Abstract
Stem cell therapy has emerged as a potential therapeutic option for cell death-related heart diseases. Application of non-invasive cell tracking approaches is necessary to determine tissue distribution and lifetime of stem cells following their injection and will likely provide knowledge about poorly understood stem cells mechanisms of tissue repair. Magnetic resonance imaging (MRI) is a potentially excellent tool for high-resolution visualization of the fate of cells after transplantation and for evaluation of therapeutic strategies. The application of MRI for in vivo cell tracking requires contrast agents to achieve efficient cell labeling without causing any toxic cellular effects or eliciting any other side effects. For these reasons clinically approved contrast agents (e.g., ferumoxides) and incorporation facilitators (e.g., protamine) are currently the preferred materials for cell labeling and tracking. Here we describe how to use superparamag-netic iron oxide nanoparticles to label cells and to monitor cell fate in several disease models.
Keywords: Stem cells, Cell labeling, Ferumoxides, Superparamagnetic iron oxide nanoparticles, Magnetic resonance imaging
1. Introduction
Stem cell therapy has emerged as a therapeutic option in several types of injury and disease. However, the lack of data concerning the distribution and engraftment of the cells presents a serious obstacle for the successful application of stem cell therapies in patients. Thus, the development of sensitive, non-invasive techniques for serially tracking cells will likely provide knowledge about the poorly understood mechanisms responsible for the reported improvement in several lesion/disease models (1–3). Magnetic resonance imaging (MRI) offers an imaging modality with high-resolution to visualize the fate of cells after transplantation and to evaluate cell-based repair, replacement, and therapeutic strategies (4–8).
Several paramagnetic contrast agents have been successfully used for in vivo cell tracking and labeling of different mammalian cell types (1, 9–12). Ferumoxides, such as Feridex IV (Advanced Magnetics Inc., USA) and Endorem (Guerbet SA, France) are dextran-coated superparamagnetic iron oxide nanoparticles (SPIONs) clinically used to provide intravenous MRI contrast in order to analyze liver pathology. The particles are biodegradable and all the degradation products can be incorporated in normal metabolic processes (13). SPIONs, however, tend to aggregate, but this can be minimized by their coating with dextran or other polymers (14). Other types of human approved contrast agents have also been used to label and track transplanted cell, such as ferucarbotran (Resovist) and gadoliniun nanoparticles (15, 16). However, there have been reports that gadolinium based agents may adversely affect the labeled cells (17, 18).
The dextran-coated SPIONs do not show sufficient cellular uptake to enable tracking of nonphagocytic cells. However, uptake of SPIONs can be facilitated by incubation with cationic compounds such as poly-L-lysine (PLL) (19–21), protamine sulfate (21–24), FuGENE (3), Superfect or Lipofectamine (12). The primary advantage of the incubation labeling method is its simplicity. The primary disadvantage is the extended incubation time (4 h or more) required (25). Thus, other approaches to induce labeling of freshly isolated cells such as magnetoelectroporation (26, 27) and magnetosonoporation (28) offer alternative protocols and are preferable when the culture system must be avoided. Nevertheless, for adherent cells that can be maintained in culture, PLL and protamine are the most frequently used agents to facilitate the incorporation of SPIONs.
PLL is a synthetic cationic polymer commonly used to enhance cell adhesion to the surface of culture dishes. Its use has not yet been approved in humans and studies have shown that complexes formed by ferumoxides and PLL (FePLL) can be large. Such large complexes do not become incorporated into endosomes and, instead, remain adhered to the cell membrane (22, 29). These complexes can affect cell proliferation (21) and differentiation (30). Protamines are low-molecular-weight arginine-rich proteins (~4,000 Da), that are purified from the mature fish testis. Protamine is a clinically approved polycationic peptide primarily used as an antidote for heparin anticoagulation (31, 32).
Approval for clinical tracking of labeled stem cells by MRI depends on efficient cell labeling that does not cause toxic cellular effects and does not elicit side effects. Ferumoxide and protamine are the currently favored materials for cell labeling and tracking because of the following advantages: Efficiency of cell tracking by MRI, commercial availability, approval for human use, and the lack of toxic effects on biological properties of cells. Besides, recent studies have shown that the number of labeled cells or the amount of intracellular SPIONs in cells exposed to FeProt complexes for 4 h is the same as at 24 h (21) and 48 h (23).
Here we describe procedures for making FeProt complexes (derivatized SPION nanoparticles), techniques for labeling adherent cells with FeProt complexes and microscopy and MRI-based methods for analysis of incorporation efficiency of the SPIONs’ incorporation efficiency.
2. Materials
All cell culture materials should be prepared and used under sterile conditions.
2.1. Labeling of Adherent Cells
Culture plates: the culture plate size should be chosen according to planned experiments. For detection of SPIONs (with Prussian Blue and/or immunostaining) use a 24-well plate and coverslips; for cell transplant in vivo and MRI detection (see Note 1) and/or for in vitro MRI use a 100 mm Petri dish.
Culture medium: use the appropriate culture medium for the cell requirements, e.g., for mesenchymal stem cells use DMEM high glucose supplemented with fetal bovine serum (FBS, see Note 2) and 100 U/mL penicillin and 100 μg/mL streptomycin, or with appropriate supplements used for the specific cell type.
Ferumoxide such as Endorem is commercially available (Guerbert SA) at 11.2 mg Fe/mL solution (particle size: 80–150 nm). The stock solution should be stored at 4 °C or at room temperature, but the solution should never be frozen. Until recently another type of ferumoxide was also available from Feridex IV (Advanced Magnetics Inc.).
Protamine sulfate (see Note 3): commercially available as 1 mL ampoules/injections containing 10 mg of protamine sulfate, 9 mg of sodium chloride in water. This solution should be maintained at room temperature.
Phosphate-buffered saline 1× (PBS): Phosphate-buffered saline 1× (PBS): 2.67 mM Potassium Chloride (KCl); 1.47 mM Potassium Phosphate monobasic (KH2PO4); 137.93 mM Sodium Chloride (NaCl) and 8.06 mM Sodium Phosphate dibasic (Na2HPO4·7H2O), pH 7.4.
2.2. Fixing Cells for Evaluation by Staining
A 24-well cell culture plate.
Round glass coverslips (diameter 13 mm).
Gelatin for coating coverslips (0.2 %): dilute 0.2 g of gelatin in 100 mL of deionized water, mix, and autoclave. This solution can be used for months, with sterile storage at 4 °C or at room temperature.
Formaldehyde for cell fixation (4 %): dilute 1 ampoule (10 mL) of 16 % formaldehyde solution in 30 mL of PBS and mix. After preparation, this solution can be stored in aliquots at −20 °C, if thawed do not refreeze.
2.3. Determining Labeling Efficiency (See Note 4)
Prepare all working solutions fresh before the staining experiment.
2.3.1. Prussian Blue Staining (See Note 5)
Deionized water.
Perl’s Solution: Prepare 20 % potassium ferrocyanide by adding 0.2 g of potassium ferrocyanide to 1 mL of deionized water. To prepare 20 % hydrochloric acid, add 200 μL of 100 % hydrochloric acid to 800 μL of deionized water (see Note 6). Mix both solutions together, cover tube with aluminum foil to protect it from the light and shake until the reagents are completely dissolved. The final volume depends on the number of wells used, e.g. for each well of a 24-well plate prepare 300 μL. The solution should be prepared fresh.
Nuclear Fast Red (1 %): dilute 0.02 g of Nuclear Fast Red in 2 mL of deionized water, mix, and then filter through filter paper to eliminate large particles.
Absolute ethanol.
Ethanol (70 and 30 %): dilute 70 or 30 mL of absolute ethanol with 30 or 70 mL of deionized water, respectively.
Xylene.
Ethanol/xylene mixture (1:1, v/v): made by mixing equal volume of absolute ethanol and xylene.
Entellan mounting medium (Merck KGaA, Darmstadt, Germany, cat. # UN 1866).
2.3.2. Immunostaining with Anti-dextran Antibody (See Note 5)
PBS with 0.1 % Triton X-100: dilute 200 μL of Triton X-100 in 200 mL of PBS and mix well.
Normal goat serum (5 %): dilute 1 mL of normal goat serum in 19 mL of PBS and mix well. This solution can be stored in aliquots at −20 °C, if thawed it can be refrozen and thawed several times.
Anti-dextran antibody (1:1,000): dilute the anti-dextran mouse monoclonal antibody (Stem Cell Technologies, Vancouver, BC, USA, cat. # 01403) 1:1,000 (v:v) with PBS/0.1 % Triton X-100. Prepare the primary antibody fresh each time, but leftover material can be stored protected from light at 4 °C and used within a week.
Goat anti-mouse IgG secondary antibody: dilute the goat-anti-mouse IgG (e.g., Alexa Fluor 488 labeled, available from Invitrogen Inc.) in PBS/0.1 % Triton X-100 at 1:400 (v:v) (see Note 7). The ratio of antibody to PBS depends on the antibody brand. Prepare the secondary antibody fresh each time, but the leftover can be stored protected from light at 4 °C and used within a week.
4′,6-Diamidino-2-phenylindole (DAPI) stock solution (1 mg/mL): dissolve 1 mg of DAPI in 1 mL of distilled water.
DAPI working solution (10 μg/mL): add 0.1 mL of 1 mg/mL DAPI stock solution into 9.9 mL of PBS (see Note 8). These solutions can be kept at 4 °C for months, protected from light.
Vectashield mounting medium for fluorescence (Vector Laboratories Inc., Burlingame, CA, USA, cat. # H-1000) (see Note 8).
Nail polish for sealing the coverslips.
2.3.3. Preparing Cells for In Vitro MRI
Use 1.5 mL tubes, 96- or 24-well plates (see Note 9).
15 % Gelatin: dilute 1.5 g of gelatin in 10 mL of distilled water. Use a water bath, at 60 °C, and the vortex to dissolve this solution. With the gelatin still hot, quickly make aliquots of 200 μL in 1.5 mL tubes, 96- or 24-well plates. Always prepare a fresh solution and only prepare it when the cells are ready to be mixed (as described in Subheading 3.3.3).
2.4. Analyzing the Labeling Efficacy by Microscopy
Light microscope equipped with a color camera for recording Prussian Blue images.
Fluorescence microscope (e.g., Axiovert 200M, Zeiss, GmbH) equipped with a camera for immunostaining experiments. For quantification, we recommend using a ×20 magnification objective.
2.5. Analyzing the Labeling Efficacy by MRI
For MRI an MRI scanner with appropriately sized imaging coils is required. We used a 9.4 T vertical bore system with a microimaging accessory but a horizontal research magnet or lower field clinical magnet could also be used (see Note 10).
Dedicated spectrometer software or other analysis programs such as Image J (from U.S. National Institutes of Health, http://rsbweb.nih.gov/ij/), and MATLAB (The Mathworks, Natick, MA) based programs are required to evaluate intensity differences between samples.
3. Methods
Carry out all procedures at room temperature unless otherwise specified.
3.1. Labeling of Adherent Cells (See Note 11)
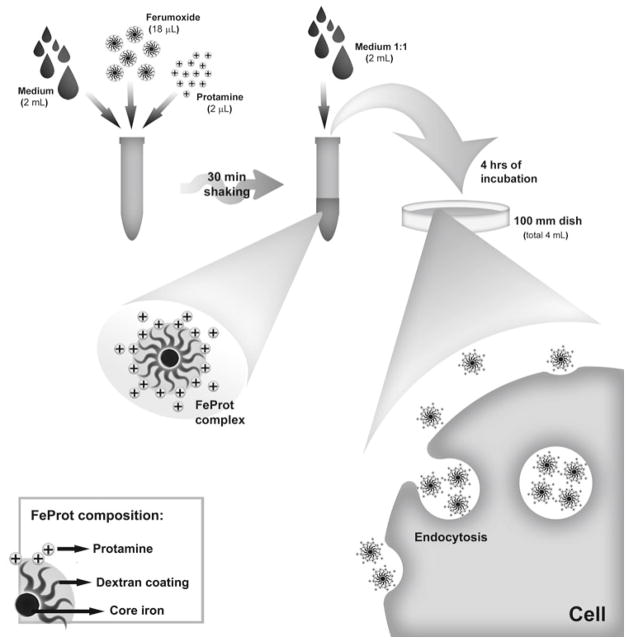
The experimental procedures are summarized in Fig. 1. The effect of protamine on cell labeling is illustrated in Fig. 2.
Fig. 1.
Derivatization of SPION nanoparticles and labeling of cells. Schematic diagram representing the steps optimizing FeProt complex formation and incorporation by cells.
Fig. 2.
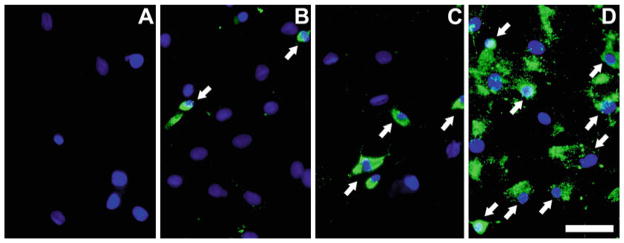
Importance of facilitator agents for labeling cells with SPIONs. Representative images showing that cationic compounds, such as protamine, are necessary for the efficient uptake of SPIONs. Detection of SPIONs is with anti-dextran antibody. (a) Mesenchymal stem cells incubated only with protamine without ferumoxides. (b) Cells incubated with ferumoxides in the absence of facilitating agent. (c) Cells exposed to FeProt complexes using low protamine concentration (1 μg/mL), which is five times less than the recommended concentration (5 μg/mL). (d ) Mesenchymal cells incubated with FeProt complexes with 5 μg/mL protamine. The arrows are showing some of the ferumoxide-labeled cells. Cell nuclei are labeled blue with DAPI; no cells with SPIONs are detected in (a ), approximately 7 % cells contain SPIONs in (b ), and 95 % in (d). We did not quantify the percentage of labeled cells in group (c). Scale bar = 50 μm.
Preparing the labeling solution: the final concentrations of SPIONs and protamine are 50 and 5 μg/mL respectively. These should always be prepared fresh as described below. Figure 2 illustrates the importance of using the recommended concentrations of SPIONs and protamine.
For preparation of 4 mL of labeling solution (see Note 12): transfer 2 mL of FBS-supplemented culture medium (see Note 2) to a 15 mL conical tube, add 18 μL of SPIONs and 2 μL of protamine. Transfer the tube containing the labeling solution to a shaker and gently shake for 30 min at room temperature. Subsequently, add 2 mL more of culture medium to the labeling solution (1:1, v:v) and very gently mix the solution using a P1000 pipette.
Replace the culture medium with labeling solution and maintain the cells in the incubator in 5 % CO2 atmosphere at 37 °C for 4 h.
Gently and quickly wash three times with PBS for elimination of non-incorporated SPIONs (no extended incubation with PBS is necessary). At this point the cells are ready to be fixed for Prussian Blue and/or immunostaining or to be transplanted (see Note 1) or detected in vitro by MRI.
3.2. Fixing Cells for Evaluation by Staining
Transfer coverslips into the wells of a 24-well plate, add 300 μL of 0.2 % gelatin to cover the coverslips and incubate for 10 min at room temperature or in the incubator at 37 °C. Aspirate the gelatin and maintain the plate for another 10 min at room temperature or in the incubator at 37 °C, at this point the plate is ready to receive the culture medium and the cells (see Note 13).
For SPION incorporation in 24-well plate use the same protocol as detailed above for 100 mm dish plate, but scale all components down. The final volumes to use are 300 μL of medium, 1.3 μL of SPIONs and 0.15 μL of protamine for each well.
To fix the cells, add 300 μL of 4 % formaldehyde to cover the coverslips in the 24-well plate and incubate for 20 min at 37 °C or at room temperature. Remove the formaldehyde and gently and quickly wash two times with PBS. At this point the cells are attached to coverslips and are ready to be stained.
To store the fixed cells, fill the wells with PBS and store plates at 4 °C for up to several months (see Note 14).
3.3. Determining Labeling Efficiency
Below we describe how to detect the SPIONs by Prussian Blue and/or immunostaining (Fig. 3), and how to prepare the cells for in vitro MRI (Fig. 4) (see Note 4).
Fig. 3.
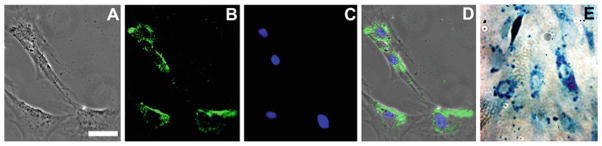
Anti-dextran and Prussian Blue staining. Representative images of cells labeled with FeProt complexes for 4 h. (a–d) Mesenchymal stem cells immunostained for anti-dextran antibody. (a) Brightfield; (b ) anti-dextran antibody; (c) nuclei counterstaining with DAPI; and (d) merged images. (e) Labeled cells stained with Prussian Blue showing the presence of iron. Note the characteristic perinuclear distribution of SPIONs revealed by both staining methods. Scale bar = 50 μm.
Fig. 4.
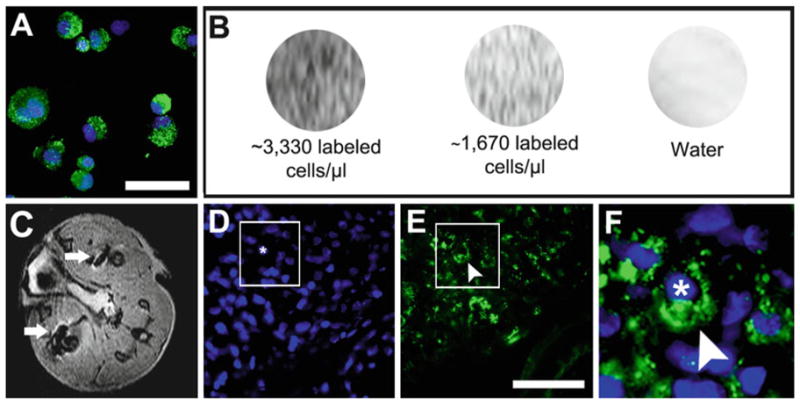
Detecting SPION labeled cells using MRI in vivo and in vitro. (a ) Appearance of FeProt labeled cells after trypsinization and immunolabeling for dextran. (b ) In vitro MRI of cells labeled for 4 h at different concentrations of cells/μL gelatin. (c) Representative image of in vivo MRI (transverse plane) showing hypointense (black) spots corresponding to ferumoxide-labeled cells injected in the leg muscles (white arrows). (d–f) Dextran immunocytochemistry confirming the presence of ferumoxide-labeled cells in sections of leg muscle; (d) nuclear counterstaining with DAPI; (e) dextran; (f) merged images in higher-magnification of the area indicated by the box in (d ) and (e ) showing a transplanted cell (nucleus marked with an asterisk) labeled with ferumoxide (arrowhead). Scale bar = 50 μm.
3.3.1. Prussian Blue Staining
Wash the coverslips with fixed cells in 24-well plate twice with deionized water for 5 min and incubate with Perl’s solution for 20 min.
Quickly wash the cells once with deionized water, incubate with 1 % Nuclear Fast Red for 5 min for nuclei counterstaining, and then quickly wash once more.
Dehydrate the cells using quick washes with 35 % ethanol, followed by 70 % ethanol and absolute ethanol. Immediately after that, quickly wash the cells once with the absolute ethanol:xylene (1:1) solution and twice with xylene alone (see Note 15) to remove the alcohol.
Mount the cover slips in slides with a drop of Entellan mounting medium, avoid having thick Entellan layer (see Note 16). Allow the mounting medium to dry (usually 20–30 min) at which time the cells are ready for imaging.
3.3.2. Immunostaining with Anti-dextran Antibody
Here we describe an immunostaining protocol adapted for immunofluorescence staining of SPIONs.
Wash the coverslips with fixed cells three times with PBS/0.1 % Triton X-100 for 5 min, incubate with 5 % normal goat serum for 30 min, and then incubate with the anti-dextran antibody overnight at 4 °C in a humid chamber to avoid the antibody drying.
Wash the coverslips three times in PBS/0.1 % Triton X-100 for 5 min and then incubate with an anti-mouse secondary antibody for 1 h 30 min at room temperature, in a humidified chamber.
Wash three times with PBS without Triton for 5 min, incubate the cells for 5 min with DAPI for nuclei counterstaining (see Note 8) and wash once more with PBS without Triton.
Remove the excess PBS from the coverslips with an absorbent paper (do not touch the cells with the paper) and mount with a drop of Vectashield mounting medium per coverslip. Remove the excess mounting medium with an absorbent paper and seal the edges of the coverslips with nail polish (Fig. 3a–d). This technique can be used for localization of labeled cells after transplant, such as demonstrated in Fig. 4d–f.
3.3.3. Preparing Cells for In Vitro MRI
All procedures are for 100 mm dish plates.
Trypsinize the cells labeled with derivatized SPION nanoparticles (as described in Subheading 3.1), collect and centrifuge cells at 300 × g for 5 min, and remove all of the supernatant taking care not to aspirate the cells.
Resuspend the cells in 1 mL of 4 % formaldehyde and incubate for 20 min at 37 °C or at room temperature.
Centrifuge the cells once more, remove all supernatant, resuspend in 300 μL of PBS and count the cells. From this point, samples with different concentrations of cells should be prepared, for example, dilute 106, 105, 104 and 103 cells per 100 μL of PBS (Fig. 4b).
Cool down the 200 μL aliquots of 15 % gelatin (see Note 9) from 60 °C down to 40 °C by keeping the tubes at room temperature.
Immediately add the 100 μL solution containing PBS and fixed cells to the gelatin aliquots and mix the solutions well. The final concentration of gelatin is 10 % and the final concentration of cells is 3,330, 333, 33 and 3.3 cells/μL.
At this point, the cells are ready to be imaged by MRI (see Note 17). Cells can be stored at 4 °C for future imaging if necessary.
3.4. Analyzing the Labeling Efficiency by Microscopy
Acquire random images of each sample (cells on coverslip) using a 20× objective (see Note 18).
Count the total number of cells within view by counting stained nuclei (nuclear fast red for Prussian Blue, and DAPI for immunofluorescence) (see Note 19).
Count the number of labeled cells. To estimate the percentage efficiency, divide the number of labeled cells by the total number of cells counted.
3.5. Analyzing the Labeling Efficiency by MRI
Contrast in MRI depends on the relaxation properties of the 1H nuclei, mainly in water, in the cells and tissues. Longitudinal relaxation, T1, is the time it takes for the 1H nuclei to relax back to their equilibrium value after perturbation with a radiofrequency pulse. Transverse or spin-spin relaxation, T2 and T2* (T2* includes additional effects caused by inhomogeneities in the static magnetic field), results from molecular interactions and causes a loss of detectable magnetization. T1, T2 and T2* weighted imaging methods are designed to detect differences in the relaxation properties, thus, a fluid filled cyst will appear bright on a T2-weighted image and dark on a T1-weighted image because it has long T2 and T1 relaxation times. On the other hand, a solid mass, having a short T2 and long T1, will appear bright on a T2-weighted image but dark on a T1-weighted image. In contrast, fat appears bright in T1-weighted images and dark in T2-weighted images because it has short T1 and T2 relaxation times. SPIONs, such as ferumox-ides, are negative contrast agents. They cause a reduction in the T2 and T2* relaxation time of the nearby water (see Note 20) and appear dark in T2-weighted images. Thus detection of SPION labeled cells by MRI is typically accomplished using T2-weighted imaging methods.
Optimize magnetic field homogeneity of the cell sample and adjust MRI parameters (echo time and repetition time for optimal contrast). Although a spin-echo imaging sequence can be used, the T2* weighted gradient echo imaging sequence is recommended, particularly at higher field (~9.4 T) (see Note 21).
Acquire several thin (0.5 mm) contiguous image slices with optimized imaging parameters (see Note 10).
Quantify mean intensity of the cells to determine labeling efficiency. Use software provided by the MRI manufacturer, or free software, such as ImageJ. The image file is read into the software and the region of interest containing the labeled cells is selected. The intensities of all pixels within the region of interest are calculated and minimum, maximum and mean intensity is reported. Depending on the sample configuration several image slices may be analyzed and the results averaged.
Footnotes
Here we focus on cell labeling. Cell transplantations techniques and in vivo cell tracking procedures as such are beyond the scope of this paper.
Most authors report preparing the labeling solution using culture medium without FBS. We routinely use culture medium supplemented with FBS, and this has not affected cell labeling.
Protamine chlorhydrate can be used instead. For example, different countries use different types of protamine salts, for example, in USA, protamine sulfate is clinically approved by Food and Drug Administration (FDA). However, in Brazil, protamine chlorhydrate is clinically approved by The National Health Surveillance Agency (ANVISA) and it has the same clinical function as protamine sulfate to rescue the heparin effect.
Different types of cells can capture different amounts of SPIONs. The incorporation rate depends on the characteristics of the cells, such as the size of the cytoplasm and capacity for endocytosis. It is important to determine whether the cells can be detected by the MRI technique.
To detect SPION incorporation into cells, either Prussian Blue and immunostaining for anti-dextran antibody are very efficient. The Prussian Blue technique detects the iron nano-particles, while anti-dextran antibody recognizes the dextran coating. For quantification we usually use the immunostaining technique because we consider it more convenient. However, it has been suggested that the dextran coating can undergo degradation when taken up by macrophages (33). Thus, a limitation of this technique is a potential underestimation of the number of labeled cells. If immunostaining is used for quantification, we recommend that Prussian Blue staining be done in some samples to confirm the iron presence inside the cells.
In this step is important to put the water into the vessel before the acid.
Other secondary antibody brands can be use but the ratio of antibody to PBS depends on the chosen antibody brand.
As an alternative for a separate DAPI solution, the Vectashield mounting medium can be replaced by Vectashield mounting medium for fluorescence containing DAPI (Vector Laboratories Inc., cat. # H-1200).
We have prepared the gelatin aliquots in 1.5 mL tubes because our MRI system is a vertical bore with a limited coil size that does not accommodate 24- or 96-well flat plates. However for a horizontal MRI system a plate may be more convenient.
For our study we used a 9.4 T magnet and imaged four 1.5 mL tubes of labeled cells at a time by mounting the tubes within a 50 mL conical tube and positioning that in a 40 mm birdcage imaging coil. We used a spin-echo sequence with a slice thickness of 0.5 mm, TR = 1 s, TE = 15 ms, and acquired four contiguous slices (Fig. 4b).
We recommend using the cells at 80–90 % confluence, regardless of the size of the culture plates.
4 mL of labeling solution is needed for each 100 mm Petri dish plate.
For immunostaining or Prussian Blue staining of cells we used a 24-well plate with gelatin-coated coverslips. Other cell types require a specialized culture plate coating, for example, PLL, laminin or fibronectin. In that case, instead of gelatin, treat the coverslips with the coating required.
There is no need to maintain sterile conditions at this stage of experiment.
. In this step, work in a fume hood exhaust system and perform the coverslip washes in a glass container using a glass pipette because xylene corrodes plastic.
. Entellan is a hydrophobic medium that preserves the reaction for a longer time. However, the Entellan layer cannot be too thick because of the objective focal distance. High magnification objectives have a quite small working distance and a thick Entellan layer can prevent obtaining focused images. To obtain a thin layer, a light pressure can be applied to the coverslips and a small weighted object (smaller than the diameter of the coverslip) can be left on the coverslips until the Entellan dries.
. We suggest that non-labeled cells be prepared for in vitro MRI as a background control.
. For labeling efficiency and statistical analyses we recommend that at least three independent experiments be performed. In each experiment two to three samples (coverslips) should be prepared. From each sample we recommend that 6–8 images from different areas be acquired for quantification.
. For immunofluorescence quantification we strongly recommend the acquisition of brightfield images. These images can be used to determine the membrane boundaries and to distinguish dextran-positive cells from the background.
. An excellent review with detailed information on the practical issues involved in the MRI detection and quantification of cells labeled with SPIO nanoparticles and other contrast agents is cited in ref. (34).
At 9.4 T Smirnov et al. showed that the gradient echo sequence was superior to the spin echo sequence which had poor sensitivity at the higher field (35).
References
- 1.Guzman R, et al. Long-term monitoring of transplanted human neural stem cells in developmental and pathological contexts with MRI. Proc Natl Acad Sci U S A. 2007;104:10211–10216. doi: 10.1073/pnas.0608519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sykova E, Jendelova P. Migration, fate and in vivo imaging of adult stem cells in the CNS. Cell Death Differ. 2007;14:1336–1342. doi: 10.1038/sj.cdd.4402140. [DOI] [PubMed] [Google Scholar]
- 3.Hoehn M, et al. Monitoring of implanted stem cell migration in vivo: a highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc Natl Acad Sci U S A. 2002;99:16267–16272. doi: 10.1073/pnas.242435499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dodd SJ, et al. Detection of single mammalian cells by high-resolution magnetic resonance imaging. Biophys J. 1999;76:103–109. doi: 10.1016/S0006-3495(99)77182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jendelova P, et al. Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J Neurosci Res. 2004;76:232–243. doi: 10.1002/jnr.20041. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro EM, et al. MRI detection of single particles for cellular imaging. Proc Natl Acad Sci U S A. 2004;101:10901–10906. doi: 10.1073/pnas.0403918101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrell E, et al. Effects of iron oxide incorporation for long term cell tracking on MSC differentiation in vitro and in vivo. Biochem Biophys Res Commun. 2008;369:1076–1081. doi: 10.1016/j.bbrc.2008.02.159. [DOI] [PubMed] [Google Scholar]
- 8.Modo M, et al. Tracking transplanted stem cell migration using bifunctional, contrast agent-enhanced, magnetic resonance imaging. Neuroimage. 2002;17:803–811. [PubMed] [Google Scholar]
- 9.Lewin M, et al. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol. 2000;18:410–414. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- 10.Dodd CH, et al. Normal T-cell response and in vivo magnetic resonance imaging of T cells loaded with HIV transactivator-peptide-derived superparamagnetic nanoparticles. J Immunol Methods. 2001;256:89–105. doi: 10.1016/s0022-1759(01)00433-1. [DOI] [PubMed] [Google Scholar]
- 11.Ahrens ET, et al. Receptor-mediated endocytosis of iron-oxide particles provides efficient labeling of dendritic cells for in vivo MR imaging. Magn Reson Med. 2003;49:1006–1013. doi: 10.1002/mrm.10465. [DOI] [PubMed] [Google Scholar]
- 12.Frank JA, et al. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003;228:480–487. doi: 10.1148/radiol.2281020638. [DOI] [PubMed] [Google Scholar]
- 13.Weissleder R, et al. Superparamagnetic iron oxide: pharmacokinetics and toxicity. AJR Am J Roentgenol. 1989;152:167–173. doi: 10.2214/ajr.152.1.167. [DOI] [PubMed] [Google Scholar]
- 14.Molday RS, MacKenzie D. Immunospecific ferromagnetic iron-dextran reagents for the labeling and magnetic separation of cells. J Immunol Methods. 1982;52:353–367. doi: 10.1016/0022-1759(82)90007-2. [DOI] [PubMed] [Google Scholar]
- 15.Kim HS, et al. The effects of clinically used MRI contrast agents on the biological properties of human mesenchymal stem cells. NMR Biomed. 2010;23:514–522. doi: 10.1002/nbm.1487. [DOI] [PubMed] [Google Scholar]
- 16.Modo M, et al. Mapping transplanted stem cell migration after a stroke: a serial, in vivo magnetic resonance imaging study. Neuroimage. 2004;21:311–317. doi: 10.1016/j.neuroimage.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 17.Brekke C, et al. The in vitro effects of a bimodal contrast agent on cellular functions and relaxometry. NMR Biomed. 2007;20:77–89. doi: 10.1002/nbm.1077. [DOI] [PubMed] [Google Scholar]
- 18.Greisberg JK, et al. Gadolinium inhibits thymidine incorporation and induces apoptosis in chondrocytes. J Orthop Res. 2001;19:797. doi: 10.1016/S0736-0266(01)00025-0. [DOI] [PubMed] [Google Scholar]
- 19.Babic M, et al. Poly(L-lysine)-modified iron oxide nanoparticles for stem cell labeling. Bioconjug Chem. 2008;19:740–750. doi: 10.1021/bc700410z. [DOI] [PubMed] [Google Scholar]
- 20.Arbab AS, et al. Intracytoplasmic tagging of cells with ferumoxides and transfection agent for cellular magnetic resonance imaging after cell transplantation: methods and techniques. Transplantation. 2003;76:1123–1130. doi: 10.1097/01.TP.0000089237.39220.83. [DOI] [PubMed] [Google Scholar]
- 21.Jasmin, et al. Optimized labeling of bone marrow mesenchymal cells with superparamagnetic iron oxide nanoparticles and in vivo visualization by magnetic resonance imaging. J Nanobiotechnology. 2011;9:4. doi: 10.1186/1477-3155-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arbab AS, et al. Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood. 2004;104:1217–1223. doi: 10.1182/blood-2004-02-0655. [DOI] [PubMed] [Google Scholar]
- 23.Janic B, et al. Optimization and validation of FePro cell labeling method. PLoS One. 2009;4:e5873. doi: 10.1371/journal.pone.0005873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Buul GM, et al. Ferumoxides-protamine sulfate is more effective than feru-carbotran for cell labeling: implications for clinically applicable cell tracking using MRI. Contrast Media Mol Imaging. 2009;4:230–236. doi: 10.1002/cmmi.289. [DOI] [PubMed] [Google Scholar]
- 25.Qiu B, Yang X. Molecular MRI of hematopoietic stem-progenitor cells: in vivo monitoring of gene therapy and atherosclerosis. Nat Clin Pract Cardiovasc Med. 2008;5:396–404. doi: 10.1038/ncpcardio1217. [DOI] [PubMed] [Google Scholar]
- 26.Walczak P, et al. Instant MR labeling of stem cells using magnetoelectroporation. Magn Reson Med. 2005;54:769–774. doi: 10.1002/mrm.20701. [DOI] [PubMed] [Google Scholar]
- 27.Tai JH, et al. Imaging islets labeled with magnetic nanoparticles at 1.5 Tesla. Diabetes. 2006;55:2931–2938. doi: 10.2337/db06-0393. [DOI] [PubMed] [Google Scholar]
- 28.Qiu B, et al. Magnetosonoporation: instant magnetic labeling of stem cells. Magn Reson Med. 2010;63:1437–1441. doi: 10.1002/mrm.22348. [DOI] [PubMed] [Google Scholar]
- 29.Arbab AS, et al. Labeling of cells with ferumoxides-protamine sulfate complexes does not inhibit function or differentiation capacity of hematopoietic or mesenchymal stem cells. NMR Biomed. 2005;18:553–559. doi: 10.1002/nbm.991. [DOI] [PubMed] [Google Scholar]
- 30.Kostura L, et al. Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed. 2004;17:513–517. doi: 10.1002/nbm.925. [DOI] [PubMed] [Google Scholar]
- 31.Bull BS, et al. Heparin therapy during extracorporeal circulation. II. The use of a dose-response curve to individualize heparin and protamine dosage. J Thorac Cardiovasc Surg. 1975;69:685–689. [PubMed] [Google Scholar]
- 32.Gervin AS. Complications of heparin therapy. Surg Gynecol Obstet. 1975;140:789–796. [PubMed] [Google Scholar]
- 33.Bourrinet P, et al. Preclinical safety and pharmacokinetic profile of ferumoxtran-10, an ultrasmall superparamagnetic iron oxide magnetic resonance contrast agent. Invest Radiol. 2006;41:313–324. doi: 10.1097/01.rli.0000197669.80475.dd. [DOI] [PubMed] [Google Scholar]
- 34.Liu W, Frank JA. Detection and quantification of magnetically labeled cells by cellular MRI. Eur J Radiol. 2009;70:258–264. doi: 10.1016/j.ejrad.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smirnov P, et al. Single-cell detection by gradient echo 9.4 T MRI: a parametric study. Contrast Media Mol Imaging. 2006;1:165–174. doi: 10.1002/cmmi.104. [DOI] [PubMed] [Google Scholar]