Abstract
This prospective study evaluated the impact that uncertainty has on quality of life as cancer patients end the active phase of their treatment. The transition from patient status to survivor may be a particularly important point in recovery because it may affect how much stress cancer survivors experience. Guided by a within-subjects design framework, 53 cancer patients (predominantly breast cancer) participated in the present study as they approached the end of adjuvant treatment and were followed for 4 months. Distress levels increased the further patients moved away from the end-of-active treatment; however, preceding these ascending scores of distress was a period characterized by little unrest—best described as a “honeymoon” phase. Using hierarchical linear modeling, greater uncertainty 1 month after treatment ended predicted more functional and physical impairment 4 months after the completion of adjuvant treatment. In both cases, uncertainty accounted for nearly 70% of the variance of these changes over time. A period of rest may emerge as active treatment ends, but that it is short-lived, particularly if uncertainty regarding health emerges. Furthermore, uncertainty may a represent a key mechanism (and target for intervention) during the transition from cancer patient to survivor.
Keywords: Cancer survivor, Emotional distress, Quality of life, Uncertainty
The successful completion of adjuvant treatment marks the beginning of the transition from cancer patient to cancer survivor. The intensity and frequency of medical support typically lessen as necessary treatments are completed. In turn, many face and must adjust to different sources of stress, treatment sequelae (eg, fatigue, physical changes in appearance, and pain), social stigmatization, changes in lifestyle, social disruption, fears about disease recurrence, and uncertainty about their disease.1–8 Although this point in the treatment of cancer has received little attention, prior research has observed a considerable level of uncertainty in light of difficulties in making sense of the disease and potential outcomes.9 To the best of our knowledge, there have been no studies that have focused on uncertainty as a determinant of quality of life (QOL) at this point in the cancer trajectory.
Of the few studies conducted, investigators have assumed a unidirectional conceptual perspective of uncertainty in that they have examined the impact of symptoms on uncertainty. This perspective is based upon the premise that uncertainty arises in response to cognitive appraisal of an ambiguous event for which the outcome is unclear.10–13 In a cross-sectional design, Tsui-Hsia and colleagues14 compared lung cancer patients with pain to those without pain and found the former to report greater levels of uncertainty. The investigators cited a moderately high level of uncertainty in their entire sample compared with other samples in which the prognoses were better established. In another cross-sectional study, Mast15 found reported fatigue to be highly linked to uncertainty in illness. Level of education appeared to moderate this relationship as women with more education had less uncertainty than did women with less education. Recent efforts have found that disease severity may influence perceived QOL.16 In each of these cases, the role of uncertainty appeared to influence symptoms that play a tremendous role in cancer survivors’ lives. However, the study design prevented the follow-up of the course of symptoms or uncertainty over time. Of the prospective studies, evidence suggests that for some cancer patients, their levels of uncertainty vary well beyond the end of treatment.17
The sparse research in this area is surprising in view of heightened levels of uncertainty after the completion of adjuvant cancer treatment. Investigation of the influence of uncertainty might assist in identifying those patients at risk for compromised well-being during long–term remission and guide the development of psychosocial interventions for patients shortly after treatment. The present study sought to investigate the impact that uncertainty has on QOL as cancer patients make the transition from patient to survivor. In particular, the aims of the present study include the following:
To determine whether uncertainty would be associated with distress and negatively associated with social support. The effects of treatment type, stage, socioeconomic status, age, coping, and other factors were considered in the initial evaluation.
To determine what psychosocial factors (ie, general distress, traumatic stress related to disease, optimism, and uncertainty) before and after the end of treatment would predict QOL 4 months after treatment has ended.
Methodology
Materials and Methods
A total of 53 patients approaching completions of active adjuvant therapy for cancer participated in this within-subjects design, prospective study. We recruited primarily from a local cancer center in which members of the treatment team identified eligible candidates and informed them of the study. Those involved with recruitment assured potential candidates that they were under no obligation to participate and that refusal to participate would not affect the quality of their care. This contact with patients took place approximately 1 month before the projected end of their treatment. We excluded patients completing treatment for recurrent disease because recurrence would have likely affected expectations and concerns regarding survival and may have reduced the homogeneity of the systematic variance. For similar reasons, we excluded patients with metastases. Although the investigators of the present study made many efforts to include minorities, the study sample was predominantly white. The racial makeup of the sample was consistent with the geographic region from which the investigators recruited. Institutional review board approval was sought and received from all participating agencies.
Procedure
Data collection took place at the medical center from where participants completed their treatment, although provisions were made to meet with patients in their homes if they could not meet with research staff at the medical center. A qualified member of the research team contacted those who agreed to receive a call regarding the study and addressed any remaining questions. If still interested, the research team member scheduled an initial appointment with the participant. This session was scheduled 2 weeks before the patient's final scheduled treatment. At that session, once we obtained informed consent, the participant completed a brief psychometric battery of instruments and provided key demographic data. Participants were then seen twice more: 1 and 4 months after the initial assessment. Each assessment took approximately 45 minutes. Participants received $30 for each completed session ($90 total) for their participation.
Materials
Participants completed several established self-report measures at each time point, including measures of uncertainty, general and trauma-related distress, and QOL. These measures have been used previously with cancer populations and, in some cases, were designed specifically to address the psychosocial issues of cancer.
TRAUMATIC STRESS
The Impact of Event Scale is a 15–item self–report scale that assesses 2 common responses to stressful events: intrusion (re–experiencing images of a stressor, uncontrollable thoughts, ideas, feelings, or unpleasant dreams related to the event) and avoidance (consciously recognized avoidance of certain ideas, feelings, or situations).18 Participants used end of treatment as the potential trauma/major stressor in responding to the measure's items. The Impact of Event Scale demonstrates good to excellent reliability (.79–.90).
PERCEIVED STRESS
The Perceived Stress Scale is a 10-item instrument developed to measure general perceptions of stress, demand, and control.19 The Perceived Stress Scale has been found to have adequate internal and test-retest reliabilities, as well as good predictive validity.
OPTIMISM
The Life Orientation Test (10-item version) was used to assess optimism.20 This is a widely used instrument with good psychometric properties.
UNCERTAINTY
The Uncertainty in Illness Scale (UIS) is a 30–item inventory that measures uncertainty and ambiguity in symptoms, diagnosis, treatment, relationship with caregivers, and planning for the future. It assesses 4 dimensions (ambiguity, complexity, inconsistency, and unpredictability) on a 5-point Likert scale (1, strongly disagree to 5, strongly agree). Reliability of the scale has been found to be adequate to excellent (.64–.91) and demonstrates good validity.12,21
QUALITY OF LIFE
The Functional Assessment of Cancer Therapy–General (FACT–G) is a 33–item general QOL measure for evaluating patients who have undergone cancer treatment.22 The FACT-G contains 4 subscales: (a) physical well-being, (b) social/family well-being, (c) emotional well-being, and (d) functional well-being. Coefficients of reliability and validity of the FACT–G are uniformly high and have been determined to be sufficient for research purposes.
Study Framework and Statistical Approach
The use of a within-subjects study design affords 2 major conceptual advantages. First, such a framework allows for increased power without having to increase the sample size, as would be required in a between-subjects design. Second, this framework reduces the amount of error variance associated with individual differences. That is, by prospectively following the same individuals over a set period of time, each participant, in a sense, is serving as his/her own control, and any observed changes in outcome variables are not due to an unidentified third variable that might plague a between-subjects framework. With a within-subjects design guiding this empirical investigation, hierarchical linear modeling (HLM) procedures were used to account for the hierarchical structure of the data to estimate the baseline and long-term models. We fit HLM models to the 2 data points spanning a 4-month period. An HLM analysis involves 2 levels: a lower level (a series of repeated measures) and an upper level (individual growth parameters become a set of outcome variables).23 This approach differs from regression in that it allows for a simultaneous estimation of both levels. The means are presented as log odds of the transition that patients make over time. For the observations of each patient, this within-unit relationship was represented by the slope for well-being (as measured by the FACT-G subscales). Subsequently, each within-unit relationship was regressed on each of the 3 time points.
Results
The present study examined variability in QOL by determining whether changes in stress and uncertainty predicted changes in life quality shortly after the end of the active phase of treatment. Analytic strategies first involved examining only female patients with breast cancer. However, when we compared these outcome results with those involving the entire sample (ie, patients with other forms of cancer), we found that the subgroup of breast cancer patients did not differ from the entire sample. Thus, we reported the study results based on the sample as a whole rather than focus exclusively on 1 cancer site.
Aim 1: Repeated-Measures Analysis of Variance
The means and standard deviations for the subscales of the FACT-G at all 3 time points are presented in Table 1. The change in score for each subscale over time is more than what would be expected. In terms of physical functioning, analyses of variance revealed a slight decline in scores (reflecting improved physical well-being) from before active treatment ended to 4 months later (F = 9.83, P < .05). Similarly, patients’ scores reflected a decline in social/family well-being the further they moved away from active treatment (F = 9.01, P < .05). However, the other 2 domains did not yield a similar pattern. Instead, scores on the functional well-being subscale of the FACT-G increased (reflecting improved functional well-being) during the first month after the end of active treatment only to significantly decrease 3 months later at the final time point (F = 12.33, P < .01). In terms of emotional well-being, scores also showed a decline (reflecting improved emotional well-being) during the first month after active treatment but increased 3 months later at the final time point (F = 11.37, P < .01).
Table 1.
Mean (SD) of the Functional Assessment of Cancer Therapy–General Subscales at Each Time Point
| Time | Physical T1 | Social T1 | Emotional T1 | Functional T1 |
|---|---|---|---|---|
| 0 | 18.75 (3.83) | 23.81 (4.54) | 18.94 (4.20) | 19.94 (5.40) |
| 1 | 19.94 (3.53) | 21.88 (4.46) | 16.66 (3.66) | 21.28 (4.79) |
| 2 | 21.41 (3.49) | 20.93 (6.48) | 19.63 (4.15) | 20.44 (6.42) |
Aim 2: Hierarchical Linear Modeling
Data analyses involved conducting HLM for each subscale of the FACT-G. At level 1, we established a baseline for each dimension of QOL. Using uncertainty, perceived stress, and trauma-related stress as predictors, and controlling for time between T0 and T1, these within-unit relationships were regressed on each of the time points. Dummy variables were used to nest data for cancer survivors at each time point.
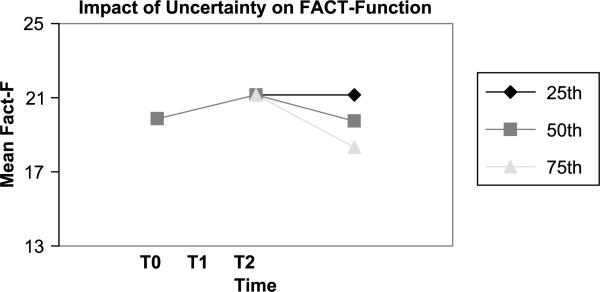
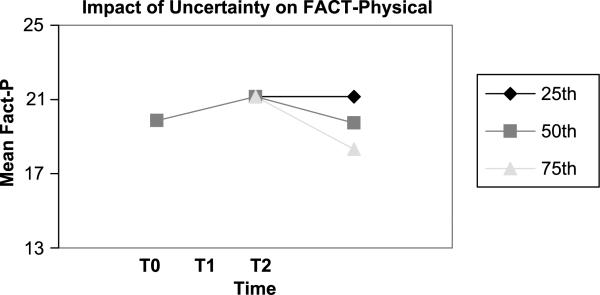
At the baseline visit, none of the predictors accounted for variation in any of the FACT-G subscales. In contrast, for both T1 (1 month after active treatment) and T2 (4 months after active treatment), uncertainty accounted for 70% of the variance for functional well-being and 60% of the variance for physical well-being (see Tables 2 and 3). The same modeling was applied to the social and emotional well-being subscales of the FACT-G; however, neither uncertainty nor any other variables predicted change in these domains of life quality after active treatment had ended. The modeling revealed that every 1.25 increase in score on the UIS resulted in a 25% decline in functional well-being (see Figure 1) and every 1.40 increase in score on the UIS resulted in a 25% decline in physical well-being.
Table 2.
Model for the Functional Well-Being Subscale of the Functional Assessment of Cancer Therapy–General
| Baseline |
Model A |
Model B |
|
|---|---|---|---|
| Coefficient (SE) | Coefficient (SE) | Coefficient (SE) | |
| Fixed effects | |||
| Baseline | 19.87 (0.91) | 19.87 (0.97)a | 19.87 (0.76)a |
| Uncertainty—T1 | – | – | -0.19 (0.03)a |
| Time 1-time 2 | 1.39 (0.62) | 1.39 (0.62)b | 1.39 (0.62)b |
| Time 2-time 3 | –1.08 (.83) | –1.25 (0.66) | –1.26 (0.66) |
| Uncertainty—T2 | – | –0.20 (0.06)a | –0.16 (0.07)b |
| Variance components | |||
| Level 1: within-person | 6.02 | 6.02 | 5.93 |
| Level 2: baseline | 19.91 | 19.91 | 10.09 |
| Level 2: slope at T2 | 6.14 | 0.65 | 0.52 |
| Deviance (DF) | 486.54 (7) | 473.05 (8) | 457.13 |
| ΔDeviance (df) | – | 13.49 (1), P < .001 | 15.92 (1), P < .000 |
Abbreviation: DF, degrees of freedom.
P < .01.
P < .05.
Table 3.
Model for the Physical Subscale of the Functional Assessment of Cancer Therapy–General
| Baseline |
Model A |
Model B |
|
|---|---|---|---|
| Coefficient (SE) | Coefficient (SE) | Coefficient (SE) | |
| Fixed effects | |||
| Baseline | 18.75 (0.88) | 18.75 (0.88)a | 18.75 (0.88)a |
| Uncertainty—T1 | – | – | 0.23 (0.02)a |
| Time 1-time 2 | 1.45 (0.37) | 1.45 (0.37)b | 1.45 (0.37)b |
| Time 2-time 3 | 1.63 (.29) | 1.51 (0.56) | 1.53 (0.83) |
| Uncertainty—T2 | – | 0.09 (0.14)a | 0.05 (0.18)b |
| Variance components | |||
| Level 1: within-person | 8.50 | 8.69 | 8.30 |
| Level 2: baseline | 18.75 | 18.75 | 12.35 |
| Level 2: slope at T2 | 8.41 | 0.75 | 0.78 |
| Deviance (DF) | 471.91 (7) | 456.11 (8) | 441.08 |
| ΔDeviance (df) | – | 15.80 (1), P < .000 | 15.03 (1), P < .001 |
P < .01.
P < .05.
Figure 1.
Decline in functional well-being per each unit increase in uncertainty.
In light of the significant amounts of variance of QOL accounted for by increases in uncertainty, further analyses were conducted to evaluate the correlations between uncertainty and the subscales of the FACT-G. These analyses revealed Pearson correlations (r) of 0.3 to 0.4 between the UIS and the FACT-G subscales.
Finally, 12 participants were lost to attrition. The reasons for withdrawing from the study generally stemmed from a lack of interest in continued participation or the time burden required by investigators. Although comparisons between those who completed all 3 time points and those who did not failed to yield any difference on any key psychosocial and/or demographic variable, the present “Results” section is based only on those who have successfully completed the entire study.
Discussion
The study was designed to evaluate the influence that uncertainty may have on life quality shortly after the end of adjuvant cancer treatment in patients without metastases, a period marking the beginning transition from being a cancer patient to being a cancer survivor. By excluding those with metastases, investigators sought to examine the effects of uncertainty among patients who may overall have a better prognosis than those whose disease had spread beyond the site of origin. The data supported the study aims to some extent. The initial assessment surprisingly showed that patients immediately prior to the completion of treatment experience little uncertainty regarding health, seemingly commensurate with what we would describe as a “honeymoon” phase as one begins to move away from the label of “patient.” Further supporting this honeymoon phase was the gradual decline in well-being as participants moved further from the end of active treatment.
Although there is growing evidence that disease–related sequelae persist well beyond treatment, the reasons for these long–term outcomes remain poorly understood. One possibility is that adjustment to diagnosis and treatment and the transition from patient status to survivor affect the quality of long–term adjustment to survival. Treatment is fraught with immediate, identifiable problems such as adverse effects or fatigue, and traumatic stress related to the diagnosis and threat to life therein complicates coping with them. In part, this is because the experience of having cancer is a traumatic stressor that often forces people to re–evaluate the assumptions they have routinely adopted. Traumatic stressors are extremely intense, require formidable coping responses, and challenge people to reorder or reassess a lifetime of experiences. These challenges can intensify to the point that they culminate into a full-blown cognitive crisis accompanied by emotional distress and hyperresponsivity. If unresolved at the end of treatment when new stressors appear, long–term adjustment may be compromised. Separation from frequent contact with the medical team may heighten overall psychological distress as well as uncertainty about the future and may reflect reduction of an important kind of social support. If this early adjustment affects long–term outcomes or predicts areas of vulnerability for cancer survivors, examination of determinants of adjustment and QOL at several points in the disease process is important.
Another common fear for cancer survivors is the threat of disease recurrence or new primary tumors. These fears are common among long–term survivors and are not unfounded. Although rates vary according to disease site, risks for second malignancies are considerable.24 This possibility suggests that cancer patients have many stressors with which they must cope during the trajectory of the disease course. Stress related to their disease appears to be most prominent. As treatment unfolds, acute distress associated with the threat of cancer and its treatment becomes more chronic and directed toward concerns about the future and different aspects of survival. Arguably, there is a strong need to understand the impact/consequence of this source of stress.
The transition from active treatment and patient status to survivor may be a particularly important point in recovery, and the quality of psychosocial adjustment at this point appears to affect the future well-being of cancer survivors. Where focused efforts to treat the disease and deal with the adverse effects of treatment had been primary stressors, survivors must now cope with more diffuse threats such as recurrence, social stigmatization, and the long–term consequences of medical treatment.17,25 Premorbid disposition, coping styles, and stress burden may also affect one's susceptibility to poor long–term adjustment. Research should address the extent to which these variables influence adjustment once treatment has ended and how well they predict long–term adjustment.
Given that the items on both the UIS and the functional and physical subscales of the FACT-G do not overlap in their content, it is possible that a causal relationship exists between uncertainty and these 2 life domains. There was considerable variability in QOL the further removed one was from the active phase of treatment, a period arguably in which there may be an attenuation of information received from the treatment team. This reduction of information may have increased the susceptibility for patients to underestimate their actual capabilities as well as misinterpret physical sensations.
Viewing all of the above findings together, uncertainty presented as a major source of distress that can compromise various domains of life quality and that often accompanies illness and its treatment.26 These data suggest that future investigations further address uncertainty as patients make the transition from patient to survivor. Recent uncertainty management interventions directed toward cancer survivors to address disease-related issues suggest that targeting uncertainty is helpful for cancer survivors.26–34 This finding bears the question as to whether the optimal time to introduce such interventions is immediately after the end of active treatment.
The study findings should be considered by taking certain of its limitations into consideration. First, despite having adequate power, the sample size was relatively small. Second, the selected methodology made no effort to compare the level of uncertainty from this sample to other cancer groups. The possibility exists that patients with already high levels of uncertainty sought to participate in this study to seek answers about their well-being and that patients with low levels of uncertainty were the least inclined to participate.
In summary, the subset of breast cancer patients had results commensurate with the entire sample that uncertainty represented a potent predictor of functional and physical well-being shortly after the active phase of treatment has ended. This finding does not minimize the heterogeneity of obstacles/challenges that cancer patients/survivors encounter during the disease trajectory, but it may speak to the global effect that uncertainty in illness may play on QOL.
Implications for Cancer Survivors and Clinicians
The present study proposes that healthcare providers should anticipate in cancer patients a brief reprieve from the distress shortly after treatment but that a decline in well-being may emerge as time passes since the end of active treatment. Healthcare providers might seek to reconsider their clinics’ policies and protocol for patients ending active treatment, specifically with regard to their follow-up procedures. Arguably, efforts shortly after treatment has ended to dampen the adverse effects of uncertainty may prolong the aforementioned reprieve.
Together, patients and healthcare providers should carefully evaluate the various domains of life quality and monitor the patterns of change within each domain after active treatment has ended. In terms of physical well-being, early difficulties (eg, fatigue, feeling ill, concerns with treatment side-effects, etc) should be monitored, as this domain may worsen over time as one experiences greater uncertainty related to one's health. A decline in social well-being (eg, unable to talk to others about one's illness and feeling distant from others) may arise from the reduced contact with a treatment team that played an immensely supportive role during an extraordinary time for the patient. Increased follow-up contact with patients may help them connect again with other members of their support system and may simultaneously reduce uncertainty by ensuring that their posttreatment questions are answered. Finally, immediate improvements in functional well-being (eg, meeting daily needs, feeling satisfied, etc) and emotional well-being (eg, improved mood, reduced anxiety, etc) are expected, but these improvements may be short-lived, and both these domains may actually worsen over time. If patients do report or exhibit signs that they are struggling with uncertainty, healthcare providers might use this as a potential marker for the need of psychosocial interventions to assist cancer patients struggling in making the transition to cancer survivor.
Figure 2.
Decreased physical well-being per each unit increase in uncertainty.
Acknowledgments
This work was supported by the Southwest Washington Medical Center/Washington State University Health Care Partnership.
References
- 1.Avis NE, Crawford S, Manuel J. Quality of life among younger women with breast cancer. J Clin Oncol. 2005;23(15):322–330. doi: 10.1200/JCO.2005.05.130. [DOI] [PubMed] [Google Scholar]
- 2.Bolin K. Health among long-term survivors of breast cancer–an analysis of 5-year survivors based on the Swedish surveys of living conditions 1979-1995 and the Swedish Cancer Registry 2000. Psychooncology. 2008;17(1):1–8. doi: 10.1002/pon.1189. [DOI] [PubMed] [Google Scholar]
- 3.Galloway SC, Graydon JE. Uncertainty, symptom distress, and information needs after surgery for cancer of the colon. Cancer Nurs. 1996;19(2):112–117. doi: 10.1097/00002820-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Ganz PA, Coscarelli A, Fred C, Kahn B, Polinsky ML, Petersen L. Breast cancer survivors: psychosocial concerns and quality of life. Breast Cancer Res Treat. 1996;38:183–199. doi: 10.1007/BF01806673. [DOI] [PubMed] [Google Scholar]
- 5.Hilton BA. The phenomenon of uncertainty in women with breast cancer. Issues Ment Health Nurs. 1988;9:217–238. doi: 10.3109/01612848809140926. [DOI] [PubMed] [Google Scholar]
- 6.Hilton BA. The relationships of uncertainty, control, commitment, and threat of recurrence to coping strategies used by women diagnosed with breast cancer. Behav Med. 1989;12(1):39–54. doi: 10.1007/BF00844748. [DOI] [PubMed] [Google Scholar]
- 7.Mishel MH. Perceived uncertainty and stress in illness. Res Nurs Health. 1984;7:163–171. doi: 10.1002/nur.4770070304. [DOI] [PubMed] [Google Scholar]
- 8.Mishel MH. Reconceptualization of the uncertainty in illness theory. Image J Nurs Sch. 1990;22(4):256–262. doi: 10.1111/j.1547-5069.1990.tb00225.x. [DOI] [PubMed] [Google Scholar]
- 9.Bailey JM, Nielsen BI. Uncertainty and appraisal of uncertainty in women with rheumatoid arthritis. Orthop Nurs. 1993;12(2):63–67. doi: 10.1097/00006416-199303000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Christman NJ. Uncertainty and adjustment during radiotherapy. Nurs Res. 1990;39(1):17–20. [PubMed] [Google Scholar]
- 11.Lazarus RS, Folkman S. Stress, Appraisal and Coping. Springer Publishing Company; New York, NY: 1984. [Google Scholar]
- 12.Mishel MH. Uncertainty in illness. Image J Nurs Sch. 1988;20(4):225–232. doi: 10.1111/j.1547-5069.1988.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 13.Wong CA, Bramwell L. Uncertainty and anxiety after mastectomy for breast cancer. Cancer Nurs. 1992;15:363–371. [PubMed] [Google Scholar]
- 14.Tsui-Hsia H, Meei-Shiow L, Tsung-Shang T, Chia-Chin L. The relationship of pain, uncertainty, and hope in Taiwanese lung cancer patients. J Pain Symptom Manage. 2003;26(3):835–842. doi: 10.1016/s0885-3924(03)00257-4. [DOI] [PubMed] [Google Scholar]
- 15.Mast ME. Survivors of breast cancer: illness uncertainty, positive reappraisal, and emotional distress. Oncol Nurs Forum. 1998;25(3):555–562. [PubMed] [Google Scholar]
- 16.Tomich PL, Helgeson VS. Is finding something good in the bad always good? Benefit finding among women with breast cancer. Health Psychol. 2004;23(1):16–23. doi: 10.1037/0278-6133.23.1.16. [DOI] [PubMed] [Google Scholar]
- 17.Nelson JP. Struggling to gain meaning: living with the uncertainty of breast cancer. Adv Nurs Sci. 1996;18(3):59–76. doi: 10.1097/00012272-199603000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Horowitz MJ, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979;41(3):209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1993;24(4):385–396. [PubMed] [Google Scholar]
- 20.Scheier MF, Carver CS. Optimism, coping and health: assessment and implications of generalized outcome expectancies. Health Psychol. 1985;4(3):219–247. doi: 10.1037//0278-6133.4.3.219. [DOI] [PubMed] [Google Scholar]
- 21.Mishel MH. The measure of uncertainty and stress in illness. Nurs Res. 1981;30(5):258–263. [PubMed] [Google Scholar]
- 22.Cella D, Tulsky D, Gray G, et al. The Functional Assessment of Cancer Therapy Scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 23.Bryk AS, Raudenbush SW, Seltzer M, Congdon RT., Jr . An Introduction to HLM: Computer Program and User's Guide. Scientific Software; Chicago, IL: 1989. [Google Scholar]
- 24.Travis LB, Curtis RE, Storm H, et al. Risk of second malignant neoplasms among long-term survivors of testicular cancer. J Natl Cancer Inst. 1997;89(19):1429–1439. doi: 10.1093/jnci/89.19.1429. [DOI] [PubMed] [Google Scholar]
- 25.Pelusi J. The lived experience of surviving breast cancer. Oncol Nurs Forum. 1997;24(8):1343–1353. [PubMed] [Google Scholar]
- 26.Mast M. Adult uncertainty in illness: a critical review of research. Sch Inq Nurs Pract. 1995;9(1):3–24. discussion 25–29. [PubMed] [Google Scholar]
- 27.Bailey DE, Jr, Mishel MH, Belyea M, Stewart JL, Mohler J. Uncertainty intervention for watchful waiting in prostate cancer. Cancer Nurs. 2004;27:339–346. doi: 10.1097/00002820-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Gil KM, Mishel MH, Germino B, Porter LS, Carlton-LaNey I, Belyea M. Uncertainty management intervention for older African American and Caucasian long-term breast cancer survivors. J Psychosoc Oncol. 2005;23(2–3):3–21. doi: 10.1300/j077v23n02_02. [DOI] [PubMed] [Google Scholar]
- 29.Mishel MH, Germino BB, Belyea M, et al. Moderators of an uncertainty management intervention: for men with localized prostrate cancer. Nurs Res. 2003;52:89–97. doi: 10.1097/00006199-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Mishel MH, Germino BB, Gil KM, et al. Benefits from an uncertainty management intervention for African-American and Caucasian older long-term breast cancer survivors. Psychooncology. 2005;14:962–978. doi: 10.1002/pon.909. [DOI] [PubMed] [Google Scholar]
- 31.Mishel MH, Hostetter T, King C, et al. Predictors of psychosocial adjustment in patients newly diagnosed with gynecological cancer. Cancer Nurs. 1984;7:291–299. [PubMed] [Google Scholar]
- 32.Mishel MH, Sorenson DS. Uncertainty in gynecological cancer: a test of the mediating functions of mastery and coping. Nurs Res. 1991;40:161–171. [PubMed] [Google Scholar]
- 33.Wonghongkul T, Moore SM, Musil C, Schneider S, Deimling G. The influence of uncertainty in illness, stress appraisal, and hope on coping in survivors of breast cancer. Cancer Nurs. 2000;23(6):422–429. doi: 10.1097/00002820-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Wonghongkul T, Dechaprom N, Phumivichuvate L, Losawatkul S. Uncertainty appraisal coping and quality of life in breast cancer survivors. Cancer Nurs. 2006;29(3):250–257. doi: 10.1097/00002820-200605000-00014. [DOI] [PubMed] [Google Scholar]