Abstract
Endometriosis is an estrogen-dependent disorder where endometrial tissue forms lesions outside the uterus. Endometriosis affects an estimated 10% of women in the reproductive-age group, rising to 30% to 50% in patients with infertility and/or pain, with significant impact on their physical, mental, and social well-being. There is no known cure, and most current medical treatments are not suitable long term due to their side-effect profiles. Endometriosis has an estimated annual cost in the United States of $18.8 to $22 billion (2002 figures). Although endometriosis was first described more than 100 years ago, current knowledge of its pathogenesis, spontaneous evolution, and the pathophysiology of the related infertility and pelvic pain, remain unclear. A consensus workshop was convened following the 10th World Congress on Endometriosis to establish recommendations for priorities in endometriosis research. One major issue identified as impacting on the capacity to undertake endometriosis research is the need for multidisciplinary expertise. A total of 25 recommendations for research have been developed, grouped under 5 subheadings: (1) diagnosis, (2) classification and prognosis, (3) treatment and outcome, (4) epidemiology, and (5) pathophysiology. Endometriosis research is underfunded relative to other diseases with high health care burdens. This may be due to the practical difficulties of developing competitive research proposals on a complex and poorly understood disease, which affects only women. By producing this consensus international research priorities statement it is the hope of the workshop participants that researchers will be encouraged to develop new interdisciplinary research proposals that will attract increased funding support for work on endometriosis.
Keywords: Endometriosis, research directions, international workshop, consensus report
INTRODUCTION
Immediately following the 10th World Congress on Endometriosis held in Melbourne in March 2008, a World Endometriosis Society (WES) and World Endometriosis Research Foundation (WERF)–endorsed workshop of interested persons was convened to develop a global consensus statement of research directions and priorities in endometriosis. Although by no means proscriptive, it is hoped that these recommendations will act as both a guide and a stimulus to the international research community, as well as the many funding agencies that may provide support for endometriosis research.
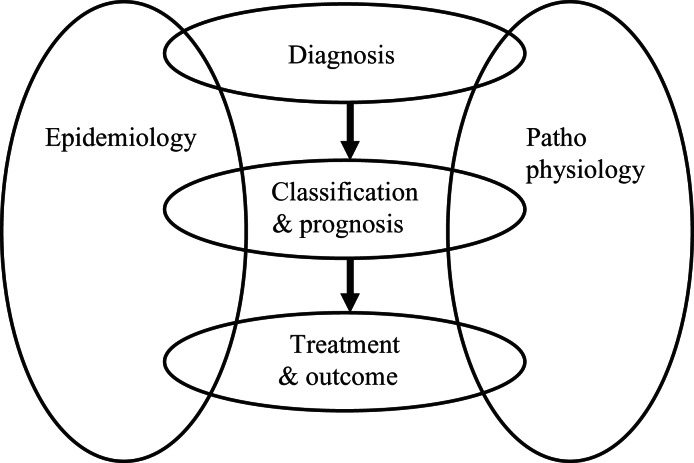
At the workshop a number of speakers were invited to introduce different endometriosis-related topics, with each presentation being followed by open discussion involving all participants. To provide a framework for the many recommendations developed during the workshop, a series of headings has been used that follows the progress of the disease from diagnosis through classification and prognosis to treatment and outcome. Impacting on each of these groupings are the major areas of disease epidemiology and pathophysiology. A representation of this framework is shown in Figure 1 .
Figure 1.
Diagram of schema used to group endometriosis research recommendations.
BACKGROUND
Endometriosis is an estrogen-dependent disorder, defined as the presence of endometrial tissue outside of the uterus in lesions of varying sizes and appearance containing endometrial glands and stroma. It may be asymptomatic or associated with symptoms of pain and/or infertility. It is found on the peritoneum and ovaries, in the recto-vaginal septum, and in other sites within and outside the pelvis. Endometriosis affects an estimated 1 in 10 women in the reproductive-age group.1 This prevalence increases up to 30% in women with infertility2 and to 50% in infertile women with a normal cycle whose partner has normal sperm.3 Although it is difficult to generate accurate figures for costs associated with endometriosis, 2 recent studies have estimated an annual cost using 2002 figures for endometriosis in the United States at $18.8 and $22 billion when direct treatment costs and indirect costs such as lost work productivity are combined.4 5 Although the existence of endometriosis has been known for more than 100 years, our current knowledge of its pathogenesis, the spontaneous evolution of the disease, and the pathophysiology of the related infertility and pelvic pain, remain unclear. One of the major issues identified as impacting on the capacity to undertake endometriosis research is the need for multidisciplinary expertise, in conjunction with sufficient funding to allow meaningful projects to be undertaken.
Recommendation
There is a need for a multidisciplinary approach to research in all aspects of endometriosis, to include reproductive medicine physicians, reproductive medicine surgeons, biologists, pathologists, oncologists, epidemiologists, geneticists, immunologists, toxicologists, pain specialists, infectious disease specialists, biostatisticians, bioinformaticians, and others to enable effective, accurate, and timely diagnosis, determination of those at risk, and prevention and treatment of endometriosis, and associated disorders.
DIAGNOSIS OF ENDOMETRIOSIS
Of the several factors that contribute to our lack of understanding of endometriosis, perhaps the most significant is the 8 to 11 years delay that typically precedes an accurate diagnosis of the disease.6 The problem of diagnosing endometriosis is further compounded by the fact that many patients suffer from comorbidities, such as adenomyosis, irritable bowel syndrome, and interstitial cystitis, which can all contribute to the symptomatology. In contrast to diagnosis of endometriosis at surgery, noninvasive diagnostic methods that can be used to effectively and economically screen for endometriosis are urgently required. Identification of biomarkers for early noninvasive diagnosis and for following the progression of endometriosis was identified as a priority for investigation as well as early clinical application. The greatest need is for noninvasive detection of minimal-mild endometriosis, given that moderate-severe forms of the disease are more likely to be identified by clinical examination and/or imaging.7 Because of the likely variable etiology of the disease, “fingerprints” rather than individual molecules will probably be required. It is also possible that different subsets of biomarkers may be required for different stages or clinical classifications of endometriosis.
Recommendation
Biomarkers are required that will provide an accurate, noninvasive method to diagnose endometriosis.
Different techniques for diagnostic and preoperative imaging of endometriosis are being explored, including ultrasound, computer tomography (CT), and magnetic resonance imaging (MRI). From a clinical point of view, the ideal is for a test with high sensitivity that does not miss any individuals with endometriosis or other pelvic conditions that might benefit from diagnostic or operative laparoscopy.7 Although the resolution of imaging techniques continues to improve, their current diagnostic accuracy remains significantly inferior to direct laparoscopic visualization. Other issues associated with imaging of endometriosis are the training required to achieve acceptable sensitivity and specificity rates, and the cost of these procedures if used as a screening tool.
Recommendation
Advances in imaging techniques should be monitored for application to diagnosis of endometriosis.
There is significant cost and expertise associated with collecting adequate numbers of well-characterized endometriotic lesions, peripheral blood samples, and other tissue specimens required for endometriosis research. Such samples have the greatest value when collected using systematic protocols and accompanied by detailed clinical classification of the patients.
Recommendation
That networks and/or biobanks and databases replete with patient clinical data are established to increase sample availability and improve study power for endometriosis research, including assessment and validation of biomarkers. Standard operating procedures should be established for tissue acquisition, processing, storage, and distribution. These activities should take account of existing databases and resources regarding patients with endometriosis.
Recommendation
That the effect of surgical sampling methods (laser, scissor, unipolar coagulation, etc) on the quality of tissues for research and influences on biomarker expression be analyzed: including the impact of anesthesia/analgesia at the time of surgery.
CLASSIFICATION AND PROGNOSIS
During the workshop, detailed discussion was held on the need for and difficulties associated with standardization of disease classification. The revised American Fertility Society (rAFS) classification of endometriosis8 and the revised American Society for Reproductive Medicine classification of endometriosis 2006 are the current gold standards.9 However, these are restricted to a limited number of criteria and are not particularly valuable for predicting pain or fertility outcomes. Although there is poor correlation between stage of disease and pain scores using the above classification systems, there is good correlation with type of lesion and pain.10–12 In the broadest sense, classification may be related to risk of endometriosis, the etiology of the disorder (including genetic and environmental factors), disorders associated with endometriosis, targeting therapies, and designing inclusion/exclusion criteria for clinical trials to evaluate diagnostics and therapeutics. Interestingly, the transcriptomes of eutopic endometrium and ectopic endometrial lesions suggest that ovarian endometriosis and peritoneal disease are different disorders13 and that, in contrast to the presence of ovarian and peritoneal disease, recto-vaginal disease does not affect gene expression in eutopic endometrium.14 These observations may give insight into developing a more comprehensive and meaningful classification system that has clinical prognostic value in determining issues like why some women develop deep infiltrating endometriosis while in others the disease remains limited. The choice of controls is crucial in studies investigating environmental risk-factors for endometriosis, but is also an important issue in genetic case-control studies. For both types of studies, use of general population controls carry the problem that they cannot be screened for absence of disease, resulting in a reduction of power of a study. In genetic studies, the additional main concern is for controls to be selected from the same ethnic background as cases, to avoid spurious findings related to population differences (population stratification).
Recommendation
Reporting standards with detailed clinical, symptom, and diagnosis information should be developed to allow better comparison between studies and to improve our ability to combine the results from different centres to increase the power of individual studies.
Recommendation
A standardized classification of endometriosis should be developed based on lesion number, size, appearance, location, pain symptoms, presence or absence of infertility, pain and infertility, age of onset, family history/genetics, associated disorders, and yet to be developed biomarkers.
TREATMENT AND OUTCOME
Current treatment options for women with endometriosis-associated pain and/or infertility include surgery, medical treatment, alternative therapies, and assisted reproduction. Professional guidelines for the clinical management of endometriosis, like the ESHRE Guidelines15 and the Practice Guidelines of the American Society for Reproductive Medicine,16 exist and it is important to ensure that these guidelines are continually reviewed and updated to reflect the latest clinical and scientific findings, and that they are adopted by health care professionals worldwide. There is a clear need for further research aimed at improving endometriosis treatment outcomes. Examples include, but are not limited to, (1) whether effective medical adjuvant therapies exist to prevent or limit the recurrence of lesions and symptoms following surgery, (2) whether laparoscopic ablation or excision of endometriosis is more effective in women with pain (3) whether all, or some categories of endometriotic lesions need to be treated prior to infertility treatment by IVF, (4) whether pain-free women with endometriosis who need in vitro fertilization (IVF) for other reasons actually benefit from being disease-free, (5) whether the introduction of advanced operative laparoscopy techniques have resulted in an increase in adverse outcomes relating to long-term bladder, bowel, or ovarian dysfunction, and whether such techniques are superior to more conservative surgery in preventing long-term recurrence of endometriosis.
Recommendation
There is a need for more well designed, adequately powered, multicenter randomized controlled trials and long-term follow-up studies comparing different endometriosis treatment options against defined outcome measures.
Development of nonhormonal medical treatments to prevent or treat endometriosis and associated symptoms is a priority.17 Such treatments should reduce pain and subfertility without suppression of ovulation, and ideally provide the option of a normal and safe pregnancy during treatment. New drug targets are currently being developed in the area of cancer and chronic inflammatory diseases that may be relevant for treatment of endometriosis. Potential mechanisms of action that could be relevant to the treatment of endometriosis include inhibition of: inflammation (tumor necrosis factor alpha [TNF-α] inhibitors, COX-2 inhibitors), fibrosis, pain, angiogenesis, and matrix metalloproteinases. Further work is also required to assess the value of selective estrogen and progesterone receptor modulators.
Recommendation
Novel medical treatments for endometriosis should be investigated.
It was also noted that surgical and clinical trials offer an excellent opportunity to obtain well characterized tissue samples for collaborative studies on the pathophysiology of endometriosis.
Recommendation
Efforts should be made to maximize the amount of data that are generated from clinical trials through add-on studies and collaboration with other relevant disciplines.
EPIDEMIOLOGY
Endometriosis is a complex disease influenced by both environmental and genetic factors.18–20 Current evidence from a range of studies supports a genetic contribution to endometriosis risk.20 The genetic contribution was questioned recently21 because of problems with the design of many studies, relating to small sample sizes, ascertainment bias, increased opportunity for diagnosis among family members of cases compared with controls, and familial aggregation of confounding risk factors such as early age at menarche and environmental exposures.21 It is certainly true that studies of endometriosis, in particular those involving candidate genes, have suffered serious methodological problems.22 However, more recent, larger studies in Australian twins,18 in the Icelandic population,23 in rhesus macaques,24 as well as the significant linkage to chromosome 10 found in a large collaborative study of 1176 families,25 address many of these concerns and together provide strong evidence for a genetic contribution to the disease.
Although the balance of evidence supports a genetic contribution to the risk of developing endometriosis, the concerns about study design21 highlight general problems for both epidemiological and genetic studies in endometriosis.22 Endometriosis can only be diagnosed following invasive procedures such as laparoscopy, and there is often a long gap between first symptoms and disease diagnosis.6 The lack of a noninvasive test means there are no good estimates of disease prevalence in the general population and there are difficulties in defining both case and control groups in systematic studies. As discussed by Di and Guo21 and in other recent reviews,26 27 the interpretation of many published genetic and epidemiological studies is problematic. There are a number of issues that must be addressed in future studies if we are to make substantive progress in understanding this disease.
Genome-wide association methods offer a powerful approach for making progress in the discovery of genes influencing risk of endometriosis. Sample size should have sufficient power to detect expected effects (genotypic odds ratios of 1.2-1.5) for a complex disease like endometriosis. For genome-wide association studies, this figure should be at least 2000 cases and 2000 controls to detect common genetic variants; when stratified analyses are anticipated (eg, on disease severity) this number needs to be increased accordingly. Minimum sample sizes for environmental or candidate gene studies should be in the range of 1000 cases and 1000 controls.28 Future studies of gene-environment interaction are likely to require sample sizes in the range of 5000-10 000 cases and an equal number of controls.
Recommendation
Collaborations be established between research groups with sufficient participant numbers and appropriate standardization of sample and information collection to identify genetic and environmental influences on endometriosis.
Recommendation
Additional samples of phenotypically well-characterized endometriosis cases and controls should be collected from different ethnic groups for replication and evaluation of positive genetic associations.
Data from animal models and from women suggest that environmental contaminants, specifically endocrine disrupting chemicals, may contribute to the pathogenesis of endometriosis. Timing of exposure appears to be important, as in utero exposure to the xenoestrogen diethylstilbesterol (DES) increases a woman’s risk of developing endometriosis as an adult by 80% (RR = 1.8, CI = 1.2-2.8).29 In addition, mice exposed in utero to the dioxin, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), on gestational day 8 have larger transplanted endometriotic lesions when combined with an adult exposure, compared to an adult exposure alone.30 Thus, it has been hypothesized that during embryogenesis, endocrine disrupting chemical (EDC) exposure has an organizational effect that increases susceptibility to endometriosis, but subsequent adult hormone/immune/EDC irregularities are required for disease onset.31
There is overwhelming evidence from nonhuman primate and rodent studies suggesting that endometriosis can be promoted by adult exposures to organochlorines (OCLs), a class of chemicals that includes the dioxin, TCDD, the pesticides methoxychlor and DDT, and polychlorinated biphenyls with dioxin-like effects.31 However, data in humans linking OCL exposure and endometriosis are equivocal, because of inherent weaknesses of observational epidemiology studies, limited sample sizes, and potential confounding variables.31
Dysfunction of the immune system influenced by endocrine disrupting chemicals (eg, TCDD) is also considered relevant because although high levels of activated macrophages and inflammatory cytokines are present in the peritoneal environment, in women with peritoneal endometriosis the immune system fails to clear the lesions.32 Thus, the progression of endometriosis is dependent on both hormonal and immune environments, but the exact etiology of endometriosis onset is unclear.
Recommendation
Further research on an environmental etiology of endometriosis is warranted, with windows of susceptibility (life stages) being an important criteria in the collection of information. Although measurements of individual and mixtures of endocrine disrupting chemicals (and other environmental contaminants) can be challenging, timing of exposure, dose, and duration are important to determine, if known, and should be included in databases, where possible.
PATHOPHYSIOLOGY
A wide range of disciplines and experimental approaches relevant to the study of endometriosis can be listed under the general heading of pathophysiology. These include physiology, pathology, immunology, endocrinology, inflammation, and pain, each of which can encompass approaches such as genomics, proteomics, and animal and in vitro models. The workshop did not attempt to develop a comprehensive set of recommendations topic by topic for each of these combinations, but rather to identify major themes and areas of importance.
The pathology of endometriosis lesions can vary widely.33 This is often not recognized or acknowledged by investigators, and raises critical questions about both the heterogeneity of endometriosis as a disease, and the normal life cycle of different endometriotic lesions. There is little understanding of whether the range of symptoms suffered by women with endometriosis can be linked to the different types of lesions. Substantial work is required to link lesion pathology to symptoms, the results of which will play a critical role in determining whether stratification of endometriosis patients into subgroups is required for epidemiological, diagnostic, prognostic, and treatment studies. Pathology studies should have broad scope and include histology, immunohistochemistry, molecular, and proteomics approaches.
Recommendation
Heterogeneity of endometriosis lesions should be investigated using the full range of pathological and analytical approaches to ascertain whether an association exists between different lesion types and any given symptomatology.
Numerous studies have reported differences between eutopic endometrium from women with endometriosis compared to those that do not have the disease.34 Studies in baboons have confirmed that major changes in eutopic endometrial gene expression occur following the induction of endometriosis,35 although the biological mechanisms driving these changes are yet to be elucidated. A crucial question is whether surgical treatment of these induced endometriotic lesions reverses the observed eutopic endometrial changes. Whether significant differences exist in eutopic endometrium between women destined to develop endometriosis and those that will not, prior to the spontaneous development of the disease, is also unknown.
Recommendation
A better understanding of the role of eutopic endometrium in the establishment and continuation of endometriosis is required.
Eutopic endometrium is generally considered the source of at least the majority of the cells that form endometriotic lesions following reflux of menstrual debris into the peritoneal cavity. In addition to tissue fragments and cells, menstrual effluent contains many leukocytes and soluble mediators released during menstruation, including cytokines and proteases such as matrix metalloproteinases. The components of this effluent and of the peritoneal fluid are likely to contribute to the fate of the endometrial tissue that reaches the peritoneum. However, the exact prevalence and quantity of endometrial tissue present in peritoneal fluid at the time of menstruation is not known. Furthermore, the capacity of the peritoneal fluid and its cellular components to “neutralize” or degrade active mediators, and of the peritoneal leukocytes to remove cellular debris, or to promote endometrial adhesion or peritoneal metaplasia needs more research, since these factors may be important in determining whether or not endometriosis can be established.
Recommendation
Research should be performed on menstrual tissue, including material obtained from the peritoneal cavity by laparoscopy performed at the time of menstruation. Differences in retrogradely shed menstrual material between women with and without endometriosis should be defined, including but not limited to soluble mediators, endometrial cells, and leucocytes.
The hypothesis that endometriosis lesions may be established by single or small groups of menstrual endometrial cells is consistent with the presence of endometrial stem or progenitor cells. Recent work by several different groups36–39 has confirmed the existence of putative endometrial epithelial and mesenchymal stem/progenitor cells. Whether these cells play a role in the establishment of endometriotic lesions remains to be discovered. However the recent identification of endometrial mesenchymal stem-like cell markers40 should facilitate examining their role in the development of endometriotic lesions. Bone marrow stem cells may also contribute to the progression of endometriotic lesions by incorporating into developing lesions and transdifferentiating into endometriotic cells.41
Recommendation
Functional properties of endometrial cells expressing stem cell markers should be investigated and menstrual endometrium examined for endometrial stem/progenitor cells using any newly identified markers. The role of bone marrow–derived cells in endometriotic lesion development should be further investigated. Developmental signaling pathways should also be examined in endometrial stem/progenitor cells and in models examining the metaplasia and fetal stem cell theories of endometriosis.
Many immune mediators (both leukocyte subsets and cytokines) have been identified as being different in the eutopic endometrium and peritoneal fluid between women without and those with endometriosis as well as within ectopic lesions. Leukocyte numbers, subsets, and particularly their activation states vary normally in the endometrium in a cycle-dependent manner, but it is not clear how closely these relate to those within the peritoneal cavity or in the lesions, or whether inflammatory responses are altered in women with endometriosis. There is an inflammatory cascade of events (Figure 2 ) where some of the mediators (such as prostaglandins) can also cause pain. Disturbance of this cascade toward a more inflammatory phenotype can occur at any point in the cascade. Furthermore, studies on individual cells or mediators are likely to be of limited value given the considerable overlap among their actions. Application of systems biology is more likely to reap rewards in terms of understanding how disturbance to this system relates to establishment of disease and its severity and to the related pain caused by the inflammation. Given that inflammation and specific immune responses differ between animal models (particularly various immune-deficient mice) these could be exploited.
Figure 2.
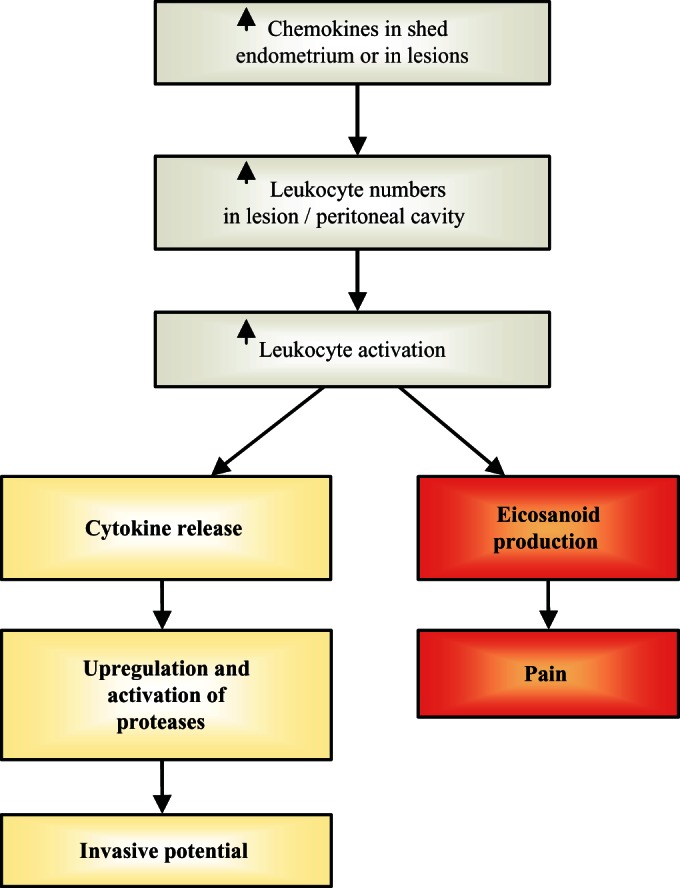
Activation of an inflammatory cascade within the peritoneal environment/endometriotic lesion can enhance the invasiveness of the lesion and contribute to pain.
Recommendation
The inflammatory response is an important avenue for further research and should focus wherever possible on a systems biology approach rather than individual components of the inflammatory pathway.
Alleviation of pain due to endometriosis is a high priority. Despite this, very little research is being undertaken in this area, possibly because of difficulties in establishing appropriate multi-disciplinary collaborative teams. The recent identification of increased nerve fiber density in endometrium from women with endometriosis42 may be highly significant in this regard.
Recommendation
That understanding the origins of the pain associated with endometriosis is a priority for endometriosis research: such work should include specialists in the pain field.
Progestins have overall anti-inflammatory activity, and there appears to be progesterone-resistance in endometriotic lesions and eutopic endometrium of women with endometriosis. Given that different progestins have different glucocorticoid and androgenic activity there may be opportunities for modifying treatments to improve outcomes. Similarly, selective progesterone receptor modulators (SPRMs) appear promising in treating endometriosis.
Recommendation
Clinical and basic studies should be undertaken to determine the effectiveness of different progestins and SPRMs as agents for treating endometriosis.
The choice of models for endometriosis research is often debated, with no single model absolutely replicating all aspects of the human disease. One relatively popular model uses the transplantation of human endometrial tissue into immunocompromized mice, resulting in steroid responsive xenografts with limited graft rejection.43–45 Other investigators have used allotransplantation of uterine endometrium from syngeneic mice.46 These models are advantageous because they have limited cost and large experimental groups can be used. Moreover, these animals are usually “inbred” and experimental results are more reproducible than usually seen in human and nonhuman primate participants. However, while rodents can provide an excellent approach to investigate endometriosis, there are limitations to these models. Despite increased ethical scrutiny, nonhuman primates (in particular the baboon and the rhesus macaque) offer several significant advantages including reproductive anatomy, endocrinology and physiology that is similar to humans, spontaneous development of endometriosis which is histologically similar to the human disease, and the ability to induce endometriosis by autologous intrapelvic injection of menstrual endometrium.47–49 Nonhuman primate models can be used to study longitudinal progression of the disease with multiple surgical procedures possible. Additionally, the close phylogenetic relationship with humans permits the use of human molecular probes and antibodies and allows the testing of potentially interesting new drugs in the prevention or treatment of endometriosis.50 51 Extended nonhuman primate pedigrees also provide an excellent opportunity to further interspecies research into genetic etiologies, especially given the recent advances in reporting of nonhuman primate genomes. Ultimately, animal and in vitro models need to be selected to most appropriately answer the scientific questions being asked. However, it is important to develop and work with models that are appropriate and that can subsequently be used for the screening and testing of future potential therapeutic agents.
Recommendation
Appropriate animal and in vitro models for studying different aspects of endometriosis pathophysiology should be agreed upon by the endometriosis research community.
The coexistence of endometriosis and ovarian cancer has been reported to range between 0.7% and 5.0% of all cases with ovarian endometriosis,52–55 and 2 case-controlled studies by Ness and colleagues have revealed endometriosis as a risk factor for ovarian cancer.56 57 In a study using the National Swedish Inpatient Register, women discharged from a hospital between 1969 and 2000 with a diagnosis of endometriosis were identified and the data linked to the National Swedish Cancer Register to find cases that developed cancer.58 Although the overall risk of cancer was not increased in a cohort of 64 492 women, an elevated risk was found for ovarian cancer (standardized incidence ratio [SIR] 1.43, 95% CI 1.19-1.71), and women with early diagnosed and long-standing endometriosis were at even higher risk (SIRs of 2.01 and 2.23, respectively). In addition, women with endometriosis had higher risks of endocrine tumors including cancer of the adrenal, thyroid, parathyroid, pituitary, and insulinoma of the pancreas (SIR 1.36, 95% CI 1.15-1.61). Increased risk was also observed for non-Hodgkin’s lymphoma (SIR 1.24, 95% CI 1.02-1.49) and brain tumors (SIR 1.22, 95% CI 1.04-1.41). Women who were hospitalized for the first time with a diagnosis coded for endometriosis between the ages of 50 and 60 had an increased risk of breast cancer (SIR 1.28, 95% CI 1.13-1.45). Women with endometriosis-associated ovarian carcinoma tend to have a lower stage of cancer, different histological subtypes compared to the general population, predominantly lower grade endometriosis lesions, and significantly better overall survival compared to women with serous adenocarcinoma, which is the most common type in the general population.59 Synchronous incidence of endometriosis with clear cell (41%) and endometrioid (38%) ovarian carcinoma suggests malignant transformation,60 and the recent mouse model of over expression of k-Ras in the ovarian epithelium and targeted deletion of PTEN with development of endometriosis and endometroid ovarian cancer provide one possible mechanism for the malignant transformation of endometriosis to ovarian cancer.
Recommendation
More basic research is needed on mechanisms and risk factors underlying transformation of ovarian endometriosis to ovarian cancer and the mechanisms underlying higher risk of developing breast cancer, non-Hodgkin’s lymphoma, and brain cancer.
Recent evidence shows that in addition to eutopic endometrium and peritoneal fluid, macroscopically normal peritoneum localized at the pelvic brim is biologically different between women with and without endometriosis.61 These data suggest that pelvic peritoneum may not be a passive recipient of endometrial tissue, but may be actively involved in the pathogenesis of endometriosis.
Recommendation
More research is needed to better understand the biology and function of macroscopically normal peritoneum in women with and without endometriosis.
The more advanced forms of endometriosis are associated with significant fibrosis and adhesions within the peritoneal cavity. It is not known why some women develop significant fibrosis and adhesions as a sequel of endometriosis while others do not.
Recommendation
A better understanding of the mechanisms that underlie fibrosis and adhesion formation in the peritoneal cavity of women with endometriosis is required.
Although diet and nutrition play a major role in lifestyle changes that many women consider when confronted with endometriosis, there is a paucity of evidence-based literature available on this topic.62 Of the studies that have been undertaken, no clear consensus recommendations have emerged on what food types to eat or avoid, to reduce the symptoms of endometriosis and/or the underlying disease.
Recommendation
Research is needed to elucidate the role/link of diet in endometriosis.
DISCUSSION
Although investigator-driven research ideas are fundamental to scientific progress, it can also be useful for groups of experts in a given field to develop consensus statements on issues of importance within their area of expertise. This document builds on earlier efforts to develop a research priorities consensus statement for endometriosis.63
The importance of a disease is often determined by its cost to society. From that perspective, cardiovascular disease, cancer, and chronic diseases such as diabetes are generally ranked highly on the scale of “priority diseases,” and subsequently research into these diseases is well funded. The cost of endometriosis to women with the disease and to society is not well known, but as stated earlier has been estimated by 2 different studies at $18.8 and $22 billion respectively in the US using 2002 data,4 5 which is substantially higher than estimated costs associated with diseases such as Crohn’s disease ($865 million) and migraine ($13-17 billion).5 These data are largely based on studies carried out in the United States, and the cost of endometriosis in other countries is less well researched. Regardless of the precise costs of endometriosis to society, there seems little doubt that research into this disease is significantly under-funded in many, if not all, countries relative to other diseases with major health care burdens. The reason for this underfunding is unclear, but may reflect to some extent the practical difficulties of developing competitive research proposals when working on such a complex and poorly understood disease, which only affects women. It is the hope of the workshop organizers and participants that this international consensus document will be a useful tool in aiding researchers to develop new interdisciplinary research proposals and obtain increased funding support from multiple disciplines for work on endometriosis. The workshop organizers also recognize the strong and active endometriosis patient advocacy groups that exist around the world, and hope that this consensus statement will provide a valuable resource for their efforts to increase awareness of, and funding support for research into, all aspects of endometriosis.
This research priorities consensus statement will have a limited life, and a revised and updated set of research priorities which builds on this document, and progress as a result of our efforts, will be developed in conjunction with the 11th World Congress on Endometriosis to be held from 4 to 7 September 2011, in Montpellier, France.
ACKNOWLEDGMENTS
The manuscript was prepared by the first author; all other authors contributed equally and are listed in alphabetical order. The workshop received direct financial support from the Monash Institute of Medical Research and the Department of Obstetrics and Gynaecology, Monash University. Thanks are due to Penny Clay, Chelsea Stoikos, and Jane Girling for transcribing and note taking during the workshop. PAWR is a National Health and Medical Research Council of Australia (NHMRC) Principal Research Fellow (Grant ID 334063). TMD’H is funded as a Fundamental Clinical Investigator by the Flemish FWO (Fonds voor Wetenschappelijk Onderzoek/Foundation for Scientific Research) and by the Leuven University Research Council, holds the Merck Serono Chair in Reproductive Medicine and is or has recently served as a consultant or has received financial support for research from Bayer Schering Biopharma, Pfizer, Organon and Merck Serono Pharmaceuticals. CEG is supported by a NHMRC RD Wright Career Development Award (Grant ID 465121). LCG is supported by NICHD/NIH through cooperative agreement 1U54HD055764–01 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research. GWM is a NHMRC Principal Research Fellow (Grant ID 339446). LAS is a NHMRC Senior Principal Research Fellow (Grant ID 388901). KTZ is a Wellcome Trust Career Development Fellow (UK).
Workshop participants and people who contributed to development of these recommendations: Mary Lou Ballweg, Endometriosis Association, Milwaukee,; Ronald Batt, State University of New York, Buffalo, NY; Judy Birch, Pelvic Pain Support Network, Poole, Dorset, England; Steve Charnock-Jones, University of Cambridge, Cambridge, England; Thomas D’Hooghe, Leuven University, Leuven, Belgium; Gerard Dunselman, Maastricht University, Maastricht, Netherlands; Asgi Fazleabas, University of Illinois, Chicago; Idhaliz Flores, Ponce School of Medicine, Ponce, Puerto Rico; Ian S Fraser, University of Sydney, Sydney, Australia; Luca Fusi, Imperial College London, London, England; Caroline Gargett, Monash University, Melbourne, Australia; Jane Girling, Monash University, Melbourne, Australia; Linda Giudice, University of California, San Francisco; Patrick Groothuis, Schering Plough, Organon, Netherlands; Ruth Grümmer, University Hospital, Essen, Germany; Natalie Hannan, Prince Henry’s Institute of Medical Research, Melbourne, Australia; David Healy, Monash University, Melbourne, Australia; Jan Hilpert, Bayer Schering Pharma AG, Berlin, Germany (now at GlaxoSmithKline, London); Louise Hull, University of Adelaide, Adelaide, Australia; Lone Hummelshoj, World Endometriosis Society and World Endometriosis Research Foundation, London, England; Grant Montgomery, Queensland Institute of Medical Research, Brisbane, Australia; Michelle Nisolle, University of Liege, Liege, Belgium; Nick Pullen, Pfizer, Sandwich, England; Peter Rogers, Monash University, Melbourne, Australia; Andrea Romano, University Hospital of Maastricht, Maastricht, The Netherlands; Luk Rombauts, Monash University, Melbourne, Australia; Lois Salamonsen, Prince Henry’s Institute of Medical Research, Melbourne, Australia; Dian Shepperson-Mills, Endometriosis & Fertility Clinic, University of Brighton, Brighton, England; Chelsea Stoikos, Prince Henry’s Institute of Medical Research, Melbourne, Australia; Kanadi Sumapradja, University of Indonesia, Jakarta, Indonesia; Robert Taylor, Emory University, Atlanta, GA; Susan Treloar, Queensland Institute of Medical Research, Brisbane, Australia; Krina Zondervan, Wellcome Trust Centre for Human Genetics, Oxford, England.
References
- 1. Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235–238 [DOI] [PubMed] [Google Scholar]
- 2. Gruppo Italiano per lo studio dell’endometriosi Prevalence and anatomical distribution of endometriosis in women with selected gynaecological conditions: results from a multicentric Italian study. Hum Reprod. 1994;9:1158–1162 [PubMed] [Google Scholar]
- 3. Meuleman C, Vandenabeele B, Fieuws S, Spiessens C, Timmerman D, D’Hooghe T. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil Steril. 2008. Epub ahead of print; [DOI] [PubMed] [Google Scholar]
- 4. Gao X, Outley J, Botteman M, Spalding J, Simon JA, Pashos CL. Economic burden of endometriosis. Fertil Steril. 2006;86:1561–1572 [DOI] [PubMed] [Google Scholar]
- 5. Simoens S, Hummelshoj L, D’Hooghe T. Endometriosis: cost estimates and methodological perspective. Hum Reprod Update. 2007;13:395–404 [DOI] [PubMed] [Google Scholar]
- 6. Sinaii N, Cleary SD, Ballweg ML, et al. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue and atopic disease among women with endometriosis: a survey analysis. Hum Reprod. 2002;17:2715–2724 [DOI] [PubMed] [Google Scholar]
- 7. D’Hooghe TM, Mihalyi AM, Simsa P, et al. Why we need a noninvasive diagnostic test for minimal to mild endometriosis with a high sensivity. Gynecol Obstet Invest. 2006;62:136–138 [DOI] [PubMed] [Google Scholar]
- 8. The American Fertility Society. Revised American Fertility Society classification of endometriosis. Fertil Steril. 1985;43: 351–352 [DOI] [PubMed] [Google Scholar]
- 9. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997; 67:817–21 [DOI] [PubMed] [Google Scholar]
- 10. Fedele L, Parazzini F, Bianchi S, Arcaini L, Candiani GB. Stage and localization of pelvic endometriosis and pain. Fertil Steril. 1990;53:155–158 [DOI] [PubMed] [Google Scholar]
- 11. Muzii L, Marana R, Pedullà S, Catalano GF, Mancuso S. Correlation between endometriosis-associated dysmenorrhea and the presence of typical or atypical lesions. Fertil Steril. 1997;68:19–22 [DOI] [PubMed] [Google Scholar]
- 12. Demco L. Mapping the source and character of pain due to endometriosis by patient-assisted laparoscopy. J Am Assoc Gynecol Laparosc. 1998;5:241–245 [DOI] [PubMed] [Google Scholar]
- 13. Wu Y, Kajdacsy-Balla A, Strawn E, et al. Transcriptional characterizations of differences between eutopic and ectopic endometrium. Endocrinology. 2006;147:232–246 [DOI] [PubMed] [Google Scholar]
- 14. Matsuzaki S, Canis M, Pouly JL, et al. Endometrial dysfunction in endometriosis—Biochemical aspects. In: Rombauts L, Tsaltas J, Maher P, Healy D. (Eds.), Endometriosis.2008;Malden, MA: Blackwell Publishing, 89–100 . 2008 (10th World Congress on Endometriosis, Melbourne, Australia, 11-14 March 2008) [Google Scholar]
- 15. Kennedy S, Bergqvist A, Chapron C, et al. ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20:2698–2704 [DOI] [PubMed] [Google Scholar]
- 16. Practice Committee of the American Society for Reproductive Medicine. Treatment of pelvic pain associated with endometriosis. Fertil Steril. 2006;86(suppl 5):S18–S27 [DOI] [PubMed] [Google Scholar]
- 17. Kyama CM, Mihalyi A, Simsa P, et al. Non-steroidal targets in the diagnosis and treatment of endometriosis. Curr Med Chem. 2008;15:1006–1017 [DOI] [PubMed] [Google Scholar]
- 18. Treloar SA, O’Connor DT, O’Connor VM, Martin NG. Genetic influences on endometriosis in an Australian twin sample. Fertil Steril. 1999;17:701–710 [DOI] [PubMed] [Google Scholar]
- 19. Zondervan KT, Cardon LR, Kennedy SH. The genetic basis of endometriosis. Curr Opin Obstet Gynecol. 2001;13:309–314 [DOI] [PubMed] [Google Scholar]
- 20. Montgomery GW, Nyholt DR, Zhao ZZ, et al. The search for genes contributing to endometriosis risk. Hum Reprod Update. 2008. Epub ahead of print; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Di W, Guo SW. The search for genetic variants predisposing women to endometriosis. Curr Opin Obstet Gynecol. 2007;19: 395–401 [DOI] [PubMed] [Google Scholar]
- 22. Zondervan KT, Cardon LR, Kennedy SH. What makes a good case-control study? Design issues for complex traits such as endometriosis. Hum Reprod. 2002;17:1415–23 [DOI] [PubMed] [Google Scholar]
- 23. Stefansson H, Geirsson RT, Steinthorsdottir V, et al. Genetic factors contribute to the risk of developing endometriosis. Hum Reprod. 2002;17:555–559 [DOI] [PubMed] [Google Scholar]
- 24. Zondervan KT, Weeks DE, Colman R, et al. Familial aggregation of endometriosis in a large pedigree of rhesus macaques. Hum Reprod. 2004;19:448–455 [DOI] [PubMed] [Google Scholar]
- 25. Treloar SA, Wicks J, Nyholt DR, Montgomery GW, et al. Genomewide linkage study in 1,176 affected sister pair families identifies a significant susceptibility locus for endometriosis on chromosome 10q26. Am J Hum Genet. 2005;77:365–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Falconer H, D’Hooghe T, Fried G. Endometriosis and genetic polymorphisms. Obstet Gynecol Surv. 2007;62:616–628 [DOI] [PubMed] [Google Scholar]
- 27. Vigano P, Somigliana E, Vignali M, et al. Genetics of endometriosis: current status and prospects. Front Biosci. 2007;12: 3247–3255 [DOI] [PubMed] [Google Scholar]
- 28. Zondervan KT, Cardon LR. The complex interplay among factors that influence allelic association. Nat Rev Genet. 2004;5:89–100 [DOI] [PubMed] [Google Scholar]
- 29. Missmer SA, Hankinson SE, Spiegelman D, et al. In utero exposures and the incidence of endometriosis. Fertil Steril. 2004;82:1501–1508 [DOI] [PubMed] [Google Scholar]
- 30. Cummings AM, Hedge JM, Birnbaum LS. Effect of prenatal exposure to TCDD on the promotion of endometriotic lesion growth by TCDD in adult female rats and mice. Toxicol Sci. 1999;52:45–49 [DOI] [PubMed] [Google Scholar]
- 31. Crain DA, Janssen SJ, Edwards TM, et al. Female reproductive disruption: the roles of endocrine disrupting compounds and developmental timing. Fertil Steril. IN PRESS; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burney RO, Giudice LC. The pathogenesis of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Gynecological Laparoscopy with Hysteroscopy: Principles and Techniques. New York, NY: Cambridge University Press, 2007; 253–259 [Google Scholar]
- 33. Clement PB. The pathology of endometriosis: a survey of the many faces of a common disease emphasizing diagnostic pitfalls and unusual and newly appreciated aspects. Adv Anat Pathol. 2007;14:241–260 [DOI] [PubMed] [Google Scholar]
- 34. Giudice LC, Talbi S, Hamilton A, Lessey BA. The endometrial transcriptome. In: J Aplin, A Fazleabas, S Glasser, L Giudice. (Eds.), The Endometrium: Molecular, Cellular & Clinical Perspectives.2nd ed 2008;London, UK: Informa Healthcare, 193–222 [Google Scholar]
- 35. Hastings JM, Fazleabas AT. A baboon model for endometriosis: implications for fertility. Reprod Biol Endocrinol. 2006;(2006;(supp1 4):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chan RWS, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738–1750 [DOI] [PubMed] [Google Scholar]
- 37. Chan RW, Gargett CE. Identification of label-retaining cells in mouse endometrium. Stem Cells. 2006;24:1529–1538 [DOI] [PubMed] [Google Scholar]
- 38. Cervello I, Martinez-Conejero JA, Horcajadas JA, Pellicer A, Simon C. Identification, characterization and co-localization of label-retaining cell population in mouse endometrium with typical undifferentiated markers. Hum Reprod. 2007;22:45–51 [DOI] [PubMed] [Google Scholar]
- 39. Kato K, Yoshimoto M, Kato K, et al. Characterization of side-population cells in human normal endometrium. Hum Reprod. 2007;22:1214–1223 [DOI] [PubMed] [Google Scholar]
- 40. Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22:2903–2911 [DOI] [PubMed] [Google Scholar]
- 41. Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007;25:2082–2086 [DOI] [PubMed] [Google Scholar]
- 42. Tokushige N, Markham R, Russell P, Fraser IS. High density of small nerve fibres in the functional layer of the endometrium in women with endometriosis. Hum Reprod. 2006;21: 782–787 [DOI] [PubMed] [Google Scholar]
- 43. Zamah NM, Dodson MG, Stephens LC, Buttram VC, Besch PK, Kaufman RH. Transplantation of normal and ectopic endometrial tissue into athymic nude mice. Am J Obstet Gynecol. 1984;149:591–597 [DOI] [PubMed] [Google Scholar]
- 44. Bruner KL, Matrisian ML, Ridgers WH, Gortein F, Osteen KG. Suppression of matrix metalloproteinases inhibits establishment of ectopic lesions by human endometrium in nude mice. J Clin Invest. 1997;99:2851–2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Awwad JT, Sayegh RA, Tao XJ, Hassan T, Awwas ST, Isaacson K. The SCID mouse: an experimental model for endometriosis. Hum Reprod. 1999;14:3107–3111 [DOI] [PubMed] [Google Scholar]
- 46. Rossi G, Somigliana E, Moschetta M, et al. Dynamic aspects of endometriosis in a mouse model through analysis of implantation and progression. Arch Gynecol Obstet. 2000;263:102–110 [DOI] [PubMed] [Google Scholar]
- 47. D’Hooghe TM, Bambra CS, Raeymaekers SCM, De Jonge I, Lauweryns JM, Koninckx PR. Intrapelvic injection of menstrual endometrium causes endometriosis in baboons (Papio cynocephalus and Papio anubis) . Am J Obstet Gynecol. 1995;173:125–134 [DOI] [PubMed] [Google Scholar]
- 48. Zondervan KT, Weeks DE, Colman R, et al. Familial aggregation of endometriosis in a large pedigree of rhesus macaques. Hum Reprod. 2004;19:448–455 [DOI] [PubMed] [Google Scholar]
- 49. D’Hooghe TM, Kyama CK, Mihalyi AM, Chai D, Falconer H, Mwenda JM. The baboon model for translational research in endometriosis. Reprod Sci. 2008; IN PRESS; [Google Scholar]
- 50. D’Hooghe TM, Nugent N, Cuneo S, et al. Recombinant human TNFRSF1A (r-hTBP-1) inhibits the development of endometriosis in baboons: a prospective, randomized, placebo- and drug-controlled study. Biol Reprod. 2006;74: 131–136 [DOI] [PubMed] [Google Scholar]
- 51. Lebovic DI, Mwenda JM, Chai DC, et al. PPAR-gamma receptor ligand induces regression of endometrial explants in baboons: a prospective, randomized, placebo- and drug-controlled study. Fertil Steril. 2007;88:1108–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Erzen M, Kovacic J. Relationship between endometriosis and ovarian cancer. Eur J Gynaecol Oncol. 1998;19:553–555 [PubMed] [Google Scholar]
- 53. Nishida M, Watanabe K, Sato N, Ichikawa Y. Malignant transformation of ovarian endometriosis. Gynecol Obstet Invest. 2000;50(suppl 1):18–25 [DOI] [PubMed] [Google Scholar]
- 54. Ogawa S, Kaku T, Amada S, et al. Ovarian endometriosis associated with ovarian carcinoma: a clinicopathological and immunohistochemical study. Gynecol Oncol. 2000;77: 298–304 [DOI] [PubMed] [Google Scholar]
- 55. Stern RC, Dash R, Bentley RC, Snyder MJ, Haney AF, Robboy SJ. Malignancy in endometriosis. Frequency and comparison of ovarian and extraovarian types. Int J Gynecol Pathol. 2001;20:133–139 [DOI] [PubMed] [Google Scholar]
- 56. Ness RB, Cramer DW, Goodman MT, Kruger Kjaer S, Mallin K. Infertility, fertility drugs, and ovarian cancer: a pooled analysis of case control studies. Am J Epidemiol. 2002;155:217–224 [DOI] [PubMed] [Google Scholar]
- 57. Ness RB, Grisso JA, Cottreau C, et al. Factors related to inflammation of the ovarian epithelium and risk of ovarian cancer. Epidemiology. 2000;11:111–117 [DOI] [PubMed] [Google Scholar]
- 58. Melin A, Sparen P, Persson I, Bergqvist A. Endometriosis and the risk of cancer with special emphasis on ovarian cancer. Hum Reprod. 2006;21:1237–1242 [DOI] [PubMed] [Google Scholar]
- 59. Erzen M, Rakar S, Klancar B, Syrjänen K. Endometriosis-associated ovarian carcinoma (EAOC): an entity distinct from other ovarian carcinomas as suggested by a nested case-control study. Gynecol Oncol. 2001;83:100108. [DOI] [PubMed] [Google Scholar]
- 60. Van Gorp T, Amant F, Neven P, Vergote I, Moerman P. Endometriosis and the development of malignant tumours of the pelvis. A review of literature. Best Pract Res Clin Obstet Gynaecol. 2004;18:349–371 [DOI] [PubMed] [Google Scholar]
- 61. Kyama CM, Overbergh L, Mihalyi A, et al. Endometrial and peritoneal mRNA expression of aromatase, cytokines, and adhesion factors in women with endometriosis. Fertil Steril. 2008;89:301–310 [DOI] [PubMed] [Google Scholar]
- 62. Fjerbaek A, Knudsen UB. Endometriosis, dysmenorrhea and diet—what is the evidence?. Eur J Obstet Gynecol Reprod Biol. 2007;132:140–147 [DOI] [PubMed] [Google Scholar]
- 63. Yoshanaga K, Parrott EC, Eds. Endometriosis: emerging research and intervention strategies. Ann NY Acad Sci. 2002; 955 pp. 1–406 11949938 [Google Scholar]