Abstract
BACKGROUND
Castration resistance occurs in most patients with metastatic hormone-sensitive prostate cancer who are receiving androgen-deprivation therapy. Replacing androgens before progression of the disease is hypothesized to prolong androgen dependence.
METHODS
Men with newly diagnosed, metastatic, hormone-sensitive prostate cancer, a performance status of 0 to 2, and a prostate-specific antigen (PSA) level of 5 ng per milliliter or higher received a luteinizing hormone–releasing hormone analogue and an antiandrogen agent for 7 months. We then randomly assigned patients in whom the PSA level fell to 4 ng per milliliter or lower to continuous or intermittent androgen deprivation, with patients stratified according to prior or no prior hormonal therapy, performance status, and extent of disease (minimal or extensive). The coprimary objectives were to assess whether intermittent therapy was noninferior to continuous therapy with respect to survival, with a one-sided test with an upper boundary of the hazard ratio of 1.20, and whether quality of life differed between the groups 3 months after randomization.
RESULTS
A total of 3040 patients were enrolled, of whom 1535 were included in the analysis: 765 randomly assigned to continuous androgen deprivation and 770 assigned to intermittent androgen deprivation. The median follow-up period was 9.8 years. Median survival was 5.8 years in the continuous-therapy group and 5.1 years in the intermittent-therapy group (hazard ratio for death with intermittent therapy, 1.10; 90% confidence interval, 0.99 to 1.23). Intermittent therapy was associated with better erectile function and mental health (P<0.001 and P = 0.003, respectively) at month 3 but not thereafter. There were no significant differences between the groups in the number of treatment-related high-grade adverse events.
CONCLUSIONS
Our findings were statistically inconclusive. In patients with metastatic hormone-sensitive prostate cancer, the confidence interval for survival exceeded the upper boundary for noninferiority, suggesting that we cannot rule out a 20% greater risk of death with intermittent therapy than with continuous therapy, but too few events occurred to rule out significant inferiority of intermittent therapy. Intermittent therapy resulted in small improvements in quality of life. (Funded by the National Cancer Institute and others; ClinicalTrials.gov number, NCT00002651.)
Prostate cancer is an androgen-dependent disease, and continuous androgen deprivation has been the standard therapy for metastatic hormone-sensitive disease. Despite a high response rate, resistance to androgen-deprivation therapy occurs in most patients, resulting in a median survival of 2.5 to 3 years.1,2
There is evidence suggesting that progression to castration resistance is adaptive in part, and pathways involving the androgen receptor, as well as cell-survival pathways independent of the androgen receptor, have been implicated.3,4 Data from an androgen-dependent tumor model have suggested that androgen withdrawal alters the ratio of putative stem cells in the tumor-cell population.5 Initially, differentiated cells are eliminated, and the proportion of tumorigenic stem cells is reduced. When the disease progresses, the proportion of stem cells is increased by a factor of 20, and the proportion of androgen-independent stem cells by a factor of 500. These data suggest that if androgens were replaced before progression of the disease, the surviving stem cells might give rise to an androgen-dependent tumor that would be susceptible to further hormonal manipulation. In an “androgen-sensitive” in vivo rat model, intermittent androgen deprivation was inferior to castration in preventing tumor growth.6 However, in an “androgen-dependent” model, intermittent androgen deprivation resulted in reinduction of apoptosis, almost tripling the mean time to castration resistance.7 Early clinical trials indicated the feasibility of intermittent androgen deprivation.8–10 The potential for improving disease control and quality of life with intermittent androgen deprivation provided the rationale for this study.
METHODS
STUDY OVERSIGHT
The primary objectives of the trial were to determine whether intermittent androgen deprivation is noninferior to continuous androgen deprivation with respect to survival in patients with metastatic hormone-sensitive prostate cancer and to assess quality of life with the two regimens at 3 months after randomization. The study was designed in 1993 by the first author and by the leaders of the genitourinary cancer and quality-of-life committees of the Southwest Oncology Group (SWOG). Approval by the institutional review board at each participating institution was required on an annual basis during the course of the trial. Investigators at the SWOG Statistical Center collected the data. The second author vouches for the integrity of the data and statistical analysis. The first three authors attest that the study was conducted and monitored as specified by the protocol. The first author wrote the first draft of the manuscript, with subsequent contributions by all coauthors. A copy of the protocol with the statistical analysis plan is available with the full text of this article at NEJM.org. AstraZeneca donated the goserelin and bicalutamide for the U.S. cooperative groups and the European Organization for Research and Treatment of Cancer (EORTC) but had no role in the design of the protocol, the collection or analysis of the data, or the preparation of the manuscript.
PATIENTS
Patients were enrolled by the SWOG, the Eastern Cooperative Oncology Group (ECOG), the Cancer and Leukemia Group B (CALGB), the National Cancer Institute of Canada–Clinical Trials Group (NCIC-CTG), and the EORTC. Patients were eligible for participation in the study if they had a pathological diagnosis of prostate cancer; radiologic evidence of metastasis; a performance status of 0, 1, or 2 (with 0 indicating that the patient is fully active and able to carry on all predisease activities without restriction, 1 indicating that the patient is ambulatory but restricted to light work, and 2 indicating that the patient is ambulatory and capable of all self-care and is up and about more than 50% of waking hours but is unable to carry out any work activities)11; and a prostate-specific antigen (PSA) level before treatment of 5 ng per milliliter or higher. Patients who had received prior neoadjuvant or adjuvant androgen-deprivation therapy were eligible if they had received the therapy for 4 months or less. Patients who had received prior finasteride therapy for prostate cancer were eligible if they had received the drug for 9 months or less. Up to 6 months of prior finasteride therapy for benign prostatic hyperplasia was allowed. Neoadjuvant or adjuvant androgen-deprivation therapy or finasteride must have been discontinued more than a year before enrollment in the trial.
Patients who began receiving luteinizing hormone–releasing hormone agonist (LHRHa) therapy with or without an antiandrogen agent a maximum of 6 months before enrollment and who met all the eligibility criteria before the therapy was initiated were eligible if the duration of LHRHa treatment did not exceed 7 months and the PSA value after 6 months of androgen-deprivation therapy was available. Concomitant radiation therapy was allowed for severe pain. North American patients who could read and understand English, Spanish, or French were required to complete quality-of-life assessments. All patients provided written informed consent in accordance with institutional and federal guidelines.
TREATMENT PLAN, STRATIFICATION, AND RANDOMIZATION
Patients underwent a 7-month induction course during which they received an LHRHa and an antiandrogen agent: goserelin and bicalutamide in the case of patients enrolled by the U.S. cooperative groups and the EORTC or similar agents at equivalent doses in patients from the NCIC-CTG or in those who started therapy before enrollment.
The PSA level was measured at months 1, 4, 6, and 7 of the induction period. Patients with stable or declining PSA levels of 4.0 ng per milliliter or lower at months 6 and 7 were eligible for randomization; this criterion was chosen to select patients with androgen-dependent disease.
Before randomization, patients were stratified according to performance status (0 or 1 vs. 2), prior hormone therapy (neoadjuvant or adjuvant androgen-deprivation therapy or finasteride therapy vs. no therapy), and extent of the disease (minimal vs. extensive). As previously defined in the SWOG trials,1,2 minimal disease was disease confined to the spine, pelvic bones, or lymph nodes, and extensive disease was disease present in the ribs, long bones, or visceral organs. Patients in whom the PSA level was higher than 4 ng per milliliter after induction therapy were not eligible for randomization but were followed for survival.
Patients who were randomly assigned to the continuous-therapy group continued to receive androgen-deprivation therapy; those randomly assigned to the intermittent-therapy group discontinued androgen-deprivation therapy. Patients in both groups underwent a clinical assessment every 3 months, and PSA levels were measured monthly. Androgen-deprivation therapy was resumed in the intermittent group when the PSA level rose to 20 ng per milliliter (or returned to baseline in the case of patients who had PSA levels of <20 ng per milliliter before enrollment). At the discretion of the investigator, treatment could be reinitiated when the PSA level reached 10 ng per milliliter or when symptoms developed. If, after another 7 months of androgen-deprivation therapy, the PSA level was 4.0 ng per milliliter or lower, another off-treatment period was initiated. If the PSA level at month 6 or 7 of an on-treatment period exceeded 4 ng per milliliter, patients continued androgen-deprivation therapy. During any off-treatment period, a rising PSA level on or before the third month required reinitiation of continuous androgen-deprivation therapy, which was continued until disease progression.
A treatment delay of more than 2 months required withdrawal of the patient from protocol treatment. All patients were treated according to the study regimen until objective disease progression and were followed for a maximum of 10 years.
QUALITY-OF-LIFE ASSESSMENTS
The quality-of-life questionnaire that was developed for SWOG trials12–15 was administered at the time of randomization and at months 3, 9, and 15 after randomization. It included five pre-specified outcomes: impotence or erectile dysfunction, libido, vitality, mental health, and physical functioning. The impotence or erectile-dysfunction outcome was assessed as the percentage of patients who reported that they had impotence or erectile dysfunction (a score of 1) or no impotence or erectile dysfunction (a score of 0); the libido outcome was assessed as the percentage of patients who reported that their interest in sexual activities was very high, high, or moderate (a score of 1) or low or very low (a score of 0). The questionnaire instructions directed patients to answer the question regarding libido only if they had had sexual activity within the previous month. However, because many men ignored this instruction while completing the questionnaire, we used all available responses for this analysis. Vitality, mental health, and physical functioning were scored on a scale of 0 to 100, with higher scores indicating better functioning.16
STATISTICAL ANALYSIS
On the basis of data from a previous SWOG trial, it was assumed that the median survival in the group receiving continuous androgen-deprivation therapy would be 35 months.1 A median survival in the intermittent-therapy group that was at least 7 months shorter than that in the continuous-therapy group was considered to be clinically unacceptable; hence the study was designed for a one-sided test of the null hypothesis that the hazard ratio for death with intermittent therapy would be 1.20. A hazard ratio of 1.00 was used as the specific alternative in calculations of the trial size. Type I and II error rates were 0.05 and 0.10, respectively. Survival was measured from the date of randomization to the date of death from any cause or to the last known contact date, at which time the data for that patient were censored.
We used a dynamic balancing algorithm that included stratification information to assign patients to continuous or intermittent androgen-deprivation therapy.17 A total of 1512 eligible patients were to undergo randomization. The enrollment period was projected to be 6.3 years. The final analysis was to be conducted 2 years after randomization was completed. A proportional-hazards regression model with stratification factors as covariates was specified in an intention-to-treat analysis. The primary test of noninferiority is reported as one-sided, and all other reported P values are two-sided tests. We evaluated the proportional-hazards assumption by checking the cumulative sums of Martingale-based residuals.18 Treatment interactions according to subgroups were evaluated with the use of the score chi-square in the proportional-hazards model, which tests the potential contribution of variables not included in the model. SAS software, version 9.2, was used for all analyses.
Our decision to include quality of life as a coprimary end point was based on the importance of improvements in quality of life even if they occur in the context of similar efficacy. The primary objective was to compare the two groups with respect to the effects of androgen-deprivation therapy on impotence, libido, and vitality and on physical functioning and mental health from randomization to 3 months. A P value of 0.01 was used for testing each of the five primary quality-of-life outcomes to control the overall type I error rate at 0.05. Between-group comparisons at months 9 and 15 were exploratory and used a P value of 0.005 (0.05/10 for five quality-of-life outcomes examined at two additional time points). We performed a pattern-mixture model analysis to evaluate the effect of missing data.19
RESULTS
PATIENTS
From May 1995 through September 2008, a total of 3040 patients were enrolled, of whom 90 were subsequently shown to be ineligible. Of the patients who completed induction therapy, 1749 were randomly assigned to a group, and 1535 were included in the primary analysis (Fig. S1 in the Supplementary Appendix, available at NEJM.org). Randomization was completed in June 2009.
The characteristics of the patients who underwent randomization were well balanced between the treatment groups (Table 1). The median duration of protocol therapy after randomization was 19 months (interquartile range, 10 to 38) in the intermittent-therapy group and 17 months (interquartile range, 8 to 33) in the continuous-therapy group. Among patients receiving intermittent therapy, the median percentage of time receiving therapy was 47% (interquartile range, 23 to 69). There were no significant between-group differences in the number of grade 3 or grade 4 treatment-related adverse events (30.4% in the intermittent-therapy group and 32.7% in the continuous-therapy group, P=0.53), including cardiovascular events, and no treatment-related grade 5 events were reported (Table S1 in the Supplementary Appendix).
Table 1.
Characteristics of Eligible Patients Who Underwent Randomization.*
| Characteristic | Intermittent Therapy (N = 770) | Continuous Therapy (N = 765) |
|---|---|---|
| Age (yr) | ||
| Median | 70 | 70 |
| Interquartile range | 39–97 | 39–92 |
| PSA level at beginning of induction period (ng/ml) | ||
| Median | 41 | 43 |
| Interquartile range | 15–132 | 15–142 |
| Race (%)† | ||
| White | 67 | 67 |
| Black | 12 | 12 |
| Other | 2 | 1 |
| Not reported | 19 | 20 |
| Cooperative Group (%) | ||
| Eastern Cooperative Oncology Group | 19 | 22 |
| Cancer and Leukemia Group B | 15 | 13 |
| National Cancer Institute of Canada Clinical Trials Group | 8 | 6 |
| European Organization for Research and Treatment of Cancer | 17 | 17 |
| Southwest Oncology Group | 40 | 39 |
| PSA level at randomization (%) | ||
| ≤0.2 ng/ml | 35.4 | 34.9 |
| >0.2–4.0 ng/ml | 64.6 | 65.1 |
| Performance status of 0 or 1 (%)‡ | 96 | 96 |
| Extent of disease (%)§ | ||
| Extensive | 49 | 47 |
| Minimal | 51 | 53 |
| Any visceral disease (%) | 7.1 | 6.3 |
| Receipt of continuous therapy before enrollment (%)¶ | 32 | 26 |
| Prior hormone therapy (%) | ||
| None | 87 | 88 |
| Neoadjuvant | 12 | 11 |
| Finasteride | 1 | 1 |
| Prior radiation therapy (%) | 30 | 28 |
| Prior radical prostatectomy (%) | 19 | 22 |
| Bone pain present at beginning of induction period (%) | 28 | 26 |
| Gleason score (%)|| | ||
| ≤6 | 16 | 17 |
| 7 | 34 | 33 |
| 8–10 | 19 | 18 |
| Missing data | 31 | 32 |
There were no significant differences between the groups in any of the characteristics listed here. PSA denotes prostate-specific antigen.
Race was self-reported.
A performance status of 0 indicates that the patient is fully active and able to carry on all predisease activities without restriction, and a performance status of 1 indicates that the patient is ambulatory but restricted to light work.
Extensive disease was considered to be disease present in the ribs, long bones, or visceral organs, and minimal disease as disease confined to the spine, pelvic bones, or lymph nodes (definitions used in the SWOG trials).
All patients who were enrolled in the study received the dose-equivalent of 7 months of continuous androgen-deprivation therapy before randomization. Patients included in this category were already receiving this therapy before enrollment in the study.
The Gleason score is used to estimate the prognosis of patients with prostate cancer. The score ranges from 2 to 10, with higher scores indicating a worse prognosis.
SURVIVAL
The median survival of all enrolled patients after initiation of androgen-deprivation therapy was 3.7 years (interquartile range, 1.8 to 7.9). The median survival of patients who did not undergo randomization was 1.7 years (interquartile range, 1.0 to 3.2).
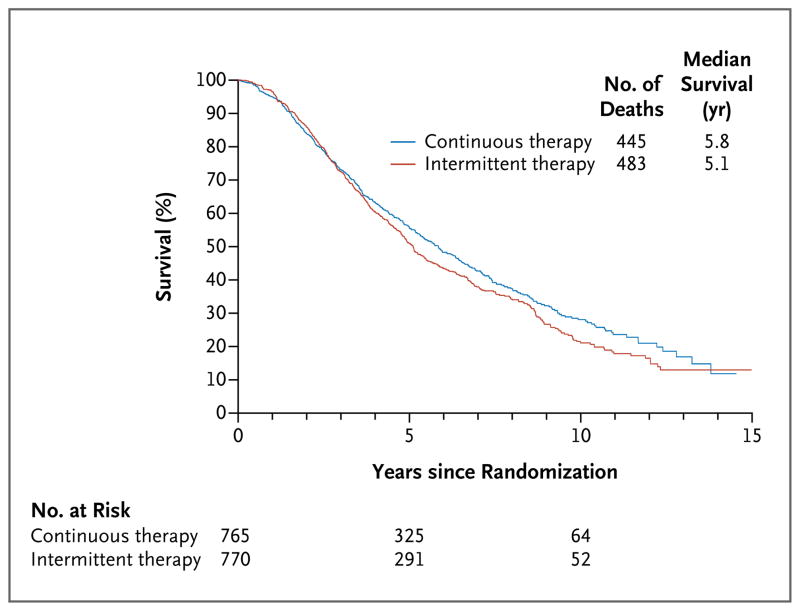
Of 928 deaths that occurred among patients who were randomly assigned to a study group, 445 occurred in the continuous-therapy group and 483 in the intermittent-therapy group. The median survival after randomization was 5.8 years in the continuous-therapy group as compared with 5.1 years in the intermittent-therapy group (5.7 years vs. 6.4 years after initial enrollment) (Fig. 1), representing a 10% relative increase in the risk of death with intermittent therapy (hazard ratio for death with intermittent therapy, adjusted for stratification factors, 1.10; 90% confidence interval [CI], 0.99 to 1.23). The hypothesis that the hazard ratio for death would be less than 1.20 was not rejected because the upper limit of the 90% confidence interval (equivalent to a one-sided 0.05 test) was 1.23, extending beyond the noninferiority threshold of 1.20. We therefore are unable to conclude that intermittent therapy was noninferior to continuous therapy with respect to survival. However, because the lower limit of the confidence interval (0.99) does not exclude 1.00, we cannot state that intermittent therapy was significantly inferior to continuous therapy. The results of the secondary analysis, a two-sided test of the noninferiority hypothesis (similar to assessment of the 95% confidence interval) led to a similar conclusion (95% CI, 0.97 to 1.25). The proportional-hazards assumption was not violated for this model (P = 0.25).
Figure 1.
Median Survival from Randomization in the Two Treatment Groups.
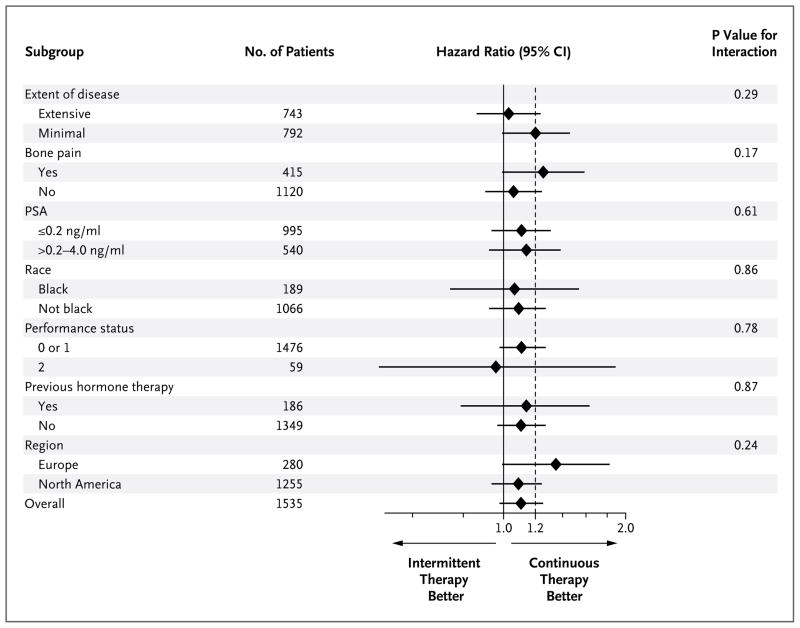
A post hoc analysis showed that the overall treatment effect was generally consistent across the subgroups of patients. The hazard-ratio estimate slightly favored continuous therapy and the 95% confidence interval included 1.20, the non-inferiority margin (Fig. 2). The P values for the interaction of treatment with various subgroup characteristics are shown in Figure 2. The median survival according to subgroups is provided in Table S2 in the Supplementary Appendix.
Figure 2. Survival According to Subgroups.
Minimal disease was considered to be disease confined to the spine, pelvic bones, or lymph nodes, and extensive disease as disease present in the ribs, long bones, or visceral organs (the definitions used in the trials of the Southwest Oncology Group). A performance status of 0 indicates that the patient is fully active and able to carry on all predisease activities without restriction; 1, that the patient is ambulatory but restricted to light work; and 2, that the patient is ambulatory and capable of all self-care and is up and about more than 50% of waking hours but is unable to carry out any work activities. Race was self-reported. PSA denotes prostate-specific antigen.
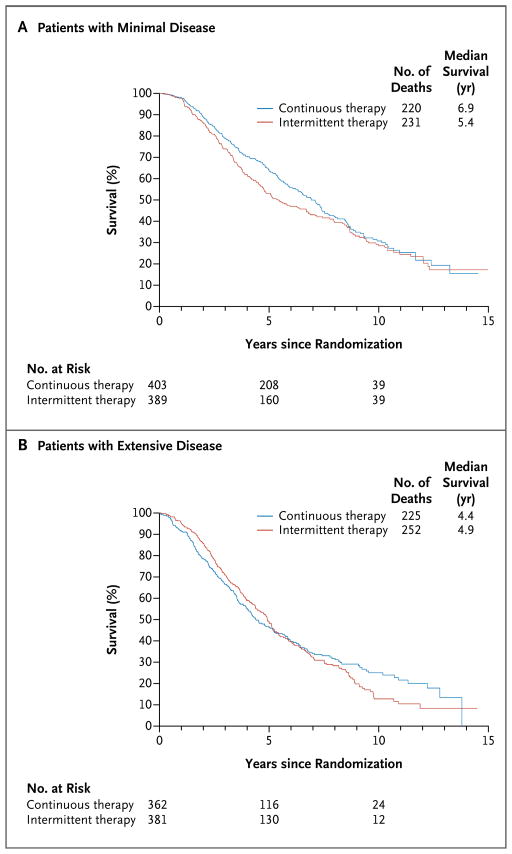
Figure 3 shows the overall survival in the two treatment groups according to the extent of disease. The median survival after randomization among patients with extensive disease was 4.9 years in the intermittent-therapy group, as compared with 4.4 years in the continuous-therapy group (hazard ratio for death with intermittent therapy, 1.02; 95% CI, 0.85 to 1.22). The median survival after randomization among patients with minimal disease was 5.4 years in the intermittent-therapy group, as compared with 6.9 years in the continuous-therapy group (hazard ratio for death with intermittent therapy, 1.19; 95% CI, 0.98 to 1.43).
Figure 3.
Median Survival from Randomization in the Two Study Groups, According to the Extent of Disease.
There was no significant difference in survival between patients who were alive before 2004 (i.e., before the approval of docetaxel) and those who were alive in 2004 or later (after approval of docetaxel in May 2004) (P = 0.54). Among men for whom we could identify the cause of death, 73% of deaths in the continuous-therapy group and 80% of deaths in the intermittent-therapy group were related to prostate cancer.
QUALITY OF LIFE
At the time of randomization, data on quality of life were available from 1162 patients: 568 in the continuous-therapy group and 594 in the intermittent-therapy group (Fig. S2 in the Supplementary Appendix). For the primary comparison 3 months after randomization, the scores for the change from baseline in quality of life indicated that as compared with patients in the continuous-therapy group, those in the intermittent-therapy group were significantly less likely to report impotence (P<0.001) and had significantly better mental health (P = 0.003); the scores for libido also favored intermittent therapy (P = 0.04) but not at the prespecified significance level (Table 2). At 9 months after randomization, the scores for four of the five quality-of-life outcomes favored intermittent therapy over continuous therapy, but at 15 months, only physical functioning scored higher in the intermittent-therapy group; none of these differences were significant at the prespecified level. The pattern-mixture models generated estimates similar to those reported in Table 2, suggesting that our results were not biased by missing data (data not shown). However, confidence intervals in both the analysis of change from baseline in Table 2 and the pattern-mixture models were wide. This variability could possibly reflect the difference in quality of life between men in the intermittent-therapy group who remained off androgen-deprivation therapy and those who resumed therapy. At 15 months, 78% of the men in the intermittent-therapy group had resumed hormone therapy (data not shown).
Table 2.
Difference in the Mean Change from Randomization to Follow-up in Primary Quality-of-Life Outcomes, According to Treatment Group.
| Outcome | Intermittent Therapy | Continuous Therapy | Difference, Intermittent-Continuous (95% CI) | P Value |
|---|---|---|---|---|
|
| ||||
| Erectile dysfunction* | ||||
|
| ||||
| Patients with erectile dysfunction at randomization (%) | 82 | 85 | ||
|
| ||||
| 3-mo analysis | ||||
| No. of patients included | 466 | 450 | ||
| Change from randomization | −7% | 2% | −10 percentage points (−14 to −5) | <0.001 |
|
| ||||
| 9-mo analysis | ||||
| No. of patients included | 438 | 393 | ||
| Change from randomization | −8% | 2% | −10 percentage points (−15 to −5) | <0.001 |
|
| ||||
| 15-mo analysis | ||||
| No. of patients included | 385 | 363 | ||
| Change from randomization | −3% | 2% | −4 percentage points (−10 to 1) | 0.12 |
|
| ||||
| High libido† | ||||
|
| ||||
| Patients with high libido at randomization (%) | 29 | 26 | ||
|
| ||||
| 3-mo analysis | ||||
| No. of patients included | 68 | 45 | ||
| Change from randomization | 16% | −2% | 18 percentage points (1 to 36) | 0.04 |
|
| ||||
| 9-mo analysis | ||||
| No. of patients included | 66 | 35 | ||
| Change from randomization | 20% | −11% | 31 percentage points (9 to 53) | 0.01 |
|
| ||||
| 15-mo analysis | ||||
| No. of patients included | 46 | 31 | ||
| Change from randomization | 13% | 3% | 10 percentage points (−16 to 36) | 0.46 |
|
| ||||
| Vitality‡ | ||||
|
| ||||
| Score at randomization | 59.7 | 59.8 | ||
|
| ||||
| 3-mo analysis | ||||
| No. of patients included | 465 | 446 | ||
| Change from randomization | −0.11 | −1.42 | 1.32 (−0.83 to 3.46) | 0.23 |
|
| ||||
| 9-mo analysis | ||||
| No. of patients included | 439 | 392 | ||
| Change from randomization | −0.36 | −3.07 | 2.71 (0.26 to 5.16) | 0.03 |
|
| ||||
| 15-mo analysis | ||||
| No. of patients included | 386 | 372 | ||
| Change from randomization | −2.02 | −3.02 | 1.00 (−1.59 to 3.59) | 0.45 |
| Mental health‡ | ||||
|
| ||||
| Score at randomization | 77.9 | 80.0 | ||
|
| ||||
| 3-mo analysis | ||||
| No. of patients included | 479 | 471 | ||
| Change from randomization | 1.92 | −0.95 | 2.88 (1.00 to 4.76) | 0.003 |
|
| ||||
| 9-mo analysis | ||||
| No. of patients included | 458 | 414 | ||
| Change from randomization | 0.08 | −1.94 | 2.01 (−0.17 to 4.19) | 0.07 |
|
| ||||
| 15-mo analysis | ||||
| No. of patients included | 402 | 386 | ||
| Change from randomization | −0.64 | −1.10 | 0.47 (−1.80 to 2.74) | 0.69 |
|
| ||||
| Physical functioning‡ | ||||
|
| ||||
| Score at randomization | 70.7 | 70.2 | ||
|
| ||||
| 3-mo analysis | ||||
| No. of patients included | 475 | 469 | ||
| Change from randomization | 0.09 | −1.74 | 1.83 (−0.31 to 3.97) | 0.09 |
|
| ||||
| 9-mo analysis | ||||
| No. of patients included | 456 | 415 | ||
| Change from randomization | −0.66 | −3.67 | 3.01 (0.50 to 5.53) | 0.02 |
|
| ||||
| 15-mo analysis | ||||
| No. of patients included | 397 | 385 | ||
| Change from randomization | −2.68 | −5.72 | 3.04 (0.13 to 5.96) | 0.04 |
Erectile dysfunction was assessed by having patients report whether they had erectile dysfunction (a score of 1) or no erectile dysfunction (a score of 0).
Libido was assessed by having patients report whether their interest in sexual activities was very high, high, or moderate (a score of 1) or low or very low (a score of 0).
This outcome was scored on a scale of 0 to 100, with higher scores indicating better functioning.
DISCUSSION
Progression to castration resistance is the major cause of death in patients with metastatic hormone-sensitive prostate cancer who are receiving androgen-deprivation therapy. Biologic, preclinical, and early clinical data fueled interest in evaluating intermittent androgen-deprivation therapy with the objective of improving disease outcomes and quality of life.
We tested the hypothesis that intermittent therapy would not be inferior to continuous therapy with respect to survival among patients with metastatic hormone-sensitive prostate cancer and that quality of life would be better with intermittent therapy. Our results failed to show that intermittent therapy was noninferior to continuous therapy with respect to survival: the median survival after randomization was 5.1 years in the intermittent-therapy group, as compared with 5.8 years in the continuous-therapy group (5.7 years vs. 6.4 years after initial enrollment). Although the overall difference in survival represented a 10% relative increase in the risk of death with intermittent therapy, a 20% increase in the risk of death could not be ruled out with 90% confidence, because the upper boundary of the confidence interval included 1.20. In addition, the lower boundary of the confidence interval (0.99) failed to exclude 1.00. According to Piaggio et al.,20 when a confidence interval includes both the noninferiority margin (1.20 in the case of this study) and 1.00, the trial results are inconclusive. However, given that nearly the entire confidence interval tends to favor continuous therapy, the result suggests that intermittent therapy may compromise survival. The lack of a significant difference between the groups does not imply similar survival.
The numbers of grade 3 or 4 adverse events were remarkably similar in the two groups, and these events were relatively infrequent (30.4% in the intermittent-therapy group and 32.7% in the continuous-therapy group). The results with respect to quality of life indicated significantly better erectile function and mental health with intermittent therapy than with continuous therapy at 3 months after randomization. However, because patients were aware of the treatment they were receiving, we cannot differentiate the emotional response of a patient receiving a treatment break from the effects of androgen recovery itself. Given the results of the pattern-mixture models, we are reasonably certain that our results were not biased by missing data.
Testosterone levels were not measured in this study, since these levels were not factored into decisions regarding therapy and because centralized testing in an international trial was deemed to be too costly. Thus, it cannot be determined whether intervening on the basis of testosterone levels would have changed the results.
Three points are worth highlighting. First, the trial was designed to prove that intermittent therapy was not inferior to continuous therapy with respect to survival. The alternative hypothesis was that survival with intermittent therapy was similar or longer; however, we failed to reject the null hypothesis. It is conceivable that the PSA trigger level for resumption of androgen-deprivation therapy affected the hazard ratio for death. Presumably, the lower the PSA threshold for retreatment, the more similar the two groups should be in terms of survival; however, study-tailored PSA triggers were based on baseline PSA levels, and therapy could be resumed at the discretion of the patient’s physician. Other trials of intermittent androgen-deprivation therapy have used other nonvalidated PSA levels to trigger resumption of therapy.8,21–25 Regardless of the PSA trigger value, we are aware of no trial to date that has shown improved survival with intermittent therapy.
Second, it took 4 to 5 years after randomization for the survival curves to separate. This shows the importance of adequate long-term follow up, and even longer follow-up is probably required for patients with earlier-stage disease. Third, we chose 1.20 as the upper limit of the hazard ratio for noninferiority because, on the basis of a median survival estimate with continuous therapy of 35 months, this hazard ratio would yield a 7-month difference in median survival. At the time the study was designed, a difference in survival of 6 months or less as a worst-case scenario was deemed to be acceptable if it was balanced by better quality of life. However, the actual median survival in both groups was longer than projected. A hazard ratio with an upper limit of 1.20 for the 95% confidence interval and a median survival of 5.8 years in the continuous-therapy group translates into an absolute difference in survival of 1 year. Had we designed this study using contemporary survival rates, a 1-year difference in survival would not be clinically acceptable as a threshold for noninferiority. Furthermore, if we had kept the same design except for choosing a hazard-ratio threshold of 1.15, we would have had to increase the sample by 1000 patients. The overall hazard ratio for treatment effect was relatively consistent across various patient subgroups. However, caution should be taken not to overinterpret the results of the subgroup analyses.
A survival-based noninferiority trial is a formidable undertaking, requiring a large sample, many disease-specific events, and long follow-up. This study was possible because of the intergroup collaboration, which resulted in a large sample with an adequate number of disease-specific events to power the trial. Other phase 3 trials testing intermittent therapy included mixed populations (patients with locally advanced disease and those with metastatic disease) or were not adequately powered to assess survival.21,23–25 By contrast, our study included only patients with metastatic disease who were preselected for androgen-dependent disease, and the study was adequately powered for an assessment of survival.
Another recent trial designed to test noninferiority, commonly known as the NCIC-CTG PR.7 trial, concluded that intermittent therapy was not inferior to continuous therapy with respect to survival (median survival, 8.8 years and 9.1 years, respectively) in patients with nonmetastatic prostate cancer and elevation of PSA levels after radiotherapy,22 a finding that contrasts with the results of our study. A plausible explanation for this apparently contradictory result is that, as compared with our study, that study targeted a population with a lower disease burden and hence a lower risk of cancer-related death. In such a population, a longer median follow-up than the reported 6.9 years may be required to observe deaths from cancer counterbalancing the deaths from other causes that are usually observed earlier in a population of older men. Only 41% of the deaths in the NCIC-CTG PR.7 trial were related to prostate cancer, with a trend toward fewer prostate cancer–related deaths in the continuous-therapy group than in the intermittent-therapy group (94 vs. 120). It is notable that the prespecified noninferiority margin in the NCIC-CTG PR.7 trial (a hazard ratio for death of 1.25) implies that a 1.8-year reduction in median survival with intermittent therapy as compared with continuous therapy would result in a finding of noninferiority for intermittent therapy.
In conclusion, in patients with metastatic hormone-sensitive prostate cancer, a 20% relative increase in the risk of death with intermittent therapy as compared with continuous therapy cannot be ruled out, according to the prespecified threshold for noninferiority (hazard ratio, 1.10; 90% CI, 0.99 to 1.23). Intermittent therapy was associated with improved erectile function and mental health at 3 months but not thereafter. These results may inform decision making about treatment for patients with metastatic hormone-sensitive prostate cancer.
Supplementary Material
Acknowledgments
Supported in part by PHS Cooperative Agreements awarded by the National Cancer Institute (CA32102, CA38926, CA14028, CA55582, CA42777, CA35192, CA46441, CA46282, CA27057, CA128567, CA45807, CA20319, CA58416, CA46113, CA04919, CA76132, CA58861, CA58686, CA68183, CA12644, CA35261, CA35431, CA46368, CA22433, CA63848, CA67575, CA76447, CA67663, CA46136, CA86780, CA35281, CA63844, CA45560, CA37981, CA11083, CA35178, CA95860, CA35176, CA21115, CA31949, CA77202, and CCSRI 015469) and in part by AstraZeneca. The contribution of EORTC to this study was supported by Fonds Cancer (FOCA) from Belgium.
Footnotes
Presented in part at the Plenary Session of the 48th Annual Meeting of the American Society of Clinical Oncology, Chicago, June 1–5, 2012.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Crawford ED, Eisenberger MA, McLeod DG, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321:419–24. doi: 10.1056/NEJM198908173210702. [Erratum, N Engl J Med 1989; 321:1420.] [DOI] [PubMed] [Google Scholar]
- 2.Eisenberger MA, Blumenstein BA, Crawford ED, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339:1036–42. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 3.Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med. 2004;351:1488–90. doi: 10.1056/NEJMp048178. [DOI] [PubMed] [Google Scholar]
- 4.Ryan CJ, Tindall DJ. Androgen receptor rediscovered: the new biology and targeting the androgen receptor therapeutically. J Clin Oncol. 2011;29:3651–8. doi: 10.1200/JCO.2011.35.2005. [DOI] [PubMed] [Google Scholar]
- 5.Bruchovsky N, Rennie PS, Coldman AJ, Goldenberg SL, To M, Lawson D. Effects of androgen withdrawal on the stem cell composition of the Shionogi carcinoma. Cancer Res. 1990;50:2275–82. [PubMed] [Google Scholar]
- 6.Trachtenberg J. Experimental treatment of prostatic cancer by intermittent hormonal therapy. J Urol. 1987;137:785–8. doi: 10.1016/s0022-5347(17)44211-x. [DOI] [PubMed] [Google Scholar]
- 7.Akakura K, Bruchovsky N, Goldenberg SL, Rennie PS, Buckley AR, Sullivan LD. Effects of intermittent androgen suppression on androgen-dependent tumors: apoptosis and serum prostate-specific antigen. Cancer. 1993;71:2782–90. doi: 10.1002/1097-0142(19930501)71:9<2782::aid-cncr2820710916>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 8.Bhandari MS, Crook J, Hussain M. Should intermittent androgen deprivation be used in routine clinical practice? J Clin Oncol. 2005;23:8212–8. doi: 10.1200/JCO.2005.03.2557. [DOI] [PubMed] [Google Scholar]
- 9.Goldenberg SL, Bruchovsky N, Gleave ME, Sullivan LD, Akakura K. Intermittent androgen suppression in the treatment of prostate cancer: a preliminary report. Urology. 1995;45:839–44. doi: 10.1016/s0090-4295(99)80092-2. [DOI] [PubMed] [Google Scholar]
- 10.Higano CS, Ellis W, Russell K, Lange PH. Intermittent androgen suppression with leuprolide and flutamide for prostate cancer: a pilot study. Urology. 1996;48:800–4. doi: 10.1016/S0090-4295(96)00381-0. [DOI] [PubMed] [Google Scholar]
- 11.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 12.Moinpour CM, Hayden KA, Thompson IM, Feigl P, Metch B. Quality of life assessment in Southwest Oncology Group trials. Oncology (Williston Park) 1990;4(5):79–84. [PubMed] [Google Scholar]
- 13.Moinpour CM, Savage MJ, Troxel A, et al. Quality of life in advanced prostate cancer: results of a randomized therapeutic trial. J Natl Cancer Inst. 1998;90:1537–44. doi: 10.1093/jnci/90.20.1537. [DOI] [PubMed] [Google Scholar]
- 14.Moinpour CM, Sawyers Triplett J, McKnight B, et al. Challenges posed by non-random missing quality of life data in an advanced-stage colorectal cancer clinical trial. Psychooncology. 2000;9:340–54. doi: 10.1002/1099-1611(200007/08)9:4<340::aid-pon466>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 15.Moinpour CM, Savage M, Hayden KA, Sawyers J, Upchurch C. Quality of life assessment in cancer clinical trials. In: Dimsdale JE, Baum A, editors. Quality of life in behavioral medicine research. Hillsdale, NJ: Lawrence Erlbaum Associates; 1995. pp. 79–95. [Google Scholar]
- 16.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–15. [PubMed] [Google Scholar]
- 18.Lin DW, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80:557–72. [Google Scholar]
- 19.Pauler DK, McCoy S, Moinpour C. Pattern mixture models for longitudinal quality of life studies in advanced stage disease. Stat Med. 2003;22:795–809. doi: 10.1002/sim.1397. [DOI] [PubMed] [Google Scholar]
- 20.Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ. Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA. 2006;295:1152–60. doi: 10.1001/jama.295.10.1152. [Erratum, JAMA 2006;296:1842.] [DOI] [PubMed] [Google Scholar]
- 21.Calais da Silva FE, Bono AV, Whelan P, et al. Intermittent androgen deprivation for locally advanced and metastatic prostate cancer: results from a randomised phase 3 study of the South European Uroncological Group. Eur Urol. 2009;55:1269–77. doi: 10.1016/j.eururo.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Crook JM, O’Callaghan CJ, Duncan G, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012;367:895–903. doi: 10.1056/NEJMoa1201546. [Erratum, N Engl J Med 2012;367:2262.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Leval J, Boca P, Yousef E, et al. Intermittent versus continuous total androgen blockade in the treatment of patients with advanced hormone-naive prostate cancer: results of a prospective randomized multicenter trial. Clin Prostate Cancer. 2002;1:163–71. doi: 10.3816/cgc.2002.n.018. [DOI] [PubMed] [Google Scholar]
- 24.Gruca D, Bacher P, Tunn U. Safety and tolerability of intermittent androgen deprivation therapy: a literature review. Int J Urol. 2012;19:614–25. doi: 10.1111/j.1442-2042.2012.03001.x. [DOI] [PubMed] [Google Scholar]
- 25.Salonen AJ, Taari K, Ala-Opas M, Viitanen J, Lundstedt S, Tammela TL. The FinnProstate Study VII: intermittent versus continuous androgen deprivation in patients with advanced prostate cancer. J Urol. 2012;187:2074–81. doi: 10.1016/j.juro.2012.01.122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.