Abstract
Recent data indicate that local translation in growth cones is critical for axon guidance. Evidence from the Drosophila midline axon guidance system suggests that the F-actin-microtubule cross-linker Short stop (Shot) might link the translation machinery to the cytoskeleton in the growth cone. The identification of a complex of translation factors attached to the actin and microtubule networks points to a mechanism by which cytoskeletal dynamics regulate translation in axons and vice versa.
Protein synthesis is often localized to a specific region of a cell. This process, known as local translation, can be achieved by targeting mRNAs to subcellular sites and provides cells with a mechanism to control the repertoire of proteins found in a particular cellular compartment (1). Over the past decade, it has become evident that local translation also plays a role in neural development and functions such as axon guidance, synaptic plasticity, and the formation of long-term memory (2, 3). The cytoskeleton plays a critical role in maintaining the structural integrity and function of the protein synthetic machinery for global translation processes (4); however, the mechanisms linking local translation to the cytoskeleton are largely unknown. A recent paper by Lee et al. (5) provides new insight into how these two processes might be coupled in Drosophila commissural neurons. The authors demonstrated that the F-actin-microtubule cross-linker protein Shot forms a platform for the translation of proteins important for midline axon guidance.
During development of the nervous system, growing axons are guided to their correct synaptic targets by their motile tip, the growth cone. Axonal growth cones respond to chemoattractive and chemorepulsive signals in the microenvironment and transduce these signals, through intracellular signaling, into directed movements that steer them along the correct pathway (6, 7). Several families of axon guidance molecules exist and some, including the Slits, the Semaphorins, and the Netrins, elicit rapid local protein synthesis that is required for directional steering (8–10). For example, blocking axonal translation inhibits Semaphorin- and Slit-induced repulsive turning of cultured Xenopus retinal axons (9).
Growth cones contain F-actin–rich filopodia and lamellipodia, and polarized arrays of microtubules. Steering requires the precise coordination of actin and microtubule dynamics, which is regulated by various actin- and microtubule-binding proteins (11). Drosophila Short stop (Shot) is a neuronally expressed rodlike protein that binds both F-actin and microtubules and belongs to the plakin family, which links cytoskeletal structures together (12, 13). Lee et al. (5) have now identified a novel Shot-interacting protein called Krasavietz (Kra) and used Drosophila mutants to demonstrate that Shot and Kra play a role in midline axon guidance, a process that depends on repulsive signaling by Slit through the Robo receptor. In embryos with loss-of-function mutations in Shot and Kra, ipsilateral commissural axons that would normally avoid the midline ectopically crossed the midline. Shot and Kra appeared to act synergistically and, moreover, Shot and Kra phenotypes were enhanced in a Robo or Slit heterozygous background. These findings indicate that Shot and Kra are part of the midline axon-repellent Slit-Robo pathway.
Kra contains a W2 domain, a motif found in translation initiation factors such as eIF2Bε and eIF5, which regulate the activity of eukaryotic initiation factor-2 (eIF2). eIF2 is a heterotrimeric GTP binding protein that, in its GTP-bound form, recruits the initiator tRNA to the small (40S) ribosomal subunit at the start codon on the mRNA to initiate translation. eIF2Bε and eIF5 mediate GDP-GTP exchange and GTP hydrolysis, respectively, and are therefore required for eIF2 activity (14). Lee et al. (5) found that Kra cosedimented with 40S ribosomal subunits and interacted with the β subunit of eIF2 (eIF2β) through its W2 domain. In vitro, Kra inhibited global translation, which suggests that Kra might compete with eIF2Bε or eIF5 or both for binding to eIF2β, thereby inhibiting initiator tRNA recruitment and initiation of translation. Mutations in the W2 domain inhibited Kra binding both to Shot and to eIF2β. These mutations revealed the importance of the Shot-Kra-eIF2β complex in midline guidance in vivo: Expression of a W2 mutant Kra failed to rescue the Kra midline guidance phenotype. Moreover, eIF2β mutant embryos also showed ectopic midline crossing defects similar to those seen with Kra mutants. Together, the findings suggest that Shot might form a platform for Kra and eIF2β, which localizes to the cytoskeleton to inhibit eIF2β-mediated translation initiation in the growth cone in response to Slit-Robo signaling and there-by directs navigation.
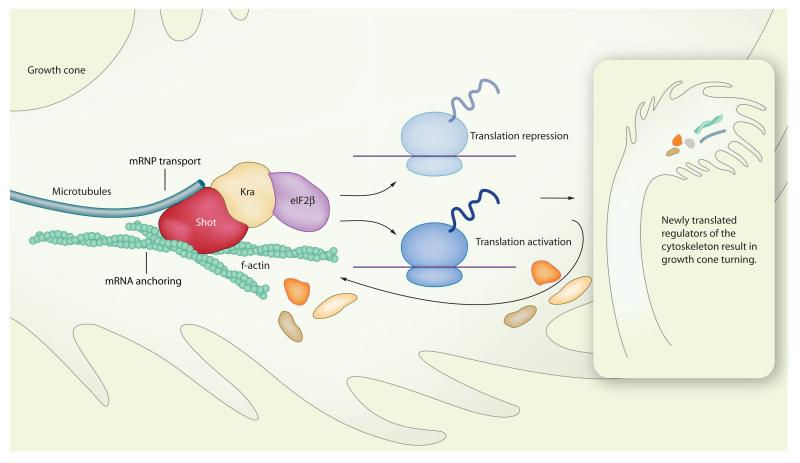
One intriguing issue raised by the study of Lee et al. (5) concerns the connection of the translation machinery to the cytoskeleton. Historically, biochemical and morphological studies showing that polysomes, mRNAs, and translation factors are associated with the cytoskeleton have fueled the hypothesis of local protein synthesis (4). More recently, it has become evident that the cytoskeleton fulfils multiple roles in the regulation of cytoplasmic translation (Fig. 1).
Fig. 1.
Model of how Shot links cytoskeletal dynamics to translational regulation, and vice versa. Shot interacts with F-actin and microtubules as well as with the translational regulators Kra and eIF2β. Cytoskeletal rearrangements can affect mRNA transport and anchoring to the Shot complex, and thereby the activity of the translation machinery. Conversely, the Shot-Kra-eIF2β complex may provide a mechanism for the translational repression—and possibly activation—of proteins involved in regulating the cytoskeleton. The identity of those mRNAs for which translation is repressed (or activated) is likely to determine the final effect of translation on growth cone steering. For instance, attractive cues could activate the synthesis of proteins that promote cytoskeletal assembly and repress the synthesis of proteins that break it down, whereas repulsive cues could have the opposite effect. Synthesis of components of the Shot-Kra-eIF2β complex or proteins that directly affect actin and microtubules attached to Shot could be part of a feedback mechanism.
First, the cytoskeleton provides a network for mRNA transport, and anchoring. mRNAs are transported in ribonucleoprotein (mRNP) complexes that consist of multiple mRNAs and proteins, including motor proteins that connect the mRNPs to the cytoskeleton and RNA binding proteins that keep the mRNAs translationally repressed during transport. Once mRNAs arrive at their cellular target site, they are released, anchored, and eventually translated (15). The mechanisms of mRNA anchoring are largely unknown. The best-known example of a mechanism through which mRNA anchoring to the cytoskeleton occurs involves elongation factor (EF) 1α. EF1α binds to F-actin and is thought to assemble actin filaments into bundles to which β-actin mRNA becomes anchored and translated in specific regions of the cell (e.g., the leading edge of fibroblasts). Growth factors that activate the Rho-family guanosine triphosphatases (Rho GTPases), RhoA and Cdc42, and the Rho effector mDia (mammalian homolog of Drosophila Diaphanous protein) mediate the interaction of EF1α to F-actin, thereby regulating mRNA recruitment (16). Similarly, Slit might regulate, through Rho GTPases, the ability of Shot to bind to and cross-link F-actin and microtubules, resulting in the sequestration of mRNAs and possibly the translational regulators Kra and eIF2β.
In addition, the cytoskeleton could provide a means for directly regulating translation. Data to support this notion have come mainly from experiments in which actin or microtubule networks (or both) have been disrupted, thereby inhibiting mRNA transport and translation (17, 18). Lee et al. (5) now show that Shot-mediated midline repulsion requires its F-actin binding domain, suggesting that the Shot-Kra-eIF2β translational complex is only functional, in other words, inhibitory, when it is connected to the F-actin network in the growth cone. However, it remains to be seen whether the ability to associate with the F-actin filaments is directly linked to the activity of the complex.
Conversely, translation can affect the cytoskeleton. Indeed, Netrin-1- and brain-derived neurotrophic factor (BDNF)– induced translation of β-actin mRNA is required for attractive turning responses (19, 20), and Semaphorin3A-induced translation of RhoA is required for growth cone collapse (21). Moreover, an attractive gradient of Netrin-1 or BDNF stimulates the asymmetrical transport and translation of β-actin mRNA, indicating that a gradient of a guidance cue induces a gradient of newly synthesized proteins across the growth cone (19, 20). It is hypothesized that attractive cues elicit the synthesis of proteins that build up the cytoskeleton, whereas proteins synthesized in response to repulsive cues break it down (2). Consistent with this view, Slit induces a protein-synthesis–dependent rise in the actin depolymerization factor cofilin in the growth cone (9).
How then does the Shot-Kra-eIF2β complex function in the Slit-Robo pathway? Lee et al. (5) suggest that Slit inhibits translation initiation by blocking the recruitment of initiator tRNA to the 40S ribosome. In contrast, studies in Xenopus retinal growth cones indicate that Slit activates translation by stimulating cap-dependent recruitment of the 40S ribosome complex (9). Studies in nonneuronal cells show that growth factors can affect all three steps of the translation process: initiation, elongation, and termination (22). It might not be surprising, therefore, if guidance cues could regulate different aspects of translation initiation, and it is possible that the relative levels of translational factors present at a given time could determine the final outcome. Alternatively, although the Kra-eIF2β complex inhibits global translation, it might activate the translation of specific mRNAs. Such a mechanism has been reported for another subunit of the eIF2 complex: eIF2α. Under basal conditions, phosphorylated eIF2α inhibits global translation but activates translation of the transcription factor ATF4. ATF4 represses the expression of genes needed for long-term memory. Neuronal activity induces eIF2α dephosphorylation, which reverses the process leading to increased global translation, decreased ATF4 translation, and subsequent improved memory function (23). The key is probably provided by the identity of the mRNAs whose translation is repressed or activated. Based on the model described above, Slit-mediated repulsion would involve the translational activation of mRNAs involved in cytoskeletal disassembly, whereas mRNAs involved in cytoskeletal assembly would be repressed. The Shot-Kra-eIF2β complex would therefore play a role in defining the balance of activation and repression during the process of midline guidance. Moreover, mRNAs identified in axonal growth cones include those encoding not only cytoskeletal proteins but also translation factors (24). The Shot-Kra-eIF2β complex could therefore also be part of a feedback mechanism in which newly translated initiation factors could shift the balance of the activity of the protein synthesis machinery to finetune the levels of translation.
Cytoskeleton-mediated mRNA transport and anchoring provides a key advantage to the cell: It allows spatially positioning mRNAs stably within the cytoplasm so that their cognate proteins are appropriately localized. The cytoskeleton also provides a framework for regulation of translation and growth cone steering: Cytoskeletal rearrangements can affect the activity of the translation machinery, and translation of cytoskeletal proteins can affect cytoskeletal rearrangements. This suggests the presence of an elegant feedback or cross-talk mechanism to control the behavior of cells in response to external stimuli. Lee et al. (5) have identified a complex that might mediate this cross-talk mechanism in axonal growth cones. Future research will be aimed at further elucidating how such cytoskeleton-based translational platforms are regulated by signaling pathways. Because Shot is the only plakin in Drosophila, it will be interesting to determine which of the vertebrate homologs fulfills a similar function. Growth cones provide an attractive model system in which to study the intimate connection between the cytoskeleton, translational control, and how these processes finally direct axonal pathfinding.
References
- 1.St Johnston D. Moving messages: The intracellular localization of mRNAs. Nat. Rev. Mol. Cell Biol. 2005;6:363–375. doi: 10.1038/nrm1643. [DOI] [PubMed] [Google Scholar]
- 2.Lin AC, Holt CE. Local translation and directional steering in axons. EMBO J. 2007;26:3729–3736. doi: 10.1038/sj.emboj.7601808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hengst U, Jaffrey SR. Function and translational regulation of mRNA in developing axons. Semin. Cell Dev. Biol. 2007;18:209–215. doi: 10.1016/j.semcdb.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hovland R, Hesketh JE, Pryme IF. The compartmentalization of protein synthesis: Importance of cytoskeleton and role in mRNA targeting. Int. J. Biochem. Cell Biol. 1996;28:1089–1105. doi: 10.1016/1357-2725(96)00059-3. [DOI] [PubMed] [Google Scholar]
- 5.Lee S, Nahm M, Lee M, Kwon M, Kim E, Zadeh AD, Cao H, Kim HJ, Lee ZH, Oh SB, Yim J, Kolodziej PA, Lee S. The F-actin-microtubule crosslinker Shot is a platform for Krasavietz-mediated translational regulation of midline axon repulsion. Development. 2007;134:1767–1777. doi: 10.1242/dev.02842. [DOI] [PubMed] [Google Scholar]
- 6.Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- 7.Erskine L, Herrera E. The retinal ganglion cell axon’s journey: Insights into molecular mechanisms of axon guidance. Dev. Biol. 2007;308:1–14. doi: 10.1016/j.ydbio.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- 9.Piper M, Anderson R, Dwivedy A, Weinl C, van Horck F, Leung KM, Cogill E, Holt C. Signaling mechanisms underlying Slit2-induced collapse of Xenopus retinal growth cones. Neuron. 2006;49:215–228. doi: 10.1016/j.neuron.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ming GL, Wong ST, Henley J, Yuan XB, Song HJ, Spitzer NC, Poo MM. Adaptation in the chemotactic guidance of nerve growth cones. Nature. 2002;417:411–418. doi: 10.1038/nature745. [DOI] [PubMed] [Google Scholar]
- 11.Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Kolodziej PA. Short Stop provides an essential link between F-actin and microtubules during axon extension. Development. 2002;129:1195–1204. doi: 10.1242/dev.129.5.1195. [DOI] [PubMed] [Google Scholar]
- 13.Sonnenberg A, Liem RK. Plakins in development and disease. Exp. Cell Res. 2007;313:2189–2203. doi: 10.1016/j.yexcr.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 14.Gebauer F, Hentze MW. Molecular mechanisms of translational control. Nat. Rev. Mol. Cell Biol. 2004;5:827–835. doi: 10.1038/nrm1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sotelo-Silveira JR, Calliari A, Kun A, Koenig E, Sotelo JR. RNA trafficking in axons. Traffic. 2006;7:508–515. doi: 10.1111/j.1600-0854.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- 16.Condeelis J, Singer RH. How and why does beta-actin mRNA target? Biol. Cell. 2005;97:97–110. doi: 10.1042/BC20040063. [DOI] [PubMed] [Google Scholar]
- 17.Gross SR, Kinzy TG. Improper organization of the actin cytoskeleton affects protein synthesis at initiation. Mol. Cell. Biol. 2007;27:1974–1989. doi: 10.1128/MCB.00832-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sotelo-Silveira J, Crispino M, Puppo A, Sotelo JR, Koenig E. Myelinated axons contain beta-actin mRNA and ZBP-1 in periaxoplasmic ribosomal plaques and depend on cyclic AMP and F-actin integrity for in vitro translation. J. Neurochem. 2008;104:545–557. doi: 10.1111/j.1471-4159.2007.04999.x. [DOI] [PubMed] [Google Scholar]
- 19.Leung KM, van Horck FP, Lin AC, Allison R, Standart N, Holt CE. Asymmetrical beta-actin mRNA translation in growth cones mediates attractive turning to netrin-1. Nat. Neurosci. 2006;9:1247–1256. doi: 10.1038/nn1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao J, Sasaki Y, Wen Z, Bassell GJ, Zheng JQ. An essential role for beta-actin mRNA localization and translation in Ca2+-dependent growth cone guidance. Nat. Neurosci. 2006;9:1265–1273. doi: 10.1038/nn1773. [DOI] [PubMed] [Google Scholar]
- 21.Wu KY, Hengst U, Cox LJ, Macosko EZ, Jeromin A, Urquhart ER, Jaffrey SR. Local translation of RhoA regulates growth cone collapse. Nature. 2005;436:1020–1024. doi: 10.1038/nature03885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Proud CG. Signalling to translation: How signal transduction pathways control the protein synthetic machinery. Biochem. J. 2007;403:217–234. doi: 10.1042/BJ20070024. [DOI] [PubMed] [Google Scholar]
- 23.Costa-Mattioli M, Gobert D, Stern E, Gamache K, Colina R, Cuello C, Sossin W, Kaufman R, Pelletier J, Rosenblum K, Krnjevic K, Lacaille JC, Nader K, Sonenberg N. eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell. 2007;129:195–206. doi: 10.1016/j.cell.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willis D, Li KW, Zheng JQ, Chang JH, Smit A, Kelly T, Merianda TT, Sylvester J, van Minnen J, Twiss JL. Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J. Neurosci. 2005;25:778–791. doi: 10.1523/JNEUROSCI.4235-04.2005. 10.1126/stke.18pe11. [DOI] [PMC free article] [PubMed] [Google Scholar]