposure to the first wave and postbirth exposure to waves 2 and 3. However, this categorization provides a useful map describing which cohorts were exposed (1) after first year of life, (2) during the first year of life, (3) late in gestation and at birth, (4) early in gestation but not at birth, or (5) were not directly exposed. A supplementary table (available as a supplement to this article at http://www.ajph.org) illustrates the exposure timing by birth cohort.
The NHIS samples from the noninstitutionalized population, but death linkages capture deaths to those institutionalized after being surveyed. Excluding the institutionalized population at baseline is unlikely to bias our results because the fraction of institutionalized population in the relevant age groups and periods is small; for example, in 1990, the fraction of institutionalized population aged 65 to 74 years was less than 2%.21
Statistical Analysis
We analyzed mortality by birth cohort. Individuals entered the risk set at the date of interview and exited at either their date of death or at the end of 2006 if they survived through the observation period. We measured all dates in quarter-year units. We used Cox proportional hazards regression22 for all-cause mortality and report the hazard ratios (HRs). For cause-specific mortality we used the Fine-Gray competing risk regression23 which is an appropriate model when one is analyzing competing causes,24 and have reported the subdistribution HRs. The interpretation of these is similar to the interpretation of the standard HRs in the Cox model. Further details of the model are available as a supplement to this article at http://www.ajph.org.
We combined the nonexposed 1920q1–1924q4 cohorts and treated them as the omitted reference category. We also combined the cohorts exposed after their first birthday (1913q1–1917q2) into a single category. Among those exposed during gestation or before first birthday (1917q3–1919q4), we used quarter and year of birth indicators to capture the exposure timing.
We controlled for age and age squared at baseline to capture nonlinearities in the association between log-mortality and age. We controlled for a cohort trend in mortality by including a continuous birth year variable. We adjusted all models for gender. We excluded lung cancer deaths from the cancer analyses because lung cancer risk is largely determined by lifetime cigarette smoking and preliminary analyses reveal that smoking behaviors did not vary across the birth cohorts examined. Removing a major cause of death that is mostly determined by adult behavior (rather than early life exposures) allows for a more accurate analysis of the remaining causes of death. We describe results from sensitivity analyses that include deaths from lung cancer, control for season of birth, and other robustness checks.
Additional methodological details are available as a supplement to this article at http://www.ajph.org. We conducted all analyses by using Stata/SE version 11.2 (StataCorp LP, College Station, TX).
RESULTS
Table 1 describes the data. The sample size was 81 571, average age at baseline was 74.5 years, and average follow-up was 9.0 years. The majority of the sample were women (47 583 vs 33 988 persons). During the follow-up, 43 808 (53.7%) persons died, 34 411 of noncancer and 9397 of cancer causes. Among noncancer causes, cardiovascular disease was the most common (19 382 cases). For the key cohorts 1917q3–1919q4 exposed in utero, at birth, or during the first year of life, the sample sizes ranged from 1490 to 1889 and number of deaths from 843 to 1030.
TABLE 1—
Descriptive Statistics of the National Health Interview Survey Data With Follow-Up Over the Years 1989–2006
| Variable | Total, No., Mean, or No. (No. Deaths) | Men, No., Mean, or No. (No. Deaths) | Women, No., Mean, or No. (No. Deaths) |
| Number of observations | 81 571 | 33 988 | 47 583 |
| Age at baseline, y | 74.5 | 74.1 | 74.7 |
| Average follow-up, y | 9.0 | 8.5 | 9.4 |
| Died during the follow-up | 43 808 | 20 823 | 22 985 |
| Noncancer causes of death | 34 411 | 15 823 | 18 588 |
| Cardiovascular disease | 19 382 | 8951 | 10 431 |
| Other noncancer | 15 029 | 6872 | 8157 |
| Cancer causes of death | 9397 | 5000 | 4397 |
| Other than lung cancer | 6734 | 3396 | 3338 |
| Lung cancer | 2663 | 1604 | 1059 |
| Birth cohort and exposure timing | |||
| 1913q1–1917q2, after 1st birthday | 23 880 (16 796) | 9162 (7212) | 14 718 (9584) |
| 1917q3, 1st y of life | 1765 (1030) | 748 (498) | 1017 (532) |
| 1917q4, 1st y of life | 1603 (953) | 727 (503) | 876 (450) |
| 1918q1, 1st y of life | 1490 (882) | 611 (421) | 879 (461) |
| 1918q2, at birth and 3rd trimester | 1508 (910) | 637 (442) | 871 (468) |
| 1918q3, 1st and 2nd trimester | 1590 (887) | 674 (418) | 916 (469) |
| 1918q4, at birth and 3rd trimester | 1495 (843) | 646 (432) | 849 (411) |
| 1919q1, at birth and 3rd trimester | 1621 (911) | 657 (428) | 964 (483) |
| 1919q2, 1st and 2nd trimester | 1558 (851) | 666 (414) | 892 (437) |
| 1919q3, 1st and 2nd trimester | 1639 (843) | 682 (418) | 957 (425) |
| 1919q4, 1st and 2nd trimester | 1889 (974) | 810 (481) | 1079 (493) |
| 1920q1–1924q4, not exposed | 41 533 (17 928) | 17 968 (9156) | 23 565 (8772) |
The fraction of dead was largest (70%; 16 796 of 23 880) for the 1913q1–1917q2 cohort that was exposed after the first birthday, and lowest (43%) for the nonexposed 1920q1–1924q4 cohort. Among those that were exposed during the first year of life, in the third trimester and at birth, or in both or either the first or second trimester, the fractions of dead were 59%, 58%, and 52%, respectively (calculations not shown). These differences show that cohorts that were born earlier in time had higher mortality than those that were born later, and tentatively suggest that the cohorts that were exposed in the third trimester or at birth may have higher mortality than those that were exposed early in gestation. However, these differences also reflect the age differences of the respective cohorts when entering our study, which we controlled for in the regressions.
Table 2 shows HRs for all-cause mortality and for causes of death by birth cohort and exposure timing. The 1918q2 and 1919q1 cohorts, which were exposed in third trimester and at birth have excess all-cause mortality, the HRs being 1.08 and 1.09, respectively (both P < .05). The excess mortality is fully attributable to noncancer causes, among which cardiovascular and respiratory diseases are the major causes of death. For the 1919q3 cohort, which was exposed to the second and third waves early in gestation, we observed decreased noncancer, in particular CVD, mortality (HR = 0.87; P < .05) and increased cancer mortality (HR = 1.26; P < .01).
TABLE 2—
All-Cause Mortality Hazard Ratios and Cause-Specific Subdistribution Hazard Ratios at Ages 63 to 95 Years by Birth Cohort and Timing of Exposure to 1918 Influenza Pandemic and Cause of Death: National Health Interview Survey, 1913–1924 Cohorts
| Noncancer Causes |
|||||
| All Causes, HR (95% CI) | All, HR (95% CI) | CVD, HR (95% CI) | Other, HR (95% CI) | Cancer,a HR (95% CI) | |
| Birth cohort, exposure timing | |||||
| 1917q3, 1st y of life | 0.99 (0.92, 1.07) | 0.98 (0.90, 1.06) | 0.90 (0.81, 1.01) | 1.11 (0.98, 1.25) | 1.07 (0.88, 1.29) |
| 1917q4, 1st y of life | 1.06 (0.99, 1.15) | 1.04 (0.95, 1.13) | 1.00 (0.89, 1.12) | 1.07 (0.94, 1.21) | 1.07 (0.88, 1.31) |
| 1918q1, 1st y of life | 1.03 (0.96, 1.11) | 1.07 (0.99, 1.16) | 1.05 (0.94, 1.17) | 1.10 (0.98, 1.25) | 1.04 (0.86, 1.26) |
| 1918q2, at birth and 3rd trimester | 1.08* (1.00, 1.16) | 1.15*** (1.06, 1.25) | 1.02 (0.91, 1.14) | 1.28*** (1.14, 1.45) | 0.96 (0.79, 1.17) |
| 1918q3, 1st and 2nd trimester | 0.97 (0.90, 1.05) | 0.95 (0.87, 1.03) | 0.99 (0.89, 1.10) | 0.93 (0.81, 1.06) | 1.09 (0.91, 1.31) |
| 1918q4, at birth and 3rd trimester | 1.02 (0.95, 1.10) | 1.00 (0.92, 1.09) | 0.97 (0.87, 1.09) | 1.04 (0.91, 1.18) | 1.14 (0.95, 1.38) |
| 1919q1, at birth and 3rd trimester | 1.09* (1.01, 1.17) | 1.13** (1.05, 1.23) | 1.07 (0.96, 1.19) | 1.20** (1.07, 1.34) | 0.97 (0.81, 1.17) |
| 1919q2, 1st and 2nd trimester | 1.05 (0.97, 1.13) | 1.05 (0.97, 1.14) | 1.06 (0.95, 1.19) | 1.05 (0.93, 1.19) | 1.06 (0.89, 1.28) |
| 1919q3, 1st and 2nd trimester | 0.97 (0.90, 1.04) | 0.92* (0.84, 1.00) | 0.87* (0.78, 0.98) | 1.02 (0.90, 1.15) | 1.26** (1.07, 1.49) |
| 1919q4, 1st and 2nd trimester | 1.00 (0.93, 1.07) | 0.99 (0.92, 1.07) | 0.93 (0.84, 1.03) | 1.08 (0.96, 1.21) | 1.07 (0.91, 1.27) |
| 1920q1–1924q4, not exposed (Ref) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| No. | 81 571 | 81 571 | 81 571 | 81 571 | 81 571 |
| Deaths | 43 808 | 34 411 | 19 382 | 15 029 | 6734 |
| Log lik | −46 5151 | −369 047 | −211 364 | −164 239 | −74 553 |
Note. CI = confidence interval; CVD = cardiovascular disease; HR = hazard ratio. We estimated results for all-cause mortality with the Cox proportional hazards model, and results for cause-specific mortality with the Fine-Gray competing risks model. All models controlled for age and age squared at baseline, gender, and linear trend in birth year.
Excludes lung cancer.
*P < .05; **P < .01; ***P < .001.
Thus, of the 3 cohorts that were exposed late in gestation and at birth, 2, (1918q2, 1919q1) experienced excess old-age mortality. The exception is the 1918q4 cohort that was born during the second wave and was exposed to the third wave soon after birth.
Our results additionally suggest that mortality HRs for cancer and noncancer causes are negatively correlated. For the cohorts that had increased noncancer mortality (1918q2 and 1919q1), the point estimates for cancer mortality were below 1.00. For other cohorts, cancer HR was above 1.00. In addition, for the only cohort with significant excess cancer mortality (1919q3), CVD mortality was significantly decreased.
Table 3 shows the HR correlations between cancer and other causes of death for the 1917q3–1919q4 cohorts. For comparison, we also show cohorts exposed after first birthday (1913q1–1917q2) and nonexposed cohorts (1920q1–1924q4). For the 1917q3–1919q4 cohorts the correlations were calculated from the HRs of Table 2. For other cohorts we estimated additional mortality regressions by birth quarter and year and calculated the correlations (see material available as a supplement to this article at http://www.ajph.org). For the 1917q3–1919q4 cohorts the cancer–noncancer HR correlation ranged from −0.70 to −0.87 (P < .05). For other cohorts the correlations were small and nonsignificant. Thus, we observed the cancer–noncancer trade-off only for the cohorts that were exposed early in life.
TABLE 3—
Correlation Coefficients Between Mortality Subdistribution Hazard Ratios for Cancer and Noncancer Mortality by Birth Cohort and Timing of Exposure to 1918 Influenza Pandemic: National Health Interview Survey
| Cause of Death by Birth Cohorts,a Timing of Exposure to the 1918 Influenza Pandemic | Correlation with Cancer, HRb (95% CI) |
| 1913q1–1917q2, after 1st birthday | |
| All noncancer | −0.16 (−0.72, 0.52) |
| CVD HR | −0.07 (−0.67, 0.59) |
| Other HR | −0.20 (−0.74, 0.49) |
| 1917q3–1919q4, in utero or during 1st y of life | |
| All noncancer | −0.87** (−0.97, -0.53) |
| CVD HR | −0.73* (−0.93, -0.19) |
| Other HR | −0.70* (−0.92, -0.13) |
| 1920q1–1924q4, not directly exposed | |
| All noncancer HR | 0.25 (−0.45, 0.76) |
| CVD HR | 0.07 (−0.56, 0.67) |
| Other HR | 0.30 (−0.41, 0.78) |
Note. CI = confidence interval; CVD = cardiovascular disease; HR = hazard ratio. The sample size was n = 81 571.
The correlations for 1917q3–1919q4 cohorts were based on mortality subdistribution hazard ratios estimated with the Fine-Gray competing risks model; all models controlled for age and age squared at baseline, gender, and linear trend in birth year. The correlations for 1913q1–1917q2 cohorts were based on analogous model with the change that birth year and quarter dummies were assigned to the 1913q1–1917q2 cohorts and 1917q3–1919q4 was controlled with a single dummy. The cohorts 1920q1–1924q4 were the reference group as in the baseline model. The correlations for 1920q1–1924q4 cohorts were based on an analogous model in which birth year and quarter dummies were assigned to the 1920q1–1924q4 cohorts, 1917q3–1919q4 was controlled with a single dummy, and cohorts 1913q1–1917q2 were the reference group.
Excludes lung cancer.
*P < .05; **P < .01.
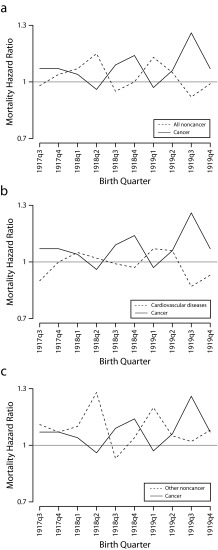
Figure 1 illustrates the mortality trade-offs for the 1917q3–1919q4 cohorts by comparing the HRs for cancer with all noncancer mortality (Figure 1a), cardiovascular disease (Figure 1b), and other noncancer mortality (Figure 1c). In each part, cancer mortality increased when noncancer mortality decreased, and vice versa.
FIGURE 1—
Mortality hazard ratios at ages 63–95 years by birth quarter among cohorts exposed to the 1918 influenza pandemic in utero or during first year of life for cancer and (a) all noncancer causes, (b) cardiovascular disease, and (c) other noncancer causes: National Health Interview Survey.
Note. Other noncancer causes include all causes other than cardiovascular disease. The sample size was n = 81 571.
Sensitivity checks are available as supplementary data at http://www.ajph.org. First, we included season of birth controls; estimated the cancer results with lung cancer; included controls for race/ethnicity and education; expanded or narrowed the cohort window from 1913–1924 to 1912–1925 or 1915–1922; and used the Cox model for cause-specific mortality. The key results did not change. Second, we estimated the results by gender. The statistical power decreased but the gender-specific results were qualitatively similar to our main results.
DISCUSSION
We studied the association between early disease exposure and old-age mortality by using the 1918 influenza pandemic as an exogenous shock. Previous research on early exposure to the 1918 pandemic has found no long-term association with mortality but has relied on annual cohorts,15 which combines cohorts exposed during different gestation stages. Unlike previous studies, we examined mortality by year and quarter of birth and examined specific causes of death. Three findings emerged. First, late gestation and at-birth exposure to the 1918 pandemic was associated with increased old-age mortality. Second, early disease exposure increased later mortality through noncancer causes, among which respiratory and cardiovascular causes were leading contributors. Third, there appears to be a trade-off between cancer and noncancer causes: cohorts with high noncancer mortality had low cancer mortality, and vice versa. The trade-off was observed only for cohorts that were exposed to the pandemic before their first birthday, not for earlier- or later-born cohorts. The trade-off was most pronounced for the 1918q2 and 1919q1 cohorts for which noncancer mortality was elevated and cancer mortality was not, and for the 1919q3 cohort for which cancer mortality was elevated and noncancer mortality was depressed.
Three cohorts were exposed to the pandemic late in gestation and at birth (1918q2, 1918q4, 1919q1). Of these, the 1918q2 and 1919q1 cohorts had 8% to 9% excess old-age all-cause mortality, corresponding to 0.6 years of decreased life expectancy at age 70 years in a population with life expectancy at birth of 75 years. This estimate should be a lower bound. Only one third of the US population was infected, and we are unable to ascertain the infection status of respondents. The excess mortality among the infected (or whose mothers were infected) is likely higher. In addition, selective mortality before the observation period may further bias the estimates downward as approximately a quarter of those born in the 1910s died before the entry age 63 years.25
The fact that we did not observe excess mortality for the 1918q4 cohort could also be attributable to earlier life selection.26 The 1918q4 cohort was exposed to the first wave early in gestation, a period during which spontaneous abortions were most common, and which is thought to be associated with increased mortality at young and middle age,27 born during the deadliest second wave, and continually exposed to the second and third waves up to 3 to 6 months of age. The 1918q2 and 1919q1 cohorts had less intense early gestation and postbirth exposures. Thus, stronger pre- and postbirth selection may bias the estimates for the 1918q4 cohort downward more so than that of the 1918q2 and 1919q1 cohorts, resulting in a null finding that is attributable to selection. Other estimates, including the observed increase in cancer mortality for the 1919q1 cohort, may also be conservative because of earlier-life selective mortality.
We found decreased noncancer mortality, in particular CVD mortality, for the 1919q3 cohort that was exposed to the deadly second and third waves early in gestation. Selection may play a role here as well. Early gestation shocks are particularly likely to result in miscarriage, and one study suggests that the risk of miscarriage or fetal death was elevated among those that were exposed to the 1918 pandemic in the first and second trimesters.28
Previous research that used epidemics other than the 1918 pandemic suggests that early disease exposure increases old-age mortality,2,29 but the causes of death have been unknown. Our result is consistent with the literature that has found excess CVD prevalence for those with late gestation exposure to the 1918 pandemic.1 Several pathways could link late gestation or postnatal disease exposure with later noncancer mortality. Developing organisms adapt to environmental signals.30 If early life environment is different from that experienced later in life, the adaptations may be harmful.9,31 For example, disease exposure may cause nutritional deprivation and permanent changes in glucose–insulin metabolism. Such adaptation might be helpful in a nutritionally deprived environment during later life but increase CVD and diabetes risk in an affluent environment.32,33 Early disease exposure may also prime the immune system to be constantly alert, leading to chronic inflammation,31 which increases CVD risk.9,34 As the third trimester is critical for lung maturation,35 late gestation exposure may increase respiratory disease mortality. Prenatal exposure may also result in preterm birth,36 increasing the risk of several health conditions.37
Our study gains leverage from jointly analyzing all 3 waves of the pandemic. Other studies have focused on the most virulent second wave, possibly because the mortality impact of the third wave was milder, and the impact, or even existence, of the first wave is not always recognized. Epidemiological studies, however, confirm that in the spring of 1918 an influenza wave with a signature W-shaped excess mortality pattern hit New York City,38 US Army camps,39 and Mexico.40 These studies provide strong evidence for a “herald” spring wave in North America.
Previous research on adverse early life exposures suggests various critical periods of exposure. Studies on the 1918 pandemic have found that late gestation or at-birth exposures are most important for later life outcomes.1,4 Analyses using historical data and population-level mortality rates as proxies for disease exposure have found that exposures at birth and during the first year of life are most important.2,29 However, some famine studies suggest that first or second trimester exposures are most important.6,27,41 Our finding that late gestation and at-birth exposures are important for later mortality is consistent with existing literature on early disease exposure and adult health and mortality. With respect to nutrition, it is possible that the mechanisms and critical periods are different.
Correlation Between Causes of Death
Collectively, our findings suggest that subtle differences in exposure timing may have important but complex implications for later mortality. Early disease exposure may trigger processes that increase later noncancer mortality but are protective against cancer, and vice versa. However, a negative correlation in HRs could also occur if the risk is increased for both cancer and noncancer causes: if the risks for both CVD and cancer are elevated, but people tend to systematically die from one cause before the other occurs, a negative correlation in HRs may arise. Unfortunately, without strong assumptions, it is not possible to test whether the negative correlation in HRs is driven by negative or positive correlations in the individual-level risks.42 We can nevertheless speculate on the likelihood that the negative HR correlation is caused by a positive versus negative correlation in the individual-level risks. Our simulations suggest that moderate positive correlation in the individual-level risks may lead to a moderately positive or negative HR correlation. Moderate negative correlation in the individual-level risk may lead to a moderate to large negative HR correlation.43 We observed a correlation of −0.87 (P < .01) between cancer and noncancer HRs. We consider this correlation to be strong, suggesting a negative rather than positive correlation in the individual-level risks.
Similar trade-offs have been documented in other settings. For example, heart disease, diabetes, and Alzheimer’s disease have been associated with decreased risk for 1 or more cancers.44,45 Low birth weight is positively associated with cardiovascular disease but negatively associated with several cancers.46 Androgen deprivation therapy treats prostate cancer47 but may increase cardiovascular disease and diabetes mortality.48,49 Our study is the first to demonstrate that early disease exposure may trigger a similar trade-off.
Inflammation and apoptosis may help in understanding the trade-off. Although it is beyond the scope of this study to test these explanations, they provide a plausible mechanism through which early disease exposure may have differential effects by cause of death. Chronic inflammation may be triggered by early disease exposure. Inflammation, in turn, is linked with apoptosis and cellular senescence, which are protective against cancer50–53 but may predispose to other aging-related diseases such as ischemic heart disease and neurodegenerative diseases.54,55 In particular, apoptosis and cellular senescence are regulated by the protein p53.50–53 In unstressed cells, p53 levels are low. DNA damage activates p53. Activated p53 may initiate cell cycle arrest, which prevents damaged DNA from replicating or allows DNA repair. When DNA is damaged beyond repair, p53 may initiate apoptosis. These processes control carcinogenesis. Indeed, increasing p53 levels may decrease cancer risk56 but also accelerate other aging-related diseases.52 Previous research documents that inflammation may influence the functioning of p53, but has not considered the role of early life exposures. Our findings suggest that early disease exposure may permanently imprint physiological processes that may result in a trade-off between cancer and other causes of death, potentially through altered functioning of p53.
Conclusions
Using the 1918 pandemic as an exogenous shock we show that early disease exposure increases old-age mortality through noncancer causes and may trigger a trade-off in the risk for cancer and noncancer causes. The findings enhance our understanding of the causes of death that contribute to the association between disease exposure early in life and adult mortality. Our study also provides suggestive evidence on why earlier research, which has only considered annual birth cohorts, has not found a mortality association for exposure to the 1918 pandemic. Early disease exposure has complex effects on later-life health, so that the magnitude and even the sign of the effect may critically depend on the timing of exposure and on the cause of death analyzed. Identification of these patterns is not possible in analyses using annual birth cohorts, wherein cohorts exposed at different stages of gestation are combined. The finding of an early disease exposure that triggers a trade-off between cancer and other causes may help further elucidate our understanding of the origins of certain cancers.
Acknowledgments
N. K. Mehta would like to thank the Robert Wood Johnson Foundation Health & Society Scholars Program for its financial support.
Human Participant Protection
No protocol approval was required because data were obtained from secondary sources.
References
- 1.Mazumder B, Almond D, Park K, Crimmins EM, Finch CE. Lingering prenatal effects of the 1918 influenza pandemic on cardiovascular disease. J Dev Orig Health Dis. 2010;1(1):26–34. doi: 10.1017/S2040174409990031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bengtsson T, Lindstrom M. Airborne infectious diseases during infancy and mortality in later life in southern Sweden, 1766–1894. Int J Epidemiol. 2003;32(2):286–294. doi: 10.1093/ije/dyg061. [DOI] [PubMed] [Google Scholar]
- 3.Schulz LC. The Dutch Hunger Winter and the developmental origins of health and disease. PNAS. 2010;107(39):16757–16758. doi: 10.1073/pnas.1012911107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almond D. Is the 1918 influenza pandemic over? Long-term effects of in utero influenza exposure in the post-1940 US population. J Polit Econ. 2006;114(4):672–712. [Google Scholar]
- 5.van den Berg GJ, Doblhammer G, Christensen K. Exogenous determinants of early-life conditions, and mortality later in life. Soc Sci Med. 2009;68(9):1591–1598. doi: 10.1016/j.socscimed.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum Dev. 2006;82(8):485–491. doi: 10.1016/j.earlhumdev.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Myrskylä M. The relative effects of shocks in early- and later-life conditions on mortality. Popul Dev Rev. 2010;36(4):803–829. doi: 10.1111/j.1728-4457.2010.00358.x. [DOI] [PubMed] [Google Scholar]
- 8.Myrskylä M. The effects of shocks in early life mortality on later life expectancy and mortality compression: a cohort analysis. Demogr Res. 2010;22:289–320. [Google Scholar]
- 9.Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305(5691):1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- 10.Costa DL. Understanding the twentieth-century decline in chronic conditions among older men. Demography. 2000;37(1):53–72. [PubMed] [Google Scholar]
- 11.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fatemi SH, Earle J, Kanodia R et al. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell Mol Neurobiol. 2002;22(1):25–33. doi: 10.1023/A:1015337611258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asp L, Beraki S, Aronsson F et al. Gene expression changes in brains of mice exposed to a maternal virus infection. Neuroreport. 2005;16(10):1111–1115. doi: 10.1097/00001756-200507130-00016. [DOI] [PubMed] [Google Scholar]
- 14.Moreno JL, Kurita M, Holloway T et al. Maternal influenza viral infection causes schizophrenia-like alterations of 5-HT2A and mGlu2 receptors in the adult offspring. J Neurosci. 2011;31(5):1863. doi: 10.1523/JNEUROSCI.4230-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen AA, Tillinghast J, Canudas-Romo V. No consistent effects of prenatal or neonatal exposure to Spanish flu on late-life mortality in 24 developed countries. Demogr Res. 2010;22(20):579–634. doi: 10.4054/DemRes.2010.22.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taubenberger J, Morens DM. 2006;12(1) doi: 10.3201/eid1201.051442. 1918 influenza: the mother of all pandemics. Emerg Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gagnon A, Mazan R. Does exposure to infectious diseases in infancy affect old-age mortality? Evidence from a pre-industrial population. Soc Sci Med. 2009;68(9):1609–1616. doi: 10.1016/j.socscimed.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. International Classification of Diseases, Ninth Revision (ICD-9) Available at: http://www.cdc.gov/nchs/icd/icd9.htm. Accessed April 10, 2012. [Google Scholar]
- 19.Centers for Disease Control and Prevention. International Classification of Diseases, Tenth Revision (ICD-10) Available at: http://www.cdc.gov/nchs/icd/icd10.htm. Accessed April 10, 2012. [Google Scholar]
- 20.Anderson R, Minino A, Hoyert D, Rosenberg H. Comparability of cause of death between ICD-9 and ICD-10: preliminary estimates. National Center for Health Statistics. National Vital Statistics Reports. 2001;49(2) [PubMed] [Google Scholar]
- 21.Anderton DL, Barrett RE, Bogue DJ. The Population of the United States. New York, NY: Free Press; 1997. [Google Scholar]
- 22.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34(2):187–220. [Google Scholar]
- 23.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 24.Andersen PK, Geskus RB, de Witte T, Putter H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol. 2012;41(3):861–870. doi: 10.1093/ije/dyr213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Human Mortality Database. University of California, Berkeley, and Max Planck Institute for Demographic Research (Germany). Available at: http://www.mortality.org or http://www.humanmortality.de. Accessed July 16, 2012.
- 26.Doblhammer G, van den Berg GJ, Lumey LH. Long-term effects of famine on life expectancy: a re-analysis of the Great Finnish Famine of 1866–1868. 2011 doi: 10.1080/00324728.2013.809140. IZA Discussion Paper No. 5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Abeelen AFM, Veenendaal MVE, Painter RC et al. Survival effects of prenatal famine exposure. Am J Clin Nutr. 2012;95(1):179–183. doi: 10.3945/ajcn.111.022038. [DOI] [PubMed] [Google Scholar]
- 28.Bloom-Feshbach K, Simonsen L, Viboud C et al. Natality decline and miscarriages associated with the 1918 influenza pandemic: the Scandinavian and United States experiences. J Infect Dis. 2011;204(8):1157–1164. doi: 10.1093/infdis/jir510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bengtsson T, Broström G. Do conditions in early life affect old-age mortality directly and indirectly? Evidence from 19th-century rural Sweden. Soc Sci Med. 2009;68(9):1583–1590. doi: 10.1016/j.socscimed.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 30.Stearns SC. The evolutionary significance of phenotypic plasticity: phenotypic sources of variation among organisms can be described by developmental switches and reaction norms. Bioscience. 1989;39(7):436–445. [Google Scholar]
- 31.Crimmins EM, Finch CE. Infection, inflammation, height, and longevity. Proc Natl Acad Sci U S A. 2006;103(2):498–503. doi: 10.1073/pnas.0501470103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barker DJP. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition. 1997;13(9):807–813. doi: 10.1016/s0899-9007(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 33.Hales CN, Barker DJP. The thrifty phenotype hypothesis. Br Med Bull. 2001;60(1):5. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 34.Roivainen M, Viik-Kajander M, Palosuo T et al. Infections, inflammation, and the risk of coronary heart disease. Circulation. 2000;101(3):252–257. doi: 10.1161/01.cir.101.3.252. [DOI] [PubMed] [Google Scholar]
- 35.Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res. 1999;46(6):641. doi: 10.1203/00006450-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Goldenberg RL, Hauth J, Andrews W. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 37.Moster D, Lie R, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med. 2008;359(3):262–273. doi: 10.1056/NEJMoa0706475. [DOI] [PubMed] [Google Scholar]
- 38.Olson DR, Simonsen L, Edelson PJ, Morse SS. Epidemiological evidence of an early wave of the 1918 influenza pandemic in New York City. Proc Nat Acad Sci U S A. 2005;102(31):11059–11063. doi: 10.1073/pnas.0408290102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barry JM, Viboud C, Simonsen L. Cross-protection between successive waves of the 1918–1919 influenza pandemic: epidemiological evidence from US Army camps and from Britain. J Infect Dis. 2008;198(10):1427–1434. doi: 10.1086/592454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chowell G, Viboud C, Simonsen L, Miller MA, Acuna-Soto R. Mortality patterns associated with the 1918 influenza pandemic in Mexico: evidence for a spring herald wave and lack of preexisting immunity in older populations. J Infect Dis. 2010;202(4):567–575. doi: 10.1086/654897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Rooij SR, Wouters H, Yonker JE, Painter RC, Roseboom TJ. Prenatal undernutrition and cognitive function in late adulthood. Proc Nat Acad Sci U S A. 2010;107(39):16881–16886. doi: 10.1073/pnas.1009459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsiatis A. A nonidentifiability aspect of the problem of competing risks. Proc Natl Acad Sci U S A. 1975;72(1):20–22. doi: 10.1073/pnas.72.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gichangi A, Vach W. The Analysis of Competing Risks Data: A Guided Tour. Odense, Denmark: University of Southern Denmark; 2005. [Google Scholar]
- 44.Yashin AI, Ukraintseva SV, Akushevich IV, Arbeev KG, Kulminski A, Akushevich L. Trade-off between cancer and aging: what role do other diseases play?: evidence from experimental and human population studies. Mech Ageing Dev. 2009;130(1-2):98–104. doi: 10.1016/j.mad.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tabarés-Seisdedos R, Dumont N, Baudot A et al. No paradox, no progress: inverse cancer comorbidity in people with other complex diseases. Lancet Oncol. 2011;12(6):604–608. doi: 10.1016/S1470-2045(11)70041-9. [DOI] [PubMed] [Google Scholar]
- 46.Burdge GC, Lillycrop KA, Jackson AA. Nutrition in Early Life, and Risk of Cancer and Metabolic Disease: Alternative Endings in an Epigenetic Tale? Cambridge, UK: Cambridge University Press; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roach M, Bae K, Speight J et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol. 2008;26(4):585–591. doi: 10.1200/JCO.2007.13.9881. [DOI] [PubMed] [Google Scholar]
- 48.Kintzel PE, Chase SL, Schultz LM, O’Rourke TJ. Increased risk of metabolic syndrome, diabetes mellitus, and cardiovascular disease in men receiving androgen deprivation therapy for prostate cancer. Pharmacotherapy. 2008;28(12):1511–1522. doi: 10.1592/phco.28.12.1511. [DOI] [PubMed] [Google Scholar]
- 49.Levine GN, D’Amico AV, Berger P et al. Androgen deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. CA Cancer J Clin. 2010;60(3):194–201. doi: 10.3322/caac.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campisi J. Cancer and aging: yin, yang, and p53. Sci Aging Knowledge Environ. 2002;2002(1) doi: 10.1126/sageke.2002.1.pe1. pe1. [DOI] [PubMed] [Google Scholar]
- 51.Donehower LA. Does p53 affect organismal aging? J Cell Physiol. 2002;192(1):23–33. doi: 10.1002/jcp.10104. [DOI] [PubMed] [Google Scholar]
- 52.Tyner SD, Venkatachalam S, Choi J et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415(6867):45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 53.Campisi J. Cancer and ageing: rival demons? Nat Rev Cancer. 2003;3(5):339–349. doi: 10.1038/nrc1073. [DOI] [PubMed] [Google Scholar]
- 54.Olivetti G, Abbi R, Quaini F et al. Apoptosis in the failing human heart. N Engl J Med. 1997;336(16):1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 55.Barinaga M. Stroke-damaged neurons may commit cellular suicide. Science. 1998;281(5381):1302–1303. doi: 10.1126/science.281.5381.1302. [DOI] [PubMed] [Google Scholar]
- 56.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]