Abstract
Objectives
To identify risk factors for failure of outpatient antibiotic therapy (OPAT) in infective endocarditis (IE).
Patients and methods
We identified IE cases managed at a single centre over 12 years from a prospectively maintained database. ‘OPAT failure’ was defined as unplanned readmission or antibiotic switch due to adverse drug reaction or antibiotic resistance. We analysed patient and disease-related risk factors for OPAT failure by univariate and multivariate logistic regression. We also retrospectively collected follow-up data on adverse disease outcome (defined as IE-related death or relapse) and performed Kaplan–Meier survival analysis up to 36 months following OPAT.
Results
We identified 80 episodes of OPAT in IE. Failure occurred in 25/80 episodes (31.3%). On multivariate analysis, cardiac or renal failure [pooled OR 7.39 (95% CI 1.84–29.66), P = 0.005] and teicoplanin therapy [OR 8.69 (95% CI 2.01–37.47), P = 0.004] were independently associated with increased OPAT failure. OPAT failure with teicoplanin occurred despite therapeutic plasma levels. OPAT failure predicted adverse disease outcome up to 36 months (P = 0.016 log-rank test).
Conclusions
These data caution against selecting patients with endocarditis for OPAT in the presence of cardiac or renal failure and suggest teicoplanin therapy may be associated with suboptimal OPAT outcomes. Alternative regimens to teicoplanin in the OPAT setting should be further investigated.
Keywords: glycopeptides, ceftriaxone, prosthetic valve endocarditis, native valve endocarditis, outcomes, teicoplanin
Introduction
Infective endocarditis (IE) is a serious and potentially life-threatening infection that requires prolonged intravenous therapy.1 Careful patient selection is necessary to identify those suitable for outpatient parenteral antibiotic therapy (OPAT).1,2 While avoiding OPAT entirely in patients with high-risk features [e.g. left-sided valve disease, staphylococcal or enterococcal infection, and prosthetic valve endocarditis (PVE)] has been recommended,1 several observational studies have reported treating high-risk patients with OPAT.3–7 Although these studies conclude that OPAT can be safely administered if patients are carefully selected, there are limited data on the factors associated with OPAT failure4 and it is unclear whether features associated with adverse outcome in inpatients1 also apply to the selected OPAT-managed population. We analysed 12 years of prospectively acquired clinical data on OPAT-managed IE in a single centre to identify factors associated with OPAT failure.
Methods
Setting
The Glasgow OPAT programme and its prospective database have been described previously.8 Patient selection and individualized OPAT management plans were the responsibility of the treating OPAT physician (R. A. S.).8 Briefly, patients received up to 6 weeks of therapy for native valve endocarditis and extended therapy for PVE. Ceftriaxone was used first-line for susceptible streptococci and Staphylococcus aureus (in combination with a second oral antibiotic), while teicoplanin plus a second oral antibiotic was used first-line for infections with coagulase-negative Staphylococcus, enterococci and methicillin-resistant S. aureus (MRSA), or those with a β-lactam antibiotic allergy. Teicoplanin dosing followed a validated protocol9 with target trough levels ≥20 mg/L.10
Data collection
The local research ethics committee granted a waiver for this study and the Caldicott guardian approved contact with general practitioners (GPs). IE cases were defined according to modified Duke criteria.11 Clinical data on demographics, comorbidities, organism, antibiotic regimen and outcome were extracted from the prospective database.8 In parallel, raw data were retrospectively reviewed (OPAT pro formas, case notes, electronic clinical and laboratory databases and death certificates). In cases without evidence of ongoing specialist review, GPs were telephoned by a member of the study team (D. A. B.) to identify outcomes of interest.
Outcomes
Failure to complete the initial OPAT regimen (OPAT failure) included any of: (i) unplanned readmission or surgery during OPAT; (ii) adverse drug reaction leading to switch/readmission; and (iii) development of antibiotic resistance.
Adverse disease outcome (ADO) was assessed for each first patient-episode, defined as IE-related death at any time or suspected relapse at the same site after OPAT completion/failure. Episodes where OPAT treatment was not intended to be curative (i.e. PVE where definitive surgery was contraindicated/refused) or where cause of death could not be determined were excluded from ADO analysis.
Statistical analysis
Continuous data were compared with the Mann–Whitney test. ORs for OPAT failure were calculated for known risk factors selected before data collection from inpatient studies. A multivariate logistic regression model was constructed with backward stepwise selection of all variables with P < 0.05 on univariate analysis. A Kaplan–Meier survival analysis of the influence of OPAT failure on ADO, censored at the date of the last specialist review, date of event or 36 months from OPAT discharge (whichever was sooner), was also performed. Two-tailed alpha <0.05 was considered significant. Analysis was done using MedCalc® version 11.6.1.0.
Results
Inclusion
Ninety-seven episodes of OPAT in IE were identified from August 2000 to September 2012. Sixteen of 97 episodes were classified ‘not IE’ by modified Duke criteria and 1 of 97 did not require parenteral treatment (Q fever). Therefore 80 episodes in 77 patients were included in the analysis: 67 were ‘definite’ or ‘possible’ IE, 4 were cardiac-device-related IE and 9 had incomplete information to fully apply modified Duke criteria, but were included based on strong supporting clinical and/or microbiological evidence for IE.2 The median duration of OPAT was 28 days (IQR 20–38 days) and all patients had a prior period of inpatient management (median 22 days, IQR 14–30 days).
Characteristics
High-risk features were common in the cohort: 65/80 (81.3%) episodes were left-sided and 26/80 (32.5%) involved a prosthetic valve. Staphylococci or enterococci were the cause of 30/80 episodes (37.5%) and planned surgery was done in 22/80 (27.5%). Septic emboli occurred pre-OPAT in 13/80 episodes (16.3%). An established prior medical history of comorbidity (including chronic kidney disease, ischaemic heart disease, chronic heart failure, adult congenital heart disease, active cancer and diabetes) was present prior to OPAT in 42/77 patients (54.5%; see Table 1).
Table 1.
Risk factors for OPAT failure
| Factor | Failed, n = 25 (31.3%) | Completed, n = 55 (68.7%) | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|---|---|
| OR | CI | P | OR | CI | P | |||
| Comorbidity (CHF or CKD) | 14 (63.6%) | 8 (36.4%) | 7.48 | 2.52–22.21 | <0.001 | 7.39 | 1.84–29.66 | 0.005 |
| Teicoplanin | 12 (67.7%) | 6 (33.3%) | 7.54 | 2.37–23.93 | <0.001 | 8.69 | 2.01–37.47 | 0.004 |
| Specialist referral | 17 (25.8%) | 49 (74.2%) | 0.26 | 0.08–0.86 | 0.027 | 0.25 | 0.06–1.11 | 0.068 |
| Prosthetic valve | 14 (53.8%) | 12 (46.2%) | 4.90 | 1.74–13.79 | 0.003 | — | — | — |
| Median delay to intravenous start, per day (IQR) | 4 (0.5–13) | 1 (0–3) | 1.07 | 1.01–1.14 | 0.026 | — | — | — |
| Glycopeptide-indicated organism | 13 (46.4%) | 15 (53.6%) | 2.89 | 1.08–7.73 | 0.035 | — | — | — |
| Intravascular device | 8 (57.1%) | 6 (42.9%) | 3.56 | 1.08–11.68 | 0.037 | — | — | — |
| Age, per yeara, median (IQR) | 67.1 (55.3–72.2) | 59.1 (51.0–68.0) | 1.01 | 0.98–1.05 | 0.466 | — | — | — |
| Male | 15 (25.4%) | 44 (74.6%) | 0.38 | 0.13–1.06 | 0.064 | — | — | — |
| Previous IE | 4 (40.0%) | 6 (60.0%) | 1.56 | 0.40–6.01 | 0.526 | — | — | — |
| Adult congenital heart disease | 2 (28.6%) | 5 (71.4%) | 0.87 | 0.16–4.82 | 0.873 | — | — | — |
| Ischaemic heart disease | 11 (45.8%) | 13 (54.2%) | 2.54 | 0.93–6.94 | 0.069 | — | — | — |
| CKD | 7 (63.6%) | 4 (36.4%) | 4.96 | 1.30–18.95 | 0.019 | — | — | — |
| CHF | 9 (60.0%) | 6 (40.0%) | 4.59 | 1.42–14.91 | 0.011 | — | — | — |
| Aortic valve | 12 (35.3%) | 22 (64.7%) | 1.45 | 0.55–3.83 | 0.448 | — | — | — |
| Left-sided | 20 (30.8%) | 45 (69.2%) | 1.00 | 0.28–3.64 | 1.00 | — | — | — |
| Emboli | 3 (23.1%) | 10 (76.9%) | 0.64 | 0.16–2.58 | 0.533 | — | — | — |
| S. aureus | 4 (30.8%) | 9 (69.2%) | 0.97 | 0.27–3.52 | 0.9674 | — | — | — |
| Streptococcalb | 6 (17.6%) | 28 (82.4%) | 0.30 | 0.11–0.88 | 0.023 | — | — | — |
| Enterococcal | 3 (60.0%) | 2 (40.0%) | 3.61 | 0.56–23.14 | 0.175 | — | — | — |
| Median inpatient stay, per day (IQR) | 30 (21–37) | 19 (12.5–32.5) | 1.03 | 1.00–1.06 | 0.060 | — | — | — |
| Median days of inpatient antibiotics (IQR) | 26 (17–33) | 17 (12–27) | 1.02 | 0.98–1.06 | 0.314 | — | — | — |
| Surgery during this episode | 7 (31.8%) | 15 (68.2%) | 1.04 | 0.36–2.98 | 0.946 | — | — | — |
| Unable to self-administer/home administer | 13 (40.6%) | 19 (59.4%) | 2.05 | 0.78–5.37 | 0.143 | — | — | — |
| Ceftriaxonec | 8 (17.4%) | 38 (82.6%) | 0.22 | 0.08–0.66 | 0.004 | — | — | — |
| Daptomycin | 3 (27.3%) | 8 (72.7%) | 0.80 | 0.19–3.32 | 0.760 | — | — | — |
CHF, chronic heart failure; CKD, chronic kidney disease.
Percentages given are the relative percentages of each factor. Factors in bold were input into the logistic regression model with stepwise backwards selection (χ2 = 30.3, full model −2 log likelihood = 63.6, P < 0.0001); factors retained in the model (P < 0.1) are displayed in the right-hand columns. Intravascular device refers to permanent pacemakers and implantable defibrillators. Comorbidities refer to an established medical diagnosis prior to OPAT. Patients with CKD had a median glomerular filtration rate of 22 mL/min (range 5–44 mL/min). Several non-significant factors in the univariate analysis occurred in six or fewer episodes (alcohol misuse, diabetes, active cancer, cardiac-device-related endocarditis and flucloxacillin) and are not displayed for clarity. Additional microbiological causes were coagulase-negative Staphylococcus (12), culture negative (8), mixed (3), Haemophilus, Actinobacillus, Cardiobacterium, Eikinella and Kingella spp. (‘HACEK’) group (2), Rothia sp. (1), Gamella sp. (1) and unknown (1).
aP = 0.018, Mann–Whitney.
bCovariate with glycopeptide-indicated organism.
cCovariate with teicoplanin.
OPAT failure
OPAT failure occurred in 25/80 (31.3%) episodes based on the composite endpoint definition (where more than one factor could apply per episode). These included readmission (OPAT/IE related, 9; unrelated, 10; and unknown, 2), adverse drug (7) or line (3) events and resistance (2). Risk factors for OPAT failure are summarized in Table 1.
ADO
Six episodes were excluded from ADO analysis (three repeat-patient episodes, one death of undetermined cause and two episodes where curative treatment was contraindicated). Eight of 74 episodes of ADO occurred during a median of 729 days of follow-up (range 35–3838 days); 7/8 were suspected relapses, at a median of 77 days (range 35–780 days) post-OPAT, and 2 of the patients died. An additional patient died of post-operative complications 47 days after unplanned readmission for surgery. OPAT failure was significantly associated with ADO by survival analysis (P = 0.016 log-rank test; Figure 1).
Figure 1.
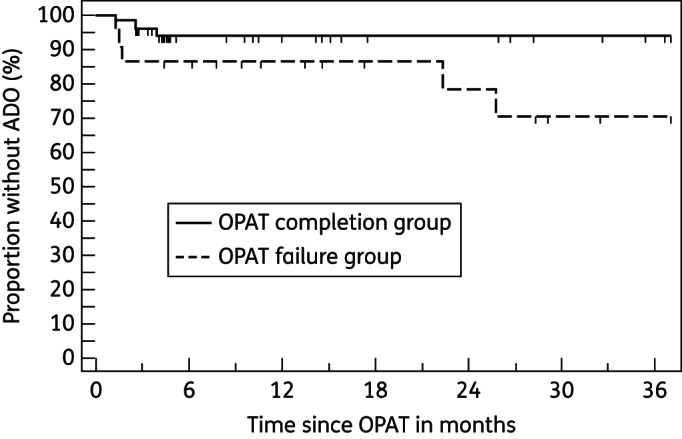
OPAT failure predicted ADO (P = 0.016 log-rank test).
Univariate analysis
Several factors were associated with the risk of OPAT failure (Table 1). An increased probability of failure was associated with comorbidities (cardiac or renal failure), presence of an intravascular device, PVE, glycopeptide-indicated organism and factors relating to the quality of pre-OPAT management (e.g. delay in starting inpatient antibiotics). Teicoplanin was associated with OPAT failure in 12/18 courses (with a total of 17 criteria for failure). Reasons for failure were readmissions [7/17 due to suspected endocarditis decompensation—clinical cardiac dysfunction or new fever—treated medically (5) or surgically (2); 4/17 non-IE related; and 1/17 unknown], adverse drug reactions leading to switch/readmission [4/17: hyperkalaemia (1); vomiting due to co-administered pristinamycin (1); acute renal dysfunction (1); and presyncope during antibiotic administration (1)] and resistance to the oral antibiotic component of the OPAT regimen (1/17)—in some cases several criteria for failure occurred in the same episode. In contrast, ceftriaxone, streptococcal IE and specialist inpatient management prior to OPAT (i.e. by infectious diseases or cardiology) were associated with OPAT completion.
Multivariate analysis
Cardiac or renal failure was independently associated with increased OPAT failure [pooled OR 7.39 (95% CI 1.84–29.66), P = 0.005] as was teicoplanin use [OR 8.69 (95% CI 2.01–37.47), P = 0.004; Table 1]. Sensitivity analysis controlling for the presence of any subtherapeutic teicoplanin concentration (<20 mg/L) did not affect the results of the multivariate analysis (data not shown). In addition, there was no significant difference in the median OPAT teicoplanin trough level in those who failed or completed OPAT [28.6 mg/L (range 16.3–40.0 mg/L) versus 34.2 mg/L (range 28.3–52.7 mg/L), P = 0.070 Mann–Whitney test].
Discussion
We devised a broad definition of ‘OPAT failure’ for patients with endocarditis in order to identify patient and treatment factors associated with failure to successfully complete a course of OPAT. This composite endpoint included unplanned readmission or surgery during OPAT, adverse drug reaction leading to a switch in antimicrobial therapy or readmission, or development of antibiotic resistance. We have previously used this approach to identify factors associated with OPAT failure for other infections,8,12 and it is supported by recently published OPAT good practice recommendations.13
Teicoplanin has been used in European OPAT programmes for several years due to its favourable pharmacokinetic properties, which permit once-daily or thrice-weekly dosing schedules.9 Concerns have been raised about the comparative efficacy of teicoplanin against vancomycin, but a recent meta-analysis showed equivalent efficacy.14 Although teicoplanin is currently recommended for inpatient or OPAT IE management,10 few data are available directly comparing glycopeptides with other treatments for IE. An open-label randomized controlled trial (RCT) of daptomycin versus standard therapy for S. aureus bacteraemia and right-sided IE suggested equivalent efficacy15 and included some OPAT patients,5 but no comparative studies have been done for other pathogens.
Teicoplanin has been associated with OPAT failure in skin and soft-tissue infections8 and with a trend towards increased failure in bone and joint infections.12 Its microbiological efficacy in complex infections is related to drug concentration,9 and the thrice-weekly dosing regimen used here has been shown to efficiently achieve therapeutic levels in the OPAT setting.9 We observed that the high rate of OPAT failure associated with teicoplanin use was independent of teicoplanin dosage and organism; however, teicoplanin MIC data were not available for all isolates. Although we cannot exclude reduced susceptibility as a potential cause of some cases of OPAT failure, our definition was not restricted to microbiological relapse or infection progression, but more broadly reflected a failure of OPAT. There was only one observed episode of microbiological relapse during OPAT in patients who received teicoplanin (the emergence of a small variant-colony Staphylococcus in a patient with PVE), although readmission with IE decompensation, which could reflect antibiotic failure, was observed in 7/18 courses. In agreement with these findings, glycopeptides were linked to increased readmissions in a retrospective OPAT IE study, although whether this association was independent of confounding factors such as organism or valve type was not tested.4
The rate of readmission we observed (26.3%) was consistent with other studies (range 10%–53%),3–7 and our data generally support the conclusion of recent European guidelines that patients with high-risk features can be managed using OPAT.2 Although PVE was associated with failure in univariate analysis, the association was lost when adjusting for other confounding factors. This was also the case for several other established high-risk features from inpatient datasets.1
There are several limitations to these data. Although prospectively recorded, they were not collected in the context of a clinical trial. Follow-up data were obtained by retrospective analysis and therefore incompleteness of data cannot be entirely excluded. This is the largest UK OPAT IE cohort involving >12 years of data, and compares favourably in size to other observational studies,3–7 yet the number of episodes was relatively small, and larger multicentre prospective studies are needed. Although we comprehensively analysed factors reported to influence outcome,1 we cannot exclude the possibility that unknown confounding factors that were not recorded may have influenced the findings. Unlike other observational studies,3–7 where the treating clinician subjectively judged clinical success, we used a conservative composite endpoint for OPAT failure that almost certainly overestimated the apparent rate of ‘failure’ in our cohort compared with other studies. However, this composite endpoint captured the important adverse outcomes associated with OPAT and predicted ADO, suggesting it is an appropriate standard by which to objectively measure OPAT performance.13
In summary, using a conservative definition of OPAT failure, we observed that patients with pre-existing cardiac and renal dysfunction were more likely to fail to complete a planned OPAT treatment course. Thus, in our experience, these comorbid conditions represent a relative contraindication to OPAT participation. Similarly, teicoplanin was associated with an increased risk of OPAT failure, despite adequate dosing and predominantly due to either IE decompensation or adverse events. Alternative regimens to teicoplanin in the OPAT setting should be further investigated.
Funding
No funding was received for the production of this report. This study was carried out as part of our routine work. C. J. A. D. is supported by a Wellcome Trust Research Training Fellowship (094449/Z/10/Z).
Transparency declarations
R. A. S. has received research funding and honoraria for consultancy and speaking at educational events from Novartis and Pfizer. All other authors: none to declare.
Author contributions
Conceived study: C. J. A. D. and R. A. S. Data collection: C. J. A. D., D. A. B., E. S., A. H. and L. S. Data analysis: D. A. B. Report writing: C. J. A. D., D. A. B. and R. A. S. The guarantor is R. A. S.
Acknowledgements
We thank Rachel Bell, Doreen Baird, Rev Raajaravi, Dr Teresa Inkster and Dr Keith Robertson for help with data collection, and Dr Claire Mackintosh for advice on data analysis. We also thank all the clinical and administrative staff working in the Glasgow OPAT Service.
References
- 1.Andrews MM, von Reyn CF. Patient selection criteria and management guidelines for outpatient parenteral antibiotic therapy for native valve infective endocarditis. Clin Infect Dis. 2001;33:203–9. doi: 10.1086/321814. [DOI] [PubMed] [Google Scholar]
- 2.Habib G, Hoen B, Tornos P, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J. 2009;30:2369–413. doi: 10.1093/eurheartj/ehp285. [DOI] [PubMed] [Google Scholar]
- 3.Partridge DG, O'Brien E, Chapman AL. Outpatient parenteral antibiotic therapy for infective endocarditis: a review of 4 years' experience at a UK centre. Postgrad Med J. 2012;88:377–81. doi: 10.1136/postgradmedj-2011-130355. [DOI] [PubMed] [Google Scholar]
- 4.Cervera C, del Rio A, Garcia L, et al. Efficacy and safety of outpatient parenteral antibiotic therapy for infective endocarditis: a ten-year prospective study. Enferm Infecc Microbiol Clin. 2011;29:587–92. doi: 10.1016/j.eimc.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Rehm S, Campion M, Katz DE, et al. Community-based outpatient parenteral antimicrobial therapy (CoPAT) for Staphylococcus aureus bacteraemia with or without infective endocarditis: analysis of the randomized trial comparing daptomycin with standard therapy. J Antimicrob Chemother. 2009;63:1034–42. doi: 10.1093/jac/dkp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larioza J, Heung L, Girard A, et al. Management of infective endocarditis in outpatients: clinical experience with outpatient parenteral antibiotic therapy. South Med J. 2009;102:575–9. doi: 10.1097/SMJ.0b013e3181a4eef2. [DOI] [PubMed] [Google Scholar]
- 7.Amodeo MR, Clulow T, Lainchbury J, et al. Outpatient intravenous treatment for infective endocarditis: safety, effectiveness and one-year outcomes. J Infect. 2009;59:387–93. doi: 10.1016/j.jinf.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Seaton RA, Sharp E, Bezlyak V, et al. Factors associated with outcome and duration of therapy in outpatient parenteral antibiotic therapy (OPAT) patients with skin and soft-tissue infections. Int J Antimicrob Agents. 2011;38:243–8. doi: 10.1016/j.ijantimicag.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Lamont E, Seaton RA, Macpherson M, et al. Development of teicoplanin dosage guidelines for patients treated within an outpatient parenteral antibiotic therapy (OPAT) programme. J Antimicrob Chemother. 2009;64:181–7. doi: 10.1093/jac/dkp147. [DOI] [PubMed] [Google Scholar]
- 10.Gould FK, Denning DW, Elliott TS, et al. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: a report of the Working Party of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother. 2012;67:269–89. doi: 10.1093/jac/dkr450. [DOI] [PubMed] [Google Scholar]
- 11.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–8. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 12.Mackintosh CL, White HA, Seaton RA. Outpatient parenteral antibiotic therapy (OPAT) for bone and joint infections: experience from a UK teaching hospital-based service. J Antimicrob Chemother. 2011;66:408–15. doi: 10.1093/jac/dkq445. [DOI] [PubMed] [Google Scholar]
- 13.Chapman AL, Seaton RA, Cooper MA, et al. Good practice recommendations for outpatient parenteral antimicrobial therapy (OPAT) in adults in the UK: a consensus statement. J Antimicrob Chemother. 2012;67:1053–62. doi: 10.1093/jac/dks003. [DOI] [PubMed] [Google Scholar]
- 14.Cavalcanti AB, Goncalves AR, Almeida CS, et al. Teicoplanin versus vancomycin for proven or suspected infection. Cochrane Database Syst Rev. 2010;issue 6:CD007022. doi: 10.1002/14651858.CD007022.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Fowler VG, Jr, Boucher HW, Corey GR, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355:653–65. doi: 10.1056/NEJMoa053783. [DOI] [PubMed] [Google Scholar]


