Abstract
We describe herein a patient presenting with bilateral ER+ breast tumors who was enrolled in a clinical trial exploring molecular aberrations associated with hormone-refractory tumor cell proliferation. Short-term (2-week) hormonal therapy with the aromatase inhibitor letrozole substantially reduced proliferation as measured by Ki67 immunohistochemistry in one tumor, while the second was essentially unchanged. Extensive molecular and genetic workup of the two tumors yielded divergent lesions in the two tumors: an activating KRAS mutation in the responsive tumor, and an amplification of the FGFR1 locus in the treatment-refractory tumor. These findings provide an insight to possible mechanisms of resistance to antiestrogen therapy in ER+ breast cancers. First, they illustrate the necessity of clinically approved assays to identify FGFR1 gene amplification, which occur in ~5% of breast tumors and have been linked to antiestrogen resistance. It is quite possible that the addition of FGFR inhibitors to ER-targeted therapy will yield a superior antitumor effect and improved patient outcome. Second, they suggest that the role of activating mutations in RAS, although rare in breast cancer, may need to be explored in the context of ER+ breast tumors.
Introduction
A 60-year-old female presented to the Vanderbilt Breast Center with an abnormal mammogram showing scattered micro-calcifications and upper-quadrant nodularity in her left breast. A diagnostic mammogram and ultrasound (US) confirmed these findings. A core biopsy revealed the lesion to be an estrogen-receptor-positive (ER+), progesterone receptor-positive (PR+), HER2-negative, low grade invasive mammary carcinoma. The patient also had a palpable abnormality in the right breast, which upon US was identified as a hypoechoic lesion suspicious for malignancy. A core biopsy revealed it to be a low grade, ER+/HER2-negative invasive mammary carcinoma.
Prior to definitive breast surgery, the patient was consented and enrolled in a pre-surgical trial of with the aromatase inhibitor letrozole (Figure 1A; Vanderbilt University NCT00651976). This trial examines the short-term cellular and molecular response to estrogen deprivation with letrozole (2.5 mg QD for 10–21 days) in stage I and II operable ER+/HER2-negative breast cancer. Molecular correlates include gene expression, proteomic, and mutational screening to identify biomarkers and effectors of resistance to estrogen deprivation in cancers that do not exhibit a change in tumor cell proliferation and/or that retain a high proliferation as measured by Ki67 immunohistochemistry (IHC). The trial design is based in large part on the results of the IMPACT study, where tumor cell proliferation (measured by Ki67 IHC) after two weeks of antiestrogen therapy was a surrogate for long-term patient disease-free survival following adjuvant endocrine therapy (1–3).
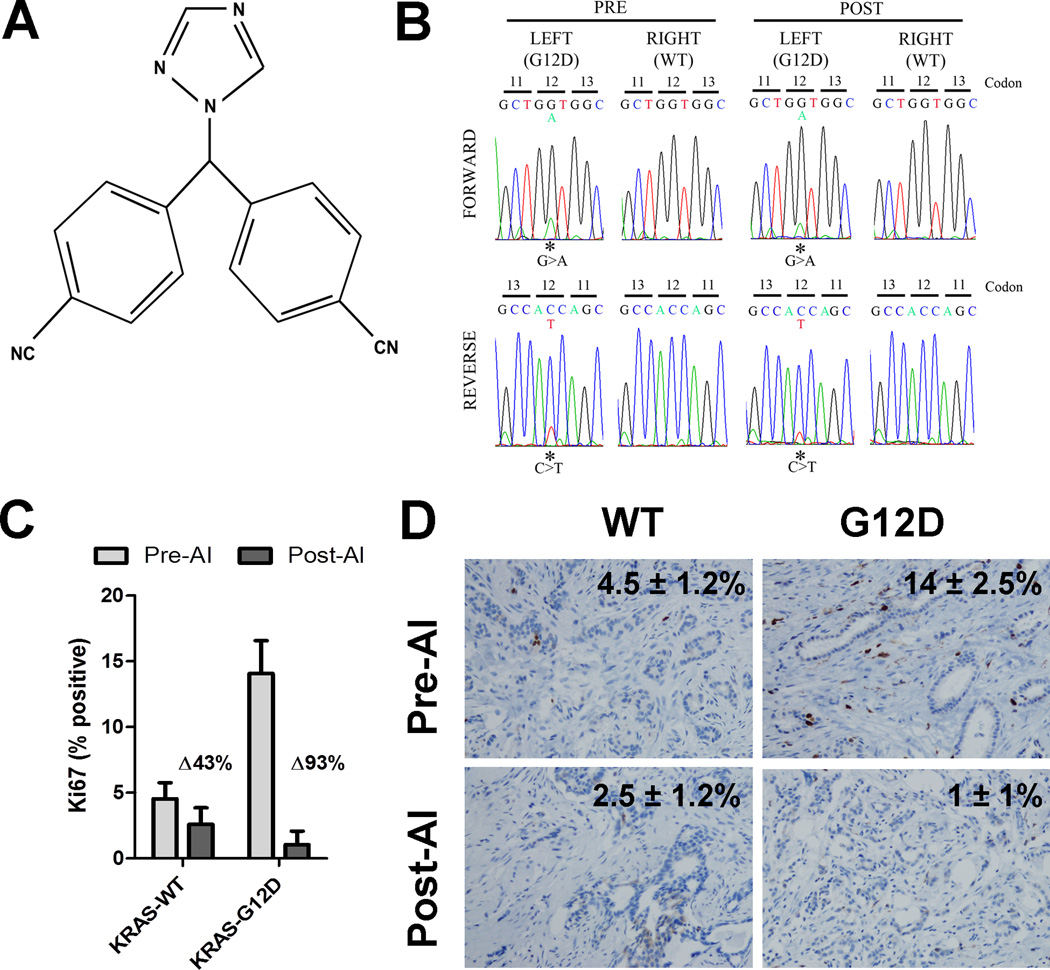
Figure 1. A breast tumor harboring mutant KRAS does not demonstrate molecular endocrine resistance.
A) Chemical structure of letrozole B) Sanger sequencing traces of KRAS codon 12 in the left and right bilateral tumors, before and after 2 weeks of letrozole therapy. C) Plot of Ki67 scores in left and right breast tumors before and after 2 weeks of letrozole therapy. Ki67 IHC was scored by 3 blinded independent pathologists to account for variability in scoring. Bars represent mean ± s.d. D) Representative IHC of Ki67 in left and right breast tumors before and after 2 weeks of letrozole therapy.
The patient received 16 days of letrozole followed by bilateral mastectomy with sentinel lymph node biopsy the day after the last dose. Her final pathology was consistent with a right invasive mammary carcinoma, stage II (T1bN1; 0.9 cm, 2 involved lymph nodes), low histological grade, ER+, PR+, HER2-negative, and a left multicentric invasive mammary carcinoma, stage I (T1bN0; 0.7 cm, 0.5 cm), low histological grade, ER+, PR+, HER2-negative tumor. A follow-up right axillary dissection revealed that none of the 15 lymph nodes removed were involved by cancer. She subsequently underwent adjuvant chemotherapy (anthracycline/taxane) followed by 5 years of endocrine therapy. The patient continues to do well without evidence of recurrence since her original diagnosis on November 2008.
Materials and Methods
Formalin-fixed, paraffin-embedded (FFPE) pre-treatment core biopsies and post-treatment surgical specimens were utilized to assess the status of >400 known somatic oncogenic mutations in 33 genes as described (4). Sanger sequencing was carried out to verify mutation status. Immunohistochemistry (IHC) was performed in both the pre-treatment biopsy and in the post-treatment surgical biopsy of both tumors for Ki67 (Dako #M7240), ERα (Santa Cruz #sc542), p-ERαS118 (Cell Signaling #2511), PR (Dako #M3569) and p-ERK1/2 (Cell Signaling, #9101). IHC for ER and PR was carried out according to the methods reported elsewhere (5). FFPE tumor sections were scanned at 100× magnification and the area containing the highest number of positive cells was selected. Positive and negative tumor cells were manually counted at 400×; the percentage of positive cells was calculated with at least 700 viable cells. Ki67 IHC was scored by 3 independent pathologists to ensure precision and estimate the standard deviation in Ki67 scoring. FISH for FGFR1 was carried out using the standard manufacturer’s protocol (Zytovision; Bremerhaven, Germany). Affymetrix U133+2.0 gene expression arrays were used to capture gene expression patterns in both post-treatment surgical specimens (deposited as GSE39387). Recurrence Score was estimated from the microarray data signal intensity values according to the methods of Paik et al (6), with rescaling in the presence of data from the additional patients in the trial.
Results
An activating KRAS mutation (G12D) was identified in the left-breast tumor, while no screened mutations were identified in the tumor from the right breast. Of note, however, the total incidence of PIK3CA mutations identified in the initial analysis was ~40% (9/20 patients), which is similar to the reported frequency on the Catalogue of Somatic Mutations in Cancer (COSMIC) database (7). The status of the G12 codon was confirmed by Sanger sequencing. Figure 1A demonstrates the c.35G>A transition resulting in the change from glycine to aspartic acid at codon 12. The mutation was confirmed in the left tumor, but not the right tumor, in both the pre-treatment core biopsy and surgical specimen in both the forward and reverse directions by PCR (primer sequence and conditions for KRAS exon 1 were previously described (8)). The same tumor block utilized for IHC was used for DNA extraction and sequencing to eliminate the potential for left/right-sided errors.
The Ki67 score in the KRAS-G12D tumor decreased from 14 ± 2.5% (pre-treatment) to 1 ± 1% after letrozole, whereas Ki67 in the KRAS-WT tumor was essentially unchanged (4.5 ± 1.2% to 2.5 ± 1.2%; Figure 1B–C). The KRAS-G12D tumor had a substantially higher Ki67 score at baseline than the KRAS-WT tumor (14% versus 4.5%, respectively). We calculated Allred scores (5) (noted in Figure 2A) for ER and PR in the pre- and post-letrozole samples. ER scores were equivalent in both tumors, suggesting that the difference in proliferative response was not due to variations in ER expression. The PR score was higher in the KRAS-G12D tumor than in the KRAS-WT tumor (ALLRED scores of 6 and 3, respectively). Consistent with estrogen deprivation, PR expression decreased significantly in both tumors in response to letrozole.
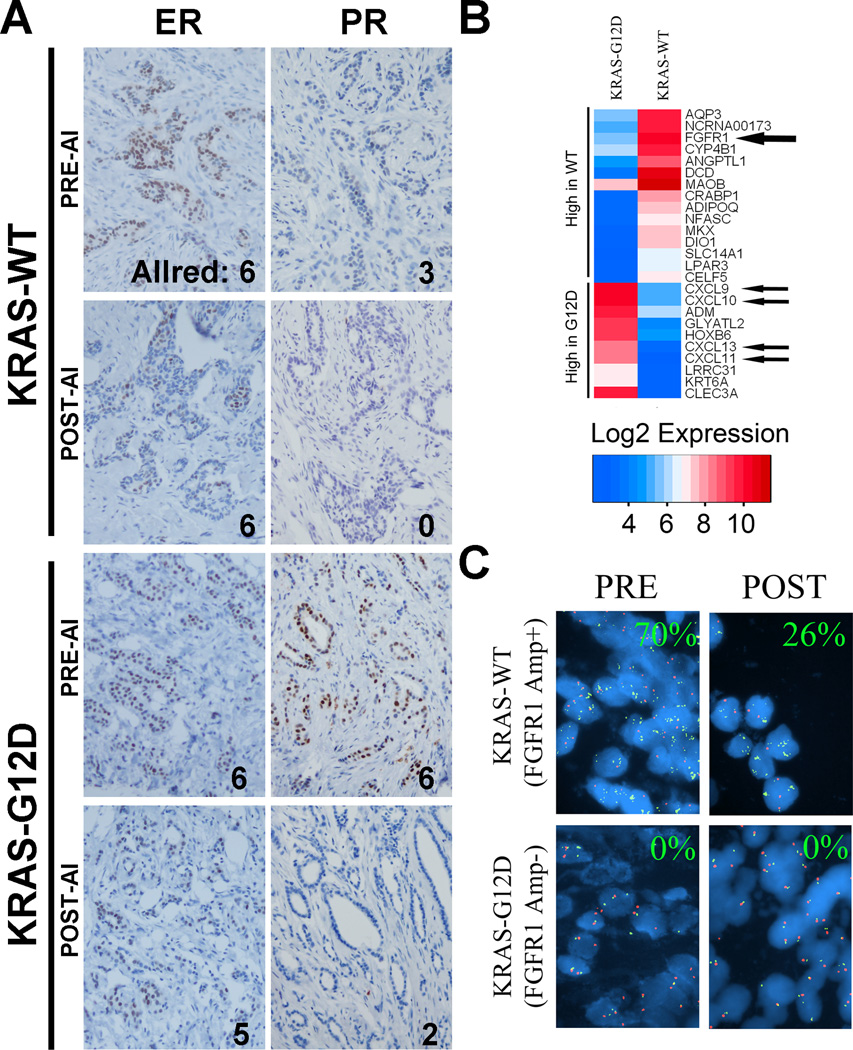
Figure 2. FGFR1 amplification in a breast tumor demonstrating molecular endocrine resistance.
A) Representative IHC and ALLRED scores for ERα (ER) and PR in the bilateral tumors, before and after 2 weeks of letrozole therapy. PR staining was substantially decrease in both tumors with letrozole therapy. B) Heatmap of the genes with the highest fold change between the left and right tumors. Among these, FGFR1 was substantially upregulated in the right tumor. C) Representative FISH for FGFR1. Percent nuclei demonstrating >5 FGFR1 gene cluster signals [green] per centromere 8 signal [red] reported in each panel.
ERα is phosphorylated at Serine-118 by ERK1/2, which lies downstream of KRAS, resulting in estrogen-independent transcriptional activity (9). Therefore, we hypothesized that the KRAS-G12D tumor may have higher phosphorylation of ERK1/2T202/Y204 and ERαS118. IHC for the phosphorylated epitopes of both proteins was performed. However, we did not observe any differences in P-ERαS118 signal between the tumors (data not shown). P-ERK1/2T202/Y204 signal was high in both pre-treatment biopsies, but essentially absent in the post-treatment specimens, possibly reflecting sample-fixation issues known to induce rapid loss of phospho-ERK1/2 immunoreactivity (10). As ERS118 is also phosphorylated by estradiol-induced CDK7 (11) it is also plausible that letrozole-induced estrogen deprivation resulted in loss of ERK phosphorylation regardless of KRAS genotype. Additionally, this could be a downstream effect of loss of PI3K pathway activity, which is down regulated in breast tumors following letrozole treatment (12). Although the tumor harboring KRAS-G12D exhibited a strong anti-proliferative response to estrogen deprivation, we questioned whether KRAS activation in this tumor may abrogate apoptotic pathways. Therefore, we also stained all 4 samples for the apoptotic marker cleaved caspase-3. Little or no cleaved caspase-3 staining was detected in any of the samples (data not shown), supporting a cytostatic but not an apoptotic effect of the aromatase inhibitor.
In examining gene expression data from the patient, we identified genes with the greatest differential expression between the KRAS-G12D and KRAS-WT tumor (Figure 2B). A microarray approximation of the ‘Recurrence Score (6)’ for the two tumors revealed that the KRAS-G12D tumor had a ‘High’ recurrence score (RS) whereas the RS in the KRAS-WT tumor was ‘Low’. This suggests KRAS mutations in breast cancer may contribute to a more invasive phenotype with higher likelihood of recurrence. Notably, genes most highly expressed in the KRAS-G12D tumor included a number of chemokine ligands, including CXCL9, 10, 11, and 13, possibly representing a strong immune response in this tumor. CXCL9, 10, and 11 are all CXCR3 ligand-chemokines which can be transcriptionally activated by TNF-α stimulation of mesenchymal stem cells, thereby promoting invasive phenotypes in breast cancer cells through paracrine activation of CXCR3 (13).
In contrast, the KRAS-WT tumor highly expressed the mRNA for the tyrosine kinase fibroblast growth factor receptor-1 (FGFR1). FISH analysis for FGFR1 confirmed genomic amplification of the 8p12 locus in the KRAS-WT, but not the KRAS-mutant tumor (Figure 2C), in both pre- and post-treatment specimens. Genomic amplification of FGFR1 has been directly linked with endocrine resistance (14). We speculate that this finding may offer an explanation for the lack of change in Ki67 in this tumor.
Discussion
Activating mutations in KRAS are common in diverse cancer types, such as melanoma, colorectal, pancreatic, and non-small cell lung cancers. In lung and colorectal cancer, KRAS mutations have been associated with resistance to EGFR-targeted therapies, primarily due to decoupling of the Ras pathway from EGFR. Activating KRAS mutations are rare in breast cancer, and no data exist on whether KRAS mutations can induce resistance to anti-estrogen therapy. Correlative studies have identified an association between Ras pathway activation as defined by high levels of P-ERK1/2Y202/T204 and P-RAFS338 and resistance to tamoxifen in patients with ER+ breast cancer (15). However, mutational status of the pathway was not assessed in this study. Ras activation coupled with estrogenic stimuli can cooperatively enhance invasive phenotypes (16). ER+ MCF-7 human breast cancer cells engineered to overexpress v-Ha-Ras were found to be less responsive to estrogen-induced proliferation and tamoxifen-induced inhibition of proliferation (17). It is intuitive that by conferring a gain of function, KRAS mutations should confer estrogen-independent growth and survival in an ER+ tumor. However, based on the cellular markers assessed in this case, the evidence does not support the hypothesis that KRAS mutations confer resistance to estrogen deprivation in ER+ breast cancer. However, it is noteworthy that the KRAS-mutant tumor demonstrated higher Ki67 staining, and thus may be a poor prognostic marker per se as is observed in other cancers.
The contribution of FGFR1 amplification to endocrine resistance in breast cancer is clearer. Recent work showed that FGFR1 amplification occurs in approximately 10% of breast cancers (predominantly ER+ and HER2-negative), is a poor prognostic factor, and can directly compensate for loss of estrogen signaling through estrogen-dependent or -independent PI3K and MAPK activation (14, 18, 19). Interestingly, Turner et al. also found that FGFR1 signaling repressed PR expression, which was lower in the FGFR1-amplified breast tumor in our patient at baseline (Figure 2A). Up to now, clinical data linking FGFR1 amplification with endocrine resistance are only correlative. With the inclusion of a matched FGFR1 gene-non-amplified control, we provide evidence supporting a role for FGFR1 in resistance to endocrine therapy. These findings support further exploration of the utility of FGFR1-targeted agents for the treatment of endocrine-resistant breast cancer.
Acknowledgements
The authors would like to acknowledge all of the patients who have taken part or are taking part in this study (NCT00651976) and the core facilities involved in this report, including the Vanderbilt Genomics Shared Resource and the Vanderbilt DNA Sequencing Core.
This work was supported by R33CA126674, the Starr Cancer Consortium (L.A. Garraway and N. Wagle) and Breast Cancer Specialized Program of Research Excellence (SPORE) grant P50CA98131 (C.L. Arteaga and J.M. Balko).
Footnotes
Conflicts of Interest – N/A
References
- 1.Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, A'Hern R, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst. 2007;99:167–170. doi: 10.1093/jnci/djk020. [DOI] [PubMed] [Google Scholar]
- 2.Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, Griffith C, et al. Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clin Cancer Res. 2005;11:951s–958s. [PubMed] [Google Scholar]
- 3.Smith IE, Dowsett M, Ebbs SR, Dixon JM, Skene A, Blohmer JU, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol. 2005;23:5108–5116. doi: 10.1200/JCO.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 4.MacConaill LE, Campbell CD, Kehoe SM, Bass AJ, Hatton C, Niu L, et al. Profiling critical cancer gene mutations in clinical tumor samples. PLoS One. 2009;4:e7887. doi: 10.1371/journal.pone.0007887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 6.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 7.Forbes SA, Bhamra G, Bamford S, Dawson E, Kok C, Clements J, et al. The Catalogue of Somatic Mutations in Cancer (COSMIC) Curr Protoc Hum Genet. 2008;Chapter 10(Unit 10):1. doi: 10.1002/0471142905.hg1011s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pao W, Wang TY, Riely GJ, Miller VA, Pan Q, Ladanyi M, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 10.Pinhel IF, Macneill FA, Hills MJ, Salter J, Detre S, A'Hern R, et al. Extreme loss of immunoreactive p-Akt and p-Erk1/2 during routine fixation of primary breast cancer. Breast Cancer Res. 12:R76. doi: 10.1186/bcr2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen D, Washbrook E, Sarwar N, Bates GJ, Pace PE, Thirunuvakkarasu V, et al. Phosphorylation of human estrogen receptor alpha at serine 118 by two distinct signal transduction pathways revealed by phosphorylation-specific antisera. Oncogene. 2002;21:4921–4931. doi: 10.1038/sj.onc.1205420. [DOI] [PubMed] [Google Scholar]
- 12.Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27:2630–2637. doi: 10.1200/JCO.2008.18.8391. [DOI] [PubMed] [Google Scholar]
- 13.Shin SY, Nam JS, Lim Y, Lee YH. TNFalpha-exposed bone marrow-derived mesenchymal stem cells promote locomotion of MDA-MB-231 breast cancer cells through transcriptional activation of CXCR3 ligand chemokines. J Biol Chem. 285:30731–30740. doi: 10.1074/jbc.M110.128124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner N, Pearson A, Sharpe R, Lambros M, Geyer F, Lopez-Garcia MA, et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res. 70:2085–2094. doi: 10.1158/0008-5472.CAN-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGlynn LM, Kirkegaard T, Edwards J, Tovey S, Cameron D, Twelves C, et al. Ras/Raf-1/MAPK pathway mediates response to tamoxifen but not chemotherapy in breast cancer patients. Clin Cancer Res. 2009;15:1487–1495. doi: 10.1158/1078-0432.CCR-07-4967. [DOI] [PubMed] [Google Scholar]
- 16.Albini A, Graf J, Kitten GT, Kleinman HK, Martin GR, Veillette A, et al. 17 beta-estradiol regulates and v-Ha-ras transfection constitutively enhances MCF7 breast cancer cell interactions with basement membrane. Proc Natl Acad Sci U S A. 1986;83:8182–8186. doi: 10.1073/pnas.83.21.8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Roy F, Mareel M, Vleminckx K, Beyaert R, Fiers W, Devleeschouwer N, et al. Hormone sensitivity in vitro and in vivo of v-ras-transfected MCF-7 cell derivatives. Int J Cancer. 1990;46:522–532. doi: 10.1002/ijc.2910460332. [DOI] [PubMed] [Google Scholar]
- 18.Kadota M, Sato M, Duncan B, Ooshima A, Yang HH, Diaz-Meyer N, et al. Identification of novel gene amplifications in breast cancer and coexistence of gene amplification with an activating mutation of PIK3CA. Cancer Res. 2009;69:7357–7365. doi: 10.1158/0008-5472.CAN-09-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elbauomy Elsheikh S, Green AR, Lambros MB, Turner NC, Grainge MJ, Powe D, et al. FGFR1 amplification in breast carcinomas: a chromogenic in situ hybridisation analysis. Breast Cancer Res. 2007;9:R23. doi: 10.1186/bcr1665. [DOI] [PMC free article] [PubMed] [Google Scholar]