Abstract
Memories that are emotionally arousing generally promote the survival of species; however, the systems that modulate emotional learning can go awry, resulting in pathological conditions such as post-traumatic stress disorders, phobias, and addiction. Understanding the conditions under which emotional memories can be targeted is a major research focus as the potential to translate these methods into clinical populations carries important implications.
It has been demonstrated that both fear and drug-related memories can be destabilised at their retrieval and require reconsolidation to be maintained. Therefore, memory reconsolidation offers a potential target period during which the aberrant memories underlying psychiatric disorders can be disrupted.
Monfils et al. in 2009 have shown for the first time that safe information provided through an extinction session after retrieval (during the reconsolidation window) may update the original memory trace and prevent the return of fear in rats. In recent years several authors have then tested the effect of post-retrieval extinction on reconsolidation of either fear or drug related memories in both laboratory animals and humans.
In this article we review the literature on post-reactivation extinction, discuss the differences across studies on the methodological ground, and review the potential boundary conditions that may explain existing discrepancies and limit the potential application of post-reactivation extinction approaches.
Keywords: Reconsolidation, Learning, Memory, Emotional, Appetitive, Retrieval, Extinction
1. Introduction
Memories that are emotionally arousing, such as those for predators, pleasure, or food, are adaptive in nature and promote the survival of a species. As a result, these memories tend to be stronger than memories for neutral events or stimuli (Cahill & McGaugh 1998) and consequently more resilient in nature. In a number of scenarios, the systems that modulate emotional learning can go awry resulting in pathological conditions such as post-traumatic stress disorder (PTSD), other anxiety disorders, and drug addiction. Understanding the conditions under which emotional memories can be targeted is a major research focus as the potential to translate these methods into clinical populations carries important implications.
Emotional memories can be created in a laboratory setting by pairing an initially neutral cue (e.g., a tone or a context) with an emotional stimulus or training the animal to perform a task to receive a positive reward or avoid a negative outcome. In fear paradigms, the training stimulus is usually some form of mild electrical shock, whereas in reward paradigms, the stimulus can be a natural, such as food, or an artificial reward, such as a drug. The strength of the memory can then be measured by quantifying the response to the previously neutral cue when it is presented alone or measuring an active response made by the animal (e.g. escaping a fear chamber or lever pressing for a drug).
Within hours after learning to associate a cue with an emotional outcome, the association becomes consolidated into long-term memory. Once consolidated, the memory can be difficult to disrupt and, until recently, was thought to be a permanently encoded memory trace (Glickman 1961; McGaugh 1966). Interrupting the consolidation process is one way to reduce the association (Schafe et al. 1999), as shown by two independent small pilot studies that showed the effects of propranolol (see below for the pharmacological rationale) in PTSD patients (Pitman et al., 2002; Vaiva et al., 2003). However, the practical application of getting to a patient in the immediate hours after a trauma or other strong emotional event occurence requires strategic planning and execution, and in most situations is not a real possibility.
In recent years, much research has focused on targeting these previously consolidated, arousing memories by initiating a labile period through a reactivation of the original memory. This labile period is termed the ‘reconsolidation phase’ and lasts up to 6 hours immediately following reactivation, in fear conditioned rodents (Nader et al. 2000). Although the discovery that memories become labile after reactivation is not new (see Misanin et al. 1968; Schneider & Sherman, 1968; Judge & Quartermain 1982) the exact conditions necessary to reactivate an emotional memory and the underlying mechanisms were not investigated until the topic saw a resurgence of interest in the last 10–12 years. Much research on reconsolidation and reconsolidation blockade has been done in laboratory animals by administering some forms of biochemical interference, such as protein synthesis inhibition by anisomycin, during the labile phase of the reconsolidation phase (for review Tronson & Taylor 2007).
In associative learning paradigms, extinction or exposure therapy is a widely used, and well-established, method to reduce emotional responding in response to a reinforcer resulting in a progressive decrease in emotional responding (Pavlov 1927; Bouton & Bolles 1979; Rescorla & Heth 1975). Although extinction reduces the response in the short-term and is entirely non-invasive, extinction procedures do not typically modify the original memory trace and instead lead to the development of a new, separate memory that inhibits responding (Pavlov 1927; Bouton 1993; Quirk et al. 2000; Milad & Quirk 2002). The result is a response that recovers over time (spontaneous recovery), when the setting is changed (renewal), or after an unsignaled stressor, conditioned cues, or reward (reinstatement).
Combining the strengths of both extinction and reconsolidation, by presenting an extinction session during the reconsolidation window, may allow researchers to persistently reduce the emotional response after conditioning in animals (Monfils et al. 2009), and develop a translational model for treatment of fear conditions as well as addictive disorders in humans. In this article we will review the literature on post-reactivation extinction, discuss the differences across studies on the methodological ground, and review the potential boundary conditions that may explain existing discrepancies and limit the potential application of post-reactivation extinction approaches.
2. Background
Over the past decade, an abundance of research has focused on disrupting emotional and appetitive memories after their consolidation into long-term storage by interrupting the reconsolidation process. Most of the early work on reconsolidation blockade and update was focused on fear learning paradigms (Misanin et al. 1968; Nader et al. 2000; Sara et al. 2000; Debiec et al. 2002), but in recent years these methods have shown efficacy in reducing responding in appetitive settings and drug addiction paradigms as well (Lee et al. 2005; Bernardi et al. 2006; Diergaarde et al. 2006; Milton et al. 2008a, 2008b). Targeting an aversive or rewarding memory while it is reactivated during this reconsolidation period provides a promising avenue for the treatment of pathogenic conditions characterised by maladaptive fear responses or drug-addiction. This reconsolidation period begins when the memory is rendered labile by cues present from the initial learning session and can be interrupted with pharmacological or behavioural intervention administered shortly after reactivation. The efficacy of such a paradigm is then put to the test by quantifying the emotional or appetitive response at a later time point, usually 24 hours after reactivation, in a scenario that previously elicited a response. Such experiments typically make use of a control group that does not get a reactivation but does undergo the pharmacological or behavioural intervention.
A major focus of reconsolidation research has been on the use of pharmacological agents injected after, or just prior to, retrieval of an emotional memory. These injections, although not without risk, provide a potentially translatable method of reducing the emotional response to stimulus as well as key information regarding the mechanisms involved in reconsolidation. Pharmacological agents that show the most promise in targeting the reconsolidation of emotional or appetitive memories include protein synthesis inhibitors (such as anisomycin or cycloheximide), kinase activity inhibitors (specifically compounds that inhibit extracellular signal-regulated kinase (ERK) and protein kinase A (PKA) activity), and beta-adrenergic antagonists (such as propranolol).
Protein synthesis is required for the consolidation of long-term memories (Davis & Squire 1984; McGaugh 2000; Flexner et al. 1965) and in a landmark study by Nader and colleagues (2000) was shown to be essential for the reconsolidation of fear memories after reactivation. Nader and colleagues blocked protein synthesis by infusing anisomycin into the basolateral amygdala of rats after reactivating a cued fear memory and found that freezing (the expression of fear) was drastically reduced to the cue when tested the next day. This result was maintained with a delay between initial learning and reactivation up to 14 days. The time between reactivation and infusion of the drug proved to be a critical factor. When anisomycin was injected immediately after the retrieval, freezing was reduced when tested to the CS the following day. However, when a delay of 6 hours was introduced between the retrieval and injection, there was no significant reduction of freezing during the test session 24 hours later. These results have since been replicated using alternative protein synthesis inhibitors, such as the drug cycloheximide (Duvarci et al. 2005), as well as alternative fear learning paradigms (Debiec et al. 2002; Runyan & Dash 2005; Frankland et al. 2006). Lack of preference for the drug-paired chamber was observed after the systemic administration of anisomycin immediately following a reactivation in conditioned place preference procedures involving morphine (Valjent et al. 2006, Milekic et al. 2006) and cocaine (Fan et al. 2010). Taken together, these results confirm that reconsolidation of emotional memories involves new protein synthesis.
The protein synthesis required for reconsolidation may have a biochemical signature that differs from that seen during the consolidation phase after initial learning (Hoeffer et al. 2011) suggesting that reconsolidation is not, as the name implies, simply the memory trace undergoing additional consolidation. This was explored by Lee and colleagues (2004) in an important study that found that in hippocampus the transcription factor zinc finger 268 (zif268) was involved in reconsolidation but not the consolidation of contextual fear memories where brain derived neurotrophic factor (BDNF) was necessary for consolidation but not reconsolidation (Lee et al. 2004).
Interfering with reconsolidation pharmacologically does not rely solely on inhibiting protein synthesis. Beta-adrenergic antagonists, including propranolol, are frequently used in humans for the treatment of hypertension, and research suggests they can reduce emotional responding after conditioned learning tasks. Infusions of propranolol into the lateral and basal nuclei of the amygdala as well as systemic injections of propranolol after a reactivation of a fear memory lead to reductions in freezing 48 hours after the injections as well as one month later (Debiec & LeDoux 2004). Propranolol can also disrupt the reconsolidation of reward memories such as those for drugs (Bernardi et al. 2006; Milton et al. 2008a; Robinson and Franklin 2010) or food (Diergaarde et al. 2006; Milton et al. 2008a).
There are some limitations on the use of pharmacological agents to interfere with reconsolidation. Milekic and Alberini (2002) showed that in an inhibitory avoidance task, anisomycin did not disrupt memory reconsolidation if the memories were older than 14 days. Further supporting the idea that the age of the initial memory is a relevant factor in the ability of protein synthesis inhibitors to block reconsolidation, Suzuki and colleagues (2004) found that memories less than 3 weeks old were subject to interruption by post retrieval anisomycin but older memories (8 weeks) were not. Not only is the age of the memory important in predicting success of pharmacological targeting of reconsolidation, but the type of learning paradigm is a key as well. In the fear setting, propranolol administered to rats only works to block the reconsolidation of cued or contextual fear memories (Muravieva & Alberini 2010; Debiec & LeDoux 2004; Abrari et al. 2008) and does not always appear to affect inhibitory avoidance memories (Muravieva & Alberini 2010). These results suggest that strength or age of the emotional memory, as well as the type of response elicited, could influence the way that reconsolidation paradigms are applied to reduce responding.
Although pharmacological interference of reconsolidation reduces emotional and appetitive responding in a number of animal learning paradigms, it’s application in humans requires further studies in addicted patients in order to assess the ratio therapeutic benefit/risk. The practice of extinction, or exposure therapy, is well established in research and clinical settings, thus providing a practical but not permanently effective method to address pathogenic emotional responses. On the other hand, reconsolidation blockade is an effective yet impractical and potentially invasive method for targeting the original memory and reducing responding permanently. In an attempt to develop a behavioural paradigm to reduce fear permanently, Monfils and colleagues (2009) combined the strengths of both reconsolidation and extinction methods into a behavioural paradigm by modifying the timing between the first and second CS presentation. It has been suggested that the reconsolidation period initiates a period of lability in which the original fear memory is open to update (Tronson & Taylor 2007, for review). By introducing an extinction session during this labile period, this behavioural paradigm, will in some cases, permanently attenuate conditioned responding in a manner that does not involve drugs or surgery. This chapter discusses the utility of post-reactivation extinction in animal models of emotional learning and provides evidence that seemingly slight differences in methodologies can yield drastically varied results.
3. Post-retrieval extinction
3.1. Animal studies
3.1.1. Studies suggesting the utility of post-retrieval extinction
The effect of post-retrieval extinction on consolidated memories was investigated for the first time by Monfils and colleagues in 2009. Based on the hypothesis that reconsolidation is an update mechanism that allows for new information available at the time of retrieval to be integrated in the original memory trace,Monfils et al. (2009) investigated the effects of extinction training applied after an isolated retrieval on conditioned fear responses. The prediction was that post-retrieval extinction would promote a re-write or updating of the original memory trace instead of creating a new one, as is usually the case with standard extinction. The return of fear after the retrieval-extinction manipulation was tested in rats under different conditions such as spontaneous recovery, renewal and reinstatement (see Box 1 for definition). To assess whether the effect of the retrieval-extinction procedure was the result of reconsolidation interference, Monfils et al. applied extinction at different intervals after an isolated CS presentation trial, including time points within and outside the labile window of the reconsolidation phase. Rats were fear–conditioned using three tone-shock pairings, with the tone serving as conditioned stimulus (CS) and shock as unconditioned stimulus (US). Twenty-four hours later, fear memory was reactivated by a single CS presentation in the absence of US, followed by extinction sessions respectively at time points 10 minutes, 1, 6 or 24 hours in a between-groups design. One month later, Monfils and colleagues showed that reduced the spontaneous recovery of fear conditioned response. Monfils et al. also found that post-retrieval-extinction prevented renewal and reinstatement, as well as led to retardation of fear re-acquisition, and reduced fear memory savings only when applied within the reconsolidation window. Importantly, other groups that received standard extinction showed re-emergence of the fear conditioned response under renewal, reinstatement and spontaneous recovery (Table 1 and 2).
BOX 1: GLOSSARY.
Consolidation: process by which new memories are stored after a new learning experience
Retrieval: return of the memory into consciousness
Destabilization: return of the memory to a labile phase due to retrieval
Reconsolidation: process by which memories are maintained after their retrieval and destabilization
Auditory fear conditioning: procedure in which a neutral tone (conditioned stimulus-CS) is repeatedly paired with a foot shock (unconditioned response-US) that causes a fear response, such as freezing (conditioned response). After the training CS elicits the conditioned response.
Contextual fear conditioning: procedure in which a neutral context is repeatedly paired with a foot shock (unconditioned response-US) that causes a fear response, such as freezing (conditioned response). After the training the conditioning context elicits the conditioned response.
Electrodermal conditioning: procedure in which a neutral stimulus (conditioned stimulus-CS) is repeatedly paired with a mild electric shock (unconditioned response-US) that causes a fear response, such as increase in skin conductance (conditioned response). After the training CS elicits the conditioned response.
Acquisition of new response: in the first phase rats are trained to self-administer a drug (or sucrose) (US) by an instrumental response, such as lever pressing. Each US presentation is paired with a presentation of a CS, such as a light. In a second phase rats are allowed to perform a different instrumental response, such as a nose poke, in order to have a CS presentation. The acquisition of the new instrumental response (nose-poke) is sustained by the CS-US associative memory.
Drug-Conditioned Place Preference (CPP): the effect of a drug (US) is repeatedly paired with one distinct context, whereas a neutral event is paired with a different context. Allowing the animal to move between the two contexts and measuring the amount of time spent on each context determine preference.
Drug self-administration: a procedure in which animals are trained to perform an instrumental response, such as lever pressing, for the administration of drug (US). Each US administration is paired with an initially neutral stimulus, such as the illumination of a lamp or a tone (CS).
Spontaneous Recovery: recovery of CS-US associative memory due to the passage of time
Renewal: recovery of CS-US associative memory due to re-exposure to the conditioning context (where CS-US association have been learned)
Reinstatement: recovery of CS-US associative memory due to an unexpected US presentation
Elevated plus maze: experimental method to measure the anxiety levels in rodents. It consists in a plus-shaped apparatus with two open and two enclosed arms, each with an open roof, elevated 40–70 cm from the floor. The model is based on rodents' aversion of open spaces. An anxiety level in the plus-maze is indicated by an increase in the proportion of time spent in the open arms (time in open arms/total time in open or closed arms), and an increase in the proportion of entries into the open arms (entries into open arms/total entries into open or closed arms).
Social Avoidance /Approach test: In this test, the time spent by a test rat in a large social compartment containing an unfamiliar stimulus rat reflects the anxiety state of the animal. It was shown that pre-stressing the test rat increased the avoidance of the social compartment as characterized by an increase in the time spent in the non-social compartment.
Table 1.
Animal studies investigating the effect of post-retrieval extinction on fear-related memories.
| TYPE OF MEMORY |
EXPERIMENTAL PARADIGM |
REFS | ANIMAL | TRAINING | RETRIEVAL-home cage-EXTINCTION | RESULTS Test on conditioned response |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RET | home | EXT | Spontaneous Recovery |
Renewal | Reinstatement | Reconditioning | |||||
| Recent fear memory | Auditory fear Conditioning | Monfils et al. 2009 | Sprague Dawley Rat | 3 (CS –US) associations CS: tone (20s, 5000Hz, 80dB) US: foot shock (500ms, 0.7mA |
1CS | 10min | 18CS | Decrease | – | – | – |
| 1CS | 1h | 18CS | Decrease | Decrease | Decrease | Decrease | |||||
| 1CS | 6h | 18CS | N.E. | – | – | – | |||||
| 1CS | 24h | 18CS | N.E. | – | – | – | |||||
| No | No | 19CS | N.E. | N.E. | N.E. | N.E. | |||||
| Clem & Huganir 2010 | S831A and S845! Mutant mouse | 6 (CS –US) associations CS: tone (20s, 2000Hz, 80dB) US: foot shock (2000ms, 1.5mA) |
1CS | 30min | 19+19 CS | Decrease | Decrease | – | – | ||
| No | 30min | 20+19 CS | N.E. | N.E. | – | – | |||||
| Chan et al. 2010 | Wistar rat | 3(CS-US) associations CS: tone (20 or 30s, 750Hz, 90dB) US: foot shock (500ms, 0.7mA) |
1CS | 10 min | 18CS | – | N.E. | – | – | ||
| 1CS | 90min | 18CS | – | Increased | – | – | |||||
| No | No | 19CS | – | N.E. | – | – | |||||
| 4(CSa-US) + 4(CSb-US) | 1CSa | 10min | 20CSa+21CSb | – | – | Increased | – | ||||
| Flavell et al. 2011 | Lister Hooded Rat | 2(CS-US) associations CS: tone (60s, 10Hz, 80dB) US: foot shock (500ms, 0,5mA) |
1CS | 1h | 10CS | – | Increased | – | – | ||
| No | No | 11CS | – | N.E. | – | – | |||||
| Contextual fear conditioning | Pérez-Cuesta et al. 2009 | Crab | 50min in CX 15US presentation US: visual danger stimulus (9s) |
15min CX | 15 min | 2h CX | N.E. | – | Decrease | – | |
| No | No | 2h CX | N.E. | – | Decrease | – | |||||
| Rao-Ruiz et al. 2011 | C57BL/6NJ Mouse | 3min in CX 2US presentation US: foot shock (2s, 0.7mA) |
3min CX | 2h | 30min CX | Decrease | – | Decrease | – | ||
| 3min CX | 24h | 30min CX | N.E | – | N.E | – | |||||
| No | No | 30min CX | N.E | – | N.E | – | |||||
| Flavell et al. 2011 | Lister Hooded Rat | 3min in CX 1US presentation US: foot shock (2s, 0.5mA) |
2min CX | 1h | 28min CX | – | – | Decrease | Decrease | ||
| No | No | 30min CX | – | – | N.E. | N.E. | |||||
| Remote fear memory r | Contextual fear conditioning | Costanzi et al. | C57BL6N mouse | 3min in CX 1US presentation US: foot shock (0.2s, 0,7mA) |
3min CX | 1h | 30min CX | N.E. | – | Decrease | – |
| No | No | 33min CX | N.E. | – | Decrease | – | |||||
| 15min CX | 1h | 30min CX | N.E. | – | Decrease | – | |||||
Abbreviations: US (unconditioned stimulus); CS (conditioned stimulus); CX (conditioning context); N.E. (no effect)
Table 2.
Animal studies investigating the effect of post-retrieval extinction on drug-related memories.
| TYPE OF MEMORY |
EXPERIMENTAL PARADIGM |
REFS | ANIMAL | TRAINING | RETRIEVAL-home cage-EXTINCTION | RESULTS How was the conditioned response on test day? |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RET. | Home | EXT | Spontaneous Recovery |
Renewal | Reinstatement | Reacquisition | |||||
| Food memory | Acquisition of new response | Flavell et al. 2011 | Lister Hooded rat | 9 daily 20min sessions FR1: 45mg food (US), 10s |
10min [FR1:CS] | 10min | 60min [FR1:CS] | – | – | – | Decrease |
| 10min CX | 10min | 60min [FR1:CS] | – | – | – | N.E. | |||||
| Drug memory | Morphine induced CPP | Ma et al. 2011 | Sprague Dawley rat | 11 daily 45min sessions morphine: 5mg/Kg IP | 15min CPP test (10 daily sessions) | 10 min | 45min CX (10 daily sessions) | Decrease | – | Decrease 24h later N.E.4 weeks later | – |
| 15min CPP test (10 daily sessions) | 3h | 45min CX. (10 daily sessions) | – | – | N.E. | – | |||||
| No | No | 45min CX (10 daily sessions) | Increase | – | Increase | – | |||||
| Xue et al. 2012 | Sprague Dawley rat | 8 daily 45min sessions morphine: 10mg/Kg SC | 10min CPP test (8 daily sessions) | 10min | 45min CX (8 daily sessions) | – | – | Decrease | – | ||
| 10min CPP test (8 daily sessions) | 1h | 45min CX (8 daily sessions) | – | ù_ | Decrease | – | |||||
| 10min CPP test (8 daily sessions) | 6h | 45min CX (8 daily sessions) | – | – | N.E. | – | |||||
| No | No | 45min CX (8 daily sessions) | – | – | N.E. | – | |||||
| Cocaine induced CPP | Xue et al. 2012 | Sprague Dawley rat | 8 daily 45min sessions Cocaine: 10 mg/Kg IP | 10min CPP test (8 daily sessions) | 10min | 45min CX (8 daily sessions) | Decrease | – | Decrease | – | |
| 10min CPP test (8 daily sessions) | 1h | 45min CX (8 daily sessions) | Decrease | – | Decrease | – | |||||
| 10min CPP test (8 daily sessions) | 6h | 45min CX (8 daily sessions) | N.E. | – | N.E. | – | |||||
| No | No | 45min CX (8 daily sessions) | N.E. | – | N.E. | – | |||||
| Heroin S/A | Xue et al.2012 | Sprague Dawley rat | 3 (1-h sessions)/day 10 days Heroin: 0,05 mg/Kg/infusion IV |
15min [FR1:CS ] (14 daily sessions) | 10min | 15min [FR1:CS ] (14 daily sessions) | – | – | Decrease | – | |
| No | No | 195min [FR1:CS] (14 daily sessions) | – | – | N.E. | – | |||||
| Cocaine S/A | Xue et al. 2012 | Sprague Dawley rat | 3 (1-h sessions)/day 10 days Cocaine: 0,75mg/Kg/infusion |
15min [FR1:CS ] (14 daily sessions) | 10min | 180min [FR1:CS ] (14 daily sessions) | Decrease | Decrease | Decrease | – | |
| 15min [FR1:CS ] (14 daily sessions) | 6h | 180min [FR1:CS ] (14 daily sessions)) | N.E. | N.E. | N.E. | – | |||||
| No | No | 195min [FR1:CS] (14 daily sessions) | N.E. | N.E. | N.E. | – | |||||
Abbreviations: CPP(Conditioned Place Preference), S/A (self-administration) FR1(fixed ratio 1); US (unconditioned stimulus); CS (conditioned stimulus); CX (conditioning context); N.E. (no effect); IP (intraperitoneal); SC(subcutaneously).
Further evidence that post-retrieval extinction may prevent the return of fear conditioned response in animals came from Rao-Ruiz et al. (2011) and Flavell et al. (2011). They investigated the effect of post-retrieval extinction on contextual fear memory in mice and rats respectively. In the work of Rao-Ruiz and colleagues (2011) mice were fear conditioned by placing them in a context were they received a mild foot shock. The day after mice received a retrieval session that consisted in re-exposure to the conditioning context for 2 minutes. Two or twenty-four hours later (within or outside the reconsolidation window), mice were placed in the conditioning context for further thirty minutes as extinction sessions. Return of fear was then tested 1 or 17 days after the retrieval- extinction manipulation by placing the mice in the conditioning context. . Only mice that received the post-retrieval extinction within the reconsolidation window showed loss of fear response on both tests (on day 1 and 17). In Flavell et al (2011) rats were trained to associate a context to a mild foot-shock and then, the day after, the contextual memory was retrieved by placing the animal in the training context for 2 minutes. One hour later, rats were returned to the same context for a 28 minutes extinction session. The next day, rats were placed in the training context and tested for contextual fear memory retention. Flavell et al. showed that extinction, only when applied in combination with an isolated retrieval, prevented the return of fear. Rats were then re-conditioned after the test in the same context using a weaker foot-shock (0.35 mA instead of 0.50 mA) in order to verify the persistence of the original contextual fear memory. Flavell et al. observed that rats that had received extinction alone significantly reacquired the contextual fear memory, whereas those who received extinction after retrieval did not. Further evidence that the effect of post-retrieval extinction was retrieval-dependent came from the observation that nimodipine, a L-type voltage-gated calcium channel blocker known to block the destabilization of memory after their retrieval, impaired the effect of retrieval-extinction in preventing the return of conditioned fear. Taken together, these data suggested that post-retrieval extinction completely prevented the return of conditioned fear, whereas extinction alone did not. Since this effect was dependent on the retrieval of the conditioned fear memory, therefore it can be argued that post-retrieval extinction operates during the reconsolidation of conditioned fear memories.
Besides the study in auditory fear conditioning paradigm, Flavell et al (2011) have also investigated the effects of post-retrieval extinction on Pavlovian appetitive memories. They have used the paradigm of acquisition of new response for stimuli previously paired to sucrose (see Box 1 for definition). In this experimental paradigm, rats were initially trained to self-administer sucrose by an instrumental response (e.g., nose poke), with each sucrose administration paired with a CS presentation (e.g., light or tone). In a second phase, rats were trained to acquire a new instrumental response (pressing a lever) in order to receive a CS presentation, so that the new instrumental behaviour was maintained by the memory of conditioned value of the CS. After a 10-minute CS retrieval session, exposure to a 1-hour extinction (during which nose poke responding was reinforced only by CS presentation) inhibited the acquisition of new response (lever press). This effect was retrieval-dependent since no effect was observed when extinction was applied, i), without previous CS retrieval, or, ii), 6 hours after the retrieval. Flavell et al. hypothesized that extinction applied within the labile phase of the retrieved memory (i.e., the reconsolidation window phase), was interfering with reconsolidation. However, they also pointed out that it was equally plausible that prior retrieval of the memory might facilitate extinction and therefore potentiate its effect. In a different group of rats, pre-extinction injection of D-cycloserine (DCS), an NMDA receptor partial agonist known to enhance extinction memory (Nic Dhonnchadha et al. 2010; Paolone et al. 2009; Torregrossa et al. 2010) did not affect subsequent acquisition of new response for CS. In conclusion, they argued that the observed post-retrieval extinction effect was more likely due to the interference with reconsolidation of sucrose-related memories, instead of enhancement of extinction.
More recently,Xue et al. (2012) have investigated the effects of post-retrieval extinction on drug-related memories by using different models. In the first set of experiments, they used the paradigm of drug conditioned place preference (CPP) in order to train two groups of rats to associate a context to cocaine or morphine. Then, the associative drug memory was retrieved by placing the rats in the drug-associated context for 10 minutes. Ten minutes, 1 or 6 hours after the end of the retrieval procedure, rats underwent an extinction session during which they were placed in the drug-associated context for 45 minutes without the drug. After repeating the retrieval-extinction paradigm for eight days, rats received a priming injection of cocaine or morphine on day 9, and CPP reinstatement was tested. Xue et al. observed that the retrieval-extinction procedure impaired drug-priming -induced reinstatement only when 10 minutes or 1 hour, but not 6 hours, elapsed between retrieval and extinction. Moreover, spontaneous recovery of cocaine CPP was still impaired 14 days later. In the second set of experiments, Xue and colleagues assessed whether post-retrieval extinction would disrupt drug instrumental memory. They trained two groups of rats to nose poke for self-administration of cocaine or heroin, with each drug infusion paired to 5-second illumination of a cue lamp and tone buzzing. After the training phase, rats were given a retrieval session (15 minutes) during which nose poke responses were associated to cue lamp and tone, or not. Ten minutes or 6 hours later, all groups underwent 180-minute extinction session during which the conditions were the same as in the retrieval session. This retrieval (or no-retrieval) -extinction procedure was repeated for approximately 14 days. On day 15, rats were tested for reinstatement of (non reinforced) nose-poke responses after an acute non-contingent injection of cocaine or heroin. The retrieval of the drug-related cues 10 minutes, but not 6 hours, before the daily extinction sessions decreased drug-priming -induced reinstatement of cocaine or heroin -seeking. Furthermore, spontaneous recovery and renewal of cocaine-seeking behaviour was also impaired. Taken together, their findings described for the first time that the retrieval-extinction manipulation may interfere with the reconsolidation of drug cue memories, as demonstrated by using two models of drug addiction behaviour, that is CPP and self-administration. Moreover, Xue et al. showed that postretrieval extinction completely blocked drug-priming –induced reinstatement in CPP, whereas only decreased reinstatement, spontaneous recovery and renewal in self-administration. Xue at al. hypothesized that the retrieval-extinction manipulation might be more effective on the reconsolidation of Pavlovian memories (responding for drug cues) that mediates CPP in rats, than on instrumental memories (drug-response outcome) that are involved in the self-administration paradigm. This hypothesis is in agreement with Hernandez & Kelley (2004) showing that the instrumental memories are not rendered labile by their retrieval and they are not susceptible to disruption by protein synthesis inhibition. Other studies reported however that instrumental memory reconsolidation may be disrupted (i.e., there is an indirect evidence of instrumental memory occurrence), for instance by Diergaarde et al., 2006 that showed propranolol inhibition of the reconsolidation of sucrose memory. Critical points about instrumental memory reconsolidation have been suggested, such as the intensity of training (i.e., weakly trained instrumental responding should be more prone to reactivation-dependent changes; Lee 2010) and shift from goal-oriented behaviour to habit-stimulus response (i.e., shift to a more strong memory; Milton & Everitt, 2012).
3.1.2. Discrepancies about the efficacy of post-retrieval extinction
Other studies have shown that the retrieval-extinction paradigm was ineffective in preventing the return of fear in fear conditioning paradigm in laboratory animals. The first evidence came from a study by Chan and colleagues (2010) who used a procedure similar to that described by Monfils et al (2009) but observed opposite results: exposure to retrieval prior to extinction increased responding on subsequent tests for renewal and reinstatement. Monfils et al. used a 5000 Hz tone, whereas Chan et al. used a 750–1200 Hz tone. This different tone frequency of the CS could possibly involve different conditioning mechanisms. Secondly, Monfils et al. in the renewal experiment have modified the original conditioning chamber (A) to create a new context (A*) for retrieval and extinction, whereas Chan et al. have used different experimental boxes (B) located in different experimental rooms. The occurrence of retrieval and extinction in a different context is important in order to assess the generalization of CS extinction when the subjects are back to the original training context for conditioned response tests. The use of a AA*A procedure in Monfils et al and of an ABA in Chan et al may be the reason for the contrasting results in renewal experiments. It should be also pointed out that the housing conditions of the animals were different between the two studies. Monfils et al (2009) rats were individually housed, whereas Chan et al.’s rats were housed in a group of eight, which may have included rats from different groups (McNally, personal communication). Even if the freezing levels at the end of the conditioning phase was similar between the two studies, it can not be excluded that placing animals from different groups (i.e. exposed to standard extinction or post-retrieval extinction) in the same cage might have a consequence on the subsequent test of conditioned response. The retrieval-extinction procedure was also tested on remote fear memory (29 days old) (Costanzi et, 2011) in a mouse model of PTSD (Siegmund and Wotjak, 2007a), which takes into consideration both the associative and the non-associative component of fear memory (i.e., sensitization process that increase the animal response to harmless, neutral stimuli independently from the CS-US association). Mice were trained to associate a foot-shock to a context, and then, the contextual memory was retrieved 29 days later by placing the animal in the conditioning context for 3 minutes without foot shock, and then, 1 hour later, in the conditioning context for 30 minutes extinction session. One day after the retrieval-extinction procedure, mice were tested in the conditioning context to evaluate the re-emergence of contextual fear (associative) memory. Four hours later, mice were placed in a novel chamber in which a neutral tone was delivered for 3 minutes. The fear response (freezing) to the neutral tone was considered as index of fear sensitization (non-associative component of fear memory). The main finding of this study is that extinction applied after retrieval of remote fear memory did not persistently attenuate the expression of fear, either in contextual memory or sensitization tests. Costanzi et al. showed that there was no difference in freezing level between the extinction and retrieval-extinction groups. The long-lasting behavioural outcome of the fear experience was also investigated. Several studies have shown an increase in anxiety level, social withdrawal, visuospatial memory deficit in patients suffering from PTSD. Given the importance of these phenomena, Costanzi et al. assessed the anxiety levels by using Elevated Plus Maze, Social Avoidance/Approach tests, and spatial memories with Morris Water Maze test. They found that neither post-retrieval extinction nor extinction alone could prevent the onset of anxiety, social withdrawal and memory deficit symptoms. Taken together, the results from Costanzi et al. suggested that extinction provided within the reconsolidation window of remote contextual fear memory did not attenuate the expression of fear. In Monfils et al.’s study (2009), the post-retrieval extinction was applied one day after the initial training (young memory), whereas in Costanzi et al. it was applied 29 days later (remote memory). Therefore, it appears that the age of the memory may be a boundary condition of reconsolidation occurrence (Milekic & Alberini, 2002) and, whether post-retrieval extinction may inhibit it or not. The relationship between age of memory and reconsolidation occurrence is still open to debate (Alberini, 2011). There are reports that showed the susceptibility to disruption of reconsolidation of remote memories (e.g., Diergaarde et al., 2006) as well as a direct relationship between age and resistance to disruption (e.g., Suzuki et al., 2004; Robinson & Franklin, 2010).
Pèrez-Cuesta (2009) investigated the effect of retrieval-extinction procedure in a memory model in the Chasmagnathus crab (Maldonado 2002). They trained crabs to associate a context to a visual danger stimulus (US), and 24 hours later they exposed crabs to training context for 15 minutes (retrieval of the conditioned context), and 15 minutes later they exposed crabs to the same context for an additional 2-hour period of extinction. The day after, crabs were placed back in the conditioning context and the occurrence of conditioned response (freezing) was measured as an index of conditioned fear recovery. If conditioned fear response was not found, the test was replicated 24 hours later (test 2) to distinguish reconsolidation impairment (supposed to be permanent) and extinction (supposed to be transient). On test 1 no fear response was observed in crabs that received the retrieval-extinction treatment, whereas on test 2 a re-emergence of memory was observed, suggesting that post-retrieval extinction induced extinction instead of reconsolidation impairment. Pèrez-Cuesta concluded that reconsolidation and extinction mutually exclude each other; therefore they would argue that post-retrieval extinction couldn’t affect the reconsolidation of retrieved memory. It should be pointed out that in the work of Perez-Cuesta and colleagues the retrieval procedure lasted 15 minutes. Even if this paradigm is different compared to the fear-conditioning paradigm in mice or rats used by the other Authors (Monfils et al. 2009; Chan et al. 2010; Costanzi et al. 2011; Flavell et al. 2011) it should be noted that the retrieval procedure is consistently longer (15 minutes in the conditioning context) compared to other studies. It is widely accepted that a long exposure to the conditioned stimulus or conditioning context triggers extinction instead of reactivation of the memory (Eisenberg et al. 2003; Suzuki et al. 2004; Power et al. 2006; Tronson and Taylor 2007), so it cannot be excluded that the retrieval procedure used by Perez-Cuesta induced extinction instead of reconsolidation. The critical role of retrieval length in the induction of reconsolidation or extinction of the memory has been highlighted also by Perez-Cuesta and colleagues in their previous paper (Pedreira & Maldonado 2003), in which the retrieval and extinction procedure lasted 5 or 60 minutes respectively. Therefore we cannot exclude that the 15-minute exposure to the conditioning context may have initiated extinction instead of reactivation of the memory.
Flavell and colleagues (2011) besides finding that post-retrieval extinction prevented reconsolidation of contextual fear memory (see above), investigated the effect of the retrieval-extinction manipulation also by using an auditory fear-conditioning paradigm similar to that described in Monfils et al. (2009). Flavell and colleagues showed that post-retrieval extinction did not prevent the return of fear in the reinstatement of fear response to an auditory cue. Some methodological issues might explain the contrasting results. First of all, the extinction procedure used by Flavell and colleagues was shorter, that is 10–11 unreinforced CS presentations rather than 18–19 in the work of Monfils et al. It can be hypothesized that the extinction provided by Flavell et al. was not sufficient to disrupt the reconsolidation of the memories for the cues. Secondly, the foot-shock used by Monfils et al. was more intense (0.7 mA) compared to 0.5 mA by Flavell et al. However, the length of tone paired with the foot-shock were different across the two studies: 60 seconds in Flavell et al. vs. 20 seconds in Monfils et al..,
Contrasting results have been also found regarding the effect of post-retrieval extinction on appetitive memories.Ma et al. (2011), showed that post-retrieval extinction procedure was effective in disrupting the reconsolidation of Pavlovian drug memory by using the paradigm of morphine-induced CPP. They showed that ten consecutive daily post-retrieval extinction sessions prevented reinstatement and spontaneous recovery of extinguished CPP. However, CPP was observed in a reinstatement test performed 4 weeks after the last extinction session. The latter finding suggested that memory trace was not erased by post-retrieval extinction. It can be hypothesized that extinction applied after retrieval did not affect the reconsolidation of memory under their conditions; otherwise, as suggested by the Authors, that reconsolidation blockade did not lead to the erasure of memory that can re-emerge by the passage of time. These data are in contrast with the findings of Xue et al. (2012) but some methodological differences between the two studies should be taken into account. In Xue et al. the dose of morphine used for the training was higher compared to the dose used by Ma and colleagues.
3.1.3. Synthesis of animal studies findings
Monfils and colleagues (2009) found that post-retrieval extinction applied within the vulnerable phase of the memory could interfere with the reconsolidation of young fear memory in the paradigm of auditory fear conditioning. Subsequently, these data were confirmed by Clem & Huganir (2010) and Flavell et al (2011) by using the paradigm of auditory fear memory, and Rao-Ruiz et al (2011) by using the paradigm of contextual fear memory, On the other hand,Chan et al. (2010) found opposite results compared to the findings of Monfils and colleagues (2009) and some methodological issues might explain the contrasting results. Moreover Costanzi and colleagues (2011) showed that post-retrieval extinction did not affect the reconsolidation of remote fear memory. Different authors have found contrasting results for appetitive (drug or food related) memories:Flavell et al. (2011) and Xue et al (2012) showed that post-retrieval extinction inhibited the reconsolidation of sugar or drug (cocaine o morphine) related memories, whereas Ma and colleagues (2011) found that extinction applied shortly after retrieval of cocaine related memories did not block the reconsolidation of cocaine-related memories.
3.2. Human studies
The effect of the retrieval-extinction procedure on the reconsolidation of fear related or drug-related memories have also been investigated in humans. The first study came from Schiller and colleagues in 2010. In the first experiment, subjects underwent a discrimination fear-conditioning paradigm with partial reinforcement on which two coloured squares were randomly presented. One of two squares (CS) was paired to a mild wrist shock (US) on about one out of three trials, whereas the other square was never paired to the shock. The following day, the CS was re-presented in order to retrieve the CS-fear memory. Ten minutes or 6 hours later, subjects were exposed to an extinction session during which the CS was presented 10 times in absence of the US. Twenty-four hours later, subjects were presented with a single CS presentation in the absence of the US and the reemergence of fear was measured (spontaneous recovery test) as increased skin conductance response. Extinction, applied 10 minutes after retrieval of conditioned fear memory, prevented the conditioned fear response in the spontaneous recovery test. About one year later, the recovery of fear was assessed in a reinstatement test during which the same subjects received four US without CS presentations, and then, the CS was presented in absence of US. In this test, extinction applied 10 minutes after retrieval impaired the return of conditioned fear response. On the other hand, extinction applied 6 hours after retrieval (outside the reconsolidation window) had effect neither on spontaneous recovery nor reinstatement tests. In a second experiment, the Schiller and colleagues trained the subjects to associate two different squares (CSa and CSb) to the shock. On day after, only one (e.g., CSa) was retrieved, and extinction session was applied 10 minutes later by using repeated presentation of both retrieved and no-retrieved CS (i.e., CSa and CSb) in the absence of US. One day later, the return of fear memory response to retrieved CSa or no-retrieved CSb presentation was assessed in a reinstatement test. Data showed that there was a return of fear only when the no-retrieved CSb was presented, whereas no recovery was observed after the presentation of the retrieved CSa. In theSchiller et al. (2010) study there are two main findings. The first is that post-retrieval extinction might interfere with the reconsolidation of conditioned fear memory in humans. Secondly, the post-retrieval extinction selectively blocks the reconsolidation of the retrieved CS-fear memory and does not affect other no-retrieved memories.
In 2011, Soeter & Kindt argued that the electrodermal conditioning used by Schiller and colleagues seems to primarily reflect only the cognitive level (declarative memory) of contingency learning (CS-US association), whereas human startle potentiation should be considered as a more reliable and specific index of fear, although it could be argued that galvanic skin resistence (GSR) is a direct physiological output. In both within- (Soeter & Kindt 2011) and between- (Kindt & Soeter 2013) subject design studies, they tested whether extinction applied during the reconsolidation window may prevent the return of an extinguished eye blink startle fear response. They also investigated the cognitive level of contingency learning through the measure of skin conductance response and US-expectancy rating (self-reported questionnaires). Subjects were trained to associate two fear-relevant pictures (e.g., pictures of spiders or gun) to an aversive electric stimulus (US). Twenty-four hours later, one of the two CS was presented once in a retrieval session. After 10 minutes, subjects underwent an extinction session during which both CSs were presented 9–10 times without US presentation. Memory retention for both CSs was tested twenty-four hour later. Soeter & Kindt found that the retrieval-extinction procedure did not affect skin conductance and US expectancy rating. Spontaneous recovery, but not reinstatement of startle fear response, was prevented by the retrieval-extinction procedure, whereas fear returned after a reacquisition session. Although these data are in contrast with those reported bySchiller et al. (2010), it should be noted that the two studies diverged in several ways, with most notable differences being the use of different type of CS (geometric figure vs. fear-relevant pictures). It has been shown that the fear relevant stimuli are more resistant to extinction than symbolic one (Mineka and Ohman, 2002) and this may account for the discrepancies between the two studies. However, this explanation is unlikely since at the end of the extinction session the skin conductance and the eye blink startle response responses were extinguished. The second procedural difference between the two findings is the reinforcing scheme used during the conditioning phase. In the work of Schiller and colleagues (2010), CSs were paired with US in 38% vs. 80% of trials in Soeter & Kindt (2011). Soeter and Kindt (2011) have hypothesized that the single CS presentation at retrieval could have triggered extinction instead of retrieval in their procedure. In Soeter & Kindt conditioning protocol CS was paired to US most of the time, therefore a single non-reinforced CS presentation at retrieval may have primed the CS-NoUS association learning. Since in Schiller at al. (2010) CS was paired to US less than half of the time, it is unlikely that a single CS presentation at retrieval could lead to extinction learning. This hypothesis is unlikely since Soeter and Kindt showed that the reconsolidation of the fear memories could be blocked by the administration of propranolol under the same conditioning and retrieval conditions. An additional important difference between the two studies is that in Soeter & Kindt subjects were asked for subjective ratings. Such a task would likely engage the prefrontal cortex (Taylor et al., 2003), and it is possible that this would interfere with an updating mechanism that is thought to primarily operate at the locus of the initial fear storage, in the amygdala (Filion et al., 1991).
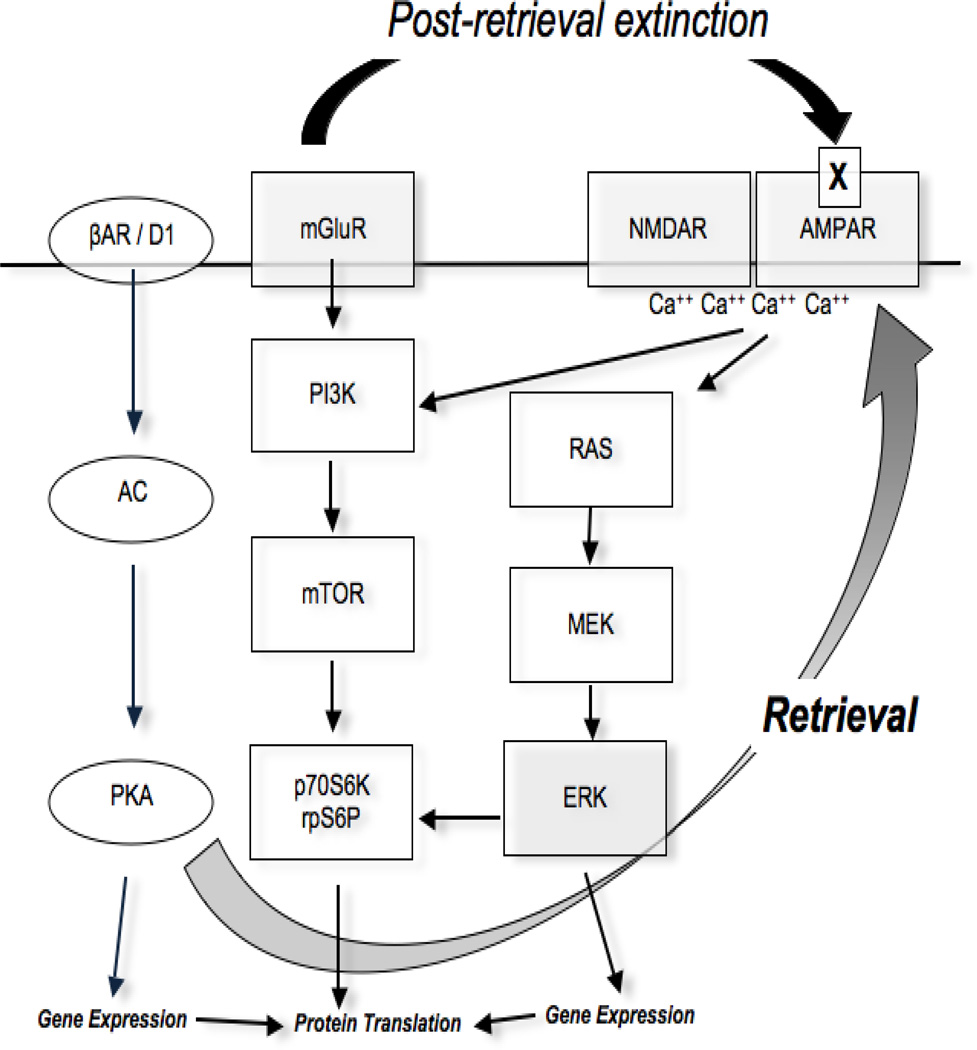
Fig. 1.
Molecular pathways of memory reconsolidation. Molecular signalling cascades downstream of β-adrenergic receptors (βAR), dopamine D1 receptor (D1) and N-methyl-d-aspartate receptors (NMDAR) have been shown to be implicated in reconsolidation. The mammalian target of rapamycin (mTOR), influenced by the activities of neuronal surface receptors and channels including NMDAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), and dopaminergic and metabotropic glutamate receptors (mGluR), also seems to play a key role in reconsolidation through the phosphorylation of kinase p70S6 (p70S6K) and ribosomal protein S6 (rpS6P). βAR and D1 activate adenylyl cyclase (AC) leading to protein kinase A (PKA) phosphorylation and gene expression. PKA also phosphorylates AMPAR after memory retrieval. Ca++ influx from NMDAR and AMPAR activates the extracellular signal-regulated kinase pathway (ERK), by rat sarcoma (RAS) and mitogen- activated protein kinase (MEK), and phosphatidylinositide 3-kinases (PI3K). Phosphorylation of ERK increases the levels of the transcription factors ets-like gene-1 (Elk-1), phosphorylated cAMP response element binding (pCREB), known to be involved in reconsolidation. PI3K activation leads to mTOR activation resulting in p70S6K and rpS6 phosphorylation and synaptic protein translation. mGluR1 receptor activation is requested after post-retrieval extinction leading to the selective removal of synaptic calcium-permeable AMPAR (X symbol in the Figure). Although the molecular details are not yet known the mGluR1-induced internalization of AMPAR may distinguish reconsolidation update from conventional extinction
Xue et al. (2012) investigated the effect of post retrieval extinction on cue reactivity in inpatient detoxified heroin addicts. The retrieval consisted in the presentation of a heroin-related videotape (5 minutes). Ten minutes or 6 hours later, subjects were exposed to an extinction session lasting approximately 45 minutes during which heroin-related slides and video were presented, followed by supervised inspection and handling of drug-related material. One, 30 and 180 days after the retrieval-extinction procedure, participants were presented with 5 minutes of heroin-related videotapes and cue-induced changes in heroin craving, heart rate and blood pressure were assessed as physiological measures of cue-reactivity. They found that extinction applied 10 minutes, but not 6 hours, after retrieval inhibited the cue-induced increase of craving and blood pressure, but not heart rate. In summary Schiller and colleagues in 2010 showed for the first time that post-retrieval extinction could interfere with the reconsolidation of fear memory by using the paradigm of electrodermal conditioning in a laboratory setting. On contrary Soeter & Kindt in 2011 and 2013 found opposite results. Concerning drug-related memories, Xue et al (2012) showed that post-retrieval extinction inhibited the cue-reactivity in ex-heroin addicts, suggesting that extinction applied shortly after retrieval might interfere with reconsolidation of heroin memories.
4. Molecular mechanisms underlying reconsolidation, extinction and possibly reconsolidation-extinction interaction
Several studies showed that different molecular pathways could be differently engaged during reconsolidation depending on the paradigm and the laboratory model used, and depending on the kind of memory that is involved (e.g., fear or drug related, young or remote). This should be taken into consideration when, despite the same treatments (pharmacological or non pharmacological, i.e. post-retrieval extinction) contrasting results were found. Memory reconsolidation and extinction are protein synthesis-dependent processes as shown by their disruption when a protein synthesis inhibitor is administered before or after the memory retrieval, or before extinction training (Nader et al., 2000; Fan et al., 2010; Milekic et al., 2006; Valjent et al., 2006; Santini et al., 2004). Several upstream receptors, signalling and transcription factors are involved in memory reconsolidation, extinction and possibly in their interaction.
4.1. α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAr)
There is considerable evidence that learning-driven increases in GluR1 subunit-containing AMPA-type glutamate receptor (GluR1-receptors) at a selected group of synapses underlie associative memory (Clem and Barth 2006; Takahashi et al. 2003). Rumpel and colleagues (2005) have shown that fear conditioning drives synaptic incorporation of GluR1-receptors in lateral amygdala neurons. Phosphorylation of GluR1-receptors at the level of Ser-831 by protein kinase C and Ser-845 by protein kinase A regulates both the channel properties (Banke et al. 2000; Derkach et al. 1999) and the synaptic incorporation of the receptor (Ehlers 1999; Esteban et al. 2003; Qin et al. 2005). Interestingly, Monfils and colleagues (2009) found increased levels of phosphorylated GluR1-receptors (pGluR1) in the lateral amygdala after fear memory retrieval and its dephosphorylation when a second CS was presented 1 hour after the first CS presentation suggesting different recruitment of pGluR1 correlated to reconsolidation or extinction of fear memory.
The molecular mechanism underlying the effect of post-retrieval extinction has been also investigated by Clem & Huganir (2010). The Authors demonstrated that post-retrieval extinction effect in preventing the return of fear was inhibited by the previous administration of 1-aminoindan- 1,5-dicarboxylic acid (AIDA) a competitive antagonist of mGluR1. Thus, they argued that effect of extinction upon retrieval required the mGluR1 activation. In further electrophysiological studies they observed a significant decrease of AMPA receptors –mediated transmission in the retrieved group compared to the no retrieved. This decrease was accompanied by the selective removal of synaptic calcium-permeable AMPA (CP-AMPAr) receptors pGluR1 in the lateral amygdala. Moreover the stability of CP-AMPAr is regulated by the activation of mGluR1. Considering post- retrieval extinction effect as a reconsolidation update author suggest that mGluR1 activation is required to update memories. Phosphorylation of the protein kinase A (PKA) target serine-845 (S845A) in GluR1 receptors has also been shown to regulate the stability of CP-AMPARs (He et al. 2009). Mutation of the PKA site S845A prevented fear-induced enhancement of CP-AMPAR currents and rats with this mutation showed no impaired reconsolidation after post-retrieval extinction manipulation compared to wild type indicating that serine-845 phosphorylation is a specific prerequisite for memory erasure during reconsolidation update (Clem & Huganir, 2010). The Authors also hypothesize that co-activation of NMDARs and mGluR1, which removes synaptic CP-AMPARs during in vitro LTD, may distinguish reconsolidation update from conventional extinction.
4.2 β-adrenergic receptor (β-AR) and dopamine receptor 1 (D1R) / protein kinase A (PKA)
It has been previously reported by many authors that β-AR and D1R are important receptors involved in memory reconsolidation (Sara, 2000; Tronson and Taylor, 2007). These receptors are G-protein-coupled receptors, stimulating adenylyl cyclase and activating cyclic AMP-dependent protein kinases such as PKA. PKA directly activates transcription factors like CREB, increases the phosphorylation of GluR1Rs (shown to be involved in fear and drug memory reconsolidation; Valjent et al. 2005; Monfils et al. 2009) and regulates the stability of CP-AMPARs (He et al. 2009). Post-retrieval inhibition of PKA by intra-BLA infusions of Rp- adenosine 3,5-cyclic monophosphorothioate hydrate triethyl- ammonium salt (Rp-cAMPs) attenuates subsequent freezing to the auditory stimulus (Tronson et al. 2006) and decreases subsequent cue-induced reinstatement and responding with a conditioned reinforcer, while having no effect on cocaine-induced reinstatement (Sanchez et al. 2010). PKA activation is required only for cue-induced memory retrieval and reconsolidation of young memories but not for motor or older memories (Kemenes et al., 2006). These studies confirm previous findings that older memories are more resistant to reconsolidation and suggest that there are some differences in the molecular mechanisms underlying reconsolidation of older and stronger compared to newer and weaker memories. PKA is implicated in learning and memory, however some discrepancies have been found for a role of this kinase in fear extinction. Szapiro and colleagues (2003) reported that infusion of the PKA inhibitor Rp-cAMPs into the CA1 region of hippocampus either before or immediately after the first of several extinction exposures in a step-down avoidance paradigm produced a persistent impairment of extinction, whereas no effect on extinction of freezing have been found after intra-BLA infusions of the PKA activator 6-BNZ-cAMP immediately following each of four daily tone extinction training sessions (Tronson et al. 2006).
5. Discussion
Several studies have shown that extinction applied shortly after the retrieval of the memory, i.e. during the reconsolidation window, prevents the re-expression of emotional memory under different conditions, i.e. spontaneous recovery, renewal or reinstatement and in different laboratory models both in animal and humans (Monfils et al. 2009; Clem & Huganir 2010; Schiller at al. 2010; Flavell et al. 2011; Rao-Ruiz et al, 2011; Xue et al, 2012).
It has been suggested that post-retrieval extinction might not be a new learning process that creates a new memory trace to compete with the original one (similar to standard extinction). This argument is supported since no re-emergence of the original memory has been observed over a number of conditions. On the contrary, it can be argued that a new learning, after the retrieval of the previous one, may interfere with memory reconsolidation of the original memory. This notion has received support from other studies targeting memory other then emotional, such as episodic, procedural and declarative memories both in human and in laboratory animals. Boccia and coworkers in 2005 showed that the exposure to a new learning task, the nose-pose habituation task (Voits et al. 1995) after retrieval of a previously acquired inhibitory avoidance task in mice, could affect the retention performance of the original learning in reinstatement, spontaneous recovery and renewal tests. Notably these effects were not observed when the nose-poke habituation task was applied without previous retrieval of the original memory. Forcato and colleagues (2007, 2009 and 2010) demonstrated that new verbal instruction, given contingently upon the retrieval of previously acquired declarative memory (learned association between cue and response syllables) might add new information to the former memory. There is evidence that also episodic memories could be selectively impaired following retrieval: Hupbach and colleagues (2007) trained subjects to memorize a list of objects, one day after the list was retrieved, and then subjects learned a second list. The day after, memory for the first list was tested. They observed that subjects exposed to the retrieval of the first list incorrectly intermixed items from the second list suggesting that new information provided within the reconsolidation window was integrated in the original memory.
Other studies have found that post-retrieval extinction did not prevent the re expression of the previously consolidated emotional memory (Chan et al. 2010; Costanzi et al. 2011; Perez Cuesta & Maldonado 2009; and Flavell et al. 2011). It should be noted that methodological issues might explain some contrasting results between studies. Different housing condition may account for different results byMonfils et al. (2009) and Chan et al (2010). Moreover different extinction length may be responsible for the lack of effect of post-retrieval extinction in preventing the return of fear in the auditory fear-conditioning paradigm in the work of Flavell and colleagues (2011).
The use of different laboratory models (e.g. CPP vs. self-administration or contextual vs. auditory fear conditioning) may lead to different memory type (e.g. Pavlovian associative memory vs. instrumental associative memory), moreover the length of conditioning phase or the time passed after the conditioning may lead to different memory strength and age. Type, age and strength of memory are boundary conditions and are important determinants of whether memory is more or less susceptible to be reactivated and, possibly, disrupted. Several studies have shown that when the memory is weakly conditioned, and long time is passed between the last conditioned session and the reactivation session, reconsolidation impairment is more unlikely to occur (Suzuki et al. 2004; Wang et al. 2009; Muravieva and Alberini 2010). These boundary conditions under which memory does not undergo reconsolidation might explain the contrasting results obtained across different studies. Typically, the occurrence of a reconsolidation process can be revealed only by its absence. When amnesia for a memory is induced by a manipulation (pharmacological or non-pharmacological) that is dependent upon retrieval of the memory, reconsolidation is said to be impaired. Therefore a retrieval-dependent impairment of a previously consolidated memory might not be observed due to a missed reconsolidation of memory under specific boundary experimental conditions, and not to a lack of effect of the treatment supposed to work on reconsolidation. It can not be excluded that post-retrieval extinction in some studies failed to impair the memory reconsolidation since this process was not occurring, perhaps due to type of memory not susceptible to reconsolidation or a retrieval protocol that induces extinction instead of reactivation of the memory.
This hypothesis is confirmed by the fact that memory age and strength, and retrieval length may results in different molecular mechanisms engaged after the retrieval. The identification of these mechanistic changes could help in the understanding of what underlies a boundary condition of memory reconsolidation
5. Closing remarks
Learning and memory processes play an important role in the development and maintenance of many psychiatric diseases, such as PTSD, phobias and drug-addiction. Therefore the blockade of reconsolidation of the maladaptive memories underlying such disorders is a promising therapeutic target. Animal studies have shown that reconsolidation could be impaired by the administration of amnestic agents; unfortunately most of them cannot be used in humans, given their limited safety. On the other hand, it has been shown that extinction, cue exposure therapy (CET) clinically, has limited efficacy in preventing the return of fear or the cue-induced relapse to drug seeking both in animals and in humans (Bouton 1993, 1979; Conklin & Tiffany 2002; Alvarez et al. 2007; Schiller et al. 2008).
The post-retrieval extinction could offer an effective, drug-free method for persistently reducing the strength and the re-emerge of selective maladaptive memories. Interestingly it has been shown that reconsolidation blockade or updating generalize from one context to another, preventing the renewal of memory when the subject is re-exposed to the conditioning context.
Much more research is needed to clarify the boundary conditions under which memories can be reconsolidated and possibly disrupted. Additionally, the length and type of retrieval and extinction procedures should be optimized (e.g. single vs. repeated retrieval-extinction sessions). This additional research is necessary in order to advance the post-retrieval extinction approach to target the disruption of pathological memories in animal model of psychiatric diseases and to ultimately translate these finding to humans.
Table 3.
Human studies investigating the effect of post-retrieval extinction on fear-related memories.
| TYPE OF MEMORY |
REF. | CONDITIONING | DEPENDENT VARIABLE |
RETRIEVAL-break-EXTINCTION | RESULTS | ||||
|---|---|---|---|---|---|---|---|---|---|
| RET | Break | EXT | Reinstatement | Spontaneous Recovery |
Reconditioning | ||||
| Fear Memory |
Schiller et al. 2010 | 10CSa-No US 10CSb-No US 6Csa-US (Intermixed) CS: yellow or blue squares US: electric shock (200ms,50 pulse/s) |
Skin conductance | 1CSa | 10min | 10CSa+11CSb | Decrease | Decrease | – |
| 1CSa | 6h | 10CSa+11CSb | N.E. | N.E. | – | ||||
| No | No | 11CSa+11CSb | N.E. | N.E. | – | ||||
| Soeter & Kindt, 2011 | 1(CSa-No US) 1(CSb-No US) 5(CSc-NoUS) 5(CSa-US) 5(CSb-No US) (Intermixed) CSa and CS b: picture of gun or spider CSc: picture of mug US: electric shock defined as uncomfortable by participant |
Eye blink startle reflex | 1CSa (or CSb) |
10min | 9CSa(or CSb)+10CSb(or CSa)+10CSc | N.E. | Decrease | N.E. | |
| Skin conductance | N.E. | N.E. | N.E | ||||||
| Distress rating | N.E. | N.E. | N.E | ||||||
| Kindt & Soeter, 2011 | 8 (CSa-US) 8(CSb-No US) CSa: spider’s picture CSb: neutral picture |
Eye blink startle reflex | 1CSa | 10min | 12CSa+12CSb | N.E. | N.E. | N.E. | |
| Skin conductance | N.E. | N.E. | N.E. | ||||||
| Distress rating | N.E. | N.E. | N.E. | ||||||
| Drug (heroin) memory | Xue et al, 2012 | — | Craving (VAS) | 5min Neutral cues | 10min | 60min heroin-cues | – | N.E. | – |
| 5min Heroin cues | 10min | 60min heroin-cues | – | Decrease | – | ||||
| 5min Heroin cues | 6h | 60min heroin-cues | – | N.E. | – | ||||
| Blood pressure | 5min Neutral cues | 10min | 60min heroin-cues | – | N.E. | – | |||
| 5min Heroin cues | 10min | 60min heroin-cues | – | Decrease | – | ||||
| 5min Heroin cues | 6h | 60min heroin-cues | – | N.E. | – | ||||
| Heart rate | 5min Neutral cues | 10min | 60min heroin-cues | – | N.E. | – | |||
| 5min Heroin cues | 10min | 60min heroin-cues | – | N.E. | – | ||||
| 5min Heroin cues | 6h | 60min heroin-cues | – | N.E. | – | ||||
Abbreviations: US (unconditioned stimulus); CS (conditioned stimulus); N.E. (no effect); VAS (visual analogue scale)
Footnotes
Authors declare no conflict of interest
References
- Abrari K, Rashidy-Pour A, Semnanian S, Fathollahi Y. Administration of corticosterone after memory reactivation disrupts subsequent retrieval of a contextual conditioned fear memory: dependence upon training intensity. Neurobiol Learn Mem. 2008;89(2):178–184. doi: 10.1016/j.nlm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Alberini CM. The Role of Reconsolidation and the Dynamic Process of Long-Term Memory Formation and Storage. Front Behav Neurosci. 2011;5:1–10. doi: 10.3389/fnbeh.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez RP, Johnson L, Grillon C. Contextual-specificity of short-delay extinction in humans: renewal of fear-potentiated startle in a virtual environment. Learn Mem. 2007;14(4):247–253. doi: 10.1101/lm.493707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi RE, Lattal KM, Berger SP. Postretrieval propranolol disrupts a cocaine conditioned place preference. Neuroreport. 2006;17:1443–1447. doi: 10.1097/01.wnr.0000233098.20655.26. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. J Exp Psychol Anim Behav Process. 1979;5(4):368–78. doi: 10.1037//0097-7403.5.4.368. PMID: 528893. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosc. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- Chan WY, Leung HT, Westbrook RF, McNally GP. Effects of recent exposure to a conditioned stimulus on extinction of Pavlovian fear conditioning. Learn Mem. 2010;17:512–521. doi: 10.1101/lm.1912510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330(6007):1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RL, Barth A. Pathway-specific trafficking of native AMPARs by in vivo experience. Neuron. 2006;49(5):663–670. doi: 10.1016/j.neuron.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. 2002;97(2):155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Costanzi M, Cannas S, Saraulli D, Rossi-Arnaud C, Cestari V. Extinction after retrieval: effects on the associative and nonassociative components of remote contextual fear memory. Learn Mem. 2011;18(8):508–518. doi: 10.1101/lm.2175811. [DOI] [PubMed] [Google Scholar]
- Davis HP, Squire LR. Protein synthesis and memory: a review. Psychological Bulletin. 1984;96:518–559. [PubMed] [Google Scholar]
- Debiec J, LeDoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:527–538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- Derkach V, Barria A, Soderling TR. Ca2+/kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci U S A. 1999;96(6):3269–3274. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diergaarde L, Schoffelmeer AN, De Vries TJ. Beta-adrenoceptor mediated inhibition of longterm reward-related memory reconsolidation. Behav Brain Res. 2006;170:333–336. doi: 10.1016/j.bbr.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Nader K, LeDoux JE. Activation of extracellular signal-regulated kinasemitogen- activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. EurJNeurosci. 2005;21:283–289. doi: 10.1111/j.1460-9568.2004.03824.x. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Synapse structure: glutamate receptors connected by the shanks. Curr Biol. 1999;9(22):R848–R850. doi: 10.1016/s0960-9822(00)80043-3. [DOI] [PubMed] [Google Scholar]
- Eisenberg M, Kobilo T, Berman DE, Dudai Y. Stability of retrieved memory: inverse correlation with trace dominance. Science. 2003;301(5636):1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- Esteban JA. AMPA receptor trafficking: a road map for synaptic plasticity. Mol Interv. 2003;3(7):375–385. doi: 10.1124/mi.3.7.375. [DOI] [PubMed] [Google Scholar]
- Fan HY, Cherng CG, Yang FY, Cheng LY, Tsai CJ, Lin LC, Yu L., 8 Systemic treatment with protein synthesis inhibitors attenuates the expression of cocaine memory. Behav Brain Res 2; 2010;208(2):522–527. doi: 10.1016/j.bbr.2009.12.034. [DOI] [PubMed] [Google Scholar]
- Filion DL, Dawson ME, Schell AM, Hazlett EA. The relationship between skin conductance orienting and the allocation of processing resources. Psychophysiology. 1991;28(4):410–424. doi: 10.1111/j.1469-8986.1991.tb00725.x. [DOI] [PubMed] [Google Scholar]
- Flavell CR, Barber DJ, Lee JL. Behavioural memory reconsolidation of food and fear memories. Nat Commun. 2011;2:504. doi: 10.1038/ncomms1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexner LB, Flexner JB, Stellar E. Memory and cerebral protein synthesis in mice as affected by graded amounts of puromycin. Exp Neurol. 1965;13:264–272. doi: 10.1016/0014-4886(65)90114-7. [DOI] [PubMed] [Google Scholar]
- Forcato C, Argibay PF, Pedreira ME, Maldonado H. Human reconsolidation does not always occur when a memory is retrieved: the relevance of the reminder structure. Neurobiol Learn Mem. 2009;91(1):50–57. doi: 10.1016/j.nlm.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Forcato C, Burgos VL, Argibay PF, Molina VA, Pedreira ME, Maldonado H. Reconsolidation of declarative memory in humans. Learn Mem. 2007;14(4):295–303. doi: 10.1101/lm.486107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcato C, Rodriguez ML, Pedreira ME, Maldonado H. Reconsolidation in humans opens up declarative memory to the entrance of new information. Neurobiol Learn Mem. 2010;93:77–84. doi: 10.1016/j.nlm.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Ding HK, Takahashi E, Suzuki A, Kida A, Silva AJ. Stability of recent and remote contextual fear memory. Learn Mem. 2006;13:451–457. doi: 10.1101/lm.183406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman S. Perseverative neural processes and consolidation of the memory trace. Psychol. Bull. 1961;58:218–233. doi: 10.1037/h0044212. [DOI] [PubMed] [Google Scholar]
- He K, Song L, Cummings LW, Goldman J, Huganir RL, Lee HK. Stabilization of Ca2+ permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc Natl Acad Sci U S A. 2009;106(47):20033–20038. doi: 10.1073/pnas.0910338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez PJ, Kelley AE. Long-term memory for instrumental responses does not undergo protein synthesis-dependent reconsolidation upon retrieval. Learn Mem. 2004;11(6):748–754. doi: 10.1101/lm.84904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Cowansage KK, Arnold EC, Banko JL, Moerke NJ, Rodriguez R, Schmidt EK, Klosi E, Chorev M, Lloyd RE, Pierre P, Wagner G, LeDoux JE, Klann E. Inhibition of the interactions between eukaryotic initiation factors 4E and 4G impairs long-term associative memory consolidation but not reconsolidation. Proc Natl Acad Sci. 2011;108:3383–3388. doi: 10.1073/pnas.1013063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupbach A, Gomez R, Hardt O, Nadel L. Reconsolidation of episodic memories: a subtle reminder triggers integration of new information. Learn Mem. 2007;14:47–53. doi: 10.1101/lm.365707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge ME, Quartermain D. Characteristics of retrograde amnesia following reactivation of memory in mice. Physiol Behav. 1982;28(4):585–590. doi: 10.1016/0031-9384(82)90034-8. [DOI] [PubMed] [Google Scholar]
- Kemenes G, Kemenes I, Michel M, Papp A, Müller U. Phase-dependent molecular requirements for memory reconsolidation: differential roles for protein synthesis and protein kinase A activity. J Neurosci. 2006;26(23):6298–6302. doi: 10.1523/JNEUROSCI.0890-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt M, Soeter M. Reconsolidation in a human fear conditioning study: A test of extinction as updating mechanism. Biol Psychol. 2011 doi: 10.1016/j.biopsycho.2011.09.016. (in press) [DOI] [PubMed] [Google Scholar]
- Lee JLC. Memory reconsolidation mediates the updating of hippocampal memory content. Frontiers in behavioral neuroscience. 2010;4:168. doi: 10.3389/fnbeh.2010.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- Ma X, Zhang JJ, Yu LC. Post-retrieval extinction training enhances or hinders the extinction of morphine-induced conditioned place preference in rats dependent on the retrieval-extinction interval. Psychopharmacology. 2011;221(1):19–26. doi: 10.1007/s00213-011-2545-4. [DOI] [PubMed] [Google Scholar]
- Maldonado H. The crustacean nervous system (sd. K Wiese) Berlin, Germany: Springer; 2002. Crustaceans as models to investigate memory illustrated by extensive behavioural and psychological studies in Chasmagnatus; pp. 314–327. [Google Scholar]
- McGaugh JL. Time-dependent processes in memory storage. Science. 1966;16:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory – a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milekic MH, Alberini CM. Temporally graded requirement for protein synthesis following memory reactivation. Neuron. 2002;36(3):521–525. doi: 10.1016/s0896-6273(02)00976-5. [DOI] [PubMed] [Google Scholar]
- Milekic MH, Brown SD, Castellini C, Alberini CM. Persistent disruption of an established morphine conditioned place preference. J Neurosci. 2006;26:3010–3020. doi: 10.1523/JNEUROSCI.4818-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Everitt BJ. The persistence of maladaptive memory: Addiction, drug memories and anti-relapse treatments. Neurosci biobehavl rev. 2012;36:1119–1139. doi: 10.1016/j.neubiorev.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Milton AL, Lee JL, Butler VJ, Gardner R, Everitt BJ. Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. J Neurosci. 2008b;28:8230–8237. doi: 10.1523/JNEUROSCI.1723-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Lee JL, Everitt BJ. Reconsolidation of appetitive memories for both natural and drug reinforcement is dependent on {ß}-adrenergic receptors. Learn Mem. 2008a;15:88–92. doi: 10.1101/lm.825008. [DOI] [PubMed] [Google Scholar]
- Mineka S, Ohman A. Phobias and preparedness: the selective, automatic, and encapsulated nature of fear. Biol Psychiatry. 2002;52(10):927–937. doi: 10.1016/s0006-3223(02)01669-4. [DOI] [PubMed] [Google Scholar]
- Misanin JR, Miller RR, Lewis DJ. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160:203–204. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;15(324(5929)):951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muravieva EV, Alberini CM. Limited efficacy of propranolol on the reconsolidation of fear memories. Learn Mem. 2010;17:306–313. doi: 10.1101/lm.1794710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406(17):722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Szalay JJ, Achat-Mendes C, Platt DM, Otto MW, Spealman RD, Kantak KM. D-cycloserine deters reacquisition of cocaine self-administration by augmenting extinction learning. Neuropsychopharmacology. 2010;35(2):357–367. doi: 10.1038/npp.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolone G, Botreau F, Stewart J. The facilitative effects of D-cycloserine on extinction of a cocaine-induced conditioned place preference can be long lasting and resistant to reinstatement. Psychopharmacology (Berl) 2009;202(1-3):403–409. doi: 10.1007/s00213-008-1280-y. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. In: Conditioned reflexes. Anrep GV, translator. London: Oxford University Press; 1927. [Google Scholar]
- Pedreira ME, Maldonado H. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron. 2003;38(6):863–869. doi: 10.1016/s0896-6273(03)00352-0. [DOI] [PubMed] [Google Scholar]
- Pèrez-Cuesta LM, Maldonado H. Memory reconsolidation and extinction in the crab: mutual exclusion or coexistence? Learn Mem. 2009;16(11):714–721. doi: 10.1101/lm.1544609. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, Cahill L, Orr SP. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002;51(2):189–192. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- Power AE, Berlau DJ, McGaugh JL, Steward O. Anisomycin infused into the hippocampus fails to block"reconsolidation" but impairs extinction: the role of re-exposure duration. Learn Mem. 2006;13(1):27–34. doi: 10.1101/lm.91206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Zhu Y, Baumgart JP, Stornetta RL, Seidenman K, Mack V, van Aelst L, Zhu JJ. State-dependent Ras signaling and AMPA receptor trafficking. Genes Dev. 2005;19(17):2000–2015. doi: 10.1101/gad.342205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosc. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao-Ruiz P, Rotaru DC, van der Loo RJ, Mansvelder HD, Stiedl O, Smit AB, Spijker S. Retrieval-specific endocytosis of GluA2-AMPARs underlies adaptive reconsolidation of contextual fear. Nat Neurosci. 2011;14(10):1302–1308. doi: 10.1038/nn.2907. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. J Exp Psychol Anim Behav Process. 1975;104(1):88–96. [PubMed] [Google Scholar]
- Robinson MJ, Franklin KB. Reconsolidation of a morphine place preference: impact of the strength and age of memory on disruption by propranolol and midazolam. Behav Brain Res. 2010;213:201–207. doi: 10.1016/j.bbr.2010.04.056. [DOI] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308(5718):83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Runyan JD, Dash PK. Inhibition of hippocampal protein synthesis following recall disrupts expression of episodic-like memory in trace conditioning. Hippocampus. 2005;15:333–339. doi: 10.1002/hipo.20055. [DOI] [PubMed] [Google Scholar]
- Sanchez H, Quinn JJ, Torregrossa MM, Taylor JR. Reconsolidation of a cocaine-associated stimulus requires amygdalar protein kinase A. J Neurosci. 2010;30(12):4401–4407. doi: 10.1523/JNEUROSCI.3149-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Mem. 2000;7(2):73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Nadel NV, Sullivan GM, Harris A, LeDoux JE. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis PKA, and MAP kinase. Learn Mem. 1999;6:97–110. [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Cain CK, Curley NG, Schwartz JS, Stern SA, Ledoux JE, Phelps EA. Evidence for recovery of fear following immediate extinction in rats and humans. Learn Mem. 2008;15(6):394–402. doi: 10.1101/lm.909208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider AM, Sherman W. Amnesia: a function of the temporal relation of footshock to electroconvulsive shock. Science. 1968;159(3811):219–221. doi: 10.1126/science.159.3811.219. [DOI] [PubMed] [Google Scholar]
- Siegmund A, Wotjak CT. A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. J Psychiatr Res. 2007a;41(10):848–860. doi: 10.1016/j.jpsychires.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Disrupting reconsolidation: pharmacological and behavioral manipulations. Learn Mem. 2011;18(6):357–366. doi: 10.1101/lm.2148511. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szapiro G, Vianna MR, McGaugh JL, Medina JH, Izquierdo I. The role of NMDA glutamate receptors PKA, MAPK, and CAMKII in the hippocampus in extinction of conditioned fear. Hippocampus. 2003;13:53–58. doi: 10.1002/hipo.10043. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Svoboda K, Malinow R. Experience strengthening transmission by driving AMPA receptors into synapses. Science. 2003;299(5612):1585–1588. doi: 10.1126/science.1079886. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage. 2003;18(3):650–659. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Sanchez H, Taylor JR. D-cycloserine reduces the context specificity of pavlovian extinction of cocaine cues through actions in the nucleus accumbens. J Neurosci. 2010;30(31):10526–10533. doi: 10.1523/JNEUROSCI.2523-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Wiseman SL, Olausson P, Taylor JR. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nature Neurosci. 2006;9:167–169. doi: 10.1038/nn1628. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular memories of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Vaiva G, Ducrocq F, Jezequel K, Averland B, Lestavel P, Brunet A, Marmar CR. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry. 2003;54(9):947–949. doi: 10.1016/s0006-3223(03)00412-8. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corbille AG, Bertran-Gonzalez J, Herve D, Girault JA. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc. Natl. Acad. Sci. 2006;103:2932–2937. doi: 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Hervé D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005;102(2):491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voits M, Fink H, Gerhardt P, Huston JP. Application of 'nose-poke habituation' validation with post-trial diazepam- and cholecystokinin-induced hypo- and hypermnesia. J Neurosci Methods. 1995;57(1):101–105. doi: 10.1016/0165-0270(94)00143-5. [DOI] [PubMed] [Google Scholar]
- Wang SH, de Oliveira Alvares L, Nader K. Cellular and systems mechanisms of memory strength as a constraint on auditory fear reconsolidation. Nat Neurosci 2009. 2009;12(7):905–912. doi: 10.1038/nn.2350. [DOI] [PubMed] [Google Scholar]
- Xue YX, Luo YX, Wu P, Shi HS, Xue LF, Chen C, Zhu WL, Ding ZB, Bao YP, Shi J, Epstein DH, Shaham Y, Lu L. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science. 2012;336(6078):241–245. doi: 10.1126/science.1215070. [DOI] [PMC free article] [PubMed] [Google Scholar]