SUMMARY
A simple method to reduce adverse effects of the chemotherapeutic agent cisplatin on animal health is described. Animals receiving normal saline (0.9% NaCl) s.c. prior to once weekly injections of cisplatin (3 mg/kg i.p.×3 or 4 weeks) exhibited failure of weight gain, lowered body temperature, elevations in creatinine and ketone levels and increased kidney weight ratios. By contrast, rats treated with sodium bicarbonate (4% NaHCO3 in saline s.c.) prior to cisplatin (3 mg/kg i.p.×3 or 4 weeks) exhibited normal weight gain, body temperature, creatinine and ketone levels, as well as normal kidney weight ratios (over 16 or 28 days, respectively). Cisplatin-induced neuropathy (i.e. mechanical and cold allodynia) developed equivalently in both groups. Our studies suggest that NaHCO3 pretreatment promotes animal health and prevents weight loss, body temperature dysregulation and signs of renal toxicity (i.e. increases in creatinine and kidney weight ratio) following repeated cisplatin treatment without altering the development of chemotherapy-induced peripheral neuropathy.
Keywords: weight loss, hypothermia, creatinine, kidney function, chemotherapy-induced peripheral neuropathy, NaHCO3 (sodium bicarbonate)
Cisplatin, a platinum-derived chemotherapeutic agent, produces both painful peripheral neuropathy and renal toxicity [1,20]. In rodent subjects, cisplatin is specifically used to model peripheral sensory neuropathies that develop in humans treated with this agent [4,5,8,20]. In animal models, cisplatin-induced mortality, attributable to damage to renal functions [22,30], ranges from 10–50% [6,27]. Attempts to minimize mortality in rodents involve reductions in frequency, duration and/or dosing of cisplatin [4 for a review]. Nonetheless, cisplatin produces changes in body temperature [6,27] and detrimental effects on body weight in rodent subjects [5,6,26,27].
Human studies have reported beneficial effects of sodium bicarbonate in reducing blood acidosis and kidney toxicity in chemotherapy patients [7,21]. Therefore, we developed a new preclinical method to minimize damage to renal functions (assessed by measurements of creatinine levels, kidney weight ratio and urine pH) and improve general health (weight gain, normal body temperature and reduced mortality) in rodent subjects. We evaluated whether NaHCO3 (4% in 0.9% NaCl (saline)), administered subcutaneously (s.c.) immediately prior to cisplatin treatment, would prevent adverse side-effects (e.g. weight loss, lowered body temperature, creatinine increases, kidney weight ratio increases and mortality) associated with repeated cisplatin dosing. We hypothesized that concurrent administration of sodium bicarbonate (4 % NaHCO3; pH 8.06 ± 0.01), an alkaline solution, would counteract acidic effects of cisplatin that underly nephrotoxicity and mortality in rodents, thereby producing a beneficial impact on animal health.
Male Sprague-Dawley rats (Harlan, Indianapolis, IN, USA), weighing 254–382 g before testing, were used. Animals were single housed in standard plastic cages with sawdust bedding in a climate-controlled room (23°C and 45% humidity), and maintained under a 12h light (7am to 7pm)/dark cycle. Rats were given free access to standard rodent chow and water. Total of 242 rats were used. Experimental protocols were approved by the Institutional Animal Care and Use Committee and followed guidelines for the treatment of animals of the International Association for the Study of Pain [31].
Cisplatin (Tocris, Ellisville, MO, USA) was administered intraperitoneally (i.p.) once a week at a dose of 3 mg/kg for 3 (cumulative dose: 9 mg/kg i.p. over 16 days) or 4 (12 mg/kg i.p. over 28 days) weeks [6,11]. Cisplatin was diluted in normal saline (0.9 % NaCl). Saline [4] or 4% sodium bicarbonate (NaHCO3 dissolved in saline) was administered (2 ml s.c.) before each i.p injection of cisplatin or saline. Injections were always performed after completion of mechanical and cold withdrawal testing.
Mechanical withdrawal thresholds were assessed using a digital Electrovonfrey Anesthesiometer (IITC Life Sciences, Woodland Hills, CA) equipped with a rigid tip [11]. Cold allodynia was assessed by applying drops of room temperature acetone to the plantar surface of the hind paw as previously described [11]. Mechanical withdrawal thresholds and cold withdrawal frequencies were measured every 4 days over 16 (for saline/cisplatin group) or 28 (for NaHCO3/cisplatin group) days. Testing took place on days 0, 4, 8, 12, 16 in all groups and continued on days 20, 24, 28 in relevant cohorts.
Rectal temperature was assessed in animals receiving NaHCO3 or saline pretreatments using a rectal probe (Physitemp RET-2 rectal probe for rats, Clifton, NJ, USA) and meter (Physitemp Model BAT-12R, Clifton, NJ, USA). Body temperature was recorded every four days. The same animals were used to evaluate mechanical and cold allodynia as well as body weight and core temperature changes. A subset (n = 8–9 per group) of these animals were used to evaluate kidney functions.
Creatinine, ketone and glucose levels (mg/dL) were measured in whole blood using the PTS CardioChek diagnostic apparatus (Cliawaived.com, San Diego, CA, USA). Urine and blood were extracted post mortem from the bladder and renal artery, respectively, using a 25 gauge needle and 1 ml syringe. Urine and blood pH was measured using a digital pH 110 meter (Oakton Instruments, Vermon Hills, IL, USA). Kidney weight ratio was also measured [9]. The experimenter was blinded to the experimental condition.
Paw withdrawal thresholds (mechanical) and frequencies (cold) were calculated for each paw and averaged. Data were analyzed using analysis of variance (ANOVA) for repeated measures or one-way ANOVA as appropriate. The Greenhouse-Geisser correction was applied to all repeated factors. The source of significant interactions was further evaluated by performing one way ANOVAs at each time point, followed by Bonferroni post hoc tests. Analyses were performed using SPSS statistical software (version 19.0; SPSS Incorporated, Chicago, IL, USA). P < 0.05 was considered significant.
No differences were observed between groups receiving saline/saline and NaHCO3/saline treatments in body weight (P > 0.526), body temperature (P > 0.942), mechanical threshold (P > 0.08) or cold withdrawal frequency (P > 0.620) in either injection paradigm. Similarly, no differences in creatinine (P > 0.6310), ketone (P > 0.5891), glucose (P > 0.2620), urine pH (P > 0.2819), blood pH (P > 0.3249) or kidney weight ratios (P > 0.0675) were observed in groups receiving saline/saline or NaHCO3/saline treatments. Therefore, these groups were pooled into a single control group (the control/saline group) for each survival time for further statistical analyses.
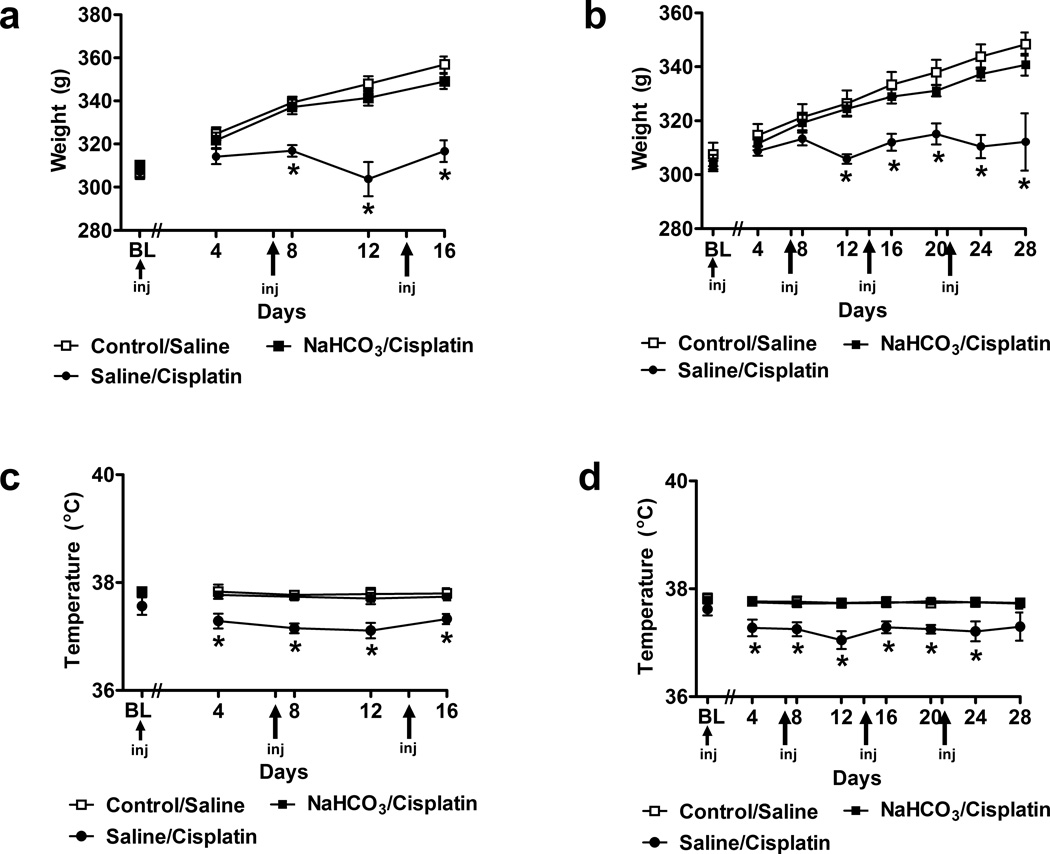
Weight gain was absent in animals receiving saline (in lieu of NaHCO3) prior to cisplatin. By contrast, both control/saline and NaHCO3/cisplatin-treated groups exhibited time-dependent increases in body weight in both injection paradigms (F8,180 = 11.31 P < 0.0001 (3 cisplatin cycles over 16 days; Fig. 1A) and (F14,567 = 5.60 P < 0.0001 (4 cisplatin cycles over 28 days; Fig. 1B). Body weight was lower in saline/cisplatin-treated groups (F2,45 = 11.55, P < 0.0001, Fig. 1A; F2,81 = 3.17, P < 0.047, Fig. 1B) relative to either control/saline or NaHCO3/cisplatin groups. The magnitude and rate of weight gain did not differ in these latter groups. Weight gain appeared on day 8 and persisted throughout the 16 day observation interval (P < 0.001) (Fig. 1A) in the NaHCO3/cisplatin group receiving 3 cycles of cisplatin. Weight gain appeared on day 12 and was maintained throughout the 28 day observation interval (P < 0.045) (Fig. 1B) in the NaHCO3/cisplatin group receiving 4 cycles of cisplatin. Saline/cisplatin-treated groups also exhibited lower body temperature relative to either control/saline or NaHCO3/cisplatin groups; lowered body temperature was observed on day 4 and was maintained throughout the study (F2,45 = 15.35, P < 0.0001; day 4–16 (P < 0.011); Fig. 1C) and (F2,81 = 12.21, P < 0.0001; day 4–24 (P < 0.037); Fig. 1D). Body temperature in NaHCO3/cisplatin groups did not differ from that observed in control/saline groups (P = 1.000) at any observation interval. Thus, sodium bicarbonate treatment was protective against hypothermic effects of cisplatin.
Fig. 1.
Pretreatment with 4% sodium bicarbonate (NaHCO3) prior to cisplatin (NaHCO3/cisplatin) prevented changes in body weight (g) and body temperature (°C) observed in saline/cisplatin-treated groups relative to control/saline groups. Animals received 3 or 4 once weekly injections of cisplatin over (a,c) 16 or (b,d) 28 days, respectively. Data are expressed as mean ± s.e.m. (n = 8–44 per group). * P < 0.045 vs. control/saline or NaHCO3/cispaltin groups.
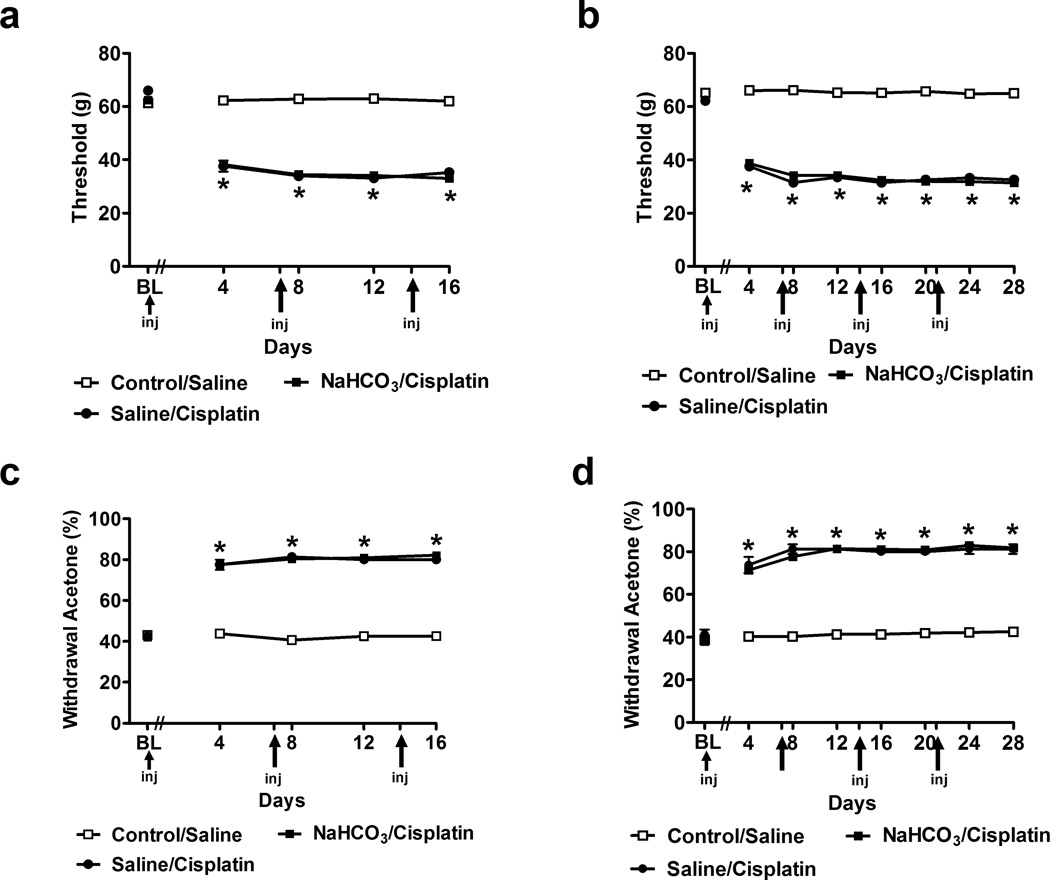
Both saline/cisplatin- and NaHCO3/cisplatin-treated groups developed equivalent levels of mechanical allodynia (F2,45 = 1686.04, P < 0.0001 (Fig. 2A) and (F2,81 = 3805.20, P < 0.0001 (Fig. 2B). Reductions in mechanical thresholds were observed in each cisplatin dosing paradigm relative to the control/saline group. Cisplatin-induced mechanical allodynia was present at all observation intervals (P < 0.0001; (Fig. 2A,B). Furthermore, both saline/cisplatin and NaHCO3/cisplatin treatments increased paw withdrawal frequencies to acetone (F2,45 = 372.87, P < 0.0001 (Fig. 2C) and (F2,81 = 1145.76, P < 0.0001 (Fig. 2D), consistent with development of cold allodynia. Cisplatin-induced cold allodynia was present (P < 0.0001) at all observation intervals relative to the control/saline group (Fig. 2C,D).
Fig. 2.
Cisplatin produces time-dependent behavioral sensitization to mechanical and cold stimulation. Time course of development of (a,b) mechanical and (c,d) cold allodynia in NaHCO3/cisplatin or saline/cisplatin-treated groups relative to control/saline-treated control group. Groups received 3 (a,c) or 4 (b,d) once weekly injections and were monitored for 16 or 28 days, respectively. Data are expressed as mean ± s.e.m. (n = 8–44 per group). * P < 0.0001 vs. control/saline group.
Mortality was not observed in animals treated with the NaHCO3/cisplatin dosing paradigm (n = 24 for 16 days; n = 44 for 28 days). By contrast, 11% (1 out of 9 rats in the 16 day dosing paradigm) and 20% (2 out of 10 rats in the 28 day dosing) died in the group receiving saline/cisplatin treatment (n = 8 for 16 days; n = 8 for 28 days), demonstrating toxicity that precluded further cisplatin dosing.
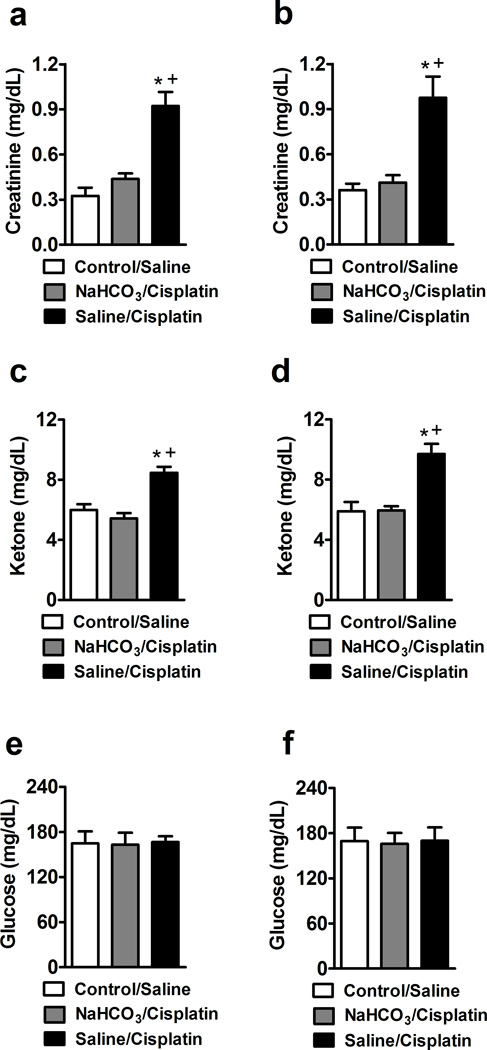
Saline/cisplatin treatment increased creatinine (F2,22 = 21.97, P < 0.0001, 16 days; F2,21 = 14.48, P < 0.0001, 28 days) (Fig. 3A,B) and ketone (F2,22 = 18.48, P < 0.0001, 16 days; F2,21 = 15.51, P < 0.0001, 28 days) (Fig. 3C,D) levels in whole blood relative to control/saline treatment. These increases were prevented by NaHCO3 pretreatment in the cisplatin-treated groups. Creatinine levels were similar in control/saline and NaHCO3/cisplatin-treated groups (P = 0.810, at 16 and P = 1.000, at 28 days). By contrast, blood glucose levels (F2,22 = 0.018, P = 0.982, 16 days; F2,21 = 0.02, P = 0.98, 28 days) (Fig. 3E,F) did not differ in any group.
Fig. 3.
Creatinine (a,b) and ketone (c,d) levels are increased in saline/cisplatin relative to control/saline or NaHCO3/cisplatin-treated groups. Animals received 3 or 4 once weekly injections over 16 (a,c,e) or 28 (b,d,f) days. Glucose levels did not differ in any group in either dosing paradigm (e,f). Data are expressed as mean ± s.e.m. (n = 8–9 per group). * P < 0.0001 vs. control/saline group; + P < 0.0001 vs NaHCO3/Cisplatin group.
Cisplatin lowered urinary pH in saline/cisplatin-treated groups relative to control/saline groups in rats receiving 4 cycles of cisplatin and killed on day 28 post initial cisplatin dosing (P < 0.001) (Table 1). Urinary pH was not reduced in saline/cisplatin groups receiving 3 cycles of cisplatin and killed at day 16 following initial cisplatin dosing (P = 0.402) (Table 1). In cisplatin-treated animals, NaHCO3 pretreatment increased the urinary pH (F2,22 = 29.06, P < 0.0001, 16 days; F2,21 = 34.77, P < 0.0001, 28 days) (Table 1) compared to either control/saline or saline/cisplatin groups in each dosing paradigm (Table 1). NaHCO3 pretreatment also blocked the cisplatin-induced increase in kidney weight ratio (Table 1). The saline/cisplatin group had a higher kidney weight ratio at both 16 (F2,22 = 18.92, P < 0.0001) and 28 (F2,21 = 8.38, P < 0.002) day survival times relative to control/saline or NaHCO3/cisplatin-treated groups. By contrast, the kidney weight ratio of NaHCO3/cisplatin-treated rats was similar to that observed in control/saline groups (P = 1.000, at 16 or 28 days). Thus, sodium bicarbonate prevented cisplatin-induced increases in kidney weight ratio. Blood pH (F2,22 = 0.291, P = 0.750, 16 days; F2,21 = 2.71, P = 0.090, 28 days) (Fig. 3E,F) did not differ in any of the groups.
Table 1.
Effect of NaHCO3 or saline pretreatment on urine pH, blood pH and kidney weight ratio in cisplatin and saline-treated rats
| Urine PH | Blood PH (renal artery) |
Kidney weight ratio (%) |
||
|---|---|---|---|---|
| Control/Saline | 16 Days | 6.95 ± 0.04 | 7.42 ± 0.03 | 0.77 ± 0.01 |
| 28 Days | 7.09 ± 0.07 | 7.37 ± 0.05 | 0.72 ± 0.02 | |
| NaHCO3/Cisplatin | 16 Days | *8.11 ± 0.07 | 7.45 ± 0.08 | 0.80 ± 0.01 |
| 28 Days | *7.84 ± 0.11 | 7.36 ± 0.03 | 0.78 ± 0.02 | |
| Saline/Cisplatin | 16 Days | 6.62 ± 0.25 | 7.39 ± 0.04 | #⊤1.11 ± 0.08 |
| 28 Days | +6.36 ± 0.17 | 7.24 ± 0.05 | #⊤1.01 ± 0.09 | |
Data are expressed as mean ± s.e.m. (n = 8–9 per group) of measurements determined 16 or 28 days following initiation of cisplatin/saline dosing.
P < 0.001 vs. control/saline or saline/cispaltin groups;
P < 0.001 vs control/saline group;
P < 0.002 vs. control/saline or NaHCO3/csipaltin groups;
P < 0.016 vs. NaHCO3/cispaltin group.
Cisplatin causes dose-dependent renal toxicity, a major dose-limiting consequence of cisplatin treatment [22,30]. Nephrotoxicity is complex and becomes more severe with repeated dosing [6,22,27]. Cisplatin produces disturbances in water permeability [16] and infiltration of inflammatory cells [18,24]. Cisplatin is partially metabolized into toxic species [30], promoting an acidic environment that produces nephrotoxicity and changes in renal functions, documented by increases in creatinine (normal creatinine between 0.2 –1.36 mg/dL) and kidney weight ratio [13,15] in our study. A change in the body pH (normal blood pH: 7.35–7.45; normal urine pH ~7.00) toward acidity (below pH 7.0) favours oxygen to be driven out of the body, thereby facilitating cancer cell survival [10,12,21]. Thus, urine, but not blood, pH would be expected to be increased by alkaline and decreased by acidic diets/treatments, as observed in our study and elsewhere [21]. Cancerous tissue is acidic because cells spill lactic acid, causing an acidic pH [29]. Thus, using systemic buffers to manipulate acidification is beneficial as a cancer treatment strategy in humans [7,19,21,25]. Our preclinical findings with NaHCO3 delivered immediately prior to cisplatin administration, confirm the clinical predictions. In our study, NaHCO3 treatment was associated with normal renal functions (normal creatinine levels, kidney weight ratio, together with alkaline urine pH) and beneficial effects on animal health (weight gain, normal body temperature and absence of mortality).
We evaluated renal functions by measuring creatinine levels, kidney weight ratio and pH of urine and blood. A similar normalization of renal function is observed in the cisplatin model following administration of the antioxidant resveratrol [9]. Indeed, creatinine levels (Fig. 3) and kidney weight ratio (Table 1) in our control group were similar to creatinine levels (0.4 mg/dL) and kidney weight ratio (0.75%) observed in the control group in this latter study [9]. In our study, NaHCO3 pretreatment prior to cisplatin was associated with normal kidney weight ratios (Table 1) and creatinine levels (Fig. 3). Following resveratrol/cisplatin treatment, normal creatinine levels (0.42 mg/dL) and kidney weight ratios (0.73%) are also observed [9]. Moreover, in our study, pretreatment with 4% sodium bicarbonate prior to cisplatin produced an alkaline urine pH (8.11 and 7.84 at 16 and 28 days, respectively), but normal blood pH (Table 1)), consistent with clinical findings [21]. Indeed, an alkali pH (above 7.5) was observed in all (n=26) patients pre-treated with 8.4% sodium bicarbonate before methotrexate treatment [21]. By comparison, pre-treatment with saline, a pretreatment commonly used in animal studies of cisplatin-induced neuropathy, was associated with an acidic urine pH (6.62 (3 cisplatin cycles over 16 days) and 6.36 (4 cisplatin cycles over 28 days) respectively) but normal blood pH (Table 1), higher kidney weight ratios (1.11 and 1.01% for 16 and 28 days, respectively) and higher creatinine levels (0.92 and 0.98 mg/dL for 16 and 28 days, respectively). Creatinine levels (1.22 mg/dL) and kidney weight ratio (1.06%) were increased by cisplatin treatment in the resveratrol study, an effect confirmed and corroborated by our findings [9].
Ketones (ketone levels (0.5–3.00 mg/dL)) are the end products of fatty acid metabolism and high ketone levels are indicative of keto-acidosis, a serious condition characterized by high acidity of bodily fluids [3,13,17]. Interestingly, ketone levels in saline/cisplatin-treated rats are increased in comparison with those observed in control/saline or NaHCO3/cisplatin-treated rats. The increase in ketone levels in saline/cisplatin-treated rats suggests that these rats could develop keto-acidosis if treated for a longer period of time [3,13]. However, glucose levels were not affected by saline/cisplatin treatment. In fact, glucose levels (50 to 135 mg/dL)) were similar between all treatment groups (see Fig. 3) [13,23]. Thus, cisplatin-induced neuropathy is not associated with high levels of glucose observed in models of diabetic neuropathy [28].
Beneficial properties of NaHCO3 pretreatment are demonstrated by improved animal health, increase in weight gain (Fig.1A,B), normal body temperature (Fig.1C,D) and absence of mortality in each cisplatin dosing paradigm. However, saline/cisplatin-treated groups exhibited failure of weight gain, lower body temperature and mortality ranging from 11 to 20% following 3 or 4 once weekly cycles of cisplatin (Fig.1A–D). Studies of renal functions altered by cisplatin do not typically report general health of animals [2,9]. To our knowledge, preclinical studies have not previously used alkalis to buffer acidic effects of cisplatin and improve animal health. Importantly, cisplatin-induced mechanical and cold allodynia was similar in the presence or absence of NaHCO3. Thus, the present model is particularly useful for maintaining animal health status in long term studies of cisplatin-induced neuropathy. More work is necessary to demonstrate the impact of sodium bicarbonate pretreatment on anti-tumor effects of cisplatin.
Subcutaneous saline has been represented to ameliorate cisplatin-induced nephrotoxicity [5,6]. However, this approach did not increase weight gain and was associated with mortality, especially when high (i.e. 3 mg/kg i.p.) cisplatin doses are employed [6,27]. Indeed, mortality rates of 50% have been reported with this saline/cisplatin dosing paradigm [27]. It is thus possible that saline hyperhydratation can only be achieved with more frequent saline injections (e.g. 3–4 times per day). However, saline lacks the alkaline properties of NaHCO3 which are beneficial in an acidic environment.
Other treatments may also be effective in preventing toxic effects of cisplatin. Indeed, antioxidants (e.g. resveratrol, vitamin C) given before or after cisplatin administration may prevent or decrease nephrotoxicity in animals [2,9]. The hypolipidemic drug simvastatin has also been reported to attenuate cisplatin-induced kidney damage [14]. The combination of our approach with an antioxidant and/or simvastatin could potentially produce synergistic beneficial effects that prevent or reduce cisplatin-induced renal toxicity.
In conclusion, our studies provide direct evidence that once weekly injection of sodium bicarbonate prior to cisplatin treatment produces a beneficial impact on animal health (i.e. normal weight gain, no change in body temperature and absence of mortality over 28 days). The lowered body temperature observed in saline/cisplatin-treated groups confirms previous reports of cisplatin-induced hypothermia in dosing paradigms that lacked sodium bicarbonate pretreatment [5,13]. Beneficial changes in renal functions (decrease in creatinine levels, normal kidney weight ratio and higher urine pH) were observed in the NaHCO3/cisplatin group relative to saline/cisplatin treated groups at each survival time. In fact, sodium bicarbonate pretreatment normalized these parameters to that observed in control groups that did not receive cisplatin. Sodium bicarbonate pretreatment also failed to alter the development of cisplatin-induced mechanical or cold allodynia. This method constitutes a valuable approach to improve general health of cisplatin-treated rodents and may also facilitate future research employing long term evaluations of chemotherapy-induced toxicities such as peripheral neuropathy.
Highlights.
NaHCO3 simple method to reduce adverse renal effects of repeated cisplatin treatment
NaHCO3/cisplatin show weight gain, normal temperature, creatinine and kidney ratio
NaCl/cisplatin show lower weight and temperature, higher creatinine and kidney ratio
Pretreatment with either NaCl or NaHCO3 does not prevent cisplatin-induced neuropathy
NaHCO3 pretreatment induce good health and normal creatinine levels and kidney ratio
Acknowledgments
Supported by DA021644 and DA028200 (AGH).
Abbreviations
- ANOVA
analysis of variance
- BL
baseline
- NaCl
sodium chloride
- NaHCO3
sodium bicarbonate
- inj
injection
- i.p.
intraperitoneal
- s.c.
subcutaneous
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors state no conflict of interest.
References
- 1.Alberts DS, Noel JK. Cisplatin-associated neurotoxicity: can it be prevented? Anticancer Drugs. 1995;6:369–383. doi: 10.1097/00001813-199506000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Antunes LM, Darin JD, Bianchi MD. Protective effects of vitamin c against cisplatin-induced nephrotoxicity and lipid peroxidation in adult rats: a dose-dependent study. Pharmacol. Res. 2000;41:405–411. doi: 10.1006/phrs.1999.0600. [DOI] [PubMed] [Google Scholar]
- 3.Askew EW, Dohm GL, Huston RL. Fatty acid and ketone body metabolism in the rat: response to diet and exercise. J. Nutri. 1975;105:1422–1432. doi: 10.1093/jn/105.11.1422. [DOI] [PubMed] [Google Scholar]
- 4.Authier N, Balayssac D, Marchand F, Ling B, Zangarelli A, Descoeur J, Coudore F, Bourinet E, Eschalier A. Animal models of chemotherapy-evoked painful peripheral neuropathies. Neurotherapeutics. 2009;6:620–629. doi: 10.1016/j.nurt.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Authier N, Fialip J, Eschalier A, Coudoré F. Assessment of allodynia and hyperalgesia after cisplatin administration to rats. Neurosci. Lett. 2000;291:73–76. doi: 10.1016/s0304-3940(00)01373-2. [DOI] [PubMed] [Google Scholar]
- 6.Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. An animal model of nociceptive peripheral neuropathy following repeated cisplatin injections. Exp. Neurol. 2003;182:12–20. doi: 10.1016/s0014-4886(03)00003-7. [DOI] [PubMed] [Google Scholar]
- 7.Bacci G, Ferrari S, Longhi A, Forni C, Loro L, Beghelli C, Tremosini M, Versari M. Delayed methotrexate clearance in osteosarcoma patients treated with multiagent regimens of neoadjuvant chemotherapy. Oncol. Rep. 2003;10:851–857. [PubMed] [Google Scholar]
- 8.Boyiadzis MM, Lebowitz PF, Frame JN, Fojo T. Hematology-Oncology Therapy. New York: McGraw Hill, Medical Publishing Division; 2007. [Google Scholar]
- 9.Do Amaral CL, Francescato HD, Coimbra TM, Costa RS, Darin JD, Antunes LM, Bianchi MdeL. Resveratrol attenuates cisplatin-induced nephrotoxicity in rats. Arch. Toxicol. 2008;82:363–370. doi: 10.1007/s00204-007-0262-x. [DOI] [PubMed] [Google Scholar]
- 10.Elwell MR, Sammons ML, Liu CT, Beisel WR. Changes in blood pH in rats after infection with Streptococcus pneumoniae. Infect. Immun. 1975;11:724–726. doi: 10.1128/iai.11.4.724-726.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guindon J, Lai Y, Takacs SM, Bradshaw HB, Hohmann AG. Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: Effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment. Pharmacol. Res. 2013;67:94–109. doi: 10.1016/j.phrs.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haylor J, Lote CJ. Urine pH and the relationship between urine flow and urinary prostaglandin E excretion in the rat. J. Endocrinol. 1986;108:247–253. doi: 10.1677/joe.0.1080247. [DOI] [PubMed] [Google Scholar]
- 13.Hillyer EV, Quesenberry KE. Ferrets, rabbits and rodents; clinical medicine and surgery. First ed. New York: Elsevier Health Science; 1996. [Google Scholar]
- 14.Işeri S, Ercan F, Gedik N, Yüksel M, Alican I. Simvastatin attenuates cisplatin-induced kidney and liver damage in rats. Toxicology. 2007;230:256–264. doi: 10.1016/j.tox.2006.11.073. [DOI] [PubMed] [Google Scholar]
- 15.Keppler A, Gretz N, Schimdt R, Kloetzer HM, Groene HJ, Lelongt B, Meyer M, Sadick M, Pill J. Plasma creatinine determination in mice and rats: an enzymatic method compares favorably with a high-performance liquid chromatography assay. Kidney Int. 2007;71:74–78. doi: 10.1038/sj.ki.5001988. [DOI] [PubMed] [Google Scholar]
- 16.Kishore BK, Krane CM, Di Iulio D, Menon AG, Cacini W. Expression of renal aquaporins 1, 2, and 3 in a rat model of cisplatin-induced polyuria. Kidney Int. 2000;58:701–711. doi: 10.1046/j.1523-1755.2000.00216.x. [DOI] [PubMed] [Google Scholar]
- 17.Koch DD, Feldbruegge DH. Optimized kinetic method for automated determination of beta-hydroxybutyrate. Clin. Chem. 1987;33:1761–1766. [PubMed] [Google Scholar]
- 18.Liu M, Chien C, Burne-Taney M, Molls RR, Racusen LC, Colvin RB, Rabb H. A pathophysiologic role for T lymphocytes in murine acute cisplatin nephrotoxicity. J. Am. Soc. Nephrol. 2006;17:765–774. doi: 10.1681/ASN.2005010102. [DOI] [PubMed] [Google Scholar]
- 19.McCarty MF, Whitaker J. Manipulating tumor acidification as a cancer treatment strategy. Altern. Med. Rev. 2010;15:264–272. [PubMed] [Google Scholar]
- 20.Perry MC. Companion Handbook to the Chemotherapy Sourcebook. Baltimore: Williams & Wilkins; 1999. [Google Scholar]
- 21.Mir O, Ropert S, Babinet A, Alexandre J, Larousserie F, Durand JP, Enkaoua E, Anract P, Goldwasser F. Hyper-alkalinization without hyper-hydration for the prevention of high-dose methotrexate acute nephrotoxicity in patients with osteosarcoma. Cancer Chemother. Pharmacol. 2010;66:1059–1063. doi: 10.1007/s00280-010-1259-3. [DOI] [PubMed] [Google Scholar]
- 22.Sánchez-González PD, López-Hernández FJ, López-Novoa JM, Morales AI. An integrative view of the pathophysiological events leading to cisplatin nephrotoxicity. Crit. Rev. Toxicol. 2011;41:803–821. doi: 10.3109/10408444.2011.602662. [DOI] [PubMed] [Google Scholar]
- 23.Schroeder HA. Serum cholesterol and glucose levels in rats fed refined and less refined sugars and chromium. J. Nutri. 1969;97:237–242. doi: 10.1093/jn/97.2.237. [DOI] [PubMed] [Google Scholar]
- 24.Sean Eardley K, Cockwell P. Macrophages and progressive tubulointerstitial disease. Kidney Int. 2005;68:437–455. doi: 10.1111/j.1523-1755.2005.00422.x. [DOI] [PubMed] [Google Scholar]
- 25.Silva AS, Yunes JA, Gillies RJ, Gatenby RA. The potential role of systemic buffers in reducing intratumoral extracellular pH and acid-mediated invasion. Cancer Res. 2009;69:2677–2684. doi: 10.1158/0008-5472.CAN-08-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vera G, Chiarlone A, Cabezos PA, Pascual D, Martín MI, Abalo R. WIN 55,212-2 prevents mechanical allodynia but not alterations in feeding behaviour induced by chronic cisplatin in the rat. Life Sci. 2007;81:468–479. doi: 10.1016/j.lfs.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Vera G, Chiarlone A, Martín MI, Abalo R. Altered feeding behaviour induced by long-term cisplatin in rats. Auton. Neurosci. 2006;126–127:81–92. doi: 10.1016/j.autneu.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Wiffen PJ. Enhanced glucose control for preventing and treating diabetic neuropathy. J. Pain Palliat. Care Pharmacother. 2012;26:380. [Google Scholar]
- 29.Wong P, Lee C, Tannock IF. Reduction of intracellular pH as a strategy to enhance the pH-dependent cytotoxic effects of melphalan for human breast cancer cells. Clin. Cancer Res. 2005;11:3553–3557. doi: 10.1158/1078-0432.CCR-04-2472. [DOI] [PubMed] [Google Scholar]
- 30.Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am. J. Med. Sci. 2007;334:115–124. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- 31.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]