Abstract
Legionella pneumophila is an intracellular bacterial pathogen that is the cause of a severe pneumonia in humans called Legionnaires’ Disease. A key feature of L. pneumophila pathogenesis is the rapid influx of neutrophils into the lungs, which occurs in response to signaling via the interleukin-1 receptor (IL-1R). Two distinct cytokines, IL-1α and IL-1β, can stimulate the IL-1R. IL-1β is produced upon activation of cytosolic sensors called inflammasomes that detect L. pneumophila in vitro and in vivo. Surprisingly, we find no essential role for IL-1β in neutrophil recruitment to the lungs in response to L. pneumophila. Instead, we show that interleukin-1α is a critical initiator of neutrophil recruitment to the lungs of L. pneumophila-infected mice. We find that neutrophil recruitment in response to virulent L. pneumophila requires the production of IL-1α specifically by hematopoietic cells. In contrast to IL-1β, the innate signaling pathways that lead to the production of IL-1α in response to L. pneumophila remain poorly defined. In particular, although we confirm a role for inflammasomes for initiation of IL-1β signaling in vivo, we find no essential role for inflammasomes in production of IL-1α. Instead, we propose that a novel host pathway, perhaps involving inhibition of host protein synthesis, is responsible for IL-1α production in response to virulent L. pneumophila. Our results establish IL-1α as a critical initiator of the inflammatory response to L. pneumophila in vivo, and point to an important role for IL-1α in providing an alternative to inflammasome-mediated immune responses in vivo.
Introduction
Legionella pneumophila is a gram-negative intracellular bacterial pathogen that is the causative agent of a severe pneumonia called Legionnaires’ disease. After inhalation of aerosolized bacteria, L. pneumophila can infect and replicate within lung alveolar macrophages. Intracellular replication of L. pneumophila in macrophages in vitro, and virulence of L. pneumophila in animal models, requires a Type IV secretion system (T4SS) called the Dot/Icm system, which secretes bacterial effector proteins into the host cytosol. These effectors, greater than 270 of which have been identified (reviewed in 1), are believed to be critical for establishment of the Legionella-containing vacuole, the specialized membrane-bound intracellular compartment in which L. pneumophila replicates. In addition to its essential role in facilitating intracellular bacterial replication, the L. pneumophila T4SS is also associated with the induction of several potent innate immune responses (reviewed in 2).
Legionnaires’ Disease is characterized by robust infiltration of neutrophils and other immune cells into the lungs (3–5). Mice depleted of neutrophils exhibit an increased burden of L. pneumophila in the lungs (6–9). Furthermore, in vivo blockade of the CXCR2 chemokine receptor reduces the number of neutrophils recruited to the lungs of L. pneumophila infected mice and increases the lethality of L. pneumophila infection (8). Despite the clear protective role of neutrophils in L. pneumophila infections, it is also believed that excessive neutrophil influx may be responsible for much of the pathology associated with Legionnaires’ Disease (3, 5). Thus, infected hosts require mechanisms to carefully regulate the influx of neutrophils into tissues such that sufficient neutrophils are recruited to mediate pathogen clearance without causing excessive immune pathology. Despite the central role of neutrophils in Legionnaires’ Disease, the mechanisms controlling neutrophil recruitment to the lung in response to L. pneumophila remain poorly understood.
Previous work has established that neutrophil recruitment to the lung in response to L. pneumophila requires bacterial expression of the Dot/Icm T4SS (6). In addition, the interleukin-1 receptor type I (IL-1R), and its downstream signaling adaptor protein, MyD88, are also required (6, 10–13). Toll-like receptors (TLRs), which also utilize the MyD88 signaling adaptor, appear to only have a modest role in neutrophil recruitment to the lung (10–12, 14), suggesting that IL-1R signaling is the main pathway leading to neutrophil recruitment to the lung in vivo. Two related cytokines, interleukin-1α (IL-1α) and interleukin-1β (IL-1β), can both signal through the IL-1R. A previous study suggested that IL-1β is critical for neutrophil recruitment in response to L. pneumophila (6). It was proposed that infected macrophages generate IL-1β that signals through the IL-1R expressed by airway epithelial cells (AECs). IL-1R signaling in AECs amplifies the initial IL-1β signal by triggering the production of chemokines, such as CXCL1 and CXCL2, which stimulate the rapid and robust recruitment of neutrophils to the lung (6). However, no study has specifically addressed a possible role for IL-1α in mediating IL-1R-dependent neutrophil recruitment in vivo, and consequently, the relative role of IL-1α and IL-1β in responses to L. pneumophila remains uncertain.
Both IL-1α and IL-1β lack classical signal peptides to target the proteins to the conventional secretory pathway, and the mechanism of their release from cells remains poorly understood. Production of IL-1β appears to require two steps. First, activation of the NF-κB transcription factor results in transcription of Il1b mRNA, which is then translated into pro-IL-1β protein. Release of mature IL-1β has then been shown, in most instances, to require the Caspase-1 protease, which cleaves and activates IL-1β into its biologically active form (15, 16). Caspase-1 is itself activated within multiprotein complexes called ‘inflammasomes’ (17, 18). L. pneumophila has been shown to stimulate IL-1β release primarily via the NAIP5/NLRC4 inflammasome that senses bacterial flagellin that is translocated into the host cell cytosol via the Dot/Icm T4SS (19–23).
In contrast to IL-1β, IL-1α does not require proteolytic processing by Caspase-1 in order to be biologically active (24, 25). Nevertheless, in certain instances, inflammasome activation can promote the extracellular release of IL-1α, perhaps as a result of inflammasome-induced cell death (24, 26, 27). However, it is still unclear if the inflammasome is required for IL-1α production in response to bacterial infections in vivo. Similar to Il1b, the Il1a gene can be transcriptionally induced by infection, but IL-1α may also be constitutively expressed in certain cell types (24, 28). Virulent (T4SS+) L. pneumophila has been shown to induce IL-1α production by macrophages in vitro as well as in lung infections in vivo (29). In contrast, ΔdotA L. pneumophila mutants, which lack an active T4SS, do not induce IL-1α in vitro or in vivo (29). Nevertheless, the precise mechanism of IL-1α production in response to L. pneumophila remains unclear. Previous studies have suggested that T4SS-dependent activation of p38 and JNK MAP kinases are required to induce Il1a transcription (29, 30). Activation of MAP kinases by L. pneumophila appears to be partially due to a T4SS-dependent inhibition of host protein synthesis (30). Five L. pneumophila T4SS-translocated effectors have been identified that inhibit host protein synthesis (31), and Myd88/Nod1/Nod2−/− macrophages infected with a strain lacking these five effectors (Δ5) exhibit diminished MAP kinase activation and reduced Il1a mRNA levels as compared to wild-type-infected macrophages (30). However, infection of WT macrophages with the Δ5 mutant still induces normal MAP kinase activation (30), implying that MyD88/Nod signaling can also contribute. The mechanism by which protein synthesis inhibition results in MAP kinase activation remains unknown, and moreover, it is not clear whether macrophages infected with the Δ5 L. pneumophila strain exhibit a defect in release of IL-1α protein.
Here we show that in response to infection with virulent L. pneumophila in vivo, IL-1α produced by hematopoietic cells is the dominant cytokine leading to neutrophil recruitment to the lung at early timepoints (0 to 12 hours) after infection. We find that IL-1α and IL-1β act redundantly at later timepoints as neither Il1a−/− nor Il1b−/− mice have defects in neutrophil recruitment or bacterial clearance in the lung 24 hours post-infection. Interestingly, IL-1α is produced normally in mice lacking both Caspase-1 and Caspase-11, strongly implying that inflammasomes are not required for IL-1α production. Mice deficient in both Casp1/11 and Il1a phenocopied Il1r−/− mice, confirming that inflammasomes can compensate for a lack of IL-1α at late timepoints. Interestingly, we did not detect a defect in IL-1α production in macrophages infected with the L. pneumophila mutant lacking 5 bacterial effectors that block host translation (Δ5). While the Δ5 mutant had no defect in IL-1α production, we find that translation inhibition in concert with TLR activation is sufficient to induce IL-1α in vitro and in vivo. Taken together with previous studies (29–31), these results suggest that an uncharacterized pathway is responsible for IL-1α production in response to L. pneumophila infection in vivo. Our results point to a critical role for IL-1α in initiating IL-1R-dependent neutrophil recruitment and inflammatory responses in vivo that is complementary to the established inflammasome/IL-1β signaling axis.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Animal Care and Use Committee at the University of California, Berkeley.
Mice
Except for bone marrow chimeras (see below), all mice were age matched at 6–8 weeks old. Il1r−/− and C57BL/6 mice were purchased from Jackson Laboratories. Casp1/11−/− mice (32) were a gift from A. Van der Elden and M. Starnbach. Il1a−/− and Il1b−/− mice have been previously described (33). Il1a/Casp1/11−/− triple knockout mice were generated from crosses at UC Berkeley. B6.SJL-Ptprca/BoyAiTac (CD45.1) mice were purchased from Taconic. For bone marrow chimeras, 5–6 week old mice were irradiated twice with 600 rad 4 hours apart and reconstituted with 1×107 donor cells by injection into the tail vein. Chimeric mice were bled 11 weeks after irradiation and reconstitution was assessed by flow cytometry of hematopoietic cells for expression of CD45.1 and CD45.2 using anti-CD45.1-FITC (eBioscience) and anti-CD45.2-PE (eBioscience) antibodies. 12 weeks after irradiation chimeric mice were infected with L. pneumophila. All mice were specific pathogen free, maintained under a 12hr light-dark cycle (7 a.m. to 7 p.m.) and given a standard chow diet (Harlan irradiated laboratory animal diet) ad libitum.
In vivo experiments
Age matched mice were anesthetized with ketamine and infected intranasally with 2×106 LP01 or LP01ΔdotA in 20µL PBS. In some experiments mice were treated intranasally with ExoA, Pam3CSK4, or both in 20µL PBS, as described before (31). Bronchoalveolar lavage was performed by introducing 800µL of PBS into the trachea with a catheter (BD Angiocath 18g, 1.3648mm). Cells in the BAL fluid were pelleted and cell free BAL fluid was analyzed by ELISA. Total host cells in the lavage fluid were counted by staining cells with Guava Viacount (Millipore) and running samples on the Guava easyCyte Plus flow cytometer running CytoSoft5.3 software (Millipore). Lavage samples were stained with anti-Gr-1-PeCy7 and anti-Ly-6G-PE (eBioscience) and analyzed on a Beckman-Coulter FC-500 analyzer. Absolute numbers of Ly-6G+Gr1+ cells were calculated by taking the percent double positive cells determined by flow cytometry and multiplying by the total number of viable cells counted by the Guava easyCyte Plus flow cytometer. Bacterial burden in lungs was enumerated by hypotonic lysis of host cells in the lavage followed by spiral plating onto charcoal BYE plates with the Autoplate 5000 spiral plating system (Spiral Biotech, Inc.). CFU/mL in BAL fluid was determined by a QCount Colony Counter (version 3.0; Advanced Instruments, Inc.). BAL fluid mass was recorded prior to processing and this mass was used to estimate the volume of recovered BAL fluid. Total CFU was then calculated by multiplying CFU/mL by the estimated volume of BAL fluid. When noted, mouse body temperature and weight were monitored after infection with LP01. Mouse body temperature was measured by rectal probe and microtherma thermometer (Braintree Scientific). The probe was lubricated with a water-based lubricant (Astroglide) before use. Temperature and weight were measured at the same time daily.
Bacterial strains
For in vitro experiments all L. pneumophila strains were derived from LP02, a streptomycin-resistant thymidine auxotroph derived from L. pneumophila LP01. The ΔdotA, ΔflaA, Δ5ΔflaA strains were generated on the LP02 background and have been described previously (21, 30, 31). Mutants lacking one or more effectors were generated from LP02 by sequential in-frame deletion using the suicide plasmid pSR47S as described (34). Sequences of primers used for constructing deletion plasmids are listed in Supplemental Table I-A. Unless otherwise noted, all strains used for in vitro infections were deficient for bacterial flagellin (ΔflaA) and thus non-motile. L. pneumophila from the ΔflaA background were utilized in vitro to avoid activation of the NAIP5/NLRC4 inflammasome (19–23). For in vivo experiments, we utilized L. pneumophila wild-type strain LP01, a non-motile streptomycin-resistant strain derived from the original Philadelphia outbreak (35). The ΔdotA LP01 strain has been previously described (36).
Infection and stimulation
Bone marrow derived macrophages were plated in 24 well plates at a density of 5×105 cells per well and infected at an MOI of 1–3 (as indicated) by centrifugation for 10min at 400 ×g. In some experiments macrophages were treated with Exotoxin A (List Biological Labs), a synthetic bacterial lipopeptide (Pam3CSK4) (Invivogen), or both. After one hour of infection, media was changed. All in vitro L. pneumophila infections were in the absence of thymidine to curtail bacterial replication.
ELISA and Cytotoxicity
At the indicated time post-treatment, supernatants or bronchoalveolar lavage fluid were collected, cleared by centrifugation and analyzed by ELISA using paired interleukin-1α antibodies (BD Biosciences and eBioscience) or paired interleukin-1β antibodies (eBioscience and BD Bioscience). Recombinant IL-1α (eBioscience) or IL-1β (eBioscience) was used as a standard for each respective ELISA. Cytotoxicity was measured by evaluation of lactate dehydrogenase (LDH) released from cells (37). Specific lysis was calculated as a percentage of LDH released by detergent-lysed macrophages.
Cell culture
Macrophages were derived from the bone marrow of C57BL/6J mice (Jackson Laboratory). Macrophages were derived by 8 days of culture in RPMI supplemented with 10% serum, 100µM streptomycin, 100U/mL penicillin, 2mM L-glutamine and 10% supernatant from 3T3-macrophage-colony stimulating factor cells, with feeding on day 5. HEK293T cells were grown in complete media (DMEM, 10% FBS, 100 U/mL penicillin, 100 µg/mL streptomycin, and 2 mM L-glutamine).
Effector library screen
The library of 259 confirmed or putative secreted effector proteins has been previously described (38). Using the Gateway cloning system (Invitrogen) the library was cloned into a Gateway compatible MSCV 2.2 retroviral expression construct. We modified the MSCV 2.2 expression construct with an in-frame 6x-Myc tag upstream of the cloned effectors to accommodate for non AUG start codon usage in prokaryotes and we removed the downstream internal ribosome entry site (IRES)- green fluorescent protein (GFP). HEK293T cells were plated at 2.5×104 cells per well in 96-well tissue culture plates. 24hrs after plating cells were co-transfected using Lipofectamine 2000 (Invitrogen), following the manufacturer’s instructions, with a single library clone and the TK-Renilla luciferase reporter construct. 24hrs after transfection cells were lysed in passive lysis buffer (Promega) for 5 min at 25°C. Cell lysates were incubated with the Renilla luciferase substrate coelentrerazine (Biotium) and luminescence was measured on a SpectraMax L microplate reader (Molecular Devices). The relative block in translation was measured by comparing Renilla luminescence in cells transfected with a control bacterial protein that does not block translation.
Quantitative RT-PCR
Macrophage RNA was isolated using an RNeasy kit (Qiagen) according to the manufacturer’s protocol. RNA samples were treated with RQ1 DNase (Promega) prior to reverse transcription (RT) with Superscript III (Invitrogen). cDNA reactions were primed with poly(dT). Quantitative PCR was performed as described previously(39) using a Step One Plus RT-PCR system (Applied Biosystems) with Platinum Taq DNA polymerase (Invitrogen) and EvaGreen (Biotium). Transcript levels were normalized to those of Rps17. The following primer sequences were used: for Il1a, Forward 5’-ATGACCTGC AACAGGAAGTAAAA-3’ and Reverse 5’-TGTGATGAGTTTTGGTGTTTCTG-3’ and for Rps17, Forward 5’-CGCCATTATCCCCAGCAAG-3’ and Reverse 5’-TGTCGGGATCCACCT CAATG-3’.
35S-methionine metabolic labeling
5×105 bone marrow derived macrophages were seeded in 24-well plates and infected with bacterial strains at an MOI of 3. 25 minutes prior to labeling, macrophages were treated with 25µg/mL chloramphenicol to inhibit bacterial translation. At 6 and 24hrs post-infection media was removed and incubated with 25µCi/mL 35S-methionine (Perkin Elmer) in RPMI without methionine supplemented with 10% serum, 2mM L-glutamine, 25µg/mL and 10% supernatant from 3T3-macrophage-colony stimulating factor cells. Cells were labeled for 1 hour, washed three times with cold PBS and then lysed with radioimmunoprecipitation assay (RIPA) buffer supplemented with 2 mM Na3VO4, 1 mM PMSF, 25 mM NaF, and 1x Roche protease inhibitor cocktail (no EDTA), pH 7.2, for 10 minutes at 4°C. Total protein levels were measured by bicinchoninic acid (BCA) assay and equal amounts of protein were mixed with SDS sample buffer (40% glycerol, 8% SDS, 2% 2-mercaptoethanol, 40 mM EDTA, 0.05% bromophenol blue and 250 mM Tris-HCl, pH 6.8), boiled for 5 min and then separated by SDS–PAGE. The gels were stained with coomassie blue to show equal protein loading, dried, and exposed to a phosphor screen and visualized using a Typhoon Trio imager (GE Healthcare)
Results
Type-I IL-1 Receptor-deficient mice are more susceptible to L. pneumophila infection
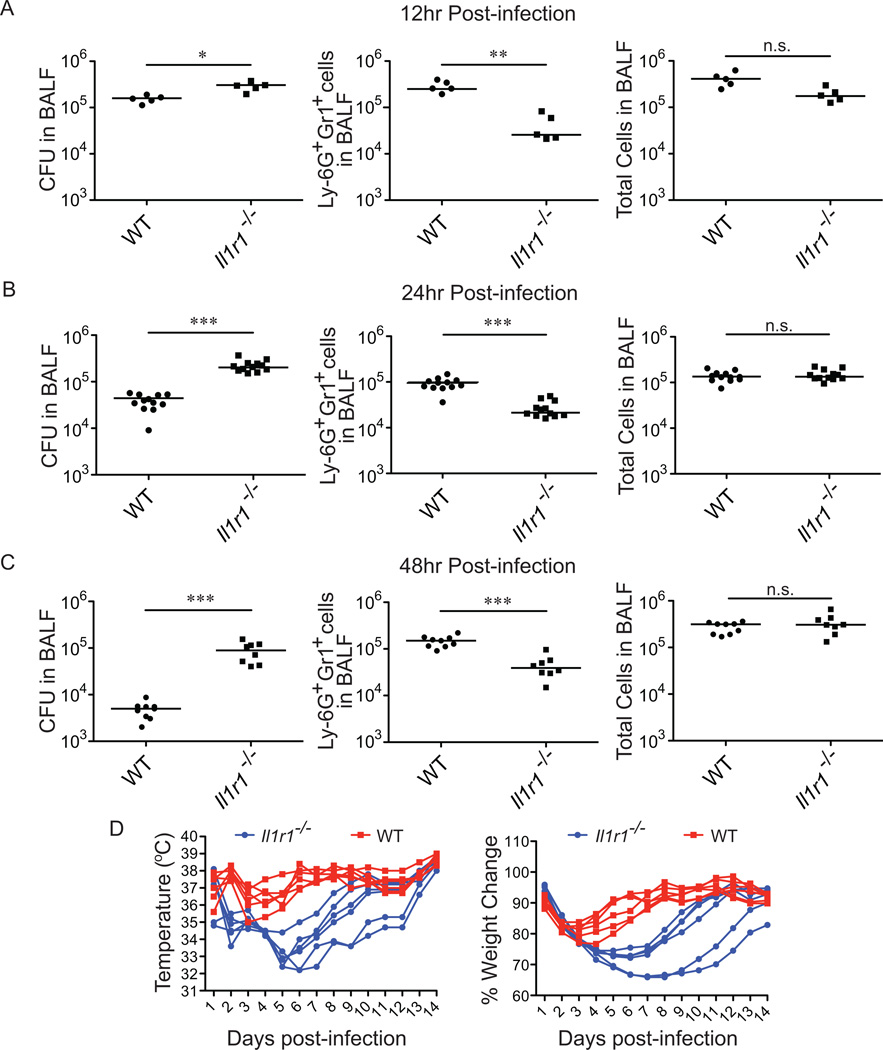
A previous report identified the type-I interleukin-1 receptor (IL-1R) as a major signaling pathway that controls the recruitment of neutrophils to the lung in response to L. pneumophila (6). This report proposed that IL-1β is the major ligand signaling through the IL-1R in L. pneumophila infections, but did not specifically address a possible role of IL-1α and also did not examine the consequences of IL-1R deficiency on host health. Before addressing the relative importance of IL-1α and IL-1β, we first set out to confirm the previously proposed role of IL-1R signaling in L. pneumophila infection. We infected IL-1R-deficient (Il1r−/−) mice with wild-type L. pneumophila and examined the mice at 12hrs, 24hrs and 48hrs post-infection. Total numbers of Ly6G+Gr1+ cells (here referred to as neutrophils) in bronchoalveolar lavage (BAL) fluid were determined by flow cytometry, and bacterial burden was measured by plating for colony forming units (CFUs). Consistent with previous reports, Il1r−/− mice recruited reduced numbers of neutrophils to the lungs in response to L. pneumophila, with approximately 10-fold, 5-fold and 4-fold fewer neutrophils in Il1r−/− mice than WT mice at 12hrs, 24hrs and 48hrs post-infection, respectively (Fig. 1A, B, C). Interestingly, while there are significant defects in the number of neutrophils recruited to the lungs of Il1r−/− mice, the total number of cells in the BAL fluid of these mice does not significantly differ from WT mice (Fig. 1A, B, C). The similarity in overall numbers of cells in the BAL appears to be because after infection with L. pneumophila, Il1r−/− mice harbor greater numbers of alveolar macrophages and CD45-negative/low cells that compensate for the decrease in neutrophils (Supplemental Fig. S1). One possible explanation for this is that in WT mice, damaged or dead alveolar macrophages and CD45-negative/low cells are normally phagocytosed and thereby eliminated by neutrophils. Thus, with decreased neutrophils in Il1r−/− mice, alveolar macrophages and CD45-negative/low cells accumulate (Supplemental Fig. S1). In addition to decreased neutrophils, Il1r−/− mice harbor approximately 5-fold and 17-fold higher CFU in BAL fluid over B6 controls at 24 and 48 hours post-infection, respectively (Fig. 1B, C). Il1r−/− mice also have a slight increase in bacterial burden measured in BAL fluid at 12hrs post-infection, but this difference is not dramatic, presumably because L. pneumophila does not have enough time to appreciably replicate or be cleared by the host at this time-point (Fig. 1A).
Figure 1.
The IL-1 Receptor Type I is essential for control of L. pneumophila infection. (A–C) IL-1R Type I-deficient mice were infected intranasally with 2×106 L. pneumophila (LP01). Bronchoalveolar lavage (BAL) was performed at12 hours (A), 24 hours (B) and 48 hours (C) post-infection. Bacterial burden in the BAL fluid was determined by plating for colony forming units. The number of Ly-6G+Gr1+ cells was determined by flow cytometry and the total number of cells in the BAL fluid as determined by Guava ViaCount assay. (D) IL-1R-deficient (blue circles) and wild-type B6 (red squares) mice were infected with 2×106 L. pneumophila (LP01) and monitored daily for temperature and weight change. Percent weight change is calculated to weight at day zero. Data are representative of two (D) or three (A, B, C) experiments. (Median in A-C). *, p<0.05. **, p<0.01. ***, p<0.005. (Statistical analysis: Mann-Whitney U test).
Although our data confirm that IL-1R signaling is critical for neutrophil recruitment and elimination of bacteria from the lung, neutrophils are also believed to be key mediators of the immune pathology of Legionnaires’ Disease. Therefore we were interested to determine whether the decreased neutrophil response in Il1r−/− mice resulted in overall increased or decreased host health. To assay host health, we followed body temperature and weight loss in wild-type and Il1r−/− mice (Fig. 1D). Although both wild-type and Il1r−/− mice eventually recover from the infection, Il1r−/− mice show more severe weight loss and temperature decreases than WT mice after being infected with L. pneumophila over a two week study (note that, in contrast to humans, mice typically exhibit a hypothermic response, rather than a fever, as a result of infection (40). We have shown that Il1r−/− mice exhibit increased bacterial burden in the lung at 12, 24 and 48 hours post-infection with L. pneumophila (Fig. 1A–D). We therefore suggest that increased bacterial burden in Il1r−/− mice over the first week of infection is likely the cause of the decreased overall health of these animals in response to L. pneumophila infection. However, after about a week of infection, compensatory innate and/or adaptive immune responses likely control the infection. Overall, our results suggest that during the course of experimental L. pneumophila infection, the beneficial function of early neutrophil influx in bacterial clearance outweighs the potentially negative effects of neutrophil-mediated immune pathology. These data also establish an important role for IL-1R signaling in host health in addition to the previously established role for IL-1R signaling in neutrophil recruitment and bacterial clearance.
IL-1α production precedes the recruitment of neutrophils to the lung
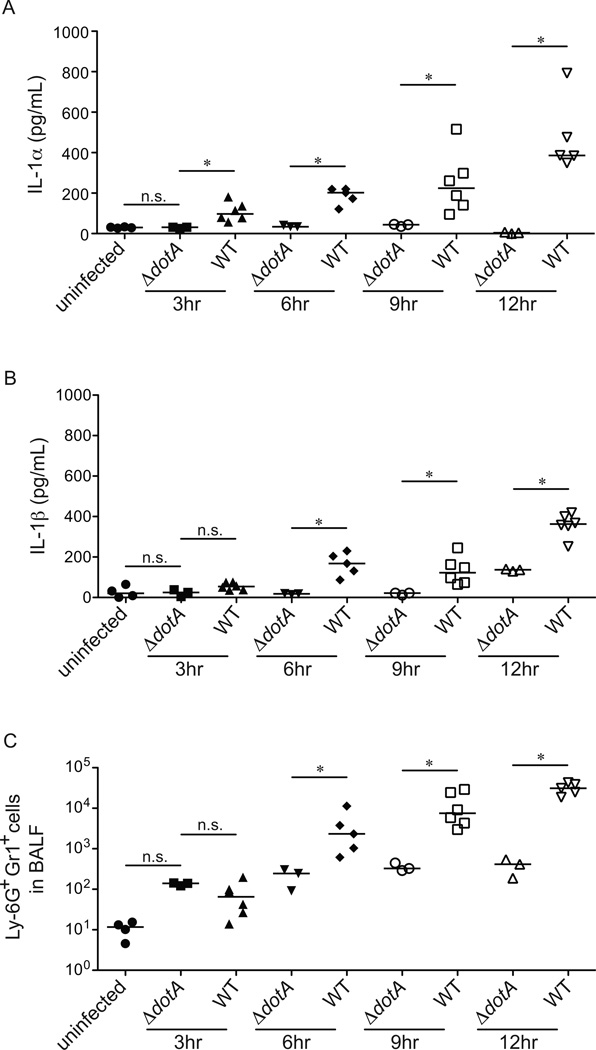
IL-1α and IL-1β are the only known agonists of the IL-1R. We therefore tested whether there was a correlation between IL-1α or IL-1β production and the recruitment of neutrophils to the lungs of infected mice. B6 mice were infected with wild-type L. pneumophila or an avirulent mutant strain of L. pneumophila that lacks a functional Type IV secretion system (ΔdotA). BAL fluid was harvested at 3, 6, 9 and 12 hours post-infection, and assessed for the presence of neutrophils, IL-1α, and IL-1β. The earliest in vivo Dot/Icm-dependent response was the production of IL-1α, which was first detectable at 3hrs post-infection (Fig. 2A). By contrast, the earliest significant production of IL-1β (above that induced by ΔdotA) was not until 6hrs post-infection (Fig. 2B), the same time that the Dot/Icm-dependent influx of neutrophils can first be detected (Fig. 2C). It is interesting to note that there seems to be an increase in the number of neutrophils found in the BAL fluid after infection with the ΔdotA L. pneumophila strain at 3hrs post-infection, although this difference is not statistically significant (Fig. 2C). Consistent with previous results, the ΔdotA L. pneumophila strain did not appreciably induce IL-1α production in the lung (Fig. 2A, B). Thus, while there may be a low level of Dot-independent neutrophil recruitment to the lung, this recruitment appears to be IL-1α independent and likely plays a minimal role in protecting the host from infection. Taken together, these data show that IL-1α production is largely Dot-dependent and occurs prior to the recruitment of neutrophils to the lung. Our findings suggest a role for IL-1α in the early IL-1R-dependent and Dot-dependent recruitment of neutrophils to the lungs of L. pneumophila infected mice.
Figure 2.
Dot/Icm T4SS-dependent IL-1α production precedes the recruitment of Ly-6G+Gr1+ cells to the lung. (A–C) B6 mice were infected intranasally with 2×106 L. pneumophila (WT) or a mutant lacking a function type IV secretion system (ΔdotA). Bronchoalveolar lavage was performed at 3, 6, 9, or 12 hours post-infection. IL-1α (A) and IL-1β (B) levels were measured by ELISA. (C) The number of Ly-6G+Gr1+ positive cells in the BAL fluid was determined by flow cytometry. Data are representative of three experiments. (Median in A-C). *, p<0.05 (Statistical analysis: Mann-Whitney U test).
IL-1α, but not IL-1β, is required for early neutrophil recruitment to the lung
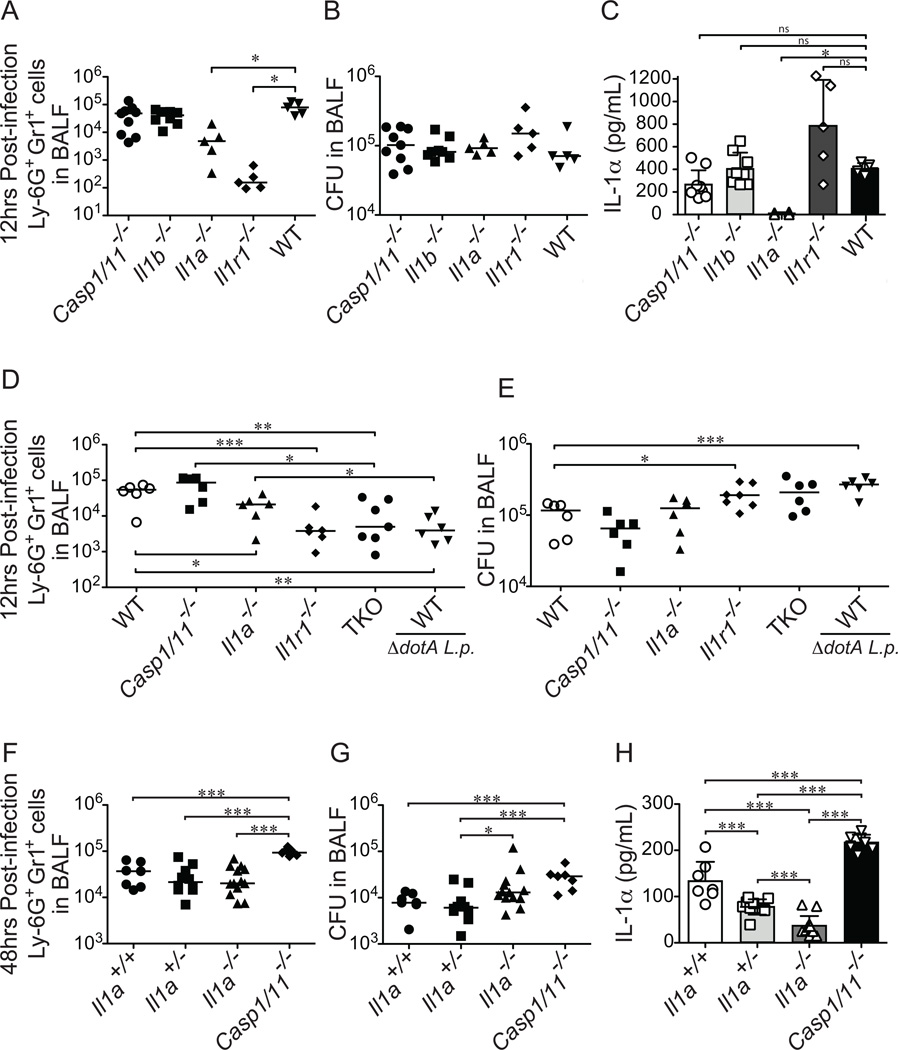
We next tested whether the loss of IL-1α or IL-1β would have an effect on neutrophil recruitment. We infected wild-type (B6), Il1a−/−, Il1b−/−, Casp1/11−/− and Il1r−/− mice with wild-type L. pneumophila and measured neutrophil recruitment and bacterial burden at 12hrs post-infection. As expected, Il1r−/− mice showed a strong defect in recruitment of neutrophils to the lung, while both the Il1b−/− and Casp1/11−/− mice showed no defect in neutrophil recruitment to the lung at 12hrs post-infection, as compared to B6 mice (Fig. 3A). However, there was approximately a 17-fold decrease in the number of neutrophils recruited to the lung of Il1a−/− mice as compared to B6 mice (Fig. 3A). Importantly, Il1a−/− mice have no significant difference in the production of IL-1β in the BAL fluid of infected mice at 12hrs post-infection (Supplemental Fig. S2A, B). These data suggest that IL-1α may be more important than IL-1β for the recruitment of neutrophils to the lung at 12hrs post-infection. Interestingly, the defect in neutrophil recruitment in Il1a−/− mice was not as pronounced as the defect seen in Il1r−/− mice. This suggests that although IL-1β is not itself essential for neutrophil recruitment, it can partially compensate for the loss of IL-1α (addressed further below). The bacterial burden in the infected mice was very similar among all of the genotypes, likely because at 12hrs post-infection L. pneumophila has not had enough time to appreciably grow or be cleared by the host immune response (Fig. 3B). As an important control, measurement of IL-1α protein levels in the BAL fluid of infected mice demonstrated that only Il1a−/− mice had defects in production of IL-1α in response to L. pneumophila infection (Fig. 3C). The amount of IL-1α detected in the BAL fluid of L. pneumophila infected Il1r−/− mice is slightly higher than WT mice, likely due to an increase in bacterial burden caused by reduced neutrophil recruitment to the lungs of these mice (Fig. 3C). The increase in bacterial burden in Il1r−/− mice likely leads to more infected macrophages and thus an increase in the production of IL-1α. Additionally, the loss of the IL-1R may result in less internalization of the IL-1α protein, resulting in higher extracellular accumulation. We also note that IL-1α is produced even in Casp1/11−/− mice, indicating that in response to L. pneumophila IL-1α production in vivo can be independent of both Caspase-1 and Caspase-11 inflammasomes (Fig. 3C).
Figure 3.
IL-1α is required for Ly-6G+Gr1+ cell recruitment to the lung in response to infection with L. pneumophila. (A–C) The indicated mouse strains were infected intranasally with 2×106 L. pneumophila (LP01). At 12hrs post-infection bronchoalveolar lavage (BAL) fluid was collected. (A) Ly-6G+Gr1+ cells in the BAL fluid were enumerated by flow cytometry. (B) Bacterial burden in the lung was determined by plating BAL fluid for CFU. (C) IL-1α levels were measured by ELISA. (D–E) The indicated mouse strains were infected intranasally with 2×106 L. pneumophila (LP01) or Dot/Icm T4SS-deficient L. pneumophila (LP01 ΔdotA) as noted. At 12 hours post-infection BAL fluid was harvested and Ly-6G+Gr1+ cell recruitment was measured by flow cytometry (D) and bacterial burden in the lung was measured by plating for CFU (E). (F–H). Il1a−/−Il1a+/−, and Il1a+/+ littermates were infected intranasally with 2×106 L. pneumophila (LP01). Non-littermate Casp1/11−/− mice were also infected with LP01. Bronchoalveolar lavage fluid was collected 48hrs post-infection and Ly-6G+Gr1+ cells were quantified by flow cytometry (F). Bacterial burden was determined by plating for CFU (G). IL-1α levels in the BAL fluid were determined by ELISA (H). Data are representative of two (F–H) or three (A–E) experiments (Median in A, B, D-G. mean ± s.d. in C, H). The low level of apparent IL-1α protein produced in Il1a−/− mice at 48h post infection appears to be due to an unknown cross-reacting protein that produced a low signal on the ELISA assay. TKO, Il1a/Casp1/11−/− triple knockout mice. *, p<0.05. **, p<0.01. ***, p<0.005. (Statistical analysis: Mann-Whitney U test).
We hypothesized that the intermediate phenotype seen in the Il1a−/− mice was due to low levels of inflammasome-dependent IL-1β production that are still capable of signaling through the IL-1R. We were unable to generate Il1a/b−/− double knockout mice as these genes are located directly next to each other on the chromosome. Thus, to test whether there is redundancy between IL-1α and IL-1β, we generated Il1a/Casp1/11−/− ‘triple’ knockout mice (TKO). These mice are predicted to be deficient in production of IL-1α and IL-1β, as production of biologically active IL-1β is generally believed to require Caspase-1. We should note that the TKO mice are not only defective in IL-1β cytokine production, but they are also unable to undergo pyroptosis, a Caspase-1/11-dependent form of lytic cell death, which has previously been shown to evict bacteria from their intracellular niche and render them susceptible to phagocytosis and killing by neutrophils (41, 42). The loss of pyroptosis could lead to an increased bacterial burden; however, at 12hrs post-infection we see very little differences in bacterial burden in the BAL fluid and thus we argue that the major defect in the TKO mice at 12hrs post-infection is the loss of IL-1β processing and release (Fig. 3D, E). Consistent with a defect in IL-1α and IL-1β production, we find that in response to L. pneumophila infection TKO mice produce almost no detectable IL-1α and very low levels of IL-1β in BAL fluid at 12 hours post-infection (Supplemental Fig. S2C, D). Interestingly, TKO mice exhibited a large defect in neutrophil recruitment to the lung; in fact, these mice were as defective in neutrophil recruitment as Il1r−/− mice (Fig. 3D). These data suggest that IL-1α is the major cytokine required to signal through the IL-1R and recruit neutrophils to the lung at 12 hours post-infection, though Casp1/11-dependent signaling through the IL-1R (presumably mediated by IL-1β) can partially compensate for the loss of IL-1α. Furthermore, Caspase-1 is usually considered to be essential for IL-1β processing (15, 16), although some previous reports have suggested that IL-1β can be generated in the absence of Casp1/11 (43, 44). Even though Il1a/Casp1/11−/− TKO mice produced very low levels of IL-1β, TKO mice were as defective in neutrophil recruitment as Il1r−/− mice, implying that, at least in response to L. pneumophila, production of biologically active IL-1β requires Caspase-1/11.
At late timepoints after infection, IL-1β compensates for the loss of IL-1α
Il1r−/− mice exhibit reduced neutrophil recruitment that is sustained until at least 48hrs post-infection (Fig. 1A). We therefore tested if the loss of IL-1α would lead to a defect in neutrophil recruitment and an increase in bacterial burden at late timepoints. We infected wild-type, Il1a+/−, and Il1a−/− littermates with wild-type L. pneumophila and compared these mice to Casp1/11−/− mice (Fig. 3F–H). At 48hrs post-infection Il1a−/− mice have no defect in neutrophil recruitment to the lung and only a modest defect in control of bacterial burden (Fig. 3F–G). Additionally we see no defect in neutrophil recruitment by Casp1/11−/− mice at 48hrs post-infection, suggesting that both IL-1α and IL-1β are capable of signaling through the IL-1R and can compensate for the loss of each other by 48h post-infection. In fact, Casp1/11−/− mice actually appeared to exhibit increased recruitment of neutrophils to the lung (Fig. 3F). However, despite the increased neutrophil recruitment, Casp1/11−/− mice also exhibited increased bacterial burdens in the lung at 48h post-infection (Fig. 3G). As mentioned previously, this counterintuitive result is likely explained by the loss of Caspase-1/11-dependent pyroptosis, which has previously been shown to evict bacteria from their intracellular niche and render them susceptible to phagocytosis and killing by neutrophils (41, 42). Importantly, we find that Casp1/11−/− mice produce IL-1α in response to L. pneumophila infection and actually induce significantly more IL-1α than wild-type mice; this increase is likely due to the loss of pyroptosis and subsequent increased bacterial burden in these mice (Fig. 3H).
IL-1α is produced by cells derived from the hematopoietic lineage
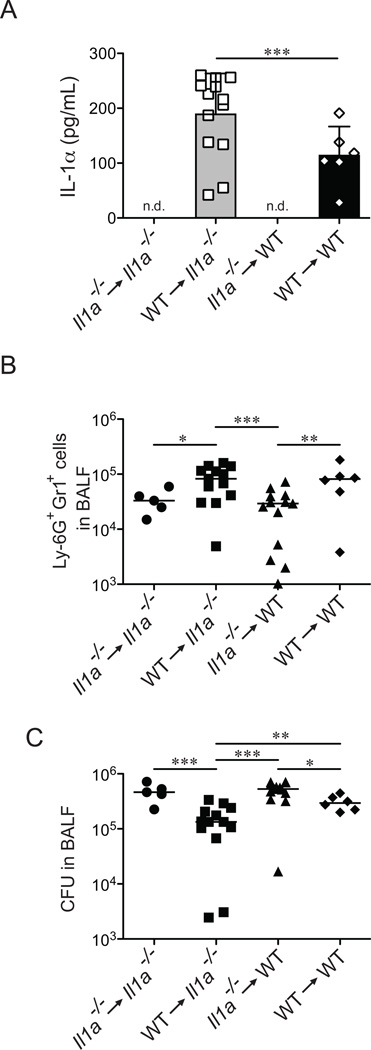
IL-1α is inducible in hematopoietic cells, but is also reported to be constitutively expressed by certain non-hematopoietic cells (24, 28). We therefore wished to determine whether the rapid production of IL-1α and the ensuing neutrophil influx required IL-1α production by hematopoietic or non-hematopoietic cells. We generated bone marrow chimeras in which wild-type B6 (CD45.1+) mice were reconstituted with bone marrow from Il1a−/− (CD45.2+) mice, and vice-versa. To confirm that our chimeras had been reconstituted to a high level, blood samples were collected and stained with antibodies for CD45.1 and CD45.2 that marked wild-type and Il1a−/− derived hematopoietic cells, respectively (Supplemental Fig. S3). Chimeric mice were infected with L. pneumophila and BAL fluid was collected 12 hours post-infection. Mice reconstituted with B6 hematopoietic cell populations produced IL-1α in response to L. pneumophila infection, whereas mice reconstituted with Il1a−/− bone marrow failed to produce IL-1α (Fig. 4A). Importantly, the production of IL-1α correlated with the recruitment of neutrophils to the lung (Fig. 4B). Consistent with our previous findings (Fig. 3) we see little difference in the total CFU found in the BAL fluid of these mice at 12h post-infection, although there was a slight increase in bacterial burden in mice that received Il1a−/− bone marrow (Fig. 4C). These chimera experiments demonstrate that hematopoietic cells in the lung, presumably macrophages that have been infected with L. pneumophila, are responsible for the early production of IL-1α and subsequent recruitment of neutrophils to the site of infection.
Figure 4.
Hematopoietic cells are responsible for IL-1α production in response to L. pneumophila. (A–C) 6 week old Il1a−/− and congenically marked B6.SJL (CD45.1) mice were lethally irradiated and reconstituted with Il1a−/− (CD45.2) or B6.SJL bone marrow as indicated. After 12 weeks of recovery, chimeric mice were infected with L. pneumophila (LP01). Bronchoalveolar lavage (BAL) fluid was collected 12hrs post-infection. (A) IL-1α levels in BAL fluid were determined by ELISA. (B) Recruitment of Ly-6G+Gr1+ cells was determined by flow cytometry. (C) Bacterial burden in the lung was determined by plating BAL fluid for bacterial CFUs. Data are representative of two (A–C) experiments. (Mean ± s.d. in A. Median in B, C). n.d., not detectable. WT, B6.SJL. *, p<0.05. **, p<0.01. ***, p<0.005. (Statistical analysis: Mann-Whitney U test).
L. pneumophila lacking effectors that block host protein synthesis still induce IL-1α
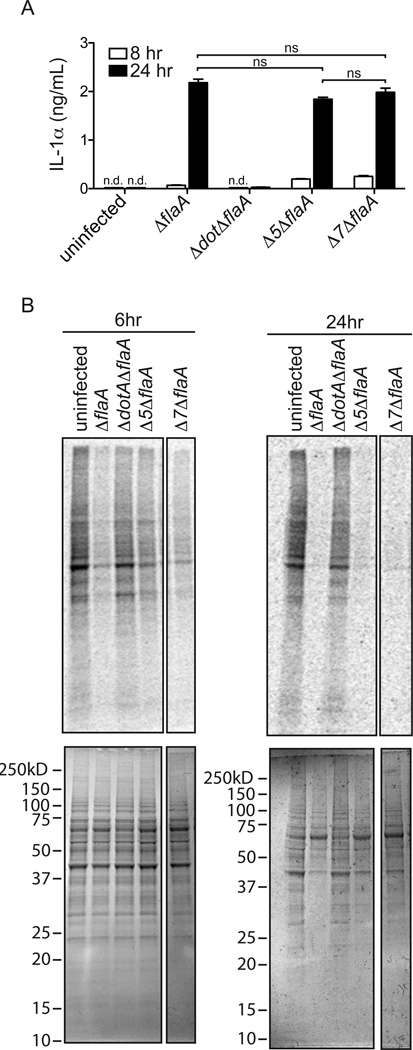
Given the major role IL-1α plays in neutrophil recruitment, we next wanted to explore the molecular mechanism of IL-1α production by macrophages. We (31) and others (34, 45, 46) previously showed that L. pneumophila encodes five Dot/Icm-secreted effectors that inhibit host protein synthesis. A strain lacking these five effectors (Δ5) was defective in the induction of a subset of inflammatory cytokines, including IL-23 and GM-CSF (31). Moreover, Δ5 was also defective in the transcriptional induction of the Il1a gene when the Toll-like receptor (TLR) and NOD-like receptor innate immune sensing pathways were severely hindered (infections of Myd88/Nod1/Nod2−/− BMDMs)(30). The overall model emerging from our previous studies was that protein synthesis inhibition by virulent L. pneumophila produces a host cell stress response that leads to the production of inflammatory cytokines. Therefore, we asked whether the Δ5 L. pneumophila strain could still induce IL-1α protein release by wild-type BMDMs. We infected macrophages with the Δ5 L. pneumophila strain on the ΔflaA background (Δ5ΔflaA) strain and measured the production of IL-1α from these cells. We utilized L. pneumophila on the ΔflaA background to avoid the confounding effects of NAIP5/NLRC4 inflammasome activation by flagellin. Interestingly, we found that the Δ5ΔflaA strain still induced production of significant amounts of IL-1α protein (Fig. 5A). This result is consistent with previous in vivo observations that showed that neutrophil recruitment is normal in response to the Δ5 mutant (30). We considered two possible explanations for the ability of the Δ5 mutant to induce IL-1α: (1) protein synthesis inhibition is not required for IL-1α production; or (2) residual protein synthesis inhibition by the Δ5 strain is sufficient to induce IL-1α. Consistent with the latter possibility, and with our previous work (31), we found that the Δ5ΔflaA strain still significantly inhibited host protein synthesis in BMDMs (as measured by incorporation of 35S-methionine) as compared to infection with ΔdotAΔflaA, which does not block translation (Fig. 5B; 31). These results raised the possibility that L. pneumophila might encode additional effectors that inhibit host protein synthesis. To identify these effectors we utilized a library of 259 known and putative secreted effectors (38), that we cloned into a mammalian expression vector. Each individual effector expression plasmid was co-transfected into 293T cells, along with a plasmid that constitutively expresses Renilla luciferase, and protein synthesis (as assessed by luminescence) was measured 24hrs after transfection (Supplemental Fig. S3; Supplemental Table I-B). As a positive control, this screen successfully identified the five previously described effectors that are known to block host translation (Lpg0437, Lpg1368, Lpg1488, Lpg2504, and Lpg2862) (31; Supplemental Table I-B). In addition, two other effectors that inhibit host protein synthesis were identified: Lpg0208, a Serine/Threonine Kinase, and Lpg1489, a putative effector of unknown function (47). Lpg0208 and Lpg1489 were confirmed to inhibit protein synthesis in 293T cells, as measured by reduced 35S-methionine incorporation upon overexpression of each effector (data not shown). However, deletion of these two additional effectors in the Δ5ΔflaA background, to generate a strain we call Δ7ΔflaA, did not significantly affect the ability of L. pneumophila to inhibit host protein synthesis in macrophages (Fig. 5B). The Δ7 strain also induced normal production of IL-1α in vitro (Fig. 5A). The residual ability of Δ7 L. pneumophila to inhibit host protein synthesis and/or induce IL-1α may therefore be due to additional effectors that were not present in our effector library. Alternatively, inhibition of host protein synthesis may result from the combined effects of multiple L. pneumophila effectors (which would not have been detected in our one-by-one effector screen), or the infection process itself.
Figure 5.
L. pneumophila mutants lacking bacterial effectors known to block translation have no defect in IL-1α production. (A) Wild-type B6 bone marrow derived macrophages were infected with the indicated strains of L. pneumophila (LP02) at a MOI of 1. 8hrs and 24hrs post-infection cell supernatants were collected and IL-1α levels were determined by ELISA. (B) Wild-type bone marrow derived macrophages were infected with the indicated strains of L. pneumophila (MOI=3) and at 6hrs (left panels) and 24hrs (right panels) post-infection cells were incubated with 35S-methionine for one hour followed by lysis in RIPA buffer. Gels were stained with coomassie blue to visualize equal loading (bottom panels) and global translation levels were determined by autoradiography (top panels). Intervening lanes on gel were removed for simplicity. Data are representative of two (B) or three (A) experiments. (Mean ± s.d. in A). n.d., not detectable. ns, not significant. (Statistical analysis: Mann-Whitney U test).
Translation inhibition together with TLR activation is sufficient to induce IL-1α production in vitro and in vivo
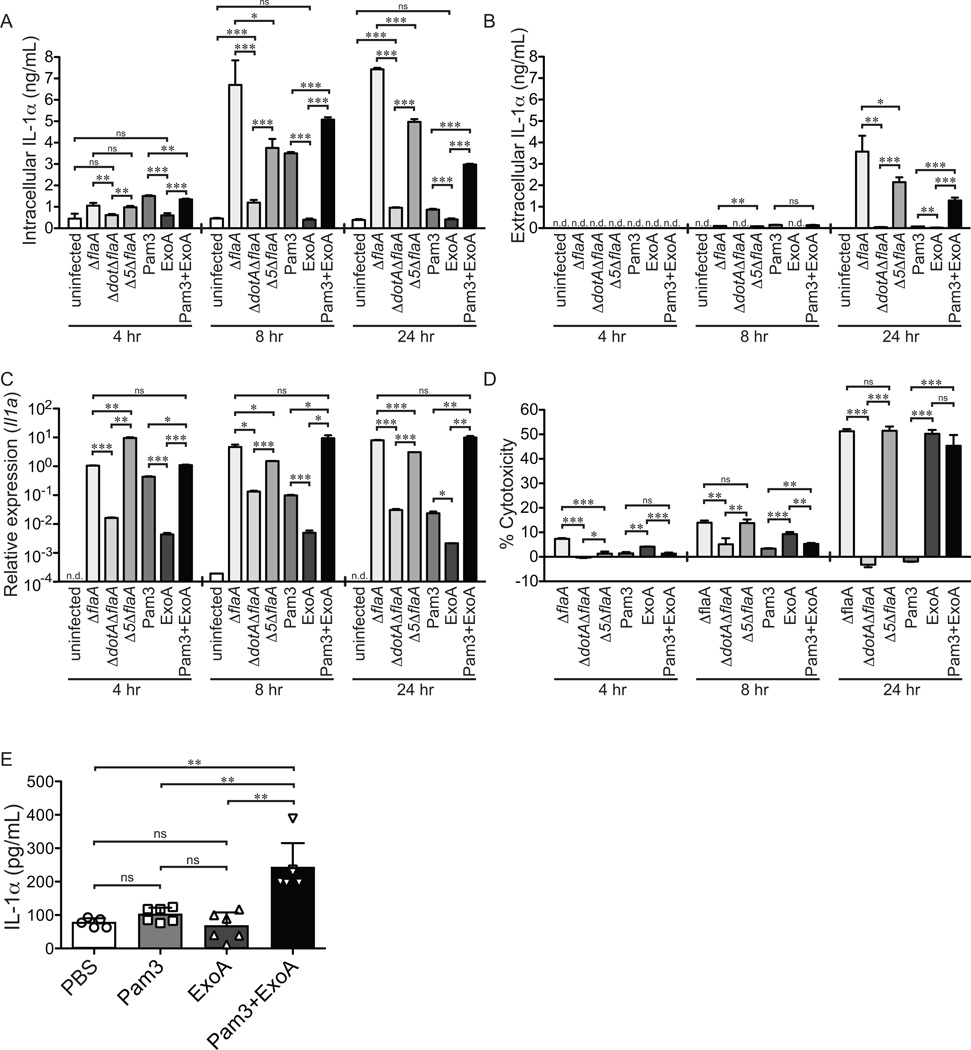
The above results showed that induction of IL-1α by Δ7 L. pneumophila correlates with inhibition of host protein synthesis. We therefore wished to determine if inhibition of host protein synthesis is sufficient to cause IL-1α release in vitro and in vivo. In order to recapitulate TLR signaling that occurs during L. pneumophila infection, bone marrow derived macrophages (BMDMs) were treated with 10ng/mL Pam3CSK4, a TLR2 ligand. This treatment induced transient intracellular IL-1α protein (Fig. 6A) but did not result in significant IL-1αrelease (Fig. 6B). BMDMs were therefore additionally treated with 50ng/mL Exotoxin A (ExoA), a toxin made by Pseudomonas aeruginosa that blocks translation by inhibiting the activity of elongation factor 2a (reviewed in 48, 49). As with TLR stimulation, ExoA treatment alone was insufficient to induce IL-1α production. However, we found that treatment of BMDMs with both Pam3CSK4 and ExoA combined to induce release of IL-1α at 24h post-infection (Fig. 6A, B). ExoA appeared to have two important effects that might explain its role in IL-1α release. First, in contrast to the transient induction of IL-1α induced by TLR signaling alone, additional treatment with ExoA caused the sustained presence of intracellular IL-1α protein at 24h post-treatment, similar to what is seen in L. pneumophila infection (Fig. 6A). The sustained production of IL-1α protein was associated with a prolonged elevation of Il1a mRNA (Fig. 6C). Second, ExoA caused cell death by 24hrs post-treatment (Fig. 6D), which may explain how intracellular accumulated IL-1α is released from these macrophages. ΔflaA and Δ5ΔflaA L. pneumophila-infected macrophages, which also experience a block in host protein synthesis, show sustained transcriptional induction, release IL-1α from the cell, and undergo cell death at 24h post-infection (Fig. 6A–D). Importantly, death of L. pneumophila infected cells does not appear to depend on bacterial replication because cell death and IL-1α release still occurred when bacterial replication was curtailed by removal of thymidine from the media. We speculate that inhibition of protein synthesis may be responsible for induction of host cell death. Protein synthesis inhibition and TLR stimulation also synergized to induce elevated IL-1α production in vivo (Fig. 6E). Taken together, these findings suggest that TLR activation in concert with translation inhibition can recapitulate IL-1α production and release in response to L. pneumophila infection and that this treatment is sufficient to induce release of IL-1α.
Figure 6.
Translation inhibition in conjunction with TLR activation is sufficient to induce the production of IL-1α both in vitro and in vivo. (A–D) Wild-type B6 bone marrow derived macrophages were infected with the indicated strains of L. pneumophila (LP02) or treated with Pam3CSK4 (10µg/mL), Exotoxin A (50ng/µL) or both Pam3CSK4 and ExoA. Samples were collected 4, 8, or 24 hours post-treatment. (A) Cells were lysed with RIPA buffer and intracellular IL-1α levels were determined by ELISA. (B) Extracellular IL-1α levels were determined by performing ELISA on cell supernatants. (C) Il1a transcript levels were assayed by quantitative reverse transcriptase PCR. (D) Cell cytotoxicity was determined by measuring the release of Lactate Dehydrogenase into cell supernatants and values were normalized to an untreated control and a 100% lysis control where cells were treated with 1% TritonX-100 for 30 minutes. (E) Wild-type B6 mice were treated intranasally with Pam3CSK4 (10µg/mouse), Exotoxin A (2µg/mouse) or both in 20 µL of PBS. Bronchoalveolar lavage was performed 24hrs post-infection. IL-1α levels in BAL fluid were determined by ELISA. Data are representative of three (A–E) experiments (mean ± s.d. in A-E). Pam3, Pam3CSK4. ExoA, Exotoxin A. n.d., not detectable. ns, not significant.*, p<0.05. **,p<0.01. ***,p<0.001. (Statistical analysis: Unpaired T-Test (A–D), Mann-Whitney U Test (E)).
Discussion
Legionnaires’ disease is an inflammatory pneumonia associated with a pronounced influx of neutrophils to the lung (3, 5). The recruitment of neutrophils to the lung is important for controlling bacterial burden; however, excessive neutrophil recruitment can also be detrimental to the host and may be responsible for immune pathologies associated with Legionnaires’ disease (3, 5). Thus, the host must tightly regulate the recruitment of neutrophils to the site of infection. In animal models of L. pneumophila infection, neutrophil recruitment has been shown to be important for protecting the host (6, 8, 9), yet the mechanism for this recruitment has remained unclear. A number of studies have demonstrated that MyD88 is an important host factor that protects mice from L. pneumophila infection (6, 10–13) and the IL-1R has been shown to be the critical receptor upstream of MyD88 that controls the recruitment of neutrophils to the lung in response to L. pneumophila infection (6). Indeed, it has been shown that IL-1R signaling is required in AECs to induce chemokines, such as CXCL1 and CXCL2, which then recruit neutrophils to the site of infection (6).
In our study, we identify the cytokine interleukin-1α (IL-1α) as a critical initiator of IL-1R-dependent neutrophil recruitment to the lungs of L. pneumophila-infected mice. We find that IL-1α, but not IL-1β, precedes neutrophil recruitment to the lung and we show that IL-1α is generated specifically by cells in the hematopoietic compartment (presumably infected macrophages). Given these data, we therefore propose a model by which IL-1α is produced by alveolar macrophages in response to virulent L. pneumophila and signals through the IL-1R on AECs, amplifying the original signal and generating chemokines which recruit the initial wave of neutrophils to the lung. Importantly, at timepoints later than 12hrs post-infection, IL-1α and IL-1β can both signal through the IL-1R and compensate for the loss of each other. Our data suggest that IL-1α is one of the earliest cytokines produced in response to L. pneumophila in vivo, and thus initiates the recruitment of neutrophils and the inflammatory response to L. pneumophila in vivo.
Similar to L. pneumophila, Streptococcus pneumoniae leads to a severe pneumonia associated with massive influx of neutrophils. In mouse models of S. pneumoniae infection in the lung, Il1a/I1b−/− double knockout and Il1b−/− mice are more susceptible to disease and have decreased clearance of bacteria from the lung (50). Moreover, Il1r−/− mice have increased bacterial burden in the lung and decreased neutrophil recruitment to the lung (51). Macrophage uptake of S. pneumoniae induces inflammasome activation and IL-1β release which can signal to epithelial cells to recruit neutrophils by releasing the chemokine CXCL8 (51). Studies with S. pneumoniae suggest a model whereby activated macrophages secrete IL-1β which signals through the IL-1R of AECs thus leading to the production of chemokines, which recruit neutrophils to the site of infection (50, 51). This proposed mechanism is similar to the mechanism that we propose for L. pneumophila infections, except that it appears that IL-1α, rather than IL-1β, is the dominant cytokine early during L. pneumophila infections. These studies with S. pneumoniae suggest that amplification of early responses to infection by IL-1R signaling in AECs may be a conserved immune strategy important for recruiting neutrophils in response to bacterial infections. Importantly, the role for IL-1α in S. pneumoniae infections remains unclear.
In addition to S. pneumoniae, IL-1R signaling has been shown to be important for host protection from numerous pathogens, including Listeria monocytogenes (52–54), Mycobacterium tuberculosis (36, 55–57), Pseudomonas aeruginosa (58), Staphylococcus aureus (59), Klebsiella pneumoniae (60) and Candida albicans (61). In many of these infections, the mechanism by which IL-1R provides protection is not clear, and the relative roles of IL-1α and IL-1β have not been elucidated. One study that dissected the relative roles of IL-1α and IL-1β during M. tuberculosis infection found each cytokine played essential and non-redundant roles in vivo (62). This study, along with our results showing that IL-1α is of primary importance in early responses to L. pneumophila in vivo, suggest it will be worthwhile to examine more carefully the relative contributions of IL-1α and IL-1β in mediating IL-1R-dependent responses to other pathogens as well.
The molecular mechanism leading to IL-1α production has remained elusive (28). This is in stark contrast to IL-1β production, where intensive effort over the past decade has defined the mechanisms leading to IL-1β release downstream of inflammasome activation (reviewed in 18). Our data suggest that equal attention should be paid to the mechanisms of IL-1α production. Indeed, IL-1α has been shown to be induced in response to a number of bacterial pathogens including L. pneumophila (29, 30), L. monocytogenes (63), S. aureus (64), and M. tuberculosis (57, 62); however, the molecular mechanism of IL-1α production in response to these pathogens remains unsettled. Classic studies showed that IL-1α can be cleaved by the Calpain family of calcium dependent proteases, but IL-1α does not appear to require processing to signal through the IL-1R (24, 25, 28). Some reports have suggested that IL-1α production in response to non-infectious stimuli such as toxins can involve activation of the Caspase-1 or Caspase-11 inflammasomes (24, 26, 27, 65). Additionally, a previous report suggests that at 4hrs post-infection Casp1/11−/− mice have defects in IL-1α production in response to L. pneumophila infection in vivo (27). In contrast to these reports, our data show that Casp1/11−/− mice have no defect in IL-1α production in response to L. pneumophila infection in vivo. This difference may be due to the different strains of L. pneumophila used in the two studies. Nevertheless, our results indicate that IL-1α and IL-1β can be produced via distinct but complementary pathways that provide alternative means to induce IL-1R signaling and immune defense in vivo. Given the critical importance of neutrophils in providing defense against numerous bacterial pathogens, it is perhaps to be expected that hosts would not rely on a single mechanism for activation of IL-1R signaling that could then be easily subverted or avoided.
Instead of a role for the inflammasome in IL-1α release, our data show that translation inhibition in concert with TLR stimulation is sufficient to induce IL-1α both in vitro and in vivo. Recent work from our lab and others have shown that in mice, and in C. elegans, translation inhibition can be sensed by the host and induce a number of immunological responses, including the production of pro-inflammatory cytokines (2, 30, 31, 66, 67). Previous research identified five L. pneumophila effectors that block host translation (31, 34, 45, 46). We previously found that this translation block induces a host stress response that can induce a subset of inflammatory cytokines, including IL-23 and GM-CSF (30, 31). Although the L. pneumophila Δ5 strain lacking the five effectors is partially defective in its ability to inhibit host protein synthesis (31) and is defective for IL-23 and GM-CSF induction, we confirmed here that cells infected with Δ5 L. pneumophila still experience a significant block in protein synthesis. Consistent with our finding that protein synthesis inhibition and TLR signaling is sufficient to induce IL-1α, we also find that Δ5-infected cells still produce IL-1α. In fact, even after identifying two novel bacterial effectors that block host translation, and generating an L. pneumophila mutant (Δ7) that lacks these effectors in addition to the original five effectors, we were still unable to abolish the Dot/Icm-dependent ability of L. pneumophila to inhibit protein synthesis and induce IL-1α. We propose several hypotheses to explain these results. First, there may be additional bacterial effectors in L. pneumophila that are not in our library of cloned effectors. Given that L. pneumophila is a generalist and has a multitude of natural hosts (68), it is possible that there is substantial additional redundancy encoded in the L. pneumophila genome. A second possibility is that there may not be a specific L. pneumophila effector that targets the host protein synthesis machinery; instead, the blockade of protein synthesis we observe may be the result of a host response to the infection process itself. Indeed, translation inhibition has long been recognized as a protective response during viral infections (69), and it is now evident that numerous bacterial infections can elicit host stress pathways that affect protein synthesis, for example via phosphorylation of eukaryotic initiation factor 2α (eIF2α) (69). Lastly, it is possible that the ability of L. pneumophila to induce IL-1α is unrelated to protein synthesis inhibition. We tend not to favor this latter possibility because we found that inhibition of protein synthesis in conjunction with TLR signaling was sufficient to induce IL-1α, and moreover, it is clear that L. pneumophila infection results in both TLR signaling and inhibition of protein synthesis. Thus, while the mechanism of IL-1α production continues to elude the field, it seems likely that translation inhibition is at least one mechanism for IL-1α induction, even if other parallel mechanisms might also exist.
Together, our data show that IL-1α is a major cytokine responsible for the early recruitment of neutrophils to the lung in response to L. pneumophila infection from 0–12 hours post-infection. We propose that a dominant role for IL-1α in protection against microbial infection may hold true for other pathogens, depending on the stage and mode of infection. Although much recent work has focused on the mechanisms of IL-1β production, our work suggests that IL-1α signaling can be as important, or indeed more important, than IL-1β signaling in vivo. Indeed, it is probably evolutionarily advantageous for hosts to encode multiple parallel pathways to induce IL-1R signaling, given the critical role that the IL-1R appears to play in orchestrating neutrophil recruitment and other immune responses in vivo.
Supplementary Material
Acknowledgements
We thank members of the Vance and Barton Labs for discussions and Norver J. Trinidad for technical assistance. We express our gratitude to April E. Price and Meghan A. Koch for experimental advice, discussion and technical assistance. We are grateful to Ralph Isberg for the L. pneumophila effector library.
Grant Support: Research in the Vance Lab is supported by Investigatorships from the Burroughs Wellcome Fund and the Cancer Research Institute and by NIH grants AI063302, AI075039, and AI080749. K.C.B. is a fellow of the National Science Foundation Graduate Research Fellowship Program.
Abbreviations used in this article
- T4SS
Type-IV Secretion System
- IL-1R
Interleukin-1 Receptor Type I
- AECs
airway epithelial cells
- BAL
bronchoalveolar lavage
- TKO
triple knockout
- BMDM
bone marrow derived macrophage
- ExoA
Exotoxin A
References
- 1.Luo ZQ. Legionella secreted effectors and innate immune responses. Cellular microbiology. 2012;14:19–27. doi: 10.1111/j.1462-5822.2011.01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fontana MF, Vance RE. Two signal models in innate immunity. Immunol Rev. 2011;243:26–39. doi: 10.1111/j.1600-065X.2011.01037.x. [DOI] [PubMed] [Google Scholar]
- 3.Winn WC, Jr, Myerowitz RL. The pathology of the Legionella pneumonias. A review of 74 cases and the literature. Human pathology. 1981;12:401–422. doi: 10.1016/s0046-8177(81)80021-4. [DOI] [PubMed] [Google Scholar]
- 4.Trisolini R, Lazzari Agli L, Cancellieri A, Procaccio L, Candoli P, Alifano M, Patelli M. Bronchoalveolar lavage findings in severe community-acquired pneumonia due to Legionella pneumophila serogroup 1. Respir Med. 2004;98:1222–1226. doi: 10.1016/j.rmed.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Yu H, Higa F, Koide M, Haranaga S, Yara S, Tateyama M, Li H, Fujita J. Lung abscess caused by Legionella species: implication of the immune status of hosts. Intern Med. 2009;48:1997–2002. doi: 10.2169/internalmedicine.48.2647. [DOI] [PubMed] [Google Scholar]
- 6.LeibundGut-Landmann S, Weidner K, Hilbi H, Oxenius A. Nonhematopoietic cells are key players in innate control of bacterial airway infection. J Immunol. 2011;186:3130–3137. doi: 10.4049/jimmunol.1003565. [DOI] [PubMed] [Google Scholar]
- 7.Sporri R, Joller N, Hilbi H, Oxenius A. A novel role for neutrophils as critical activators of NK cells. J Immunol. 2008;181:7121–7130. doi: 10.4049/jimmunol.181.10.7121. [DOI] [PubMed] [Google Scholar]
- 8.Tateda K, Moore TA, Newstead MW, Tsai WC, Zeng X, Deng JC, Chen G, Reddy R, Yamaguchi K, Standiford TJ. Chemokine-dependent neutrophil recruitment in a murine model of Legionella pneumonia: potential role of neutrophils as immunoregulatory cells. Infection and immunity. 2001;69:2017–2024. doi: 10.1128/IAI.69.4.2017-2024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tateda K, Moore TA, Deng JC, Newstead MW, Zeng X, Matsukawa A, Swanson MS, Yamaguchi K, Standiford TJ. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J Immunol. 2001;166:3355–3361. doi: 10.4049/jimmunol.166.5.3355. [DOI] [PubMed] [Google Scholar]
- 10.Archer KA, Alexopoulou L, Flavell RA, Roy CR. Multiple MyD88-dependent responses contribute to pulmonary clearance of Legionella pneumophila. Cellular microbiology. 2009;11:21–36. doi: 10.1111/j.1462-5822.2008.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Archer KA, Roy CR. MyD88-dependent responses involving toll-like receptor 2 are important for protection and clearance of Legionella pneumophila in a mouse model of Legionnaires' disease. Infection and immunity. 2006;74:3325–3333. doi: 10.1128/IAI.02049-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Archer KA, Ader F, Kobayashi KS, Flavell RA, Roy CR. Cooperation between multiple microbial pattern recognition systems is important for host protection against the intracellular pathogen Legionella pneumophila. Infection and immunity. 2010;78:2477–2487. doi: 10.1128/IAI.00243-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawn TR, Smith KD, Aderem A, Skerrett SJ. Myeloid differentiation primary response gene (88)- and toll-like receptor 2-deficient mice are susceptible to infection with aerosolized Legionella pneumophila. The Journal of infectious diseases. 2006;193:1693–1702. doi: 10.1086/504525. [DOI] [PubMed] [Google Scholar]
- 14.Fuse ET, Tateda K, Kikuchi Y, Matsumoto T, Gondaira F, Azuma A, Kudoh S, Standiford TJ, Yamaguchi K. Role of Toll-like receptor 2 in recognition of Legionella pneumophila in a murine pneumonia model. J Med Microbiol. 2007;56:305–312. doi: 10.1099/jmm.0.46913-0. [DOI] [PubMed] [Google Scholar]
- 15.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 16.Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T, Cannizzaro LA, et al. Molecular cloning of the interleukin-1 beta converting enzyme. Science. 1992;256:97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- 17.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 18.von Moltke J, Ayres JS, Kofoed EM, Chavarria-Smith J, Vance RE. Recognition of Bacteria by Inflammasomes. Annu Rev Immunol. 2012 doi: 10.1146/annurev-immunol-032712-095944. [DOI] [PubMed] [Google Scholar]
- 19.Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, Dunipace EA, Henry T, Sun YH, Cado D, Dietrich WF, Monack DM, Tsolis RM, Vance RE. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nature immunology. 2008;9:1171–1178. doi: 10.1038/ni.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozoren N, Brady G, Meshinchi S, Jagirdar R, Gewirtz A, Akira S, Nunez G. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. The Journal of biological chemistry. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 21.Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS pathogens. 2006;2:e18. doi: 10.1371/journal.ppat.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zamboni DS, Kobayashi KS, Kohlsdorf T, Ogura Y, Long EM, Vance RE, Kuida K, Mariathasan S, Dixit VM, Flavell RA, Dietrich WF, Roy CR. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nature immunology. 2006;7:318–325. doi: 10.1038/ni1305. [DOI] [PubMed] [Google Scholar]
- 23.Molofsky AB, Byrne BG, Whitfield NN, Madigan CA, Fuse ET, Tateda K, Swanson MS. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. The Journal of experimental medicine. 2006;203:1093–1104. doi: 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gross O, Yazdi AS, Thomas CJ, Masin M, Heinz LX, Guarda G, Quadroni M, Drexler SK, Tschopp J. Inflammasome activators induce interleukin-1alpha secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity. 2012;36:388–400. doi: 10.1016/j.immuni.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Mosley B, Urdal DL, Prickett KS, Larsen A, Cosman D, Conlon PJ, Gillis S, Dower SK. The interleukin-1 receptor binds the human interleukin-1 alpha precursor but not the interleukin-1 beta precursor. The Journal of biological chemistry. 1987;262:2941–2944. [PubMed] [Google Scholar]
- 26.Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 27.Fettelschoss A, Kistowska M, LeibundGut-Landmann S, Beer HD, Johansen P, Senti G, Contassot E, Bachmann MF, French LE, Oxenius A, Kundig TM. Inflammasome activation and IL-1beta target IL-1alpha for secretion as opposed to surface expression. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:18055–18060. doi: 10.1073/pnas.1109176108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 29.Shin S, Case CL, Archer KA, Nogueira CV, Kobayashi KS, Flavell RA, Roy CR, Zamboni DS. Type IV secretion-dependent activation of host MAP kinases induces an increased proinflammatory cytokine response to Legionella pneumophila. PLoS pathogens. 2008;4 doi: 10.1371/journal.ppat.1000220. e1000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fontana MF, Shin S, Vance RE. Activation of host mitogen-activated protein kinases by secreted Legionella pneumophila effectors that inhibit host protein translation. Infection and immunity. 2012;80:3570–3575. doi: 10.1128/IAI.00557-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fontana MF, Banga S, Barry KC, Shen X, Tan Y, Luo ZQ, Vance RE. Secreted bacterial effectors that inhibit host protein synthesis are critical for induction of the innate immune response to virulent Legionella pneumophila. PLoS pathogens. 2011;7 doi: 10.1371/journal.ppat.1001289. e1001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 33.Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. The Journal of experimental medicine. 1998;187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen X, Banga S, Liu Y, Xu L, Gao P, Shamovsky I, Nudler E, Luo ZQ. Targeting eEF1A by a Legionella pneumophila effector leads to inhibition of protein synthesis and induction of host stress response. Cellular microbiology. 2009;11:911–926. doi: 10.1111/j.1462-5822.2009.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berger KH, Merriam JJ, Isberg RR. Altered intracellular targeting properties associated with mutations in the Legionella pneumophila dotA gene. Molecular microbiology. 1994;14:809–822. doi: 10.1111/j.1365-2958.1994.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 36.Zuckman DM, Hung JB, Roy CR. Pore-forming activity is not sufficient for Legionella pneumophila phagosome trafficking and intracellular growth. Molecular microbiology. 1999;32:990–1001. doi: 10.1046/j.1365-2958.1999.01410.x. [DOI] [PubMed] [Google Scholar]
- 37.Decker T, Lohmann-Matthes ML. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. Journal of immunological methods. 1988;115:61–69. doi: 10.1016/0022-1759(88)90310-9. [DOI] [PubMed] [Google Scholar]
- 38.Losick VP, Haenssler E, Moy MY, Isberg RR. LnaB: a Legionella pneumophila activator of NF-kappaB. Cellular microbiology. 2010;12:1083–1097. doi: 10.1111/j.1462-5822.2010.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monroe KM, McWhirter SM, Vance RE. Identification of host cytosolic sensors and bacterial factors regulating the type I interferon response to Legionella pneumophila. PLoS pathogens. 2009;5 doi: 10.1371/journal.ppat.1000665. e1000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karp CL. Unstressing intemperate models: how cold stress undermines mouse modeling. The Journal of experimental medicine. 2012;209:1069–1074. doi: 10.1084/jem.20120988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, Dixit VM, Monack DM. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature. 2012;490:288–291. doi: 10.1038/nature11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nature immunology. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karmakar M, Sun Y, Hise AG, Rietsch A, Pearlman E. Cutting Edge: IL-1beta Processing during Pseudomonas aeruginosa Infection Is Mediated by Neutrophil Serine Proteases and Is Independent of NLRC4 and Caspase-1. J Immunol. 2012;189:4231–4235. doi: 10.4049/jimmunol.1201447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guma M, Ronacher L, Liu-Bryan R, Takai S, Karin M, Corr M. Caspase 1-independent activation of interleukin-1beta in neutrophil-predominant inflammation. Arthritis and rheumatism. 2009;60:3642–3650. doi: 10.1002/art.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belyi Y, Tabakova I, Stahl M, Aktories K. Lgt: a family of cytotoxic glucosyltransferases produced by Legionella pneumophila. Journal of bacteriology. 2008;190:3026–3035. doi: 10.1128/JB.01798-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belyi Y, Niggeweg R, Opitz B, Vogelsgesang M, Hippenstiel S, Wilm M, Aktories K. Legionella pneumophila glucosyltransferase inhibits host elongation factor 1A. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16953–16958. doi: 10.1073/pnas.0601562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chien M, Morozova I, Shi S, Sheng H, Chen J, Gomez SM, Asamani G, Hill K, Nuara J, Feder M, Rineer J, Greenberg JJ, Steshenko V, Park SH, Zhao B, Teplitskaya E, Edwards JR, Pampou S, Georghiou A, Chou IC, Iannuccilli W, Ulz ME, Kim DH, Geringer-Sameth A, Goldsberry C, Morozov P, Fischer SG, Segal G, Qu X, Rzhetsky A, Zhang P, Cayanis E, De Jong PJ, Ju J, Kalachikov S, Shuman HA, Russo JJ. The genomic sequence of the accidental pathogen Legionella pneumophila. Science. 2004;305:1966–1968. doi: 10.1126/science.1099776. [DOI] [PubMed] [Google Scholar]
- 48.Deng Q, Barbieri JT. Molecular mechanisms of the cytotoxicity of ADP-ribosylating toxins. Annual review of microbiology. 2008;62:271–288. doi: 10.1146/annurev.micro.62.081307.162848. [DOI] [PubMed] [Google Scholar]
- 49.Yates SP, Jorgensen R, Andersen GR, Merrill AR. Stealth and mimicry by deadly bacterial toxins. Trends in biochemical sciences. 2006;31:123–133. doi: 10.1016/j.tibs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 50.Kafka D, Ling E, Feldman G, Benharroch D, Voronov E, Givon-Lavi N, Iwakura Y, Dagan R, Apte RN, Mizrachi-Nebenzahl Y. Contribution of IL-1 to resistance to Streptococcus pneumoniae infection. International immunology. 2008;20:1139–1146. doi: 10.1093/intimm/dxn071. [DOI] [PubMed] [Google Scholar]
- 51.Marriott HM, Gascoyne KA, Gowda R, Geary I, Nicklin MJ, Iannelli F, Pozzi G, Mitchell TJ, Whyte MK, Sabroe I, Dockrell DH. Interleukin-1beta regulates CXCL8 release and influences disease outcome in response to Streptococcus pneumoniae, defining intercellular cooperation between pulmonary epithelial cells and macrophages. Infection and immunity. 2012;80:1140–1149. doi: 10.1128/IAI.05697-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rogers HW, Sheehan KC, Brunt LM, Dower SK, Unanue ER, Schreiber RD. Interleukin 1 participates in the development of anti-Listeria responses in normal and SCID mice. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:1011–1015. doi: 10.1073/pnas.89.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Havell EA, Moldawer LL, Helfgott D, Kilian PL, Sehgal PB. Type I IL-1 receptor blockade exacerbates murine listeriosis. J Immunol. 1992;148:1486–1492. [PubMed] [Google Scholar]
- 54.Labow M, Shuster D, Zetterstrom M, Nunes P, Terry R, Cullinan EB, Bartfai T, Solorzano C, Moldawer LL, Chizzonite R, McIntyre KW. Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J Immunol. 1997;159:2452–2461. [PubMed] [Google Scholar]
- 55.Guler R, Parihar SP, Spohn G, Johansen P, Brombacher F, Bachmann MF. Blocking IL-1alpha but not IL-1beta increases susceptibility to chronic Mycobacterium tuberculosis infection in mice. Vaccine. 2011;29:1339–1346. doi: 10.1016/j.vaccine.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 56.Juffermans NP, Florquin S, Camoglio L, Verbon A, Kolk AH, Speelman P, van Deventer SJ, van Der Poll T. Interleukin-1 signaling is essential for host defense during murine pulmonary tuberculosis. The Journal of infectious diseases. 2000;182:902–908. doi: 10.1086/315771. [DOI] [PubMed] [Google Scholar]
- 57.Mayer-Barber KD, Barber DL, Shenderov K, White SD, Wilson MS, Cheever A, Kugler D, Hieny S, Caspar P, Nunez G, Schlueter D, Flavell RA, Sutterwala FS, Sher A. Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol. 2010;184:3326–3330. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mijares LA, Wangdi T, Sokol C, Homer R, Medzhitov R, Kazmierczak BI. Airway epithelial MyD88 restores control of Pseudomonas aeruginosa murine infection via an IL-1-dependent pathway. J Immunol. 2011;186:7080–7088. doi: 10.4049/jimmunol.1003687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller LS, Cho JS. Immunity against Staphylococcus aureus cutaneous infections. Nature reviews Immunology. 2011;11:505–518. doi: 10.1038/nri3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cai S, Batra S, Wakamatsu N, Pacher P, Jeyaseelan S. NLRC4 inflammasome-mediated production of IL-1beta modulates mucosal immunity in the lung against gram-negative bacterial infection. J Immunol. 2012;188:5623–5635. doi: 10.4049/jimmunol.1200195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bellocchio S, Montagnoli C, Bozza S, Gaziano R, Rossi G, Mambula SS, Vecchi A, Mantovani A, Levitz SM, Romani L. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J Immunol. 2004;172:3059–3069. doi: 10.4049/jimmunol.172.5.3059. [DOI] [PubMed] [Google Scholar]
- 62.Mayer-Barber KD, Andrade BB, Barber DL, Hieny S, Feng CG, Caspar P, Oland S, Gordon S, Sher A. Innate and adaptive interferons suppress IL-1alpha and IL-1beta production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity. 2011;35:1023–1034. doi: 10.1016/j.immuni.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dewamitta SR, Nomura T, Kawamura I, Hara H, Tsuchiya K, Kurenuma T, Shen Y, Daim S, Yamamoto T, Qu H, Sakai S, Xu Y, Mitsuyama M. Listeriolysin O-dependent bacterial entry into the cytoplasm is required for calpain activation and interleukin-1 alpha secretion in macrophages infected with Listeria monocytogenes. Infection and immunity. 2010;78:1884–1894. doi: 10.1128/IAI.01143-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olaru F, Jensen LE. Staphylococcus aureus stimulates neutrophil targeting chemokine expression in keratinocytes through an autocrine IL-1alpha signaling loop. The Journal of investigative dermatology. 2010;130:1866–1876. doi: 10.1038/jid.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng Y, Humphry M, Maguire JJ, Bennett MR, Clarke MC. Intracellular interleukin-1 receptor 2 binding prevents cleavage and activity of interleukin-1alpha, controlling necrosis-induced sterile inflammation. Immunity. 2013;38:285–295. doi: 10.1016/j.immuni.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dunbar TL, Yan Z, Balla KM, Smelkinson MG, Troemel ER. C. elegans detects pathogen-induced translational inhibition to activate immune signaling. Cell host & microbe. 2012;11:375–386. doi: 10.1016/j.chom.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McEwan DL, Kirienko NV, Ausubel FM. Host translational inhibition by Pseudomonas aeruginosa Exotoxin A Triggers an immune response in Caenorhabditis elegans. Cell host & microbe. 2012;11:364–374. doi: 10.1016/j.chom.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O'Connor TJ, Adepoju Y, Boyd D, Isberg RR. Minimization of the Legionella pneumophila genome reveals chromosomal regions involved in host range expansion. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14733–14740. doi: 10.1073/pnas.1111678108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohr I, Sonenberg N. Host translation at the nexus of infection and immunity. Cell host & microbe. 2012;12:470–483. doi: 10.1016/j.chom.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.