Abstract
BACKGROUND
The recruitment of immune cells by chemokines and the regulation of endometrial cell apoptosis are critical aspects of endometriosis biology. Here, we review the local (paracrine) and systemic hormone (endocrine) modulation of these two specific, but highly related phenomena.
METHODS
We searched Pubmed for items published in English between September 1991 and September 2011 and selected the studies evaluating the effects of hormones on chemokines or apoptosis in normal human endometrium and endometriosis.
RESULTS
Estradiol has proinflammatory and antiapoptotic effects in endometrial cells, and these effects appear to be exacerbated in women with endometriosis. In these women, physiological estradiol concentrations are able to induce an enhanced inflammatory response mediated by local chemokine production and to reinforce mechanisms of cell survival mediated by extracellular signal-regulated kinases and Bcl-2. The main effect of progestogens is to inhibit interleukin-8 and other chemokines in stromal cells from both eutopic and ectopic endometrium. Progesterone is also effective in inducing apoptosis in endometrial and endometriotic cells through the inhibition of Bcl-2 and nuclear factor-κB.
CONCLUSIONS
Estrogens and progestogens modulate chemotaxis and apoptosis in human endometrium and endometriotic cells and tissues. These endocrine and paracrine pathways are perturbed in women with endometriosis, contributing to inflammatory responses, abnormal tissue remodeling, therapeutic refractoriness and disease persistence. Ultimately, they promote adhesion formation and the clinical symptoms of pelvic pain and infertility. A more detailed understanding of the molecular mechanisms involved will offer new opportunities for novel pharmacological strategies to diagnose and treat endometriosis.
Keywords: endometriosis, endometrium, chemokines, apoptosis, hormones
Introduction
Endometriosis is a common gynecologic disorder characterized by the presence of hormonally responsive, ectopic implants of endometrial epithelium and stroma dispersed in extrauterine locations. It is estimated that >175 million women worldwide suffer from this disease and the symptoms of pelvic pain and infertility that commonly accompany its surgical findings (Adamson et al., 2010). The precise etiology and pathogenesis of endometriosis remain controversial, however, many hypotheses have been put forward and several are considered in detail in this review. The primary objective of our report is to summarize two critical and related processes in the progression of endometriosis: chemotaxis, the recruitment of immune cells to anatomic sites of ectopic lesion invasion; and apoptosis, the regulation of programmed cell death leading to the remodeling of these dynamic tissues. Both phenomena have been uncovered in recent years and gaining an understanding of them promises to expand our comprehension of the pathogenesis of endometriosis.
Endometriosis as an inflammatory syndrome
In his definitive historical review, Batt (2011) reports that John Sampson himself attributed von Rokitansky (1861) as the first to comprehensively describe the appearance of an ovarian endometrioma. However, it was Meigs (1922) who originally appreciated that neoangiogenesis, fibrosis and hemosiderin accumulation were intrinsic microscopic characteristics of the lesion. His description of the infiltration of ‘endothelial leukocytes’ ushered in a modern understanding of the inflammatory response evoked by endometriosis. These findings set the stage for our consideration of the mechanisms and consequences of immune cell recruitment and activation within ectopic foci of endometrial tissue.
As argued from the turn of the 20th century, by Meyer (1924), Halban (1925) and Sampson (1927), respectively, it remains controversial whether the derivation of endometriotic lesions is metaplastic, lymphovascular or via retrograde menstruation. It is possible that a combination of mechanisms contributes to their genesis (Taylor, 2010). Nevertheless, even from early records in our medical literature, the histology and pathogenesis of endometriosis have been linked to its presumptive source and ‘mother’ of all the implants, the eutopic endometrium. When one considers the dramatic physiological remodeling that is required for normal cyclical endometrial function and gestational support in Old World primates, it is not surprising that this plastic tissue has evolved a remarkable capacity for ectopic translocation. In this review, we consider two specific, but highly related, aspects of endometriosis biology: the recruitment of immune cells and the regulation of mucosal apoptosis, including local (paracrine) and systemic hormone (endocrine) modulation of these phenomena. We contend that a fundamental understanding of the molecular pathways that mediate immune cell chemotaxis and programmed cell death in endometriosis lesions is required before devising rational new therapeutic strategies to reduce lesion burden and mitigate the symptoms associated with endometriosis.
Overview of immune response
As noted above, inflammation in the setting of endometriosis has been recognized for decades (reviewed in Batt, 2011), but the critical role of leukocytes, particularly macrophages and their products, did not receive attention until the 1980s.
Two arms of the immune system are currently recognized. The adaptive immune arm, composed of specialized T and B lymphocytes and antibody-producing plasma cells, recognizes specific antigens. Its characteristic anamnestic response allows the host to mount more avid attacks when the same pathogen is encountered subsequently.
In contrast, the innate immune arm is evolutionarily more ancient, lacking antigen specificity and memory, but providing a generic line of defense. Granulocytes, eosinophils, mast cells, natural killer (NK) cells and macrophages are recruited to sites of infection and injury, where they produce biochemical mediators, including histamine, leukotrienes and prostaglandins. These cells also secrete cytokines and complement, but we will focus on their production of chemokines, the primary stimuli for leukocyte attraction (Hornung et al., 1997).
Peritoneal inflammation and endometriosis: a historical perspective
Although an association of inflammation with endometriotic histopathology was recognized and reported by Meigs (1922) and Sampson (1927) in their pioneering treatises, the intimate relationship between immune cell activation and endometriosis was not generally accepted until 30 years ago. In their seminal publication, Weed and Arquembourg (1980) extended Meigs' observation of perivascular leukocytes and proposed that endometriosis was an autoimmune phenomenon. Their microscopic observations of lymphocyte infiltration of endometriomas, and evidence of complement C3 deposition in ectopic and eutopic biopsies from affected women, transformed the thinking about the pathogenesis of endometriosis. Moreover, their hypothesis that endometriosis-associated infertility might be due to ‘the rejection of early implantation of embryos’ was highly insightful. This proposal now is supported by a decade of recent transcriptomic and proteomic data focused on the secretory differentiation of the eutopic endometrium (Kao et al., 2003; Klemmt et al., 2006).
Immune cell infiltration of normal endometrium and endometriotic lesions
Insight into the immune cell biology of normal human endometrium also was beginning to emerge by the late 1980s, particularly with respect to pregnancy (Bulmer et al., 1988). Cluster determination (CD)14+ macrophages were noted to be prominent in the decidua and localized in close association with invading extravillous trophoblasts, where they were thought to play an immunoprotective role. Endometrial leukocytes were observed by confocal scanning laser microscopy to be concentrated in lymphoid aggregates consisting of a core of B cells surrounded by T cells and a circumferential halo of macrophages. The aggregates were small or absent during the early proliferative stage of the menstrual cycle, increasing in size during the secretory phase, and absent in post-menopausal women, suggesting that their development was hormonally influenced (Yeaman et al., 1997). Leukocyte populations in women with endometriosis were evaluated with respect to steroid hormone receptors, proliferative activity and apoptosis markers in eutopic (intrauterine) and ectopic tissues. These studies revealed high estrogen receptor (ER) and Bcl-2 (an antiapoptotic gene product discussed in more detail below) expression and increased density of leukocytes in ectopic lesions (Jones et al., 1998).
As the innate cell-mediated immune response became better understood, it was postulated that deficits in this function might compromise the ability to eliminate misplaced autologous cells in endometriosis. Further, it was suggested that impaired macrophage and NK cell function might facilitate ectopic implantation and the growth of endometriotic lesions (Dmowski et al., 1994). Retrograde refluxed cells were thought to irritate the peritoneum, and elaborate chemotactic, angiogenic and immunosuppressive cytokines (discussed below), creating a vicious cycle (Vinatier et al., 1996; Lebovic et al., 2001b).
As described above, several laboratories contributed fundamental insights into the role of macrophage accumulation within the pelvic fluid of women with endometriosis. In particular, the studies of Haney et al. (1981) and Halme et al. (1983) indicated that macrophage concentration and degree of biochemical activation were higher in endometriosis cases compared with fertile controls. By the early 1990s investigators began to query how these peritoneal macrophages were recruited into the pelvic fluid.
Our laboratory in San Francisco took a candidate molecule approach, based on the discovery that members of the CC chemokine family attract monocytes to sites of chronic inflammation (Schall et al., 1988). Using a newly developed, enzyme-linked immunosorbent assay, the first chemokine we characterized in the peritoneal fluid of subjects with endometriosis was RANTES (regulated on activation, normal T cell expressed and secreted, CCL5) and we observed that its concentration was correlated directly with the extent of active disease as assessed by laparoscopic staging (Khorram et al., 1993). A few years later, another CC chemokine, MCP-1 (monocyte chemoattractant protein-1, CCL2), was identified in the pelvic fluid of endometriosis patients (Akoum et al., 1996; Arici et al., 1997). The CC chemokines comprise the largest family of these bioactive peptides and will be described in greater detail below. We went on to demonstrate that RANTES protein (Hornung et al., 1997) and mRNA (Hornung et al., 2001b) were synthesized de novo within the stromal cells of endometriotic lesions and that the protein was biologically active as a monocyte chemokine (Hornung et al., 2001a).
In the same year as our first report, Lyttle and his collaborators at the University of Pennsylvania used a bioassay strategy and demonstrated that a histiocyte chemotactic protein was present at increased concentrations in peritoneal fluid specimens from women with endometriosis compared with controls (Leiva et al., 1993). Subsequent work from that group suggested that a 20 kDa immunophilin-like protein might contribute to the chemotactic activity (Weil et al., 1997).
Chemokines and their receptors in endometriosis
Chemokines (a term derived from the contraction of ‘chemoattractant cytokines’) are low molecular mass (8–10 kDa) peptides that recruit leukocytes from the circulation to sites of inflammation. These molecules operate by binding to transmembrane receptors expressed on the surface of inflammatory cells (Fig. 1). Ligation by its cognate chemokine typically activates one of several heptahelical G-protein-coupled receptors (GPCRs), resulting in inositol 1,4,5-trisphosphate (IP3) generation and calcium release from intracellular compartments, facilitating cell motility. Leukocyte stimulation by chemokines also results in phosphatidic acid accumulation (Sozzani et al., 1994), which stimulates a transient peak of diacylglycerol kinase activity. Chemokine receptor activation results in a respiratory burst, relaxation of leukocyte adhesion, cellular shape remodeling and migration of the immune cell toward a gradient of the chemotactic substance (Baggiolini et al., 1989). While these steps are becoming appreciated, a sophisticated understanding of the biochemical structure–activity relationships of specific chemokines with their GPCRs remains obscure.
Figure 1.
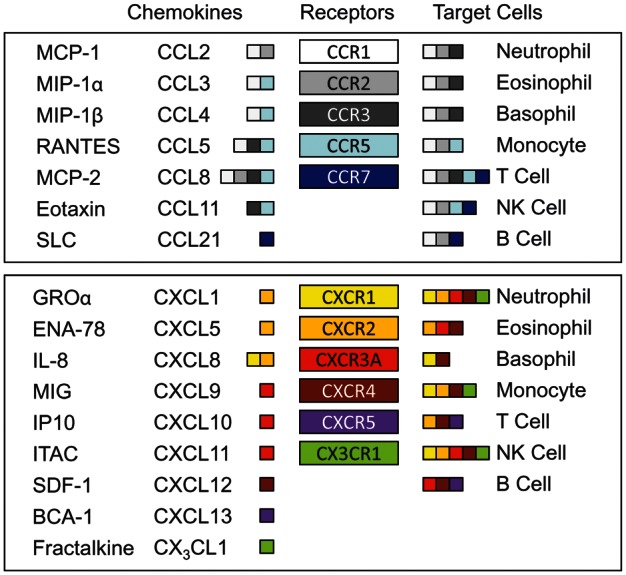
Chemokines identified in endometriosis. The upper box shows members of the CC family and the lower box shows members of the CXC family and one member of the CX3C family. Each receptor is represented by a different color, which is reproduced in its corresponding, high-affinity ligands and target cells. The chemokines are identified by their old names or abbreviations (first column) and by the current nomenclature (second column). MCP, monocyte chemoattractant protein; MIP, monocyte inflammatory protein; RANTES, regulated on activation, normal T cell expressed and secreted; SLC, secondary lymphoid tissue chemokine; GRO, growth-related oncogene; ENA, epithelial neutrophil-activating peptide; IL, interleukin; MIG, monokine induced by gamma interferon; IP, interferon-inducible protein; ITAC, Interferon-inducible T-cell alpha chemoattractant; SDF, stromal cell-derived factor; BCA, B-cell-attracting chemokine.
Approximately 50 human chemokines have been identified and these can be categorized into four distinct families based on their structure. Members of three of the four families have been reported in studies of endometriosis (Fig. 1). The largest family consists of the CC chemokines, so named for the adjacent location of the first two of four canonical cysteine residues near the carboxy terminus. Representatives of this family, which have been detected in cases of endometriosis, include MCP-1 (CCL2) (Akoum et al., 1995), MIP-1α (monocyte inflammatory protein-1α, CCL3) (Na et al., 2011), RANTES (CCL5) (Khorram et al., 1993) and eotaxin (CCL11) (Hornung et al., 2000). The primary targets of this class of chemokines are monocytes, T cells and eosinophils.
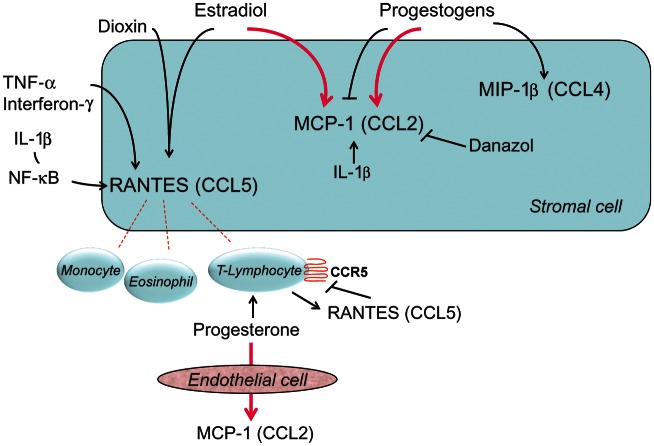
The regulation of CC chemokines in endometriosis is well exemplified by two representative molecules, which appear to be consistently altered in the disease: MCP-1 and RANTES. Endometrial glands from women with endometriosis express high mRNA and protein levels of MCP-1 (Jolicoeur et al., 1998). These cells, when isolated and incubated in vitro with proinflammatory cytokines, also release MCP-1 to a much greater extent than endometrial epithelial cells obtained from normal individuals (Akoum et al., 1995).
Endometrial RANTES protein and mRNA are mostly confined to the stromal compartment of endometrial and endometriotic tissues. In vitro, stromal cell cultures synthesize RANTES mRNA and secrete protein when stimulated by proinflammatory cytokines, whereas epithelial cells synthesize neither transcripts nor protein. In endometriosis, the pattern of RANTES protein distribution was similar to that found in normal endometrium, however, the production of this chemokine in endometriotic cultures was significantly greater than from cells derived from normal tissue (Hornung et al., 1997; Wieser et al., 2005). This phenomenon of enhanced chemokine production may provide a mechanism by which peritoneal implants increase pelvic fluid concentrations of RANTES in patients with moderate and severe endometriosis and manifest more monocyte chemotactic bioactivity. Expression of the RANTES gene is up-regulated in endometriotic stromal cells in response to tumor necrosis factor (TNF)-α, interferon-γ and interleukin (IL)-1β (Hornung et al., 1997, 2001b; Lebovic et al., 2001a; Fig. 2). The transcription factor nuclear factor (NF)-κB plays a critical role in the regulation of RANTES expression (Lebovic et al., 2001a). These findings are consistent with a feed-forward inflammatory loop whereby cytokines secreted from activated macrophages can lead to RANTES production and stimulation of further monocyte chemotaxis into the peritoneal cavity. In addition to confirming many of the above data, Wang et al. (2010) demonstrated that recombinant RANTES can induce surface markers of macrophage tolerance in vitro and inhibits the apoptotic effects of macrophage-like U937 cells on endometrial stromal cells. The findings suggest that despite more immune cell recruitment, macrophages in the vicinity of endometriotic lesions may be less capable of phagocytosing and clearing the ectopic implants.
Figure 2.
CC chemokines: endocrine and paracrine regulation in human endometrium and endometriosis. ↑ stimulation; ⊥ inhibition. The bold, pink signs indicate abnormal responses observed in endometriosis. Note that leukocytes are attracted by chemokines released by the endometrial or endometriotic cell in response to estradiol and/or proinflammatory cytokines, such as TNF-α. In endometriotic cells, sex steroids may abnormally stimulate, rather than inhibit MCP-1. NF, nuclear factor; CCR, CC chemokine receptor; MIP, monocyte inflammatory protein.
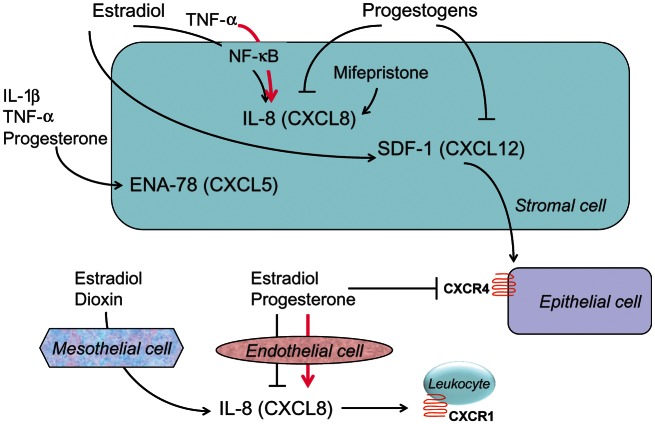
The next most numerous family of chemokines is the CXC family, in which a single, variable amino acid is interposed between the two conserved cysteines. Growth regulated oncogene (GRO)-α (CXCL1) (Oral et al., 1996), epithelial cell-derived neutrophil-activating peptide (ENA)-78 (CXCL5) (Mueller et al., 2003), IL-8 (CXCL8) (Ryan et al., 1995; Arici et al., 1996) and stromal cell-derived factor (SDF)-1 (CXCL12) (Ruiz et al., 2010) are members of this family (Fig. 1). Primary targets for the CXC chemokines include monocytes and neutrophils. An important characteristic of this group of proteins is that they also stimulate angiogenesis (Strieter et al., 2004), a process intimately linked with the pathogenesis of endometriosis (Taylor et al., 2009). The recruitment of macrophages and neutrophils to sites of endometriosis may contribute indirectly to angiogenesis within the lesions and both these inflammatory cells are known to be potent sources of vascular endothelial growth factor production and release (McLaren et al., 1996; Mueller et al., 2000). The distribution of ENA-78 and IL-8 within endometriotic lesions tends to be more prominent in the epithelial compartment. The elevated concentrations of ENA-78 in the pelvic fluid of women with endometriosis (Mueller et al., 2003) were confirmed by others (Suzumori et al., 2004). As we observed for RANTES gene activation, the stimulation of endometrial cells with IL-1β and TNF-α also induces ENA-78 production (Bersinger et al., 2011; Fig. 3).
Figure 3.
CXC chemokines: endocrine and paracrine regulation in endometrial cells (stromal, epithelial and endothelial) and mesothelial cells. ↑ stimulation; ⊥ inhibition. The bold, pink signs indicate abnormal responses observed in endometriosis. Observe that both stromal cells and peritoneal (mesothelial) cells release IL-8 after estradiol stimulation, which recruits leukocytes and enhances the local inflammatory response in endometriosis. TNF, tumor necrosis factor; CXCR, CXC chemokine receptor.
Apoptosis in human endometrium and endometriosis
Programmed cell death, or apoptosis, is a physiological process required for the balanced growth, differentiation and renewal of many tissues. It is mediated by selective internucleosomal cleavage of chromatin, leading to cell blebbing, shrinkage and nuclear pyknosis (Kerr et al., 1972). In human endometrium, apoptosis is particularly important due to the dynamic cycles of proliferation and shedding, where the characteristic nuclear DNA fragmentation has been clearly documented (Tabibzadeh, 1996; Nasu et al., 2011). The presumed role of apoptosis in the normal endometrium is to eliminate senescent or dysfunctional cells and pave the way for tissue repair at each menstrual cycle. Apoptotic cells predominate in the glandular epithelium but also can be found to a lesser extent in the stroma. The number of apoptotic cells in the functional layer of the normal endometrium increases in the late secretory phase and peaks at menstruation. This is followed by proliferation of new cells from the basal layer during the proliferative phase of the subsequent cycle (Tabibzadeh, 1996; Vaskivuo et al., 2000; Nasu et al., 2011).
One of the most intriguing aspects of endometriosis is the perturbation of the apoptotic mechanism (Gebel et al., 1998; Izawa et al., 2006). In women with endometriosis, endometrial cells regurgitated into the peritoneal cavity lack the appropriate mechanisms of programmed cell death and therefore escape clearance and survive to invade the peritoneum, induce neovascularization and establish an endometriotic implant. This abnormal survivability has been associated with concomitant overexpression of antiapoptotic factors and underexpression of proapoptotic factors (Nasu et al., 2011). Microarray studies revealed down-regulation of several apoptosis-related genes in endometriotic tissues. However, the changes observed in apoptosis gene expression vary markedly according to the localization or type of endometriosis (Harada et al., 2004). For instance, the Bcl-2 gene family is variably altered in peritoneal versus ovarian endometriosis (Nezhat and Kalir, 2002; Dufournet et al., 2006; Table I).
Table I.
Abnormal expression of Bcl-2 family proteins in women with endometriosis.
| Protein | Lesion Localization | Compartment | Phase | Expression | References |
|---|---|---|---|---|---|
| Bcl-2 | Eutopic, peritoneal, ovarian, deep | Glands (+++) and stroma (+) | Proliferative and secretory | Increased in eutopic endometrium and further increased in endometriotic lesions, except cysts | Braun et al. (2007), Dufournet et al. (2006), Goumenou et al. (2004), Hassa et al. (2009), Nezhat and Kalir (2002) |
| Bcl-XL | Eutopic, peritoneal, ovarian | Undefined | Proliferative and secretory | Increased in eutopic endometrium and in endometriotic lesions | Braun et al. (2007), Nishida et al. (2005) |
| Bax | Eutopic, peritoneal, ovarian, deep | Glands (+++) and stroma (+) | Proliferative and secretory | Stronger in ovarian cysts versus other endometriotic lesions | Goumenou et al. (2004), Zubor et al. (2009) |
| Bcl-XS | Eutopic | Undefined | Proliferative | Increased in women with endometriosis | Zubor et al. (2009) |
Bcl-2 and Bcl-XL prevent apoptosis and increase cell survival, whereas Bax and Bcl-XS induce apoptosis.
+++, high levels of protein.
+, low levels of protein.
According to the implantation theory, intrinsic characteristics of the eutopic endometrium in women with endometriosis will be carried into the peritoneal endometriotic implants and contribute to abnormal cell survival in ectopic sites. The physiological increase in the apoptotic rate in the late secretory phase is missing in the eutopic endometrium of women with endometriosis (Szymanowski, 2007). This abnormal characteristic of the intrauterine endometrium is probably retained by the ectopic tissue, which partly explains the excess proliferation and insufficient apoptosis of endometriotic cells.
Methods
We searched Pubmed for items published in the English language between September 1991 and September 2011, including clinical and experimental, in vivo and in vitro studies but restricted to the human species, using the following search terms: ‘Chemokines’[Mesh] AND (endometrium OR endometriosis) AND (hormone OR steroid OR estradiol OR estrogen OR progesterone OR progestogen). This search returned 94 articles. Reference lists of the preselected articles and from other reviews were also searched. After detailed screening of titles, abstracts and full texts, we selected the studies evaluating the effects of hormones on chemokines in endometrial or endometriotic cells or tissues, and excluded the studies performed only in pregnancy, resulting in 38 articles being reviewed.
A second search was performed using the same criteria but substituting ‘Apoptosis’[Mesh] for; Chemokines [Mesh], which returned 143 items. We then selected the studies evaluating the effects of hormones on apoptosis in endometrial or endometrium-like cells or tissues, and excluded studies performed only in pregnancy or only in endometrial cancer, which resulted in 44 articles meeting the inclusion criteria. The data were then extracted, interpreted and summarized by all authors. No quantitative or statistical analysis was performed.
Results
Endocrine and paracrine regulation of chemokines in endometriosis
CC Chemokines
The endocrine and paracrine modifiers of RANTES in endometriosis have been evaluated by several investigative groups (Fig. 2). Despite higher concentrations of immunodetectable RANTES in secretory phase biopsies in situ, our group reported that endometrial and endometriotic stromal cells in vitro failed to respond directly to acute stimulation with estradiol, with or without progestogens (Hornung et al., 1997). Similar negative findings were noted with estradiol for RANTES secretion from isolated endometrial epithelial cells (Caballero-Campo et al., 2002). However, in subsequent reports, Akoum et al. (2002) and Yu et al. (2008) showed that combinations of estradiol plus IL-1β and estradiol plus the endocrine disruptor dioxin, respectively, indeed increased stromal cell RANTES synthesis. Interestingly, when stromal cells were exposed to chronic progestogen treatment, RANTES mRNA and protein levels were decreased; this effect appeared to be mediated via the NF-κB pathway (Zhao et al., 2002). Conversely, in cultured T lymphocytes isolated from the endometrium, progesterone stimulated RANTES expression, while recombinant RANTES down-regulated its own receptor CCR5 (Ramhorst et al., 2006).
In cultured endometrial stromal cells, both estradiol and dioxin induced MIP-1α (CCL3) release. This chemokine appeared to be involved in promoting endometrial cell invasiveness (Yu et al., 2008), which is an important mechanism in the onset of peritoneal endometriotic lesions. In addition, progesterone stimulated the release of MIP-1β (CCL4), a ligand of the CCR5 receptor (Kitaya et al., 2003).
Estradiol, progesterone and medroxyprogesterone acetate did not modify the MCP-1 (CCL2) output from normal endometrial stromal cells (DeLoia et al., 2000; Matta et al., 2007). However, in cells from women with endometriosis, estradiol (Akoum et al., 2000) and progesterone increased IL-1β-induced MCP-1 synthesis and release (Boucher et al., 2000b). Luk et al. (2010) reported that ovarian steroids directly up-regulated MCP-1 mRNA and protein in cultured human endometrial endothelial cells. Interestingly, this effect was only present in cells obtained from women with endometriosis (Luk et al., 2010). Danazol, a synthetic steroid with anti-estrogenic and androgenic activity, inhibited MCP-1 release by endometrial and endometriotic cells in vitro (Boucher et al., 2000a; Jolicoeur et al., 2001), indicating a direct anti-inflammatory action (Fig. 2).
It emerges from these studies that women with endometriosis have an aberrant CC chemokine response to sex steroids in their endometrial stroma and microvascular endothelium, which might explain the therapeutic failure and inadequate pain relief in some women with endometriosis who are treated with progestogens or hormonal contraceptives.
CXC Chemokines
The endocrine and paracrine control of the CC chemokine family in human endometrium has been addressed in several in vitro models. SDF-1 mRNA and protein have been detected in primary stromal cells, whereas its receptor CXCR4 was abundant in epithelial cells (Tsutsumi et al., 2011). In cultured endometrial stromal cells, estradiol stimulated SDF-1 production dose-dependently. This chemokine promoted the proliferation of an epithelial cell line, suggesting that it may be one of the paracrine mediators of the proliferative action of estrogen in the endometrium (Tsutsumi et al., 2011). According to this hypothesis, estrogen stimulation of stromal cells induces SDF-1 release, which acts in a paracrine fashion to increase epithelial/glandular cell proliferation (Fig. 3). Recent reports indicate that progesterone inhibits the expression of SDF-1 in an endometrial epithelial cell line (Ruiz et al., 2010) and this hormone as well as synthetic progestogens reduced SDF-1 secretion from primary endometrial stromal cells (Okada et al., 2011). In epithelial cells, CXCR4 was down-regulated by treatment with estradiol, progesterone or estradiol plus progesterone. Interestingly, in vivo assessment of CXCR4 showed that this chemokine receptor was more abundant in endometriotic lesions than in normal endometrium (Ruiz et al., 2010). Altogether, this body of evidence suggests that the chemotactic signal produced by SDF-1 through the receptor CXCR4 is potentially sensitive to inhibition by anti-estrogenic and/or progestogenic compounds used to treat endometriosis.
IL-8 concentrations in situ were observed to be highest in premenstrual endometrium (Dominguez et al., 2003) and in vivo administration of the progesterone antagonist mifepristone induced its up-regulation (Critchley et al., 2003). In vitro studies in endometriotic stromal cells showed that the combination of TNF-α and estradiol increased IL-8 mRNA and protein, and that this effect was mediated by NF-κB activation and could be reversed in the presence of natural progesterone, danazol and dienogest (Horie et al., 2005). Moreover, IL-8 production by endometrial stromal cells markedly decreases during progesterone-induced decidualization (Lockwood et al., 2004). A similar down-regulation of IL-8 release was induced by progesterone in endometrial tissue explants (Kelly et al., 1994). As expected, progesterone withdrawal leads to IL-8 release by endometrial stromal cells in vitro (Kizilay et al., 2008). In addition, estradiol inhibits aminopeptidase N activity in cultured endometrial stromal cells, an enzyme which limits the bioavailability of IL-8 in the endometrium (Seli et al., 2001). Thus, inhibition of aminopeptidase N might be a mechanism by which estradiol could increase IL-8 activity independent from regulating its gene expression or translation.
In addition to hormone effects on epithelial and stromal cells, the endocrine regulation of IL-8 and its receptor also has been evaluated in mesothelial and endothelial cells. While not extensively characterized in this field, these two cell types represent critical barriers to the invasion and nourishment, respectively, of nascent peritoneal endometriotic implants. Estradiol plus dioxin stimulated IL-8 release by human pelvic mesothelial cells, while the IL-8 receptor CXCR1 was shown to be highly expressed in endometriotic tissue, suggesting that IL-8 secreted by peritoneal mesothelial cells in response to estrogen could act on CXCR1 receptors in the endometriotic implants to maintain the inflammatory status in the peritoneum of women with endometriosis (Shi et al., 2006). The expression and release of IL-8 by normal human endometrial endothelial cells was inhibited by estradiol and progesterone, whereas both steroids paradoxically stimulated IL-8 release from the same cell type isolated from women with endometriosis (Luk et al., 2005).
While it is premature to extrapolate these in vitro data to a therapeutic context, they consistently point to an antagonistic relationship between estrogens and progestogens in the regulation of IL-8 in endometrial stromal cells, with stimulation by estrogens and inhibition by progestogens. This is not the case in endothelial cells, where the effect of natural progesterone appears to be synergistic with that of estradiol and to be reversed in women with endometriosis. Therefore, the effects of progestogenic compounds designed to treat or prevent endometriosis should be tested not only in endometrial cells but also in endothelial cells before any conclusion is reached about their potential clinical effectiveness in blocking IL-8 production at critical sites.
CX3C chemokines
Fractalkine (CX3CL1) is a member of a third family of chemokines, wherein three amino acids separate the two canonical cysteines. In a single report that described fractalkine in the peritoneal fluid of women with endometriosis, its concentrations were lower than in control samples (Shimoya et al., 2005). This chemokine was noted to be present in endometrial epithelium and stroma, with the latter prominent in the secretory phase of the normal cycle and further stimulated after therapeutic treatment with progestogen contraceptives (Hannan et al., 2004). To date, in vitro manipulation with ovarian steroids has not been reported, but several studies indicate that fractalkine can be suppressed in respiratory cells by glucocorticoids (Bhavsar et al., 2008). IL-1β and interferon-γ both are potent activators of fractalkine in lung fibroblasts (Isozaki et al., 2011).
In vivo evidence for endocrine modulation of endometrial chemokines
A study of endometrial fluid samples collected during controlled ovarian hyperstimulation for IVF showed the up-regulation of several endometrial cytokines and chemokines in stimulated cycles compared with previous natural cycles. However, it is not possible to determine in this model if the intrauterine milieu might have been altered by direct effects of GnRH analog or gonadotropins, or by excess estradiol or other ovarian products (Boomsma et al., 2010). In a similar study of endometrial biopsies, B-cell-attracting chemokine (BCA)-1 (CXCL13) was up-regulated in stimulated versus natural cycles, but again the endocrine pathway involved could not be determined (Macklon et al., 2008). In women receiving donor oocytes and treated with ovarian steroids to prepare the endometrium for embryo transfer, the immunoreactive levels of endometrial MCP-1 increased following the administration of progesterone (Caballero-Campo et al., 2002).
Several chemokines were found to be overexpressed in the endometrium of women using contraceptive subdermal implants or intrauterine systems containing levonorgestrel, showing correlations with increased leukocyte recruitment and, hypothetically, endometrial atrophic changes and susceptibility to irregular bleeding (Hannan et al., 2004; Jones et al., 2005). In levonorgestrel implant users, uterine washings revealed a progressive increase in ENA-78 concentrations over time (Chegini et al., 2007). This is consistent with in vitro findings that progesterone stimulates ENA-78 expression in endometrial stromal cells (Nasu et al., 2001). Long-term users of the levonorgestrel-releasing intrauterine system also manifest high expression of chemokines with six cysteines (6Ckine, CCL21) and IL-8 in the endometrial glands and stroma (Peloggia et al., 2006). In contrast, MCP-1 and MCP-2 (CCL8) appear to be down-regulated in levonorgestrel implant users (Hampton et al., 2001). Although the lack of RCTs limits any conclusion about the impact of levonorgestrel itself on endometrial chemokine production, the persistence of these chemokines in levonorgestrel-releasing contraceptive users suggests that leukocyte recruitment leads to a low-grade chronic inflammatory response.
From the endometriosis perspective, these studies might suggest that long-term levonorgestrel-releasing systems induce increased chemokine production and leukocyte recruitment to the endometrium, which could counter the therapeutic goal of reducing the proinflammatory environment in endometriotic lesions. At this point we should remember that eutopic endometrium does not necessarily mirror the endometriotic implants. Thus, it remains to be investigated directly whether chronic treatment with progestogens modulate chemokine production and leukocyte infiltration within the endometriotic tissue. This hypothesis is difficult to test in humans because endometriotic lesions cannot be sampled repeatedly to compare chemokine expression before and after progestogen treatment, while parallel controls taking placebo or other treatments would be hard to match due to the large variability in endometriotic lesions. Thus, the in vitro culture of endometriotic cells, despite their intrinsic limitations, may provide the most expeditious answers. In these cells, progesterone, danazol and dienogest all counteracted estradiol-induced IL-8 production (Horie et al., 2005). In a similar in vitro model, estradiol enhanced but progesterone did not affect RANTES expression (Akoum et al., 2002). In other studies, exposure of endometrial stromal cells to 100 nM medroxyprogesterone acetate for 8 days resulted in significant repression of RANTES protein secretion and gene expression, whereas no effect was seen in cells exposed to the same dose for only 2 days (Zhao et al., 2002). It seems clear that more clinical research is needed to assess the chemokine profile of endometriotic lesions in women treated with progestogens and combined contraceptive pills, in order to elucidate the effects of such hormonal interventions on the local inflammatory response, and their relationship to the cardinal endometriotic symptoms, pain and infertility.
Endocrine and paracrine regulation of apoptosis in endometriosis
Sex steroid hormones and apoptosis
In most human tissues and under most physiological conditions, the activation of ER by estradiol or other ligands results in the inhibition of apoptosis (Amaral et al., 2009). This is particularly true for the endometrium, as suggested by the following evidence. In natural cycles, unopposed estrogen stimulation in the proliferative phase is characterized by absence of apoptosis in endometrial glandular cells (Otsuki et al., 1994). Furthermore, estrogen stimulation in vitro increases endometrial cell viability, and this effect is strongly reversed by estrogen withdrawal (Song et al., 2002). The antiapoptotic effects of estrogens follow several pathways (Fig. 4) and appear to involve both nuclear and extranuclear ER signaling. For instance, through the classical nuclear mechanism, estrogen increases the transcription of the antiapoptotic protein Bcl-2, while the activation of extranuclear kinases by the estrogen-ER complex triggers a rapid non-genomic signaling cascade resulting in apoptosis inhibition (Amaral et al., 2009).
Figure 4.
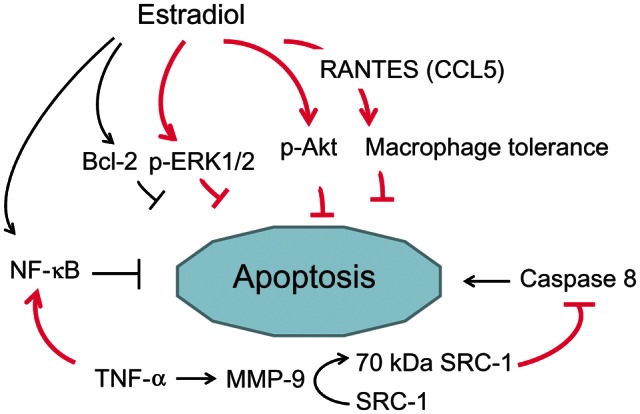
Apoptosis regulation by estradiol in human endometrium and endometriosis. ↑ stimulation; ⊥ inhibition. The bold, pink signs indicate abnormal responses observed in endometriosis. These include an enhanced activation (phosphorylation) of protein kinases (ERK1/2, Akt), increased levels of NF-κB and the cleavage of SRC-1, all converging to inhibit apoptosis. Bcl, B-cell leukemia/lymphoma; p-ERK, phosphorylated extracellular signal-regulated kinase; p-Akt, phosphorylated serine/threonine protein kinase B; MMP, matrix metalloproteinase.
The effects of progesterone, conversely, are more complex. In healthy volunteer women, the in vivo administration of progesterone decreases endometrial apoptosis observed in the late secretory phase (Lovely et al., 2005). The data suggest that hormone withdrawal in the late luteal phase triggers apoptosis and that progesterone supplementation may prolong endometrial epithelial cell survival and prevent the premenstrual surge of apoptosis. However, the same strategy of progesterone supplementation, when used in endometrial explants, does not affect apoptosis, despite effectively maintaining the histological integrity of explanted tissue (Li et al., 2005). In isolated endometrial cells in culture, progesterone actually induces apoptosis (Li et al., 2001; Choksuchat et al., 2009; Tang et al., 2009), an effect that may be consistent with reports of antiapoptotic actions of mifepristone in endometrial biopsies from progestogen users (Jain et al., 2006). Furthermore, progesterone physiologically down-regulates endometrial Bcl-2 expression, which decreases at the early secretory phase (von Rango et al., 1998) and increases upon mifepristone administration (Critchley et al., 1999; Fig. 5).
Figure 5.
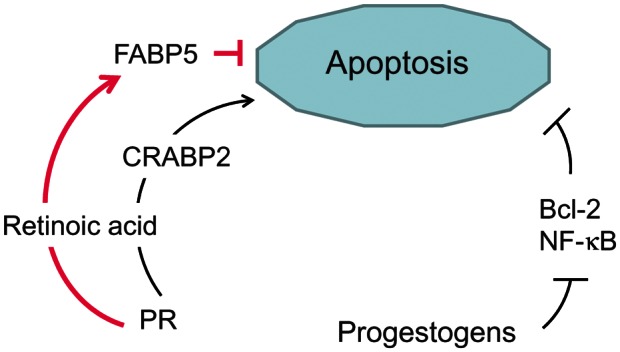
Apoptosis regulation by progestogens in human endometrium and endometriosis. ↑ stimulation; ⊥ inhibition. The bold, pink signs indicate abnormal responses observed in endometriosis. FABP, fatty acid-binding protein; CRABP, cellular retinoic acid-binding protein; PR, progesterone receptor.
To reconcile these findings, we suggest that part of the growth-limiting effect of progesterone on the human endometrium includes the modulation of apoptosis-related genes in favor of increased apoptosis (Tao et al., 1998; von Rango et al., 1998), but the process of glandular cell death is somehow precipitated by progesterone withdrawal at the late secretory phase (Lovely et al., 2005).
Effects of sex steroid hormones on apoptosis in endometriosis
Considering that endometriotic cells are resistant to apoptosis, and that sex steroids modulate apoptosis in the endometrium, the next question is whether endometriotic cells respond abnormally to the apoptosis modulation by sex steroids and, if so, which mechanisms underlie this abnormal response. Indirect in vitro evidence suggests that endometriotic cells respond to estrogen-induced antiapoptotic signaling more intensely than normal cells (Fig. 4). For example, the antiapoptotic protein extracellular signal-regulated kinase (ERK)1/2 is highly activated (i.e. phosphorylated) in vivo in eutopic and ectopic glandular endometrial cells from women with endometriosis throughout the menstrual cycle (Murk et al., 2008). Moreover, estrogen stimulates ERK1/2 phosphorylation in eutopic endometrial stromal cells isolated from patients with endometriosis, but not in endometrial stromal cells from women without endometriosis. This abnormal response to estrogen probably explains the excess ERK1/2 activation and contributes to apoptosis resistance in endometriotic cells (Murk et al., 2008; Fig. 4).
The serine/threonine protein kinase B, or Akt, is a pleiotropic regulator of cell proliferation and apoptosis. Estrogen induces a rapid phosphorylation of Akt in endometrial stromal cells, which is indicative of non-genomic action (Cinar et al., 2009). Akt activity (phosphorylation) is increased in both eutopic and ectopic endometrial cells from women with endometriosis (Cinar et al., 2009). Akt may play a central role in endometriosis by increasing cell survival through decreased apoptosis (Fig. 4). One of the putative factors responsible for higher Akt phosphorylation in endometriotic cells may be the continuous local stimulation of ERs due to intralesional production of estradiol, as suggested by studies showing local expression of aromatase (Bulun et al., 2012).
The increased sensitivity of endometriotic cells to the survival message mediated by estradiol does not appear to be related to the abundance of ERα, which is expressed in endometriotic lesions at normal or reduced levels (Fujishita et al., 1997; Brandenberger et al., 1999; Morsch et al., 2009). In contrast, ERβ is up-regulated in endometriosis and seems to contribute to implant survival (Cavallini et al., 2011; Bulun et al., 2012). A recent study tested the hypothesis that endometriotic cells overexpress estrogen-related receptors (ERRs), a subfamily of orphan receptors that shares sequence homology with ERs. However, the study reached the opposite conclusion, that ERRα and ERRγ mRNA and protein levels are significantly lower in endometriotic lesions compared with eutopic endometrium from women with endometriosis and normal endometrium (Cavallini et al., 2011).
Recently, Han and coworkers (2012) discovered a novel potential mediator of resistance to apoptosis in endometriosis. They observed that endometriotic tissues contain a 70-kDa truncated isoform of steroid receptor coactivator (SRC)-1, which is generated by matrix metalloproteinase 9 cleavage of full-length SRC-1 (Fig. 4). The proteolytic process is induced by TNF-α released by macrophages and NK cells as part of the inflammatory response to endometriotic implants. The truncated SRC-1 isoform still acts as a transcriptional coactivator and boosts nuclear ER-β effects, but also has the unique ability of stabilizing procaspase-8 and thereby blocking apoptosis (Dyson and Bulun, 2012).
Progesterone resistance is one of the pathogenic mechanisms potentially involved in the survival of endometriotic implants. Progesterone receptor (PR) mRNA levels, particularly those of transcripts encoding the PR-B isoform, are reduced in extraovarian ectopic lesions relative to matched eutopic endometrium from the same subjects. These effects also are manifested at the level of PR-B protein as detected by western blots (Attia et al., 2000). Immunohistochemical studies also suggested that PR levels tended to be low and without menstrual cycle variation in peritoneal ectopic lesions (Beliard et al., 2004). In addition, the establishment of endometriotic lesions in the pelvis is able to induce long-lasting progesterone resistance in the eutopic endometrium (Fazleabas, 2010; Al-Sabbagh et al., 2012).
The apoptotic index in mid-luteal phase endometrium is reduced in women with endometriosis (Szymanowski, 2007), suggesting a subnormal response to progesterone. Conversely, the number of apoptotic cells in endometrial biopsies from women with endometriosis increased following treatment with combined oral contraceptives (Meresman et al., 2002) or the levonorgestrel-releasing intrauterine system (Gomes et al., 2009). However, from a therapeutic perspective, it is relevant to know if endometriotic lesions, rather than the eutopic endometrium, are refractory to progestogen-induced apoptosis. As noted above, ethical constraints prevent the investigation of this response in vivo, which would require performing two laparoscopic biopsies of the same lesion, before and after a progestogen treatment course. Hence, we are left to extrapolate experimental findings obtained with stromal cells derived from endometriotic cysts.
In normal cells, PR stimulates the apoptotic pathway triggered by retinoic acid nuclear signaling, through the up-regulation of the retinoic acid shuttling protein CRABP2 (Fig. 5). Endometriotic cells have deficient retinoic acid production due to insufficient retinol uptake. In addition, they have an aberrant profile of retinoic acid shuttling proteins, leading to a paradoxical retinoic acid action mediated by fatty acid-binding protein 5 (FABP5), a prosurvival nuclear receptor (Pavone et al., 2010). Wieser et al. (2005) demonstrated the constitutive activation of NF-κB, a master transcriptional regulator of inflammatory responses, in endometriotic cells. It has been suggested that NF-κB may promote the growth and survival of endometriotic lesions, since NF-κB inhibitors block the proliferation of endometriotic stromal cells and induce apoptosis (Gonzalez-Ramos et al., 2008). NF-κB activation in endometriotic stromal cells is induced by TNF-α and estradiol (Fig. 4) and inhibited by progestogens (Horie et al., 2005; Fig. 5), which is an additional downstream mechanism involved in steroid hormone control of apoptosis in endometriosis.
Genomic variants may also be implicated in the progesterone resistance of women with endometriosis. The PR gene polymorphism PROGINS codifies a variant PR that is less responsive to progestogens, when compared with wild-type PR, resulting in reduced biological activity. As predicted, in vitro experiments have demonstrated that progesterone fails to induce apoptosis in endometrial cells harboring the PROGINS variant allele (D'Amora et al., 2009). This finding is relevant because the PROGINS variant has been reported to be more common in women with surgically confirmed endometriosis in some studies, compared with general populations (Wieser et al., 2002; Lattuada et al., 2004; De Carvalho et al., 2007). However, these findings may not be consistent, as another larger collaborative survey by Near et al. (2011) failed to find an association, but it should be noted that in the latter study, the endometriosis diagnosis was based on self-report alone.
We conclude that normal apoptosis mechanisms are suppressed in endometrial cells from women with endometriosis and within endometriotic lesions. Increased estrogen availability due to local estradiol synthesis and increased estrogen sensitivity due to ERβ and SRC-1 lead to exaggerated protein kinase activation and apoptosis inhibition. Progesterone resistance further contributes to endometriotic cell survival, and may reduce the therapeutic effects of progestogens.
Effects of peptide hormones on apoptosis in endometriosis
GnRH receptors are not exclusive to the pituitary gland, but also are found in peripheral reproductive tissues, including the human endometrium (Wu et al., 2009). Biopsies of rectovaginal endometriotic lesions obtained before and during treatment with the GnRH agonist leuprolide acetate showed increased apotosis during treatment (Mizutani et al., 1999). However, this approach does not allow a distinction between systemic (ovarian suppression) and local action of the GnRH analog. To address this question, leuprolide acetate was incubated with human endometrial cells in vitro and directly stimulated apoptosis (Imai et al., 2000; Meresman et al., 2003). This effect was observed in cells from women with endometriosis as well as from normal controls, and was reversed by a GnRH antagonist (Meresman et al., 2003). If confirmed, these experiments suggest that GnRH agonists may have a unique peripheral mechanism of inhibiting endometriosis by directly inducing apoptosis, but they question the role of GnRH antagonists in this setting.
Summary and conclusions
The mechanisms underlying the onset, progression and maintenance of endometriosis include an abnormal inflammatory response, which is established in the early stages of the disease and sustained by persistent immune cell recruitment to the endometriotic foci. Production of proteases, mitogens, angiogenic factors and activating cytokines and chemokines by infiltrating leukocytes appears to promote lesion invasiveness. A complementary and possibly synergistic mechanism of endometriosis formation derives from an imbalanced regulation of cell fate, with reduced susceptibility to apoptosis. Some chemokines, such as RANTES and IL-8, may be involved not only in the amplification of the local inflammatory response but also in the survival of endometriotic cells through increased proliferation and/or attenuation of apoptosis (Selam et al., 2006; Wang et al., 2010). Despite the unequivocal recognition that sex steroids play a central role in endometriosis biology and remain the first-line targets for medical therapies, the effects of estrogens and progestogens on chemotaxis and apoptosis in endometriotic cells are complex and only partially known.
As reviewed here, considerable evidence indicates that estrogen is not only proliferative but also proinflammatory and antiapoptotic in endometrial epithelial cells and stromal cells. These effects appear to be exacerbated in women with endometriosis, in whom local estradiol reinforces both inflammation and cell survival, mediated by chemokines, kinases and Bcl-2. Although endothelial cells from the endometrium of women with endometriosis may release IL-8 in response to progestogens, it is reassuring that, in stromal cells from both eutopic and ectopic endometrium, the predominant effect of progestogens is to inhibit IL-8 and other chemokines. Progestogens also are effective in inducing apoptosis in endometrial epithelial cells through the inhibition of Bcl-2 and the stimulation of Bax and ERK pathways. Finally, the role of peptide hormones, in particular GnRH, in regulating endometrial apoptosis deserves further investigation in order to clarify whether their direct proapoptotic effects on endometrial cells, indicated by some experiments in vitro, might be clinically relevant in vivo. Further elucidation of the fundamental mechanisms of leukocyte chemotaxis and endometrial and immune cell apoptosis will undoubtedly lead to the future development of adjuvant medical therapies for the clinical management of endometriosis.
Authors' roles
All authors (F.M.R., F.P. and R.N.T.) participated in the design, bibliographic search and selection, data analyses, manuscript writing and final manuscript approval.
Funding
Research supported by the Italian Ministry of University and Scientific Research (to F.P.), Brazilian National Institute of Hormones and Women's Health (to F.M.R.) and the Eunice Kennedy Shriver NICHD through cooperative agreements U01HD66439 and U54HD55787 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (to R.N.T.).
Conflict of interest
None declared.
Acknowledgements
We thank Helen Del Puerto, PhD for help with reference screening and data extraction.
References
- Adamson GD, Kennedy SH, Hummelshoj L. Creating solutions in endometriosis: global collaboration through the World Endometriosis Research Foundation. J Endometriosis. 2010;2:3–6. [Google Scholar]
- Akoum A, Lemay A, Brunet C, Hebert J. Secretion of monocyte chemotactic protein-1 by cytokine-stimulated endometrial cells of women with endometriosis. Le groupe d'investigation en gynecologie. Fertil Steril. 1995;63:322–328. doi: 10.1016/s0015-0282(16)57363-4. [DOI] [PubMed] [Google Scholar]
- Akoum A, Lemay A, McColl S, Turcot-Lemay L, Maheux R. Elevated concentration and biologic activity of monocyte chemotactic protein-1 in the peritoneal fluid of patients with endometriosis. Fertil Steril. 1996;66:17–23. [PubMed] [Google Scholar]
- Akoum A, Jolicoeur C, Boucher A. Estradiol amplifies interleukin-1-induced monocyte chemotactic protein-1 expression by ectopic endometrial cells of women with endometriosis. J Clin Endocrinol Metab. 2000;85:896–904. doi: 10.1210/jcem.85.2.6348. doi:10.1210/jc.85.2.896. [DOI] [PubMed] [Google Scholar]
- Akoum A, Lemay A, Maheux R. Estradiol and interleukin-1beta exert a synergistic stimulatory effect on the expression of the chemokine regulated upon activation, normal T cell expressed, and secreted in endometriotic cells. J Clin Endocrinol Metab. 2002;87:5785–5792. doi: 10.1210/jc.2002-020106. doi:10.1210/jc.2002-020106. [DOI] [PubMed] [Google Scholar]
- Al-Sabbagh M, Lam EW, Brosens JJ. Mechanisms of endometrial progesterone resistance. Mol Cell Endocrinol. 2012;358:208–215. doi: 10.1016/j.mce.2011.10.035. doi:10.1016/j.mce.2011.10.035. [DOI] [PubMed] [Google Scholar]
- Amaral JD, Sola S, Steer CJ, Rodrigues CM. Role of nuclear steroid receptors in apoptosis. Curr Med Chem. 2009;16:3886–3902. doi: 10.2174/092986709789178028. doi:10.2174/092986709789178028. [DOI] [PubMed] [Google Scholar]
- Arici A, Tazuke SI, Attar E, Kliman HJ, Olive DL. Interleukin-8 concentration in peritoneal fluid of patients with endometriosis and modulation of interleukin-8 expression in human mesothelial cells. Mol Hum Reprod. 1996;2:40–45. doi: 10.1093/molehr/2.1.40. doi:10.1093/molehr/2.1.40. [DOI] [PubMed] [Google Scholar]
- Arici A, Oral E, Attar E, Tazuke SI, Olive DL. Monocyte chemotactic protein-1 concentration in peritoneal fluid of women with endometriosis and its modulation of expression in mesothelial cells. Fertil Steril. 1997;67:1065–1072. doi: 10.1016/s0015-0282(97)81440-9. doi:10.1016/S0015-0282(97)81440-9. [DOI] [PubMed] [Google Scholar]
- Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85:2897–2902. doi: 10.1210/jcem.85.8.6739. doi:10.1210/jc.85.8.2897. [DOI] [PubMed] [Google Scholar]
- Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–1049. doi: 10.1172/JCI114265. doi:10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batt RE. A History of Endometriosis. London: Springer; 2011. [Google Scholar]
- Beliard A, Noel A, Foidart JM. Reduction of apoptosis and proliferation in endometriosis. Fertil Steril. 2004;82:80–85. doi: 10.1016/j.fertnstert.2003.11.048. doi:10.1016/j.fertnstert.2003.11.048. [DOI] [PubMed] [Google Scholar]
- Bersinger NA, Gunthert AR, McKinnon B, Johann S, Mueller MD. Dose–response effect of interleukin (IL)-1beta, tumour necrosis factor (TNF)-alpha, and interferon-gamma on the in vitro production of epithelial neutrophil activating peptide-78 (ENA-78), IL-8, and IL-6 by human endometrial stromal cells. Arch Gynecol Obstet. 2011;283:1291–1296. doi: 10.1007/s00404-010-1520-3. doi:10.1007/s00404-010-1520-3. [DOI] [PubMed] [Google Scholar]
- Bhavsar PK, Sukkar MB, Khorasani N, Lee KY, Chung KF. Glucocorticoid suppression of CX3CL1 (fractalkine) by reduced gene promoter recruitment of NF-kappaB. FASEB J. 2008;22:1807–1816. doi: 10.1096/fj.07-094235. doi:10.1096/fj.07-094235. [DOI] [PubMed] [Google Scholar]
- Boomsma CM, Kavelaars A, Eijkemans MJ, Fauser BC, Heijnen CJ, Macklon NS. Ovarian stimulation for in vitro fertilization alters the intrauterine cytokine, chemokine, and growth factor milieu encountered by the embryo. Fertil Steril. 2010;94:1764–1768. doi: 10.1016/j.fertnstert.2009.10.044. doi:10.1016/j.fertnstert.2009.10.044. [DOI] [PubMed] [Google Scholar]
- Boucher A, Lemay A, Akoum A. Effect of hormonal agents on monocyte chemotactic protein-1 expression by endometrial epithelial cells of women with endometriosis. Fertil Steril. 2000a;74:969–975. doi: 10.1016/s0015-0282(00)01540-5. doi:10.1016/S0015-0282(00)01540-5. [DOI] [PubMed] [Google Scholar]
- Boucher A, Mourad W, Mailloux J, Lemay A, Akoum A. Ovarian hormones modulate monocyte chemotactic protein-1 expression in endometrial cells of women with endometriosis. Mol Hum Reprod. 2000b;6:618–626. doi: 10.1093/molehr/6.7.618. doi:10.1093/molehr/6.7.618. [DOI] [PubMed] [Google Scholar]
- Brandenberger AW, Lebovic DI, Tee MK, Ryan IP, Tseng JF, Jaffe RB, Taylor RN. Oestrogen receptor (ER)-alpha and ER-beta isoforms in normal endometrial and endometriosis-derived stromal cells. Mol Hum Reprod. 1999;5:651–655. doi: 10.1093/molehr/5.7.651. doi:10.1093/molehr/5.7.651. [DOI] [PubMed] [Google Scholar]
- Braun DP, Ding J, Shaheen F, Willey JC, Rana N, Dmowski WP. Quantitative expression of apoptosis-regulating genes in endometrium from women with and without endometriosis. Fertil Steril. 2007;87:263–268. doi: 10.1016/j.fertnstert.2006.06.026. doi:10.1016/j.fertnstert.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Bulmer JN, Pace D, Ritson A. Immunoregulatory cells in human decidua: morphology, immunohistochemistry and function. Reprod Nutr Dev. 1988;28:1599–1613. doi: 10.1051/rnd:19881006. doi:10.1051/rnd:19881006. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Monsavais D, Pavone ME, Dyson M, Xue Q, Attar E, Tokunaga H, Su EJ. Role of estrogen receptor-beta in endometriosis. Semin Reprod Med. 2012;30:39–45. doi: 10.1055/s-0031-1299596. doi:10.1055/s-0031-1299596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero-Campo P, Dominguez F, Coloma J, Meseguer M, Remohi J, Pellicer A, Simon C. Hormonal and embryonic regulation of chemokines IL-8, MCP-1 and RANTES in the human endometrium during the window of implantation. Mol Hum Reprod. 2002;8:375–384. doi: 10.1093/molehr/8.4.375. doi:10.1093/molehr/8.4.375. [DOI] [PubMed] [Google Scholar]
- Cavallini A, Resta L, Caringella AM, Dinaro E, Lippolis C, Loverro G. Involvement of estrogen receptor-related receptors in human ovarian endometriosis. Fertil Steril. 2011;96:102–106. doi: 10.1016/j.fertnstert.2011.04.032. doi:10.1016/j.fertnstert.2011.04.032. [DOI] [PubMed] [Google Scholar]
- Chegini N, Luo X, Pan Q, Rhoton-Vlasak A, Archer DF. Endometrial expression of epithelial neutrophil-activating peptide-78 during the menstrual cycle or in progestin-only contraceptive users with breakthrough bleeding and the influence of doxycycline therapy. Hum Reprod. 2007;22:427–433. doi: 10.1093/humrep/del398. doi:10.1093/humrep/del398. [DOI] [PubMed] [Google Scholar]
- Choksuchat C, Zhao S, Deutch TD, Kimble TD, Archer DF. Effects of progesterone, levonorgestrel and medroxyprogesterone acetate on apoptosis in human endometrial endothelial cells. Contraception. 2009;79:139–145. doi: 10.1016/j.contraception.2008.08.008. doi:10.1016/j.contraception.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Cinar O, Seval Y, Uz YH, Cakmak H, Ulukus M, Kayisli UA, Arici A. Differential regulation of Akt phosphorylation in endometriosis. Reprod Biomed Online. 2009;19:864–871. doi: 10.1016/j.rbmo.2009.10.001. doi:10.1016/j.rbmo.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Critchley HO, Tong S, Cameron ST, Drudy TA, Kelly RW, Baird DT. Regulation of bcl-2 gene family members in human endometrium by antiprogestin administration in vivo. J Reprod Fertil. 1999;115:389–395. doi: 10.1530/jrf.0.1150389. doi:10.1530/jrf.0.1150389. [DOI] [PubMed] [Google Scholar]
- Critchley HO, Kelly RW, Brenner RM, Baird DT. Antiprogestins as a model for progesterone withdrawal. Steroids. 2003;68:1061–1068. doi: 10.1016/j.steroids.2003.07.001. doi:10.1016/j.steroids.2003.07.001. [DOI] [PubMed] [Google Scholar]
- D'Amora P, Maciel TT, Tambellini R, Mori MA, Pesquero JB, Sato H, Girao MJ, Guerreiro da Silva ID, Schor E. Disrupted cell cycle control in cultured endometrial cells from patients with endometriosis harboring the progesterone receptor polymorphism PROGINS. Am J Pathol. 2009;175:215–224. doi: 10.2353/ajpath.2009.080966. doi:10.2353/ajpath.2009.080966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Carvalho CV, Nogueira-De-Souza NC, Costa AM, Baracat EC, Girao MJ, D'Amora P, Schor E, da Silva ID. Genetic polymorphisms of cytochrome P450cl7alpha (CYP17) and progesterone receptor genes (PROGINS) in the assessment of endometriosis risk. Gynecol Endocrinol. 2007;23:29–33. doi: 10.1080/09513590601024707. doi:10.1080/09513590601024707. [DOI] [PubMed] [Google Scholar]
- DeLoia JA, Stewart-Akers AM, Brekosky J, Kubik C. Stimulation of uterine cell cytokine production by ovarian hormones. Am J Reprod Immunol. 2000;44:16–21. doi: 10.1111/j.8755-8920.2000.440103.x. doi:10.1111/j.8755-8920.2000.440103.x. [DOI] [PubMed] [Google Scholar]
- Dmowski WP, Gebel HM, Braun DP. The role of cell-mediated immunity in pathogenesis of endometriosis. Acta Obstet Gynecol Scand Suppl. 1994;159:7–14. [PubMed] [Google Scholar]
- Dominguez F, Galan A, Martin JJ, Remohi J, Pellicer A, Simon C. Hormonal and embryonic regulation of chemokine receptors CXCR1, CXCR4, CCR5 and CCR2B in the human endometrium and the human blastocyst. Mol Hum Reprod. 2003;9:189–198. doi: 10.1093/molehr/gag024. doi:10.1093/molehr/gag024. [DOI] [PubMed] [Google Scholar]
- Dufournet C, Uzan C, Fauvet R, Cortez A, Siffroi JP, Darai E. Expression of apoptosis-related proteins in peritoneal, ovarian and colorectal endometriosis. J Reprod Immunol. 2006;70:151–162. doi: 10.1016/j.jri.2005.11.003. doi:10.1016/j.jri.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Dyson MT, Bulun SE. Cutting SRC-1 down to size in endometriosis. Nat Med. 2012;18:1016–1018. doi: 10.1038/nm.2855. doi:10.1038/nm.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazleabas AT. Progesterone resistance in a baboon model of endometriosis. Semin Reprod Med. 2010;28:75–80. doi: 10.1055/s-0029-1242997. doi:10.1055/s-0029-1242997. [DOI] [PubMed] [Google Scholar]
- Fujishita A, Nakane PK, Koji T, Masuzaki H, Chavez RO, Yamabe T, Ishimaru T. Expression of estrogen and progesterone receptors in endometrium and peritoneal endometriosis: an immunohistochemical and in situ hybridization study. Fertil Steril. 1997;67:856–864. doi: 10.1016/s0015-0282(97)81397-0. doi:10.1016/S0015-0282(97)81397-0. [DOI] [PubMed] [Google Scholar]
- Gebel HM, Braun DP, Tambur A, Frame D, Rana N, Dmowski WP. Spontaneous apoptosis of endometrial tissue is impaired in women with endometriosis. Fertil Steril. 1998;69:1042–1047. doi: 10.1016/s0015-0282(98)00073-9. doi:10.1016/S0015-0282(98)00073-9. [DOI] [PubMed] [Google Scholar]
- Gomes MK, Rosa-e-Silva JC, Garcia SB, de Sa Rosa-e-Silva AC, Turatti A, Vieira CS, Ferriani RA. Effects of the levonorgestrel-releasing intrauterine system on cell proliferation, Fas expression and steroid receptors in endometriosis lesions and normal endometrium. Hum Reprod. 2009;24:2736–2745. doi: 10.1093/humrep/dep288. doi:10.1093/humrep/dep288. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Ramos R, Van Langendonckt A, Defrere S, Lousse JC, Mettlen M, Guillet A, Donnez J. Agents blocking the nuclear factor-kappaB pathway are effective inhibitors of endometriosis in an in vivo experimental model. Gynecol Obstet Invest. 2008;65:174–186. doi: 10.1159/000111148. doi:10.1159/000111148. [DOI] [PubMed] [Google Scholar]
- Goumenou AG, Matalliotakis IM, Tzardi M, Fragouli YG, Mahutte NG, Arici A. Apoptosis and differential expression of apoptosis-related proteins in endometriotic glandular and stromal cells. J Soc Gynecol Investig. 2004;11:318–322. doi: 10.1016/j.jsgi.2004.02.005. doi:10.1016/j.jsgi.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Halban J. Hysteroadenosis metastatica. Zentralb Gynäkol. 1925;7:387–391. [Google Scholar]
- Halme J, Becker S, Hammond MG, Raj MH, Raj S. Increased activation of pelvic macrophages in infertile women with mild endometriosis. Am J Obstet Gynecol. 1983;145:333–337. doi: 10.1016/0002-9378(83)90720-2. [DOI] [PubMed] [Google Scholar]
- Hampton AL, Rogers PA, Affandi B, Salamonsen LA. Expression of the chemokines, monocyte chemotactic protein (MCP)-1 and MCP-2 in endometrium of normal women and Norplant users, does not support a central role in macrophage infiltration into endometrium. J Reprod Immunol. 2001;49:115–132. doi: 10.1016/s0165-0378(00)00082-6. doi:10.1016/S0165-0378(00)00082-6. [DOI] [PubMed] [Google Scholar]
- Han SJ, Hawkins SM, Begum K, Jung SY, Kovanci E, Qin J, Lydon JP, Demayo FJ, O'Malley BW. A new isoform of steroid receptor coactivator-1 is crucial for pathogenic progression of endometriosis. Nat Med. 2012;18:1102–1111. doi: 10.1038/nm.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney AF, Muscato JJ, Weinberg JB. Peritoneal fluid cell populations in infertility patients. Fertil Steril. 1981;35:696–698. doi: 10.1016/s0015-0282(16)45567-6. [DOI] [PubMed] [Google Scholar]
- Hannan NJ, Jones RL, Critchley HO, Kovacs GJ, Rogers PA, Affandi B, Salamonsen LA. Coexpression of fractalkine and its receptor in normal human endometrium and in endometrium from users of progestin-only contraception supports a role for fractalkine in leukocyte recruitment and endometrial remodeling. J Clin Endocrinol Metab. 2004;89:6119–6129. doi: 10.1210/jc.2003-031379. doi:10.1210/jc.2003-031379. [DOI] [PubMed] [Google Scholar]
- Harada T, Kaponis A, Iwabe T, Taniguchi F, Makrydimas G, Sofikitis N, Paschopoulos M, Paraskevaidis E, Terakawa N. Apoptosis in human endometrium and endometriosis. Hum Reprod Update. 2004;10:29–38. doi: 10.1093/humupd/dmh007. doi:10.1093/humupd/dmh007. [DOI] [PubMed] [Google Scholar]
- Hassa H, Tanir HM, Tekin B, Artan S, Dundar E, Kirilmaz SD, Sahin Mutlu F. Apoptosis patterns in eutopic and ectopic endometrium, adhesions and normal-looking peritoneum from women with or without endometriosis. Arch Gynecol Obstet. 2009;280:195–199. doi: 10.1007/s00404-008-0895-x. doi:10.1007/s00404-008-0895-x. [DOI] [PubMed] [Google Scholar]
- Horie S, Harada T, Mitsunari M, Taniguchi F, Iwabe T, Terakawa N. Progesterone and progestational compounds attenuate tumor necrosis factor alpha-induced interleukin-8 production via nuclear factor kappa B inactivation in endometriotic stromal cells. Fertil Steril. 2005;83:1530–1535. doi: 10.1016/j.fertnstert.2004.11.042. doi:10.1016/j.fertnstert.2004.11.042. [DOI] [PubMed] [Google Scholar]
- Hornung D, Ryan IP, Chao VA, Vigne JL, Schriock ED, Taylor RN. Immunolocalization and regulation of the chemokine RANTES in human endometrial and endometriosis tissues and cells. J Clin Endocrinol Metab. 1997;82:1621–1628. doi: 10.1210/jcem.82.5.3919. doi:10.1210/jc.82.5.1621. [DOI] [PubMed] [Google Scholar]
- Hornung D, Dohrn K, Sotlar K, Greb RR, Wallwiener D, Kiesel L, Taylor RN. Localization in tissues and secretion of eotaxin by cells from normal endometrium and endometriosis. J Clin Endocrinol Metab. 2000;85:2604–2608. doi: 10.1210/jcem.85.7.6665. doi:10.1210/jc.85.7.2604. [DOI] [PubMed] [Google Scholar]
- Hornung D, Bentzien F, Wallwiener D, Kiesel L, Taylor RN. Chemokine bioactivity of RANTES in endometriotic and normal endometrial stromal cells and peritoneal fluid. Mol Hum Reprod. 2001a;7:163–168. doi: 10.1093/molehr/7.2.163. doi:10.1093/molehr/7.2.163. [DOI] [PubMed] [Google Scholar]
- Hornung D, Klingel K, Dohrn K, Kandolf R, Wallwiener D, Taylor RN. Regulated on activation, normal T-cell-expressed and -secreted mRNA expression in normal endometrium and endometriotic implants: assessment of autocrine/paracrine regulation by in situ hybridization. Am J Pathol. 2001b;158:1949–1954. doi: 10.1016/S0002-9440(10)64664-0. doi:10.1016/S0002-9440(10)64664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai A, Takagi A, Tamaya T. Gonadotropin-releasing hormone analog repairs reduced endometrial cell apoptosis in endometriosis in vitro. Am J Obstet Gynecol. 2000;182:1142–1146. doi: 10.1067/mob.2000.104804. doi:10.1067/mob.2000.104804. [DOI] [PubMed] [Google Scholar]
- Isozaki T, Otsuka K, Sato M, Takahashi R, Wakabayashi K, Yajima N, Miwa Y, Kasama T. Synergistic induction of CX3CL1 by interleukin-1beta and interferon-gamma in human lung fibroblasts: involvement of signal transducer and activator of transcription 1 signaling pathways. Transl Res. 2011;157:64–70. doi: 10.1016/j.trsl.2010.11.007. doi:10.1016/j.trsl.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Izawa M, Harada T, Deura I, Taniguchi F, Iwabe T, Terakawa N. Drug-induced apoptosis was markedly attenuated in endometriotic stromal cells. Hum Reprod. 2006;21:600–604. doi: 10.1093/humrep/dei372. doi:10.1093/humrep/dei372. [DOI] [PubMed] [Google Scholar]
- Jain JK, Li A, Yang W, Minoo P, Felix JC. Effects of mifepristone on proliferation and apoptosis of human endometrium in new users of medroxyprogesterone acetate. Hum Reprod. 2006;21:798–809. doi: 10.1093/humrep/dei383. doi:10.1093/humrep/dei383. [DOI] [PubMed] [Google Scholar]
- Jolicoeur C, Boutouil M, Drouin R, Paradis I, Lemay A, Akoum A. Increased expression of monocyte chemotactic protein-1 in the endometrium of women with endometriosis. Am J Pathol. 1998;152:125–133. [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur C, Lemay A, Akoum A. Comparative effect of danazol and a GnRH agonist on monocyte chemotactic protein-1 expression by endometriotic cells. Am J Reprod Immunol. 2001;45:86–93. doi: 10.1111/j.8755-8920.2001.450204.x. doi:10.1111/j.8755-8920.2001.450204.x. [DOI] [PubMed] [Google Scholar]
- Jones RK, Bulmer JN, Searle RF. Phenotypic and functional studies of leukocytes in human endometrium and endometriosis. Hum Reprod Update. 1998;4:702–709. doi: 10.1093/humupd/4.5.702. doi:10.1093/humupd/4.5.702. [DOI] [PubMed] [Google Scholar]
- Jones RL, Morison NB, Hannan NJ, Critchley HO, Salamonsen LA. Chemokine expression is dysregulated in the endometrium of women using progestin-only contraceptives and correlates to elevated recruitment of distinct leukocyte populations. Hum Reprod. 2005;20:2724–2735. doi: 10.1093/humrep/dei140. doi:10.1093/humrep/dei140. [DOI] [PubMed] [Google Scholar]
- Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Osteen K, Lessey BA, Giudice LC. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144:2870–2881. doi: 10.1210/en.2003-0043. doi:10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- Kelly RW, Illingworth P, Baldie G, Leask R, Brouwer S, Calder AA. Progesterone control of interleukin-8 production in endometrium and chorio-decidual cells underlines the role of the neutrophil in menstruation and parturition. Hum Reprod. 1994;9:253–258. doi: 10.1093/oxfordjournals.humrep.a138491. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. doi:10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorram O, Taylor RN, Ryan IP, Schall TJ, Landers DV. Peritoneal fluid concentrations of the cytokine RANTES correlate with the severity of endometriosis. Am J Obstet Gynecol. 1993;169:1545–1549. doi: 10.1016/0002-9378(93)90433-j. [DOI] [PubMed] [Google Scholar]
- Kitaya K, Nakayama T, Okubo T, Kuroboshi H, Fushiki S, Honjo H. Expression of macrophage inflammatory protein-1beta in human endometrium: its role in endometrial recruitment of natural killer cells. J Clin Endocrinol Metab. 2003;88:1809–1814. doi: 10.1210/jc.2002-020980. doi:10.1210/jc.2002-020980. [DOI] [PubMed] [Google Scholar]
- Kizilay G, Cakmak H, Yen CF, Atabekoglu C, Arici A, Kayisli UA. Expression and regulation of c-Jun N-terminal kinase (JNK) in endometrial cells in vivo and in vitro. Histochem Cell Biol. 2008;130:761–771. doi: 10.1007/s00418-008-0421-z. doi:10.1007/s00418-008-0421-z. [DOI] [PubMed] [Google Scholar]
- Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril. 2006;85:564–572. doi: 10.1016/j.fertnstert.2005.08.046. doi:10.1016/j.fertnstert.2005.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattuada D, Somigliana E, Vigano P, Candiani M, Pardi G, Di Blasio AM. Genetics of endometriosis: a role for the progesterone receptor gene polymorphism PROGINS? Clin Endocrinol (Oxf) 2004;61:190–194. doi: 10.1111/j.1365-2265.2004.02076.x. doi:10.1111/j.1365-2265.2004.02076.x. [DOI] [PubMed] [Google Scholar]
- Lebovic DI, Chao VA, Martini JF, Taylor RN. IL-1beta induction of RANTES (regulated upon activation, normal T cell expressed and secreted) chemokine gene expression in endometriotic stromal cells depends on a nuclear factor-kappaB site in the proximal promoter. J Clin Endocrinol Metab. 2001a;86:4759–4764. doi: 10.1210/jcem.86.10.7890. doi:10.1210/jc.86.10.4759. [DOI] [PubMed] [Google Scholar]
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001b;75:1–10. doi: 10.1016/s0015-0282(00)01630-7. doi:10.1016/S0015-0282(00)01630-7. [DOI] [PubMed] [Google Scholar]
- Leiva MC, Hasty LA, Pfeifer S, Mastroianni L, Jr, Lyttle CR. Increased chemotactic activity of peritoneal fluid in patients with endometriosis. Am J Obstet Gynecol. 1993;168:592–598. doi: 10.1016/0002-9378(93)90500-i. [DOI] [PubMed] [Google Scholar]
- Li HY, Chang SP, Yuan CC, Chao HT, Ng HT, Sung YJ. Nitric oxide induces extensive apoptosis in endometrial epithelial cells in the presence of progesterone: involvement of mitogen-activated protein kinase pathways. Mol Hum Reprod. 2001;7:755–763. doi: 10.1093/molehr/7.8.755. doi:10.1093/molehr/7.8.755. [DOI] [PubMed] [Google Scholar]
- Li A, Felix JC, Hao J, Minoo P, Jain JK. Menstrual-like breakdown and apoptosis in human endometrial explants. Hum Reprod. 2005;20:1709–1719. doi: 10.1093/humrep/deh824. doi:10.1093/humrep/deh824. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Kumar P, Krikun G, Kadner S, Dubon P, Critchley H, Schatz F. Effects of thrombin, hypoxia, and steroids on interleukin-8 expression in decidualized human endometrial stromal cells: implications for long-term progestin-only contraceptive-induced bleeding. J Clin Endocrinol Metab. 2004;89:1467–1475. doi: 10.1210/jc.2003-030141. doi:10.1210/jc.2003-030141. [DOI] [PubMed] [Google Scholar]
- Lovely LP, Fazleabas AT, Fritz MA, McAdams DG, Lessey BA. Prevention of endometrial apoptosis: randomized prospective comparison of human chorionic gonadotropin versus progesterone treatment in the luteal phase. J Clin Endocrinol Metab. 2005;90:2351–2356. doi: 10.1210/jc.2004-2130. doi:10.1210/jc.2004-2130. [DOI] [PubMed] [Google Scholar]
- Luk J, Seval Y, Kayisli UA, Ulukus M, Ulukus CE, Arici A. Regulation of interleukin-8 expression in human endometrial endothelial cells: a potential mechanism for the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2005;90:1805–1811. doi: 10.1210/jc.2004-1813. doi:10.1210/jc.2004-1813. [DOI] [PubMed] [Google Scholar]
- Luk J, Seval Y, Ulukus M, Ulukus EC, Arici A, Kayisli UA. Regulation of monocyte chemotactic protein-1 expression in human endometrial endothelial cells by sex steroids: a potential mechanism for leukocyte recruitment in endometriosis. Reprod Sci. 2010;17:278–287. doi: 10.1177/1933719109352380. doi:10.1177/1933719109352380. [DOI] [PubMed] [Google Scholar]
- Macklon NS, van der Gaast MH, Hamilton A, Fauser BC, Giudice LC. The impact of ovarian stimulation with recombinant FSH in combination with GnRH antagonist on the endometrial transcriptome in the window of implantation. Reprod Sci. 2008;15:357–365. doi: 10.1177/1933719107311781. doi:10.1177/1933719107311781. [DOI] [PubMed] [Google Scholar]
- Matta P, Lockwood CJ, Schatz F, Krikun G, Rahman M, Buchwalder L, Norwitz ER. Thrombin regulates monocyte chemoattractant protein-1 expression in human first trimester and term decidual cells. Am J Obstet Gynecol. 2007;196:268e261–268. doi: 10.1016/j.ajog.2006.09.008. [DOI] [PubMed] [Google Scholar]
- McLaren J, Prentice A, Charnock-Jones DS, Millican SA, Muller KH, Sharkey AM, Smith SK. Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. J Clin Invest. 1996;98:482–489. doi: 10.1172/JCI118815. doi:10.1172/JCI118815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meigs JV. Endometrial hematomas of the ovary. Boston Med Surg J. 1922;187:1–13. doi:10.1056/NEJM192207061870101. [Google Scholar]
- Meresman GF, Auge L, Baranao RI, Lombardi E, Tesone M, Sueldo C. Oral contraceptives suppress cell proliferation and enhance apoptosis of eutopic endometrial tissue from patients with endometriosis. Fertil Steril. 2002;77:1141–1147. doi: 10.1016/s0015-0282(02)03099-6. doi:10.1016/S0015-0282(02)03099-6. [DOI] [PubMed] [Google Scholar]
- Meresman GF, Bilotas M, Buquet RA, Baranao RI, Sueldo C, Tesone M. Gonadotropin-releasing hormone agonist induces apoptosis and reduces cell proliferation in eutopic endometrial cultures from women with endometriosis. Fertil Steril. 2003;80(Suppl 2):702–707. doi: 10.1016/s0015-0282(03)00769-6. doi:10.1016/S0015-0282(03)00769-6. [DOI] [PubMed] [Google Scholar]
- Meyer R. Zur Frage der heterotopen Epithelwucherung, insbesondere des Peritonealepithels und in die Ovarien. Virch Arch Path Anat Phys. 1924;250:595–610. [Google Scholar]
- Mizutani T, Sugihara A, Nakamuro K, Suehara N, Terada N. The gonadotropin-releasing hormone agonist leuprolide acetate induces apoptosis and suppresses cell proliferative activity in rectovaginal endometriosis. Am J Obstet Gynecol. 1999;181:750–751. doi: 10.1016/s0002-9378(99)70523-5. doi:10.1016/S0002-9378(99)70523-5. [DOI] [PubMed] [Google Scholar]
- Morsch DM, Carneiro MM, Lecke SB, Araujo FC, Camargos AF, Reis FM, Spritzer PM. c-fos gene and protein expression in pelvic endometriosis: a local marker of estrogen action. J Mol Histol. 2009;40:53–58. doi: 10.1007/s10735-009-9212-7. doi:10.1007/s10735-009-9212-7. [DOI] [PubMed] [Google Scholar]
- Mueller MD, Lebovic DI, Garrett E, Taylor RN. Neutrophils infiltrating the endometrium express vascular endothelial growth factor: potential role in endometrial angiogenesis. Fertil Steril. 2000;74:107–112. doi: 10.1016/s0015-0282(00)00555-0. doi:10.1016/S0015-0282(00)00555-0. [DOI] [PubMed] [Google Scholar]
- Mueller MD, Mazzucchelli L, Buri C, Lebovic DI, Dreher E, Taylor RN. Epithelial neutrophil-activating peptide 78 concentrations are elevated in the peritoneal fluid of women with endometriosis. Fertil Steril. 2003;79(Suppl 1):815–820. doi: 10.1016/s0015-0282(02)04828-8. doi:10.1016/S0015-0282(02)04828-8. [DOI] [PubMed] [Google Scholar]
- Murk W, Atabekoglu CS, Cakmak H, Heper A, Ensari A, Kayisli UA, Arici A. Extracellularly signal-regulated kinase activity in the human endometrium: possible roles in the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2008;93:3532–3540. doi: 10.1210/jc.2007-2051. doi:10.1210/jc.2007-2051. [DOI] [PubMed] [Google Scholar]
- Na YJ, Lee DH, Kim SC, Joo JK, Wang JW, Jin JO, Kwak JY, Lee KS. Effects of peritoneal fluid from endometriosis patients on the release of monocyte-specific chemokines by leukocytes. Arch Gynecol Obstet. 2011;283:1333–1341. doi: 10.1007/s00404-010-1583-1. doi:10.1007/s00404-010-1583-1. [DOI] [PubMed] [Google Scholar]
- Nasu K, Arima K, Kai K, Fujisawa K, Nishida M, Miyakawa I. Expression of epithelial neutrophil-activating peptide 78 in cultured human endometrial stromal cells. Mol Hum Reprod. 2001;7:453–458. doi: 10.1093/molehr/7.5.453. doi:10.1093/molehr/7.5.453. [DOI] [PubMed] [Google Scholar]
- Nasu K, Nishida M, Kawano Y, Tsuno A, Abe W, Yuge A, Takai N, Narahara H. Aberrant expression of apoptosis-related molecules in endometriosis: a possible mechanism underlying the pathogenesis of endometriosis. Reprod Sci. 2011;18:206–218. doi: 10.1177/1933719110392059. doi:10.1177/1933719110392059. [DOI] [PubMed] [Google Scholar]
- Near AM, Wu AH, Templeman C, Van Den Berg DJ, Doherty JA, Rossing MA, Goode EL, Cunningham JM, Vierkant RA, Fridley BL, et al. Progesterone receptor gene polymorphisms and risk of endometriosis: results from an international collaborative effort. Fertil Steril. 2011;95:40–45. doi: 10.1016/j.fertnstert.2010.06.059. doi:10.1016/j.fertnstert.2010.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezhat FR, Kalir T. Comparative immunohistochemical studies of endometriosis lesions and endometriotic cysts. Fertil Steril. 2002;78:820–824. doi: 10.1016/s0015-0282(02)03345-9. doi:10.1016/S0015-0282(02)03345-9. [DOI] [PubMed] [Google Scholar]
- Nishida M, Nasu K, Ueda T, Fukuda J, Takai N, Miyakawa I. Endometriotic cells are resistant to interferon-gamma-induced cell growth inhibition and apoptosis: a possible mechanism involved in the pathogenesis of endometriosis. Mol Hum Reprod. 2005;11:29–34. doi: 10.1093/molehr/gah133. doi:10.1093/molehr/gah133. [DOI] [PubMed] [Google Scholar]
- Okada H, Okamoto R, Tsuzuki T, Tsuji S, Yasuda K, Kanzaki H. Progestins inhibit estradiol-induced vascular endothelial growth factor and stromal cell-derived factor 1 in human endometrial stromal cells. Fertil Steril. 2011;96:786–791. doi: 10.1016/j.fertnstert.2011.06.048. doi:10.1016/j.fertnstert.2011.06.048. [DOI] [PubMed] [Google Scholar]
- Oral E, Seli E, Bahtiyar MO, Olive DL, Arici A. Growth-regulated alpha expression in the peritoneal environment with endometriosis. Obstet Gynecol. 1996;88:1050–1056. doi: 10.1016/s0029-7844(96)00361-4. doi:10.1016/S0029-7844(96)00361-4. [DOI] [PubMed] [Google Scholar]
- Otsuki Y, Misaki O, Sugimoto O, Ito Y, Tsujimoto Y, Akao Y. Cyclic bcl-2 gene expression in human uterine endometrium during menstrual cycle. Lancet. 1994;344:28–29. doi: 10.1016/s0140-6736(94)91051-0. [DOI] [PubMed] [Google Scholar]
- Pavone ME, Reierstad S, Sun H, Milad M, Bulun SE, Cheng YH. Altered retinoid uptake and action contributes to cell survival in endometriosis. J Clin Endocrinol Metab. 2010;95:E300–E309. doi: 10.1210/jc.2010-0459. doi:10.1210/jc.2010-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peloggia A, Petta CA, Bahamondes L, Oliveira-Ribeiro M, Zhang J, Salamonsen L. Endometrial chemokines, uterine natural killer cells and mast cells in long-term users of the levonorgestrel-releasing intrauterine system. Hum Reprod. 2006;21:1129–1134. doi: 10.1093/humrep/dei476. doi:10.1093/humrep/dei476. [DOI] [PubMed] [Google Scholar]
- Ramhorst R, Patel R, Corigliano A, Etchepareborda JJ, Fainboim L, Schust D. Induction of maternal tolerance to fetal alloantigens by RANTES production. Am J Reprod Immunol. 2006;56:302–311. doi: 10.1111/j.1600-0897.2006.00430.x. doi:10.1111/j.1600-0897.2006.00430.x. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Salvo VA, Ruiz LA, Baez P, Garcia M, Flores I. Basal and steroid hormone-regulated expression of CXCR4 in human endometrium and endometriosis. Reprod Sci. 2010;17:894–903. doi: 10.1177/1933719110379920. doi:10.1177/1933719110379920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan IP, Tseng JF, Schriock ED, Khorram O, Landers DV, Taylor RN. Interleukin-8 concentrations are elevated in peritoneal fluid of women with endometriosis. Fertil Steril. 1995;63:929–932. [PubMed] [Google Scholar]
- Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–429. [Google Scholar]
- Schall TJ, Jongstra J, Dyer BJ, Jorgensen J, Clayberger C, Davis MM, Krensky AM. A human T cell-specific molecule is a member of a new gene family. J Immunol. 1988;141:1018–1025. [PubMed] [Google Scholar]
- Selam B, Kayisli UA, Akbas GE, Basar M, Arici A. Regulation of FAS ligand expression by chemokine ligand 2 in human endometrial cells. Biol Reprod. 2006;75:203–209. doi: 10.1095/biolreprod.105.045716. doi:10.1095/biolreprod.105.045716. [DOI] [PubMed] [Google Scholar]
- Seli E, Senturk LM, Bahtiyar OM, Kayisli UA, Arici A. Expression of aminopeptidase N in human endometrium and regulation of its activity by estrogen. Fertil Steril. 2001;75:1172–1176. doi: 10.1016/s0015-0282(01)01779-4. doi:10.1016/S0015-0282(01)01779-4. [DOI] [PubMed] [Google Scholar]
- Shi YL, Luo XZ, Zhu XY, Hua KQ, Zhu Y, Li DJ. Effects of combined 17beta-estradiol with TCDD on secretion of chemokine IL-8 and expression of its receptor CXCR1 in endometriotic focus-associated cells in co-culture. Hum Reprod. 2006;21:870–879. doi: 10.1093/humrep/dei414. doi:10.1093/humrep/dei414. [DOI] [PubMed] [Google Scholar]
- Shimoya K, Zhang Q, Temma-Asano K, Hayashi S, Kimura T, Murata Y. Fractalkine in the peritoneal fluid of women with endometriosis. Int J Gynaecol Obstet. 2005;91:36–41. doi: 10.1016/j.ijgo.2005.06.018. doi:10.1016/j.ijgo.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Song J, Rutherford T, Naftolin F, Brown S, Mor G. Hormonal regulation of apoptosis and the Fas and Fas ligand system in human endometrial cells. Mol Hum Reprod. 2002;8:447–455. doi: 10.1093/molehr/8.5.447. doi:10.1093/molehr/8.5.447. [DOI] [PubMed] [Google Scholar]
- Sozzani S, Agwu DE, Ellenburg MD, Locati M, Rieppi M, Rojas A, Mantovani A, McPhail LC. Activation of phospholipase D by interleukin-8 in human neutrophils. Blood. 1994;84:3895–3901. [PubMed] [Google Scholar]
- Strieter RM, Belperio JA, Burdick MD, Sharma S, Dubinett SM, Keane MP. CXC chemokines: angiogenesis, immunoangiostasis, and metastases in lung cancer. Ann N Y Acad Sci. 2004;1028:351–360. doi: 10.1196/annals.1322.041. doi:10.1196/annals.1322.041. [DOI] [PubMed] [Google Scholar]
- Suzumori N, Katano K, Suzumori K. Peritoneal fluid concentrations of epithelial neutrophil-activating peptide-78 correlate with the severity of endometriosis. Fertil Steril. 2004;81:305–308. doi: 10.1016/j.fertnstert.2003.08.011. doi:10.1016/j.fertnstert.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Szymanowski K. Apoptosis pattern in human endometrium in women with pelvic endometriosis. Eur J Obstet Gynecol Reprod Biol. 2007;132:107–110. doi: 10.1016/j.ejogrb.2006.04.008. doi:10.1016/j.ejogrb.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Tabibzadeh S. The signals and molecular pathways involved in human menstruation, a unique process of tissue destruction and remodelling. Mol Hum Reprod. 1996;2:77–92. doi: 10.1093/molehr/2.2.77. doi:10.1093/molehr/2.2.77. [DOI] [PubMed] [Google Scholar]
- Tang L, Zhang Y, Pan H, Luo Q, Zhu XM, Dong MY, Leung PC, Sheng JZ, Huang HF. Involvement of cyclin B1 in progesterone-mediated cell growth inhibition, G2/M cell cycle arrest, and apoptosis in human endometrial cell. Reprod Biol Endocrinol. 2009;7:144. doi: 10.1186/1477-7827-7-144. doi:10.1186/1477-7827-7-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao XJ, Sayegh RA, Tilly JL, Isaacson KB. Elevated expression of the proapoptotic BCL-2 family member, BAK, in the human endometrium coincident with apoptosis during the secretory phase of the cycle. Fertil Steril. 1998;70:338–343. doi: 10.1016/s0015-0282(98)00129-0. doi:10.1016/S0015-0282(98)00129-0. [DOI] [PubMed] [Google Scholar]
- Taylor RN. Classical and neo-classical concepts in the etiology of endometriosis. In: Hédon B, Mettler L, Tinneberg H-R, editors. 20th World Congress on Fertility and Sterility. Germany: Lukon-Verlagsgesellschaft; 2010. pp. 70–73. [Google Scholar]
- Taylor RN, Yu J, Torres PB, Schickedanz AC, Park JK, Mueller MD, Sidell N. Mechanistic and therapeutic implications of angiogenesis in endometriosis. Reprod Sci. 2009;16:140–146. doi: 10.1177/1933719108324893. doi:10.1177/1933719108324893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi A, Okada H, Nakamoto T, Okamoto R, Yasuda K, Kanzaki H. Estrogen induces stromal cell-derived factor 1 (SDF-1/CXCL12) production in human endometrial stromal cells: a possible role of endometrial epithelial cell growth. Fertil Steril. 2011;95:444–447. doi: 10.1016/j.fertnstert.2010.08.037. doi:10.1016/j.fertnstert.2010.08.037. [DOI] [PubMed] [Google Scholar]
- Vaskivuo TE, Stenback F, Karhumaa P, Risteli J, Dunkel L, Tapanainen JS. Apoptosis and apoptosis-related proteins in human endometrium. Mol Cell Endocrinol. 2000;165:75–83. doi: 10.1016/s0303-7207(00)00261-6. doi:10.1016/S0303-7207(00)00261-6. [DOI] [PubMed] [Google Scholar]
- Vinatier D, Dufour P, Oosterlynck D. Immunological aspects of endometriosis. Hum Reprod Update. 1996;2:371–384. doi: 10.1093/humupd/2.5.371. doi:10.1093/humupd/2.5.371. [DOI] [PubMed] [Google Scholar]
- von Rango U, Classen-Linke I, Krusche CA, Beier HM. The receptive endometrium is characterized by apoptosis in the glands. Hum Reprod. 1998;13:3177–3189. doi: 10.1093/humrep/13.11.3177. doi:10.1093/humrep/13.11.3177. [DOI] [PubMed] [Google Scholar]
- von Rokitansky C. Lehrbuch der pathologischen anatomie. Wien: Braunmüller; 1861. [Google Scholar]
- Wang XQ, Yu J, Luo XZ, Shi YL, Wang Y, Wang L, Li DJ. The high level of RANTES in the ectopic milieu recruits macrophages and induces their tolerance in progression of endometriosis. J Mol Endocrinol. 2010;45:291–299. doi: 10.1677/JME-09-0177. doi:10.1677/JME-09-0177. [DOI] [PubMed] [Google Scholar]
- Weed JC, Arquembourg PC. Endometriosis: can it produce an autoimmune response resulting in infertility? Clin Obstet Gynecol. 1980;23:885–893. [PubMed] [Google Scholar]
- Weil SJ, Wang S, Perez MC, Lyttle CR. Chemotaxis of macrophages by a peritoneal fluid protein in women with endometriosis. Fertil Steril. 1997;67:865–869. doi: 10.1016/s0015-0282(97)81398-2. doi:10.1016/S0015-0282(97)81398-2. [DOI] [PubMed] [Google Scholar]
- Wieser F, Schneeberger C, Tong D, Tempfer C, Huber JC, Wenzl R. PROGINS receptor gene polymorphism is associated with endometriosis. Fertil Steril. 2002;77:309–312. doi: 10.1016/s0015-0282(01)02984-3. doi:10.1016/S0015-0282(01)02984-3. [DOI] [PubMed] [Google Scholar]
- Wieser F, Vigne JL, Ryan I, Hornung D, Djalali S, Taylor RN. Sulindac suppresses nuclear factor-kappaB activation and RANTES gene and protein expression in endometrial stromal cells from women with endometriosis. J Clin Endocrinol Metab. 2005;90:6441–6447. doi: 10.1210/jc.2005-0972. doi:10.1210/jc.2005-0972. [DOI] [PubMed] [Google Scholar]
- Wu HM, Wang HS, Huang HY, Soong YK, MacCalman CD, Leung PC. GnRH signaling in intrauterine tissues. Reproduction. 2009;137:769–777. doi: 10.1530/REP-08-0397. doi:10.1530/REP-08-0397. [DOI] [PubMed] [Google Scholar]
- Yeaman GR, Guyre PM, Fanger MW, Collins JE, White HD, Rathbun W, Orndorff KA, Gonzalez J, Stern JE, Wira CR. Unique CD8+ T cell-rich lymphoid aggregates in human uterine endometrium. J Leukoc Biol. 1997;61:427–435. [PubMed] [Google Scholar]
- Yu J, Wang Y, Zhou WH, Wang L, He YY, Li DJ. Combination of estrogen and dioxin is involved in the pathogenesis of endometriosis by promoting chemokine secretion and invasion of endometrial stromal cells. Hum Reprod. 2008;23:1614–1626. doi: 10.1093/humrep/den125. doi:10.1093/humrep/den125. [DOI] [PubMed] [Google Scholar]
- Zhao D, Lebovic DI, Taylor RN. Long-term progestin treatment inhibits RANTES (regulated on activation, normal T cell expressed and secreted) gene expression in human endometrial stromal cells. J Clin Endocrinol Metab. 2002;87:2514–2519. doi: 10.1210/jcem.87.6.8526. doi:10.1210/jc.87.6.2514. [DOI] [PubMed] [Google Scholar]
- Zubor P, Hatok J, Galo S, Dokus K, Klobusiakova D, Danko J, Racay P. Anti-apoptotic and pro-apoptotic gene expression evaluated from eutopic endometrium in the proliferative phase of the menstrual cycle among women with endometriosis and healthy controls. Eur J Obstet Gynecol Reprod Biol. 2009;145:172–176. doi: 10.1016/j.ejogrb.2009.04.024. doi:10.1016/j.ejogrb.2009.04.024. [DOI] [PubMed] [Google Scholar]