Abstract
The cell body has classically been considered the exclusive source of axonal proteins. However, significant evidence has accumulated recently to support the view that protein synthesis can occur in axons themselves, remote from the cell body. Indeed, local translation in axons may be integral to aspects of synaptogenesis, long-term facilitation, and memory storage in invertebrate axons, and for growth cone navigation in response to environmental stimuli in developing vertebrate axons. Here we review the evidence supporting mRNA translation in axons and discuss the potential roles that local protein synthesis may play during development and subsequent neuronal function. We advance the view that local translation provides a rapid supply of nascent proteins in restricted axonal compartments that can potentially underlie long-term responses to transient stimuli.
Keywords: mRNA, ribosome, protein synthesis, growth cone
INTRODUCTION
The extreme polarity exhibited by many neuronal cells imposes a number of unique constraints with respect to development, growth, and survival. The axon, for example, must navigate through a complex environment during its development and must form and maintain specific synaptic connections at its target, often at a significant distance from the cell body. What is the source of axonal proteins? One of the tenets of neuronal cell biology has been that the perikaryon is the exclusive site of protein synthesis within the cell, with transport of newly synthesized proteins from here to distant cellular locations accounting for needs accrued through maintenance and growth. However, evidence challenging this theory has been gradually accumulating. The discovery of polyribosomes located beneath postsynaptic sites on dendrites (Steward & Fass 1983, Steward & Levy 1982) and the subsequent identification of various mRNA species specifically localized within dendrites (Garner et al. 1988, Racca et al. 1997) suggest that compartmentalized synthesis of proteins occurs in neurons. This hypothesis has been supported experimentally (for review, see Steward & Schuman 2003), indicating that local mRNA translation within the dendritic domain plays a vital role in protein turnover, regulation of activity, and synapse plasticity. Axonal protein synthesis, however, has been an altogether more controversial area. The reason for this seems to date back to the failure of biochemical investigations in the 1970s to detect translational machinery and mRNA in axons. The purpose of this review is to summarize the data concerning localized RNA translation in axons and to highlight the potential role this may play in both developing and mature axons.
Historical Perspective
The prevailing view of axonal proteins being synthesized exclusively in the cell body arose primarily from a failure of early studies to detect ribosomes in axons (Lasek et al. 1973), coupled with the identification of axonal transport as a means of supplying axonal proteins (Droz & Leblond 1963). This source material has been comprehensively reviewed recently (see Alvarez et al. 2000). Axonally transported proteins can be divided into two rate groups. Fast axonal transport occurs with proteins associated with vesicles and membranous organelles and is mediated via microtubules at a rate of 50–200 mm/day. The majority of proteins, including many cytoskeletal and cytosolic proteins, fall into the slow axonal transport group, traveling at 0.1–3 mm/day (for review, see Campenot & Eng 2000). Given that proteins have half-lives ranging from a few seconds to many days (Varshavsky 1996), the speed of slow axonal transport immediately raises a fundamental issue: How do proteins destined for the most distal part of an axon survive a journey that may be greater than 1 m in length? Tubulin, for example, has been reported to have a half-life of 1 week in rat brain neurons (Forgue & Dahl 1978); moving at 3 mm a day, little would survive a journey of more than a few centimeters. The hypothesis that axonal proteins are stable during transit has not been demonstrated experimentally; indeed, radiolabeled axoplasmic proteins have average half-lives similar to those of cellular proteins (Nixon 1980). Moreover, Nixon (1980) reported that released amino acids were reused locally, a phenomenon that would require local axonal protein synthesis. The theory that all axonal proteins are produced in the cell body also overlooked a number of electron microscopy (EM) studies providing evidence for the existence of protein-synthesizing machinery in developing vertebrate growth cones from the rabbit spinal cord (Tennyson 1970, Yamada et al. 1971) and mature rat spinal axons (Zelena 1970). Axonal protein synthesis could reconcile the inconsistencies inherent in the slow axonal transport model, possibly providing an efficient mechanism for replenishing axonal proteins and decentralizing control from the cell body (for review, see Alvarez et al. 2000). However, the maintenance of axoplasmic mass is only one of the potential roles for local mRNA translation. In this review we focus on other functions that axonal protein synthesis may mediate, both in vertebrates and invertebrates.
AXONAL RNA TRANSLATION IN INVERTEBRATES
Two model systems have primarily been used to investigate RNA translation in mature invertebrate axons: the squid giant axon and cultured neurons from marine gastropods such as Aplysia. All of the necessary components for RNA translation, including tRNA (Black & Lasek 1977), rRNA (Martin et al. 1998), aminoacyl-tRNA synthetases (Giuditta et al. 1977), polypeptide elongation factors (Giustetto et al. 2003), and a variety of mRNA species (Table 1), have been identified in invertebrate axons (Giuditta et al. 1977). Although there have been in vitro biochemical demonstrations of axonal protein synthesis in fractions isolated from squid axons (Ingoglia et al. 1983) and synaptosomes (Benech et al. 1999, Crispino et al. 1997, Ingoglia et al. 1983), no data yet exist to reveal a functional role for axonal protein synthesis in these axons.
TABLE 1.
Examples of mRNAs isolated from invertebrate axons
| mRNA | Species | Tissue | Reference |
|---|---|---|---|
| β-Actin | Squid | Giant axon | (Gioio et al. 1994) |
| CDCH | Snail | CNS | (Dirks et al. 1993) |
|
Cytochrome oxidase
subunit 17 |
Squid | Photoreceptor presynaptic terminals |
(Gioio et al. 2001) |
| eEIF1α | Snail | Sensory neurons | (Giustetto et al. 2003) |
| ELH | Snail | CNS | (Lee et al. 2002) |
| Enolase | Squid | Giant axon | (Chun et al. 1996) |
| hsp70 | Squid | Giant axon | (Gioio et al. 2001) |
| Kinesin | Squid | Giant axon | (Gioio et al. 1994) |
| MAPH1 | Squid | Giant axon | (Chun et al. 1996) |
| Neurofilament | Squid | CNS | (Giuditta et al. 1991) |
| Neuropeptide | Snail | CNS | (Landry et al. 1992, van Minnen 1994) |
| Sensorin A | Snail | Sensory neurons | (Hu et al. 2002, Schacher et al. 1999) |
| Syntaxin | Snail | Sensory neurons | (Hu et al. 2003) |
| β-Tubulin | Squid | Giant axon | (Gioio et al. 1994) |
Axonal protein synthesis has been directly demonstrated in mature cultured neurons from the mollusc Lymnaea stagnalis. van Minnen et al. (1997) injected mRNA encoding the peptide egg-laying hormone (ELH) into isolated axons from pedal A cluster cells. These cells do not express the endogenous ELH gene in vivo. Within 2 h the axons were shown via immunohistochemistry to express ELH protein (van Minnen et al. 1997). The ability to post-translationally process and secrete the hormone was not investigated, but this issue has been addressed in a recent study of the capacity of axons from Lymnaea neurosecretory cells to synthesize proteins (Spencer et al. 2000). The authors analyzed the ability of surgically isolated axons to synthesize the conopressin receptor, CPR-2. Using axons from visceral F neurons, which do not express the CPR-2 gene, CPR-2 mRNA was injected into isolated axons. The tagged protein was detected 24 h later, and furthermore, subsequent application of conopressin, the cognate CPR-2 ligand, to the axons produced a prolonged depolarizing response. These data imply that the conopressin receptor mRNA is locally translated within the axon and that the protein is functionally inserted into the plasma membrane (Spencer et al. 2000).
What role does axonal protein synthesis play in invertebrate neurons? This has been investigated primarily using cultured neurons from the mollusc Aplysia. Schacher et al. (1999), using mechanosensory neurons (SN) that specifically form synapses with L7 motor cells, demonstrated that rRNA and certain mRNAs were exported in a branch-specific manner to those SN axons forming synapses with L7 targets. Furthermore, when the SN cell body was removed, synaptic connections with L7 cells could still be formed. This was dependent on local RNA translation in distal synaptic sites, as synaptogenesis was reversibly blocked by the translational inhibitor, anisomycin (Schacher & Wu 2002). These data suggest that axonal protein synthesis plays an important role in synapse formation and maintenance. Whereas target interactions between SN axons and L7 targets correlate with the synaptic stability of the neuropeptide sensorin mRNA after SN cell body removal (Hu et al. 2002), the identity of the proteins that are required to be synthesized locally to facilitate synaptogenesis remains largely unknown.
In addition to synapse biosynthesis, a number of recent experiments have pointed to local translation contributing to synapse function. An elegant experimental model with a single Aplysia SN, possessing a bifurcated axon that makes synapses with two spatially separated motor neurons, was used to investigate protein synthesis during long-term synaptic plasticity and memory storage (Martin et al. 1997). Application of serotonin to the synapse made on one motor neuron produced branch-specific long-term facilitation (LTF), an effect reliant on transcription in the sensory cell nucleus and local translation in the presynaptic region (Martin et al. 1997). Local translation can also enable transient, cell-wide LTF within the SN to be stabilized at specific synapses in response to subsequent localized serotonin application (Casadio et al. 1999), and coincident application of serotonin to the SN somata and the synaptic region can produce LTF, which is dependent on immediate presynaptic protein synthesis and delayed synthesis in the cell body (Sherff & Carew 1999, 2002). A similar dependence on local, presynaptic RNA translation for LTF in crayfish has also been reported (Beaumont et al. 2001). These studies underline an important role for local protein synthesis in the ability of a neuron to regulate its synaptic connections in response to activation. As each neuron may make over 1000 connections (Martin et al. 1997), decentralizing control from the perikaryon through local RNA translation provides an effective way of specifically strengthening those connections receiving stimuli, thereby conferring greater flexibility during plasticity and memory storage.
The next challenge will be the identification of the mRNA species being locally translated in the presynaptic region and characterization of just how their protein products act to modulate synaptogenesis, LTF, and memory storage. Indeed, the mRNA population in Aplysia sensory neurites has been partially elucidated and contains mRNAs encoding components of the translational machinery and cytoskeletal mRNAs such as α1-tubulin (Moccia et al. 2003). Furthermore, the cytoplasmic polyadenylation element-binding (CPEB) protein was recently shown to be locally upregulated in Aplysia SNs synapsing on motor neurons, demonstrating one mechanism by which local protein synthesis can complement maintenance of LTF (Si et al. 2003). However, an important caveat is that invertebrate neurons, including the squid giant neuron and Aplysia SNs, generally lack the polarity displayed by vertebrate neurons and possess only one type of process. Although this process is called an axon, it has both receptive and transmissive surfaces and thus combines the functions of vertebrate dendrites and axons (for review, see Mohr & Richter 2000). As such, direct comparisons between invertebrate and vertebrate axons should be made with care.
AXONAL RNA TRANSLATION IN VERTEBRATES
Much of the controversy surrounding RNA translation in vertebrate axons stems from early reports that failed to identify ribosomes via EM. However, because ribosomes have electron opacity similar to other cellular components, they can be inconspicuous in EM unless regularly arranged on membranes or grouped as polyribosomes (Martin et al. 1989). Furthermore, the doctrinal view of axons being incapable of translation evolved despite a number of early studies that identified sporadic ribosomes in axons and growth cones of developing neurons (Bunge 1973, Tennyson 1970, Yamada et al. 1971) and also in mature axons (Zelena 1970). More recently, ribosomes have been identified in both the initial axon segment (Steward & Ribak 1986) and intermittently along the axon shaft (Koenig et al. 2000, Pannese & Ledda 1991) using both biochemical and immunohistochemical methods (Bassell et al. 1998; Campbell & Holt 2001; Giuditta et al. 1991, 2002; Koenig 1979; Koenig & Martin 1996; Zheng et al. 2001). Other components necessary for translation such as tRNA (Koenig 1979), initiation factors (Zheng et al. 2001), and mRNA (Bassell et al. 1998, Gioio et al. 1994, Giuditta et al. 1980, Koenig 1979) (Table 2) have all been identified in vertebrate axons. Is axonal protein synthesis important for vertebrate axons? Although this appears to be a simple question, experimental difficulties, such as purifying presynaptic regions without contamination from other cellular debris, have proven difficult to overcome.
TABLE 2.
Examples of mRNAs isolated from vertebrate axons
| Age | mRNA | Species | Tissue | Reference |
|---|---|---|---|---|
| Embryonic | β-Actin | Chick, rat | Sympathetic | (Olink-Coux & neurons, cortex Hollenbeck 1996) |
| Embryonic | ADF | Chick | Sympathetic | (Lee & Hollenbeck neurons 2003) |
| Embryonic | CGRP | Rat | Olfactory bulb | (Denis-Donini et al. 1998) |
| Embryonic | EphB2 | Chick | Retina | (Brittis et al. 2002) |
| Embryonic | MAP Tau | Rat | Cortex | (Litman et al. 1993) |
| Embryonic | N-CAM | Chick | Retina | (Brittis et al. 2002) |
| Embryonic | Vasopressin | Rat | Hypothalamus | (Mohr et al. 1991) |
| Adult | L7 | Mouse | Cerebellum | (Wanner et al. 1997) |
| Adult | Neurofilament-M | Goldfish | Mauthner axon | (Weiner et al. 1996) |
| Adult |
Neurofilament-L, -M,-H |
Rat | Sciatic nerve | (Sotelo-Silveira et al. 2000) |
| Adult |
Olfactory marker
protein |
Rat | Sensory neurons | (Vassar et al. 1994, Wensley et al. 1995) |
| Adult | Oxytocin | Rat | Hypothalamus | (Jirikowski et al. 1990) |
| Adult | Prodynorphin | Rat | Hypothalamus | (Mohr & Richter 1992) |
| Adult | VR1 | Rat | Sensory neurons | (Tohda et al. 2001) |
Mature Axons
The data supporting axonal and presynaptic translation of RNA in mature vertebrate neurons are fragmentary. In cultured hippocampal and cortical neurons, the presence of rRNA and mRNA in developing axons seems to be transient, with both disappearing from axons as neurons mature, differentiate, and form synapses (Bassell et al. 1994, Kleiman et al. 1990). However, mRNA is present in the axons of a number of mature vertebrate nerve cells, including the goldfish Mauthner neuron (Weiner et al. 1996), hypothalamic magnocellular neurons (Trembleau et al. 1996), and sensory neurons projecting to the olfactory bulb (Ressler et al. 1994, Vassar et al. 1994). Apart from the Mauthner neuron (Koenig & Martin 1996), ribosomes have not been identified in these specific axons and have rarely been identified in myelinated axons (Koenig et al. 2000), leaving their functional significance in doubt.
The potential for local RNA translation to occur in mature axons has been investigated using metabolic labeling experiments in both isolated axonal fields (Koenig 1967, 1989; Koenig & Adams 1982) and presynaptic membrane preparations (Gambetti et al. 1972, Gilbert 1972, Morgan & Austin 1968), although contributions from other cellular contaminants make these data difficult to interpret. A more recent report demonstrated that local protein synthesis was needed for neurotrophin-induced synaptic plasticity in adult hippocampal slices (Kang & Schuman 1996), but whether synthesis took place in the axonal or dendritic compartment was not addressed. Compelling data demonstrating the existence and functional significance of local intra-axonal protein synthesis in regenerating axons has been provided by studies of regenerating adult sensory axons (Twiss et al. 2000, Zheng et al. 2001) (Figure 1). Mammalian dorsal root ganglia (DRG) axons regenerate robustly in vitro after conditioning induced by axonal crush and, in the absence of their cell bodies, possess and translate multiple RNAs (Twiss et al. 2000, Zheng et al. 2001). Indeed, Zheng et al. (2001) demonstrated that rat DRG axons regenerating after axonal crush conditioning contained ribosome-bound β-actin and neurofilament mRNAs and that local protein synthesis was necessary for growth cone integrity during regeneration. Twiss and colleagues also showed that motor axons of ventral spinal roots, 7 days after nerve crush in vivo, contain rRNA, translation initiation factors, and ribosomal proteins (Zheng et al. 2001). These results clearly demonstrate that adult mammalian axons, at least in some situations, are capable of mRNA translation and suggest that local protein synthesis plays a role in successful regeneration. Future studies are needed to discover the exact role that intra-axonal protein synthesis plays in regeneration and to determine whether local protein synthesis has a functional role in noninjured axons.
Figure 1.
Regenerating sensory axons require local protein synthesis (yellow) to maintain growth cone advancement. Model based on Zheng et al. (2001).
Developing Axons
Developing neurons tend to be more accessible to experimental manipulation; therefore the situation regarding axonal RNA translation is more clear-cut than that in mature axons. Growth cones of developing cortical and hippocampal neurons in culture contain both rRNA and poly(A) mRNA (Bassell et al. 1994, Kleiman et al. 1990). Of interest is the finding that the embryonic mRNAs identified to date have often been those encoding cytoskeletal proteins (Table 2) such as β-actin (Bassell et al. 1998), the microtubule-associated protein tau (Litman et al. 1993), and actin-depolymerizing factor (ADF; Lee & Hollenbeck 2003). The presence of cytoskeletal mRNAs in the axon suggests that their local translation could play a functional role in axonogenesis.
To investigate this possibility, Campenot and colleagues used a simple, yet powerful, compartmented culture system in which rat sympathetic neurons were grown in a central division of a compartmented dish. In these “Campenot chambers,” axons extend into compartments isolated from the cell bodies, allowing the addition of reagents exclusively to the tips of growing axons. By incubating the axon-containing compartment with [35S]methionine and then purifying proteins, the authors demonstrated that axons could synthesize both actin and β-tubulin, even when the cell bodies had been removed prior to the commencement of the assay (Eng et al. 1999). Importantly, translation of actin and β-tubulin mRNAs was not essential for axonal elongation, as inhibition of translation with cycloheximide during the assay period (4 h) did not inhibit axon growth. Although these findings do not preclude a role for axonal protein synthesis in supplying cytoskeletal proteins for growth and elongation, they do raise the possibility that local RNA translation in the axonal compartment may fulfill an entirely different role.
In vitro studies of cultured chick embryonic hippocampal neurons provided an insight into what this function may be. Stimulation of these neurons with the neurotrophin NT-3 elicited a rapid localization of β-actin mRNA to axonal growth cones (Zhang et al. 1999). Moreover, β-actin mRNA localization to the growth cone required the formation of a complex between zipcode binding protein 1 (ZBP1) and a region in the 3′UTR of the β-actin mRNA, called the zipcode. Disruption of complex formation, using antisense oligonucleotides complementary to the zipcode region, caused a decrease in growth cone motility when assayed with NT-3 over the course of 1 h (Zhang et al. 2001). These findings highlighted two important concepts. First, the dependence of directed mRNA transport upon ZBP1 focuses attention on the role of RNA-binding proteins in mRNA localization. This has also been shown with the RNA-binding protein, HuD, which mediates axonal localization of tau mRNA (Aronov et al. 2002). The importance of these proteins in RNA transport, localization, and extrasomatic translation is becoming apparent, although many questions remain regarding formation of mRNA/protein complexes, axonal transport, and mRNA localization and tethering within the growth cone. Second, the findings of Zhang and colleagues suggest that regulated axonal localization of mRNA may mediate growth cone dynamics in response to environmental cues. In turn this raises further questions that were not addressed in these studies: Does local axonal translation occur in response to extracellular signals? Does inhibition of translation interfere with the ability of growth cones to respond to these cues?
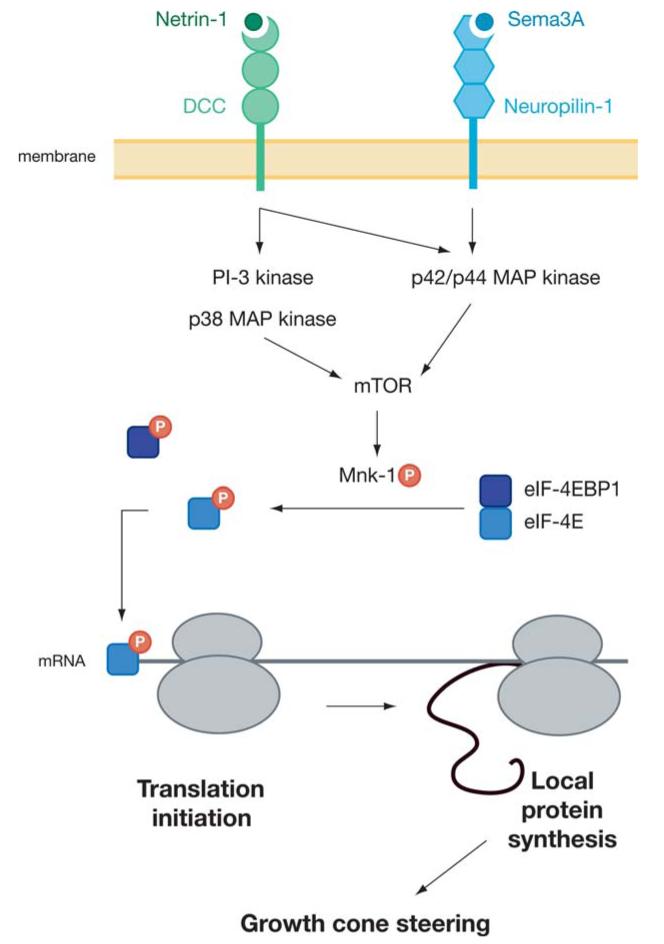
These fundamental issues were addressed in a recent in vitro study of Xenopus retinal ganglion cells (RGCs). Using axons from cultured embryonic RGCs that had been separated from their cell bodies, Campbell & Holt (2001) demonstrated that bath-application of the guidance cues Sema3A or netrin-1 elicited a rapid (10 min) increase in protein synthesis, as measured by incorporation of trichloroacetic acid-precipitated tritiated leucine. With the use of specific antibodies, the authors also demonstrated an abundance of ribosomal proteins, capped-RNA, and translation initiation factors (eIF-4E, eIF-4EBP1) in RGC growth cones and, furthermore, showed that guidance cues rapidly (5 min) stimulate a rise in the phosphorylation of eIF-4E, eIF-4EBP1, and Mnk-1 (Campbell & Holt 2001, 2003). To determine if translation played a functional role in axon guidance, the authors performed two types of chemotropic guidance assays on growth cones (turning and collapse) in the presence of various protein synthesis inhibitors (anisomycin, cycloheximide, rapamycin). Xenopus RGC growth cones turn and grow toward a gradient of attractant (e.g., netrin-1) and away from a gradient of repellent (e.g., Sema3A; Campbell & Holt 2001, de la Torre et al. 1997, Hopker et al. 1999) over a period of 60 min and collapse within 10 min when chemorepellents are added globally to the bathing medium (Campbell et al. 2001). Remarkably, inhibition of RNA translation even in axons separated from their cell bodies completely blocked the repulsive turning and collapse responses induced by Sema3A and the attractive and repulsive turning elicited by netrin-1 (Campbell & Holt 2001). Similarly, the attractive turning responses of Xenopus spinal neurons to the neuropeptide, pituitary adenylate cyclase-activating polypeptide (PACAP), which signals through a G-coupled receptor, depend on protein synthesis (Guirland et al. 2003). In agreement with findings of Eng et al. (1999), axonal elongation is not significantly affected in the presence of translation inhibitors during the 60-min course of the turning assay, indicating that local protein synthesis is not needed for extension over the short term (Campbell & Holt 2001). This is likely fueled by proteins and lipids already being trafficked to the axon tip from the soma. These findings were pivotal in ascribing a functional role for axonal protein synthesis in the mediation of growth cone steering in response to environmental cues. A further interesting finding of this study and a subsequent study (Campbell & Holt 2003) is that different guidance cues elicit protein synthesis through different intracellular signaling pathways (Figure 2). For example, wortmannin blocks netrin-1- but not Sema3A-induced protein synthesis in growth cones, implicating the involvement of PI3 (phosphatidylinositol-3) kinase in one pathway and not the other. Both pathways are sensitive to rapamycin, an inhibitor of mTOR (target of rapamycin), indicating that translation in growth cones is likely to be mostly cap-dependent. These studies raise the possibility that different guidance cues initiate the translation of distinct mRNAs. In the future it will be important to discover whether this is the case and also determine whether a single cue initiates the translation of a fixed array of mRNAs and whether the composition of the array changes with age and cell type.
Figure 2.
Intracellular pathways leading to initiation of RNA translation in the growth cone. Sema3A and netrin-1 signal through different mitogen-activated protein kinase (MAPK) molecules that converge on mTOR and lead to an increase in phosphorylation of translation initiation factors and local protein synthesis, which subsequently mediates growth cone steering responses to these guidance cues (adapted from Campbell & Holt 2003).
A role for local protein synthesis in response to the attractants netrin-1 and brain-derived neurotrophic factor (BDNF) has also been suggested in a slightly different context (Ming et al. 2002). In turning assays, the presence of background levels of either molecule in the bathing medium was found to attenuate the turning responses of Xenopus spinal neurons to applied gradients of the attractants. This desensitization is thought to underlie the mechanism of growth cone adaptation. Removal of the chemoattractant from the bath resulted in resensitization, with the growth cones resuming normal turning behavior within 30 min (Ming et al. 2002). Importantly, this process of resensitization was found to be dependent on local protein synthesis, revealing that local synthesis of RNAs plays a role in growth cone adaptation.
How the involvement of local protein synthesis in initial growth cone turning (Campbell & Holt 2001) relates to its role in resensitization of growth cones (Ming et al. 2002) is not yet clear. Indeed, these functions may not be mutually exclusive, as Campbell & Holt (2001) did not address the issue of desensitization/resensitization, and Ming et al. (2002) did not investigate if exposure to guidance molecules also triggers a rapid increase in protein synthesis. However, the activation of MAPK pathways was implicated by both studies as an essential component of local protein synthesis (Campbell & Holt 2001, Ming et al. 2002). The intracellular pathways leading from receptor activation to protein synthesis within the growth cone are gradually being elucidated, demonstrating that the growth cone utilizes signaling cascades similar to those of the cell body to link receptor activation with translational responses (Figure 2) (Campbell & Holt 2003).
The above studies, all conducted in vitro, leave open questions about the in vivo relevance of protein synthesis. Growing axons use intermediate targets during development and can change responsiveness to guidance cues once past these targets (Dickson 2002, Shewan et al. 2002, Shirasaki et al. 1998, Tessier-Lavigne & Goodman 1996, Zou et al. 2000). Translation of RNA in axons could provide an ideal means of accomplishing this. For example, guidance cues at an intermediate target may trigger the local synthesis of new receptors, endowing the growth cone with an additional sensitivity. This possibility has been addressed in vivo using embryonic chick commissural neurons. A subset of these neurons upregulates expression of the EphA2 receptor in the distal segment of the axon after crossing the midline (Brittis et al. 2002). When green fluorescent protein (GFP) mRNA was linked with the 3′UTR of the EphA2 receptor and electroporated into chick spinal cords, expression of GFP was observed only in the distal part of the commissural axons that had crossed the midline (Brittis et al. 2002). Although this study did not demonstrate the presence of EphA2 mRNA in chick commissural axons, these data beautifully demonstrate the ability of growing axons to switch on the synthesis of a protein in a spatially regulated way and imply that distinct regions of the axon may express specific receptors, conferring the ability to locally regulate responsiveness to guidance cues along the pathway. Unlike dendrites (Torre & Steward 1996), there have been no reports of post-translational glycosylation or of conventional Golgi apparatus in axons to date. However, as Brittis et al. (2002) demonstrated that axons were capable of exporting and inserting proteins to the cell surface, it is possible that the Golgi is present in an unconventional organization (Yamada et al. 1971).
On the basis of these recent studies, it is interesting to speculate that protein synthesis may play at least two roles in developing neurons based on the latency and type of proteins synthesized locally. For example, a guidance cue (or mixture of guidance cues at an intermediate target) expressed at a distinct choice point in the pathway (e.g., optic nerve head or midline) might trigger the rapid translation (5–10 min) of mRNAs that are for immediate use in steering. Candidate mRNAs for this rapid translation might encode cytoskeletal/binding proteins and/or proteins that derepress translation. Alternatively, or in addition, the translation of other mRNAs may occur with a longer latency (>30 min) for later use, endowing growth cones with a new responsiveness to distally expressed pathway cues (see Figure 3). Candidate mRNAs for these longer-term changes might be surface receptors or new signaling components. By this means, a brief stimulus may lead to both transient and long-term changes in the protein composition of the growth cone.
Figure 3.
Diagram illustrating hypothesized roles of axonal protein synthesis in developing axons. (a) Growth cones approach a directional cue (blue) such as Sema3A at an angle (1); filopodia on the cue-side of the growth cone are stimulated first and translation (purple spots) is elicited asymmetrically (2), leading to local collapse on the cue-side and subsequent repulsive turning (3). Model based on Campbell & Holt (2001). (b) Growth cones approach (1) and extend through (2) an intermediate target (green) expressing a cue that elicits the translation of RNAs encoding new proteins (red) needed for responding to a second cue (blue) encountered later in the journey (3). Model based on Brittis et al. (2002).
Developing growth cones can behave autonomously in that they can correctly navigate through the optic tract and recognize their target in vivo when separated from the cell body (Harris et al. 1987). The findings discussed above give a compelling indication of how this is possible, showing embryonic axons to be capable of RNA translation. The uncoupling from the dependence on the cell body for new proteins enables a far greater flexibility in the growth cone, allowing rapid, local alterations in cytoskeletal dynamics and guidance receptor expression, thereby modulating responsiveness to external stimuli. A major challenge to neurobiologists will be to understand how local synthesis of proteins is integrated into functional responses within the growth cone (for review, see van Horck et al. 2004). The first step toward achieving this will be the thorough cataloging of the mRNA species within axonal domains, as has been done with dendrites (Crino & Eberwine 1996), which will identify those proteins the axon can synthesize and thus the full range of processes it is potentially able to mediate locally. The goal for the future will then be to identify which mRNAs are translated in response to different guidance cues. Once these are known, experiments can begin to test the functional role of specific mRNA candidates in axonal guidance, synapse formation, and repair.
CONCLUSION
A significant body of evidence now exists to suggest the doctrinal view that axons are incapable of protein synthesis (reviewed in Alvarez et al. 2000) is unfounded. The ability to translate RNA within the axon provides distinct advantages to the neuron, allowing rapid, focal synthesis of proteins from template mRNAs. As each mRNA can be tethered locally and used multiple times, production of proteins in this way is flexible and efficient when compared with bulk axoplasmic transport and, perhaps crucially, allows spatially restricted responses to focal external stimuli. When these findings are coupled with reports that aspects of lipid biosynthesis occur in axons (Posse de Chaves et al. 1995), it seems justifiable to consider the axon as a complex, metabolically active region of the cell that is sensitive and responsive to its surroundings. The challenges for the future are many and include determining which mRNAs are translated in response to stimuli such as guidance cues, neurotrophic factors, and electrical activity. Are the same mRNAs translated or do different stimuli trigger translation of different sets of mRNAs? Are different mRNAs localized spatially to specific axonal compartments, as occurs in dendrites, and how is their translation precisely regulated? The potential role of defects in axonal RNA translation contributing to neuronal-based diseases such as spinal muscular atrophy (for review, see Jablonka et al. 2004) and Fragile X syndrome (Antar & Bassell 2003) gives an exciting insight into how protein synthesis within the axon may be functionally relevant in disease.
ACKNOWLEDGMENTS
The authors thank Douglas Campbell, Francis van Horck, and Sarah Penning for critical comments on the manuscript. C.E.H is supported by a Wellcome Trust Programme Grant.
LITERATURE CITED
- Alvarez J, Giuditta A, Koenig E. Protein synthesis in axons and terminals: significance for maintenance, plasticity and regulation of phenotype. With a critique of slow transport theory. Prog. Neurobiol. 2000;62:1–62. doi: 10.1016/s0301-0082(99)00062-3. [DOI] [PubMed] [Google Scholar]
- Antar LN, Bassell GJ. Sunrise at the synapse: the FMRP mRNP shaping the synaptic interface. Neuron. 2003;37:555–58. doi: 10.1016/s0896-6273(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Aronov S, Aranda G, Behar L, Ginzburg I. Visualization of translated tau protein in the axons of neuronal P19 cells and characterization of tau RNP granules. J. Cell Sci. 2002;115:3817–27. doi: 10.1242/jcs.00058. [DOI] [PubMed] [Google Scholar]
- Bassell GJ, Singer RH, Kosik KS. Association of poly(A) mRNA with microtubules in cultured neurons. Neuron. 1994;12:571–82. doi: 10.1016/0896-6273(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Bassell GJ, Zhang H, Byrd AL, Femino AM, Singer RH, et al. Sorting of beta-actin mRNA and protein to neurites and growth cones in culture. J. Neurosci. 1998;18:251–65. doi: 10.1523/JNEUROSCI.18-01-00251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont V, Zhong N, Fletcher R, Froemke RC, Zucker RS. Phosphorylation and local presynaptic protein synthesis in calcium- and calcineurin-dependent induction of crayfish long-term facilitation. Neuron. 2001;32:489–501. doi: 10.1016/s0896-6273(01)00483-4. [DOI] [PubMed] [Google Scholar]
- Benech JC, Crispino M, Kaplan BB, Giuditta A. Protein synthesis in presynaptic endings from squid brain: modulation by calcium ions. J. Neurosci. Res. 1999;55:776–81. doi: 10.1002/(SICI)1097-4547(19990315)55:6<776::AID-JNR12>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Black MM, Lasek RJ. The presence of transfer RNA in the axoplasm of the squid giant axon. J. Neurobiol. 1977;8:229–37. doi: 10.1002/neu.480080306. [DOI] [PubMed] [Google Scholar]
- Brittis PA, Lu Q, Flanagan JG. Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell. 2002;110:223–35. doi: 10.1016/s0092-8674(02)00813-9. [DOI] [PubMed] [Google Scholar]
- Bunge MB. Fine structure of nerve fibers and growth cones of isolated sympathetic neurons in culture. J. Cell Biol. 1973;56:713–35. doi: 10.1083/jcb.56.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–26. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- Campbell DS, Holt CE. Apoptotic pathway and MAPKs differentially regulate chemotropic responses of retinal growth cones. Neuron. 2003;37:939–52. doi: 10.1016/s0896-6273(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Campbell DS, Regan AG, Lopez JS, Tannahill D, Harris WA, Holt CE. Semaphorin 3A elicits stage-dependent collapse, turning, and branching in Xenopus retinal growth cones. J. Neurosci. 2001;21:8538–47. doi: 10.1523/JNEUROSCI.21-21-08538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot RB, Eng H. Protein synthesis in axons and its possible functions. J. Neurocytol. 2000;29:793–98. doi: 10.1023/a:1010939307434. [DOI] [PubMed] [Google Scholar]
- Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, et al. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell. 1999;99:221–37. doi: 10.1016/s0092-8674(00)81653-0. [DOI] [PubMed] [Google Scholar]
- Chun JT, Gioio AE, Crispino M, Giuditta A, Kaplan BB. Differential compartmentalization of mRNAs in squid giant axon. J. Neurochem. 1996;67:1806–12. doi: 10.1046/j.1471-4159.1996.67051806.x. [DOI] [PubMed] [Google Scholar]
- Crino PB, Eberwine J. Molecular characterization of the dendritic growth cone: regulated mRNA transport and local protein synthesis. Neuron. 1996;17:1173–87. doi: 10.1016/s0896-6273(00)80248-2. [DOI] [PubMed] [Google Scholar]
- Crispino M, Kaplan BB, Martin R, Alvarez J, Chun JT, et al. Active polysomes are present in the large presynaptic endings of the synaptosomal fraction from squid brain. J. Neurosci. 1997;17:7694–702. doi: 10.1523/JNEUROSCI.17-20-07694.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre JR, Hopker VH, Ming GL, Poo MM, Tessier-Lavigne M, et al. Turning of retinal growth cones in a netrin-1 gradient mediated by the netrin receptor DCC. Neuron. 1997;19:1211–24. doi: 10.1016/s0896-6273(00)80413-4. [DOI] [PubMed] [Google Scholar]
- Denis-Donini S, Branduardi P, Campiglio S, Carnevali MD. Localization of calcitonin gene-related peptide mRNA in developing olfactory axons. Cell Tissue Res. 1998;294:81–91. doi: 10.1007/s004410051158. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–64. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Dirks RW, van Dorp AG, van Minnen J, Fransen JA, van der Ploeg M, Raap AK. Ultrastructural evidence for the axonal localization of caudodorsal cell hormone mRNA in the central nervous system of the mollusc Lymnaea stagnalis. Microsc. Res. Tech. 1993;25:12–18. doi: 10.1002/jemt.1070250104. [DOI] [PubMed] [Google Scholar]
- Droz B, Leblond CP. Axonal migration of proteins in the central nervous system and peripheral nerves as ahown by radioautography. J. Comp. Neurol. 1963;121:325–46. doi: 10.1002/cne.901210304. [DOI] [PubMed] [Google Scholar]
- Eng H, Lund K, Campenot RB. Synthesis of beta-tubulin, actin, and other proteins in axons of sympathetic neurons in compartmented cultures. J. Neurosci. 1999;19:1–9. doi: 10.1523/JNEUROSCI.19-01-00001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgue ST, Dahl JL. The turnover rate of tubulin in rat brain. J. Neurochem. 1978;31:1289–97. doi: 10.1111/j.1471-4159.1978.tb06254.x. [DOI] [PubMed] [Google Scholar]
- Gambetti P, Autilio-Gambetti LA, Gonatas NK, Shafer B. Protein synthesis in synaptosomal fractions. Ultrastructural radioautographic study. J. Cell Biol. 1972;52:526–35. doi: 10.1083/jcb.52.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner CC, Tucker RP, Matus A. Selective localization of messenger RNA for cytoskeletal protein MAP2 in dendrites. Nature. 1988;336:674–77. doi: 10.1038/336674a0. [DOI] [PubMed] [Google Scholar]
- Gilbert JM. Evidence for protein synthesis in synaptosomal membranes. J. Biol. Chem. 1972;247:6541–50. [PubMed] [Google Scholar]
- Gioio AE, Chun JT, Crispino M, Capano CP, Giuditta A, Kaplan BB. Kinesin mRNA is present in the squid giant axon. J. Neurochem. 1994;63:13–18. doi: 10.1046/j.1471-4159.1994.63010013.x. [DOI] [PubMed] [Google Scholar]
- Gioio AE, Eyman M, Zhang H, Lavina ZS, Giuditta A, Kaplan BB. Local synthesis of nuclear-encoded mitochondrial proteins in the presynaptic nerve terminal. J. Neurosci. Res. 2001;64:447–53. doi: 10.1002/jnr.1096. [DOI] [PubMed] [Google Scholar]
- Giuditta A, Cupello A, Lazzarini G. Ribosomal RNA in the axoplasm of the squid giant axon. J. Neurochem. 1980;34:1757–60. doi: 10.1111/j.1471-4159.1980.tb11271.x. [DOI] [PubMed] [Google Scholar]
- Giuditta A, Kaplan BB, van Minnen J, Alvarez J, Koenig E. Axonal and presynaptic protein synthesis: new insights into the biology of the neuron. Trends Neurosci. 2002;25:400–4. doi: 10.1016/s0166-2236(02)02188-4. [DOI] [PubMed] [Google Scholar]
- Giuditta A, Menichini E, Perrone Capano C, Langella M, Martin R, et al. Active polysomes in the axoplasm of the squid giant axon. J. Neurosci. Res. 1991;28:18–28. doi: 10.1002/jnr.490280103. [DOI] [PubMed] [Google Scholar]
- Giuditta A, Metafora S, Felsani A, Del Rio A. Factors for protein synthesis in the axoplasm of squid giant axons. J. Neurochem. 1977;28:1393–95. doi: 10.1111/j.1471-4159.1977.tb12339.x. [DOI] [PubMed] [Google Scholar]
- Giustetto M, Hegde AN, Si K, Casadio A, Inokuchi K, et al. Axonal transport of eukaryotic translation elongation factor 1alpha mRNA couples transcription in the nucleus to long-term facilitation at the synapse. Proc. Natl. Acad. Sci. USA. 2003;100:13680–85. doi: 10.1073/pnas.1835674100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirland C, Buck KB, Gibney JA, DiCicco-Bloom E, Zheng JQ. Direct cAMP signaling through G-protein-coupled receptors mediates growth cone attraction induced by pituitary adenylate cyclase-activating polypeptide. J. Neurosci. 2003;23:2274–83. doi: 10.1523/JNEUROSCI.23-06-02274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris WA, Holt CE, Bonhoeffer F. Retinal axons with and without their somata, growing to and arborizing in the tectum of Xenopus embryos: a time-lapse video study of single fibres in vivo. Development. 1987;101:123–33. doi: 10.1242/dev.101.1.123. [DOI] [PubMed] [Google Scholar]
- Hopker VH, Shewan D, Tessier-Lavigne M, Poo M, Holt C. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature. 1999;401:69–73. doi: 10.1038/43441. [DOI] [PubMed] [Google Scholar]
- Hu JY, Meng X, Schacher S. Target interaction regulates distribution and stability of specific mRNAs. J. Neurosci. 2002;22:2669–78. doi: 10.1523/JNEUROSCI.22-07-02669.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JY, Meng X, Schacher S. Redistribution of syntaxin mRNA in neuronal cell bodies regulates protein expression and transport during synapse formation and long-term synaptic plasticity. J. Neurosci. 2003;23:1804–15. doi: 10.1523/JNEUROSCI.23-05-01804.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingoglia NA, Giuditta A, Zanakis MF, Babigian A, Tasaki I, et al. Incorporation of [3H]-amino acids into proteins in a partially purified fraction of axoplasm: evidence for transfer RNA-mediated, post-translational protein modification in squid giant axons. J. Neurosci. 1983;3:2463–73. doi: 10.1523/JNEUROSCI.03-12-02463.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonka S, Wiese S, Sendtner M. Axonal defects in mouse models of motoneuron disease. J. Neurobiol. 2004;58:272–86. doi: 10.1002/neu.10313. [DOI] [PubMed] [Google Scholar]
- Jirikowski GF, Sanna PP, Bloom FE. mRNA coding for oxytocin is present in axons of the hypothalamo-neurohypophysial tract. Proc. Natl. Acad. Sci. USA. 1990;87:7400–4. doi: 10.1073/pnas.87.19.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Schuman EM. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science. 1996;273:1402–6. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- Kleiman R, Banker G, Steward O. Differential subcellular localization of particular mRNAs in hippocampal neurons in culture. Neuron. 1990;5:821–30. doi: 10.1016/0896-6273(90)90341-c. [DOI] [PubMed] [Google Scholar]
- Koenig E. Synthetic mechanisms in the axon. IV. In vitro incorporation of [3H]precursors into axonal protein and RNA. J. Neurochem. 1967;14:437–46. doi: 10.1111/j.1471-4159.1967.tb09542.x. [DOI] [PubMed] [Google Scholar]
- Koenig E. Ribosomal RNA in Mauthner axon: implications for a protein synthesizing machinery in the myelinated axon. Brain Res. 1979;174:95–107. doi: 10.1016/0006-8993(79)90806-0. [DOI] [PubMed] [Google Scholar]
- Koenig E. Cycloheximide-sensitive [35S]methionine labeling of proteins in goldfish retinal ganglion cell axons in vitro. Brain Res. 1989;481:119–23. doi: 10.1016/0006-8993(89)90491-5. [DOI] [PubMed] [Google Scholar]
- Koenig E, Adams P. Local protein synthesizing activity in axonal fields regenerating in vitro. J. Neurochem. 1982;39:386–400. doi: 10.1111/j.1471-4159.1982.tb03960.x. [DOI] [PubMed] [Google Scholar]
- Koenig E, Martin R. Cortical plaque-like structures identify ribosome-containing domains in the Mauthner cell axon. J. Neurosci. 1996;16:1400–11. doi: 10.1523/JNEUROSCI.16-04-01400.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig E, Martin R, Titmus M, Sotelo-Silveira JR. Cryptic peripheral ribosomal domains distributed intermittently along mammalian myelinated axons. J. Neurosci. 2000;20:8390–400. doi: 10.1523/JNEUROSCI.20-22-08390.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry C, Crine P, DesGroseillers L. Differential expression of neuropeptide gene mRNA within the LUQ cells of Aplysia californica. J. Neurobiol. 1992;23:89–101. doi: 10.1002/neu.480230109. [DOI] [PubMed] [Google Scholar]
- Lasek RJ, Dabrowski C, Nordlander R. Analysis of axoplasmic RNA from invertebrate giant axons. Nat. New Biol. 1973;244:162–65. doi: 10.1038/newbio244162a0. [DOI] [PubMed] [Google Scholar]
- Lee SK, Hollenbeck PJ. Organization and translation of mRNA in sympathetic axons. J. Cell Sci. 2003;116:4467–78. doi: 10.1242/jcs.00745. [DOI] [PubMed] [Google Scholar]
- Lee W, Jones AM, Ono JK, Wayne NL. Regional differences in processing of locally translated prohormone in peptidergic neurons of Aplysia californica. J. Neurochem. 2002;83:1423–30. doi: 10.1046/j.1471-4159.2002.01252.x. [DOI] [PubMed] [Google Scholar]
- Litman P, Barg J, Rindzoonski L, Ginzburg I. Subcellular localization of tau mRNA in differentiating neuronal cell culture: implications for neuronal polarity. Neuron. 1993;10:627–38. doi: 10.1016/0896-6273(93)90165-n. [DOI] [PubMed] [Google Scholar]
- Martin KC, Casadio A, Zhu H, Yaping E, Rose JC, et al. Synapse-specific, long-term facilitation of aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell. 1997;91:927–38. doi: 10.1016/s0092-8674(00)80484-5. [DOI] [PubMed] [Google Scholar]
- Martin R, Fritz W, Giuditta A. Visualization of polyribosomes in the postsynaptic area of the squid giant synapse by electron spectroscopic imaging. J. Neurocytol. 1989;18:11–18. doi: 10.1007/BF01188419. [DOI] [PubMed] [Google Scholar]
- Martin R, Vaida B, Bleher R, Crispino M, Giuditta A. Protein synthesizing units in presynaptic and postsynaptic domains of squid neurons. J. Cell Sci. 1998;111:3157–66. doi: 10.1242/jcs.111.21.3157. [DOI] [PubMed] [Google Scholar]
- Ming GL, Wong ST, Henley J, Yuan XB, Song HJ, et al. Adaptation in the chemotactic guidance of nerve growth cones. Nature. 2002;417:411–18. doi: 10.1038/nature745. [DOI] [PubMed] [Google Scholar]
- Moccia R, Chen D, Lyles V, Kapuya E, E Y, et al. An unbiased cDNA library prepared from isolated Aplysia sensory neuron processes is enriched for cytoskeletal and translational mRNAs. J. Neurosci. 2003;23:9409–17. doi: 10.1523/JNEUROSCI.23-28-09409.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr E, Fehr S, Richter D. Axonal transport of neuropeptide encoding mRNAs within the hypothalamo-hypophyseal tract of rats. EMBO J. 1991;10:2419–24. doi: 10.1002/j.1460-2075.1991.tb07781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr E, Richter D. Diversity of mRNAs in the axonal compartment of peptidergic neurons in the rat. Eur. J. Neurosci. 1992;4:870–76. doi: 10.1111/j.1460-9568.1992.tb00197.x. [DOI] [PubMed] [Google Scholar]
- Mohr E, Richter D. Axonal mRNAs: functional significance in vertebrates and invertebrates. J. Neurocytol. 2000;29:783–91. doi: 10.1023/a:1010987206526. [DOI] [PubMed] [Google Scholar]
- Morgan IG, Austin L. Synaptosomal protein synthesis in a cell-free system. J. Neurochem. 1968;15:41–51. doi: 10.1111/j.1471-4159.1968.tb06172.x. [DOI] [PubMed] [Google Scholar]
- Nixon RA. Protein degradation in the mouse visual system. I. Degradation of axonally transported and retinal proteins. Brain Res. 1980;200:69–83. doi: 10.1016/0006-8993(80)91095-1. [DOI] [PubMed] [Google Scholar]
- Olink-Coux M, Hollenbeck PJ. Localization and active transport of mRNA in axons of sympathetic neurons in culture. J. Neurosci. 1996;16:1346–58. doi: 10.1523/JNEUROSCI.16-04-01346.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannese E, Ledda M. Ribosomes in myelinated axons of the rabbit spinal ganglion neurons. J. Submicrosc. Cytol. Pathol. 1991;23:33–38. [PubMed] [Google Scholar]
- Posse de Chaves E, Vance DE, Campenot RB, Vance JE. Axonal synthesis of phosphatidylcholine is required for normal axonal growth in rat sympathetic neurons. J. Cell Biol. 1995;128:913–18. doi: 10.1083/jcb.128.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racca C, Gardiol A, Triller A. Dendritic and postsynaptic localizations of glycine receptor alpha subunit mRNAs. J. Neurosci. 1997;17:1691–700. doi: 10.1523/JNEUROSCI.17-05-01691.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell. 1994;79:1245–55. doi: 10.1016/0092-8674(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Schacher S, Wu F. Synapse formation in the absence of cell bodies requires protein synthesis. J. Neurosci. 2002;22:1831–39. doi: 10.1523/JNEUROSCI.22-05-01831.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacher S, Wu F, Panyko JD, Sun ZY, Wang D. Expression and branch-specific export of mRNA are regulated by synapse formation and interaction with specific postsynaptic targets. J. Neurosci. 1999;19:6338–47. doi: 10.1523/JNEUROSCI.19-15-06338.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherff CM, Carew TJ. Coincident induction of long-term facilitation in Aplysia: cooperativity between cell bodies and remote synapses. Science. 1999;285:1911–14. doi: 10.1126/science.285.5435.1911. [DOI] [PubMed] [Google Scholar]
- Sherff CM, Carew TJ. Coincident induction of long-term facilitation at sensory-motor synapses in Aplysia: presynaptic and postsynaptic factors. Neurobiol. Learn. Mem. 2002;78:498–507. doi: 10.1006/nlme.2002.4092. [DOI] [PubMed] [Google Scholar]
- Shewan D, Dwivedy A, Anderson R, Holt CE. Age-related changes underlie switch in netrin-1 responsiveness as growth cones advance along visual pathway. Nat. Neurosci. 2002;5:955–62. doi: 10.1038/nn919. [DOI] [PubMed] [Google Scholar]
- Shirasaki R, Katsumata R, Murakami F. Change in chemoattractant responsiveness of developing axons at an intermediate target. Science. 1998;279:105–7. doi: 10.1126/science.279.5347.105. [DOI] [PubMed] [Google Scholar]
- Si K, Giustetto M, Etkin A, Hsu R, Janisiewicz AM, et al. A neuronal isoform of CPEB regulates local protein synthesis and stabilizes synapse-specific long-term facilitation in aplysia. Cell. 2003;115:893–904. doi: 10.1016/s0092-8674(03)01021-3. [DOI] [PubMed] [Google Scholar]
- Sotelo-Silveira JR, Calliari A, Kun A, Benech JC, Sanguinetti C, et al. Neurofilament mRNAs are present and translated in the normal and severed sciatic nerve. J. Neurosci. Res. 2000;62:65–74. doi: 10.1002/1097-4547(20001001)62:1<65::AID-JNR7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Spencer GE, Syed NI, van Kesteren E, Lukowiak K, Geraerts WP, van Minnen J. Synthesis and functional integration of a neurotransmitter receptor in isolated invertebrate axons. J. Neurobiol. 2000;44:72–81. doi: 10.1002/1097-4695(200007)44:1<72::aid-neu7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Steward O, Fass B. Polyribosomes associated with dendritic spines in the denervated dentate gyrus: evidence for local regulation of protein synthesis during reinnervation. Prog. Brain Res. 1983;58:131–36. doi: 10.1016/S0079-6123(08)60013-8. [DOI] [PubMed] [Google Scholar]
- Steward O, Levy WB. Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. J. Neurosci. 1982;2:284–91. doi: 10.1523/JNEUROSCI.02-03-00284.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Ribak CE. Polyribosomes associated with synaptic specializations on axon initial segments: localization of protein-synthetic machinery at inhibitory synapses. J. Neurosci. 1986;6:3079–85. doi: 10.1523/JNEUROSCI.06-10-03079.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Schuman EM. Compartmentalized synthesis and degradation of proteins in neurons. Neuron. 2003;40:347–59. doi: 10.1016/s0896-6273(03)00635-4. [DOI] [PubMed] [Google Scholar]
- Tennyson VM. The fine structure of the axon and growth cone of the dorsal root neuroblast of the rabbit embryo. J. Cell Biol. 1970;44:62–79. doi: 10.1083/jcb.44.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–33. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Tohda C, Sasaki M, Konemura T, Sasamura T, Itoh M, Kuraishi Y. Axonal transport of VR1 capsaicin receptor mRNA in primary afferents and its participation in inflammation-induced increase in capsaicin sensitivity. J. Neurochem. 2001;76:1628–35. doi: 10.1046/j.1471-4159.2001.00193.x. [DOI] [PubMed] [Google Scholar]
- Torre ER, Steward O. Protein synthesis within dendrites: glycosylation of newly synthesized proteins in dendrites of hippocampal neurons in culture. J. Neurosci. 1996;16:5967–78. doi: 10.1523/JNEUROSCI.16-19-05967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trembleau A, Morales M, Bloom FE. Differential compartmentalization of vasopressin messenger RNA and neuropeptide within the rat hypothalamo-neurohypophysial axonal tracts: light and electron microscopic evidence. Neuroscience. 1996;70:113–25. doi: 10.1016/0306-4522(95)00328-g. [DOI] [PubMed] [Google Scholar]
- Twiss JL, Smith DS, Chang B, Shooter EM. Translational control of ribosomal protein L4 mRNA is required for rapid neurite regeneration. Neurobiol. Dis. 2000;7:416–28. doi: 10.1006/nbdi.2000.0293. [DOI] [PubMed] [Google Scholar]
- van Horck F, Weinl C, Holt C. Retinal axon guidance: novel mechanisms for steering. Curr. Opin. Neurobiol. 2004;14:61–66. doi: 10.1016/j.conb.2004.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Minnen J. Axonal localization of neuropeptide-encoding mRNA in identified neurons of the snail Lymnaea stagnalis. Cell. Tissue Res. 1994;276:155–61. doi: 10.1007/BF00354795. [DOI] [PubMed] [Google Scholar]
- van Minnen J, Bergman JJ, Van Kesteren ER, Smit AB, Geraerts WP, et al. De novo protein synthesis in isolated axons of identified neurons. Neuroscience. 1997;80:1–7. doi: 10.1016/s0306-4522(97)00137-1. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. The N-end rule: functions, mysteries, uses. Proc. Natl. Acad. Sci. USA. 1996;93:12142–49. doi: 10.1073/pnas.93.22.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Chao SK, Sitcheran R, Nunez JM, Vosshall LB, Axel R. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79:981–91. doi: 10.1016/0092-8674(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Wanner I, Baader SL, Brich M, Oberdick J, Schilling K. Subcellular localization of specific mRNAs and their protein products in Purkinje cells by combined fluorescence in situ hybridization and immunocytochemistry. Histochem. Cell Biol. 1997;108:345–57. doi: 10.1007/s004180050175. [DOI] [PubMed] [Google Scholar]
- Weiner OD, Zorn AM, Krieg PA, Bittner GD. Medium weight neurofilament mRNA in goldfish Mauthner axoplasm. Neurosci. Lett. 1996;213:83–86. doi: 10.1016/0304-3940(96)12860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensley CH, Stone DM, Baker H, Kauer JS, Margolis FL, Chikaraishi DM. Olfactory marker protein mRNA is found in axons of olfactory receptor neurons. J. Neurosci. 1995;15:4827–37. doi: 10.1523/JNEUROSCI.15-07-04827.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KM, Spooner BS, Wessells NK. Ultrastructure and function of growth cones and axons of cultured nerve cells. J. Cell Biol. 1971;49:614–35. doi: 10.1083/jcb.49.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelena J. Ribosome-like particles in myelinated axons of the rat. Brain Res. 1970;24:359–63. doi: 10.1016/0006-8993(70)90120-4. [DOI] [PubMed] [Google Scholar]
- Zhang HL, Eom T, Oleynikov Y, Shenoy SM, Liebelt DA, et al. Neurotrophin-induced transport of a beta-actin mRNP complex increases beta-actin levels and stimulates growth cone motility. Neuron. 2001;31:261–75. doi: 10.1016/s0896-6273(01)00357-9. [DOI] [PubMed] [Google Scholar]
- Zhang HL, Singer RH, Bassell GJ. Neurotrophin regulation of beta-actin mRNA and protein localization within growth cones. J. Cell Biol. 1999;147:59–70. doi: 10.1083/jcb.147.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JQ, Kelly TK, Chang B, Ryazantsev S, Rajasekaran AK, et al. A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J. Neurosci. 2001;21:9291–303. doi: 10.1523/JNEUROSCI.21-23-09291.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Stoeckli E, Chen H, Tessier-Lavigne M. Squeezing axons out of the gray matter: a role for slit and semaphorin proteins from midline and ventral spinal cord. Cell. 2000;102:363–75. doi: 10.1016/s0092-8674(00)00041-6. [DOI] [PubMed] [Google Scholar]