Abstract
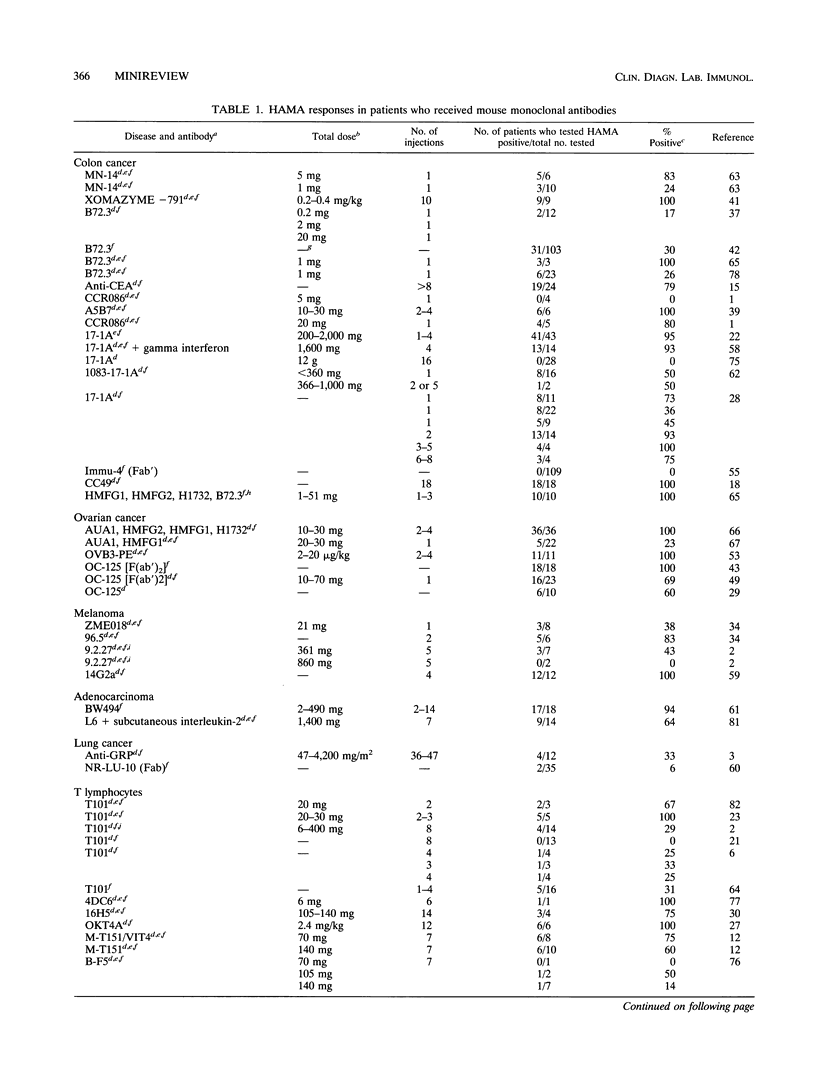
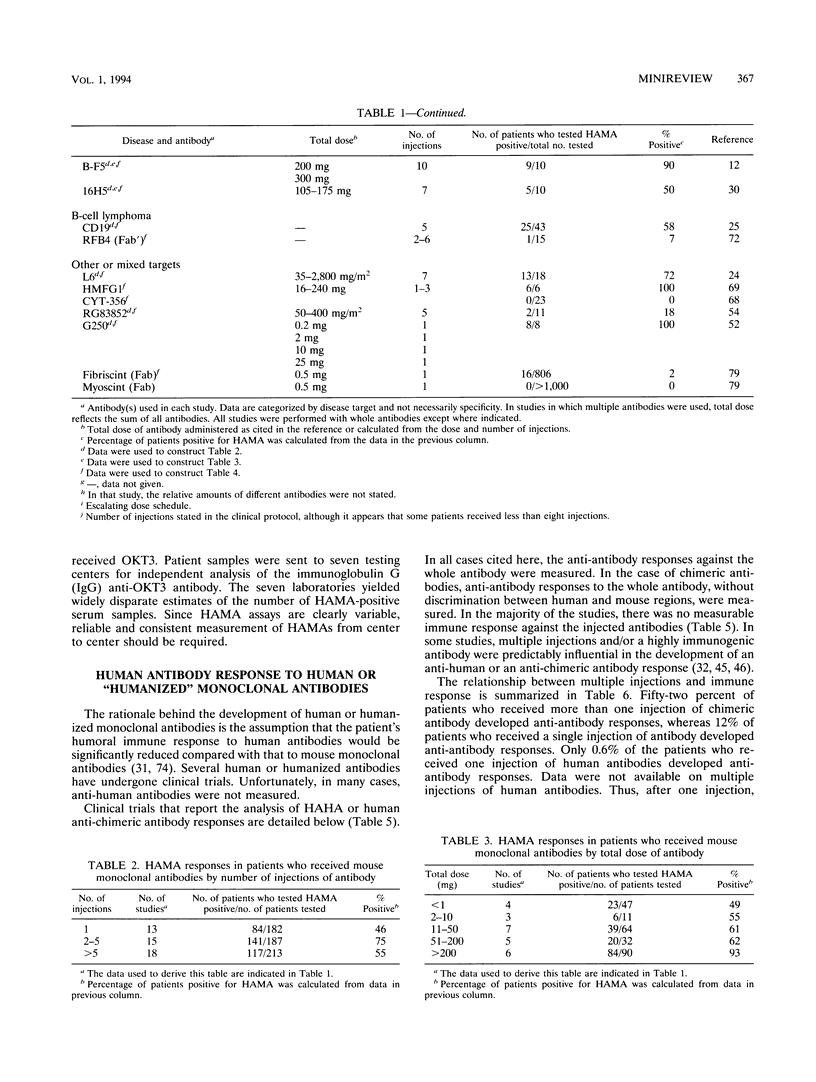
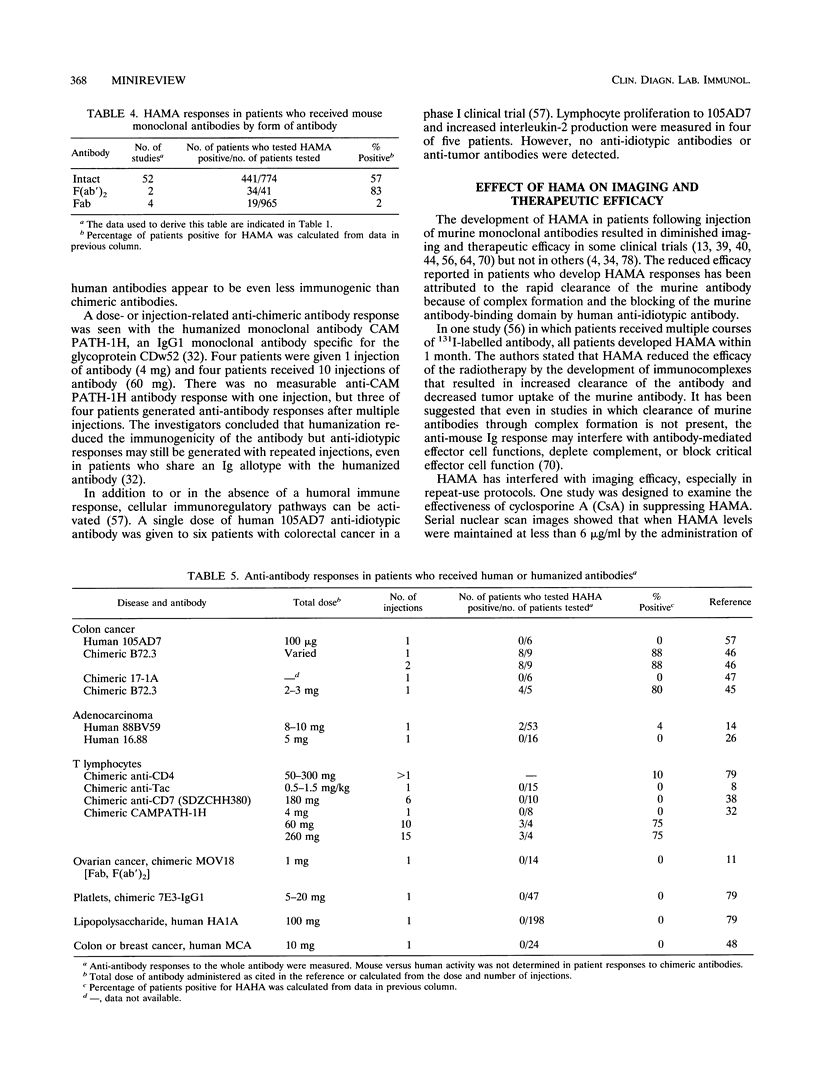
While monoclonal antibodies show promise for use in the treatment of a variety of disease states, including cancer, autoimmune disease, and allograft rejection, generation of anti-antibody responses still remains a problem. For example, 50% of the patients who receive OKT3 produce blocking antibodies that interfere with its binding to T cells, thus decreasing the therapeutic effect (51). HAMA responses have also interfered with tumor imaging (39,40) and radioimmunotherapy (56). The generation of an anti-antibody response is dependent on many factors. These include the dose of antibody, the number of injections of antibody, the immunogenicity of the antibody, the form of the antibody, and the immunocompetence of the recipient. Predictably, both the number of injections of antibody and the dosage are influential in the generation of an anti-antibody response. It is apparent that human antibodies, chimeric antibodies, and mouse Fab fragments are much less likely to induce anti-antibody responses than intact mouse monoclonal antibodies or mouse F(ab')2 fragments when one injection is administered. Injections of human or chimeric antibodies appears to reduce immunogenicity, but the probability that anti-antibody responses can still be induced on multiple injections must be considered and appropriately evaluated. Several areas demand extensive investigation to enhance the clinical utility of monoclonal antibodies. First, results of thorough clinical trials with human or chimeric antibodies need to be evaluated for the induction of anti-antibodies after multiple injections of antibodies. Second, less immunogenic forms of antibodies (Fab, Fv) need to be studied for their clinical efficacies and for their abilities to induce anti-antibody responses.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Nabi H. H., Levine G., Lamki L. M., Murray J. L., Tauxe W. N., Shah A. N., Patt Y. Z., Doerr R. J., Klein H. A., Gona J. Colorectal carcinoma metastases: detection with In-111-labeled monoclonal antibody CCR 086. Radiology. 1990 Jul;176(1):117–122. doi: 10.1148/radiology.176.1.2353080. [DOI] [PubMed] [Google Scholar]
- Avner B., Swindell L., Sharp E., Liao S. K., Ogden J. R., Avner B. P., Oldham R. K. Evaluation and clinical relevance of patient immune responses to intravenous therapy with murine monoclonal antibodies conjugated to adriamycin. Mol Biother. 1991 Mar;3(1):14–21. [PubMed] [Google Scholar]
- Bartlett R. R., Anagnostopulos H., Zielinski T., Mattar T., Schleyerbach R. Effects of leflunomide on immune responses and models of inflammation. Springer Semin Immunopathol. 1993;14(4):381–394. doi: 10.1007/BF00192310. [DOI] [PubMed] [Google Scholar]
- Bertram J. H., Gill P. S., Levine A. M., Boquiren D., Hoffman F. M., Meyer P., Mitchell M. S. Monoclonal antibody T101 in T cell malignancies: a clinical, pharmacokinetic, and immunologic correlation. Blood. 1986 Sep;68(3):752–761. [PubMed] [Google Scholar]
- Bhattacharya-Chatterjee M., Foon K. A., Köhler H. Anti-idiotype monoclonal antibodies as vaccines for human cancer. Int Rev Immunol. 1991;7(4):289–302. doi: 10.3109/08830189109114876. [DOI] [PubMed] [Google Scholar]
- Brüggemann M., Winter G., Waldmann H., Neuberger M. S. The immunogenicity of chimeric antibodies. J Exp Med. 1989 Dec 1;170(6):2153–2157. doi: 10.1084/jem.170.6.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester G. R., Horneff G., Emmrich F. Management of early inflammatory arthritis. Intervention with immunomodulatory agents: monoclonal antibody therapy. Baillieres Clin Rheumatol. 1992 Jun;6(2):415–434. doi: 10.1016/s0950-3579(05)80183-9. [DOI] [PubMed] [Google Scholar]
- Carrasquillo J. A., Krohn K. A., Beaumier P., McGuffin R. W., Brown J. P., Hellström K. E., Hellström I., Larson S. M. Diagnosis of and therapy for solid tumors with radiolabeled antibodies and immune fragments. Cancer Treat Rep. 1984 Jan;68(1):317–328. [PubMed] [Google Scholar]
- De Jager R., Abdel-Nabi H., Serafini A., Pecking A., Klein J. L., Hanna M. G., Jr Current status of cancer immunodetection with radiolabeled human monoclonal antibodies. Semin Nucl Med. 1993 Apr;23(2):165–179. doi: 10.1016/s0001-2998(05)80096-0. [DOI] [PubMed] [Google Scholar]
- Distasio J. A., Cheung N. K. Current therapies using monoclonal antibodies: I. Organ transplantation, bacterial sepsis, bone marrow transplantation. Compr Ther. 1992 Apr;18(4):33–39. [PubMed] [Google Scholar]
- Eugui E. M., Allison A. C. Immunosuppressive activity of mycophenolate mofetil. Ann N Y Acad Sci. 1993 Jun 23;685:309–329. doi: 10.1111/j.1749-6632.1993.tb35881.x. [DOI] [PubMed] [Google Scholar]
- Foon K. A., Schroff R. W., Bunn P. A., Mayer D., Abrams P. G., Fer M., Ochs J., Bottino G. C., Sherwin S. A., Carlo D. J. Effects of monoclonal antibody therapy in patients with chronic lymphocytic leukemia. Blood. 1984 Nov;64(5):1085–1093. [PubMed] [Google Scholar]
- Goldman-Leikin R. E., Kaplan E. H., Zimmer A. M., Kazikiewicz J., Manzel L. J., Rosen S. T. Long-term persistence of human anti-murine antibody responses following radioimmunodetection and radioimmunotherapy of cutaneous T-cell lymphoma patients using 131I-T101. Exp Hematol. 1988 Nov;16(10):861–864. [PubMed] [Google Scholar]
- Goodman G. E., Hellström I., Brodzinsky L., Nicaise C., Kulander B., Hummel D., Hellström K. E. Phase I trial of murine monoclonal antibody L6 in breast, colon, ovarian, and lung cancer. J Clin Oncol. 1990 Jun;8(6):1083–1092. doi: 10.1200/JCO.1990.8.6.1083. [DOI] [PubMed] [Google Scholar]
- Henell K. R., Cheever J. M., Kimball J. A., Lye W. C., Munar M. Y., Misiti J., Vitow C., Norman D. J. OKT4A (a murine IgG2a anti-CD4 monoclonal antibody) in human organ transplantation: pharmacokinetics and peripheral pharmacodynamics. Transplant Proc. 1993 Feb;25(1 Pt 1):800–801. [PubMed] [Google Scholar]
- Herlyn D., Lubeck M., Sears H., Koprowski H. Specific detection of anti-idiotypic immune responses in cancer patients treated with murine monoclonal antibody. J Immunol Methods. 1985 Dec 17;85(1):27–38. doi: 10.1016/0022-1759(85)90271-6. [DOI] [PubMed] [Google Scholar]
- Horneff G., Winkler T., Kalden J. R., Emmrich F., Burmester G. R. Human anti-mouse antibody response induced by anti-CD4 monoclonal antibody therapy in patients with rheumatoid arthritis. Clin Immunol Immunopathol. 1991 Apr;59(1):89–103. doi: 10.1016/0090-1229(91)90084-n. [DOI] [PubMed] [Google Scholar]
- Isaacs J. D. The antiglobulin response to therapeutic antibodies. Semin Immunol. 1990 Nov;2(6):449–456. [PubMed] [Google Scholar]
- Isaacs J. D., Watts R. A., Hazleman B. L., Hale G., Keogan M. T., Cobbold S. P., Waldmann H. Humanised monoclonal antibody therapy for rheumatoid arthritis. Lancet. 1992 Sep 26;340(8822):748–752. doi: 10.1016/0140-6736(92)92294-p. [DOI] [PubMed] [Google Scholar]
- Jaffe R. M., Alderson P. O., Seldin D. W., Esser P. D., Bartholomew R. M., Hyman G. A. Evaluation of metastatic melanoma with planar and SPECT scintigraphy using ZME 018 and 96.5 radiolabeled monoclonal antibodies. Cancer Detect Prev. 1988;12(1-6):303–312. [PubMed] [Google Scholar]
- Jaffee B. D., Jones E. A., Loveless S. E., Chen S. F. The unique immunosuppressive activity of brequinar sodium. Transplant Proc. 1993 Jun;25(3 Suppl 2):19–22. [PubMed] [Google Scholar]
- Kimball J. A., Norman D. J., Shield C. F., Schroeder T. J., Lisi P., Garovoy M., O'Connell J. B., Stuart F., McDiarmid S. V., Wall W. OKT3 antibody response study: comparative testing of human antimouse antibody. Transplant Proc. 1993 Apr;25(2 Suppl 1):74–76. [PubMed] [Google Scholar]
- Koprowski H., Herlyn D., Lubeck M., DeFreitas E., Sears H. F. Human anti-idiotype antibodies in cancer patients: Is the modulation of the immune response beneficial for the patient? Proc Natl Acad Sci U S A. 1984 Jan;81(1):216–219. doi: 10.1073/pnas.81.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamki L. M., Buzdar A. U., Singletary S. E., Rosenblum M. G., Bhadkamkar V., Esparza L., Podoloff D. A., Zukiwski A., Hortobagyi G. N., Murray J. L. Indium-111-labeled B72.3 monoclonal antibody in the detection and staging of breast cancer: a phase I study. J Nucl Med. 1991 Jul;32(7):1326–1332. [PubMed] [Google Scholar]
- Lazarovits A. I., Rochon J., Banks L., Hollomby D. J., Muirhead N., Jevnikar A. M., White M. J., Amlot P. L., Beauregard-Zollinger L., Stiller C. R. Human mouse chimeric CD7 monoclonal antibody (SDZCHH380) for the prophylaxis of kidney transplant rejection. J Immunol. 1993 Jun 1;150(11):5163–5174. [PubMed] [Google Scholar]
- Ledermann J. A., Begent R. H., Bagshawe K. D., Riggs S. J., Searle F., Glaser M. G., Green A. J., Dale R. G. Repeated antitumour antibody therapy in man with suppression of the host response by cyclosporin A. Br J Cancer. 1988 Nov;58(5):654–657. doi: 10.1038/bjc.1988.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitha T., Walter R., Schlick W., Dudczak R. 99mTc-anti-CEA radioimmunoscintigraphy of lung adenocarcinoma. Chest. 1991 Jan;99(1):14–19. doi: 10.1378/chest.99.1.14. [DOI] [PubMed] [Google Scholar]
- Maher V. E., Drukman S. J., Kinders R. J., Hunter R. E., Jennings J., Brigham C., Stevens S., Griffin T. W. Human antibody response to the intravenous and intraperitoneal administration of the F(ab')2 fragment of the OC125 murine monoclonal antibody. J Immunother (1991) 1992 Jan;11(1):56–66. doi: 10.1097/00002371-199201000-00007. [DOI] [PubMed] [Google Scholar]
- Meeker T. C., Lowder J., Maloney D. G., Miller R. A., Thielemans K., Warnke R., Levy R. A clinical trial of anti-idiotype therapy for B cell malignancy. Blood. 1985 Jun;65(6):1349–1363. [PubMed] [Google Scholar]
- Meredith R. F., LoBuglio A. F., Plott W. E., Orr R. A., Brezovich I. A., Russell C. D., Harvey E. B., Yester M. V., Wagner A. J., Spencer S. A. Pharmacokinetics, immune response, and biodistribution of iodine-131-labeled chimeric mouse/human IgG1,k 17-1A monoclonal antibody. J Nucl Med. 1991 Jun;32(6):1162–1168. [PubMed] [Google Scholar]
- Muto M. G., Finkler N. J., Kassis A. I., Lepisto E. M., Knapp R. C. Human anti-murine antibody responses in ovarian cancer patients undergoing radioimmunotherapy with the murine monoclonal antibody OC-125. Gynecol Oncol. 1990 Aug;38(2):244–248. doi: 10.1016/0090-8258(90)90049-q. [DOI] [PubMed] [Google Scholar]
- Nisonoff A. Idiotypes: concepts and applications. J Immunol. 1991 Oct 15;147(8):2429–2438. [PubMed] [Google Scholar]
- Norman D. J., Chatenoud L., Cohen D., Goldman M., Shield C. F., 3rd Consensus statement regarding OKT3-induced cytokine-release syndrome and human antimouse antibodies. Transplant Proc. 1993 Apr;25(2 Suppl 1):89–92. [PubMed] [Google Scholar]
- Oosterwijk E., Bander N. H., Divgi C. R., Welt S., Wakka J. C., Finn R. D., Carswell E. A., Larson S. M., Warnaar S. O., Fleuren G. J. Antibody localization in human renal cell carcinoma: a phase I study of monoclonal antibody G250. J Clin Oncol. 1993 Apr;11(4):738–750. doi: 10.1200/JCO.1993.11.4.738. [DOI] [PubMed] [Google Scholar]
- Pai L. H., Bookman M. A., Ozols R. F., Young R. C., Smith J. W., 2nd, Longo D. L., Gould B., Frankel A., McClay E. F., Howell S. Clinical evaluation of intraperitoneal Pseudomonas exotoxin immunoconjugate OVB3-PE in patients with ovarian cancer. J Clin Oncol. 1991 Dec;9(12):2095–2103. doi: 10.1200/JCO.1991.9.12.2095. [DOI] [PubMed] [Google Scholar]
- Riva P., Marangolo M., Lazzari S., Agostini M., Sarti G., Moscatelli G., Franceschi G., Spinelli A., Vecchietti G. Locoregional immunotherapy of human ovarian cancer: preliminary results. Int J Rad Appl Instrum B. 1989;16(6):659–666. doi: 10.1016/0883-2897(89)90092-5. [DOI] [PubMed] [Google Scholar]
- Robins R. A., Denton G. W., Hardcastle J. D., Austin E. B., Baldwin R. W., Durrant L. G. Antitumor immune response and interleukin 2 production induced in colorectal cancer patients by immunization with human monoclonal anti-idiotypic antibody. Cancer Res. 1991 Oct 1;51(19):5425–5429. [PubMed] [Google Scholar]
- Schroff R. W., Foon K. A., Beatty S. M., Oldham R. K., Morgan A. C., Jr Human anti-murine immunoglobulin responses in patients receiving monoclonal antibody therapy. Cancer Res. 1985 Feb;45(2):879–885. [PubMed] [Google Scholar]
- Schulz G., Büchler M., Muhrer K. H., Klapdor R., Kübel R., Harthus H. P., Madry N., Bosslet K. Immunotherapy of pancreatic cancer with monoclonal antibody BW 494. Int J Cancer Suppl. 1988;2:89–94. doi: 10.1002/ijc.2910410721. [DOI] [PubMed] [Google Scholar]
- Sears H. F., Herlyn D., Steplewski Z., Koprowski H. Effects of monoclonal antibody immunotherapy on patients with gastrointestinal adenocarcinoma. J Biol Response Mod. 1984;3(2):138–150. [PubMed] [Google Scholar]
- Shawler D. L., Bartholomew R. M., Smith L. M., Dillman R. O. Human immune response to multiple injections of murine monoclonal IgG. J Immunol. 1985 Aug;135(2):1530–1535. [PubMed] [Google Scholar]
- Stern H., Reilly R., Gallinger S., Kirsh J., DeAngelis C., Papa M., Hay K., Polihronis J., Schmoker B. Radioimmunodetection of colorectal cancer metastases with 131I-labeled monoclonal antibody B72.3: a pilot study to determine efficacy of detection and pharmacokinetics. Cancer Invest. 1993;11(2):129–134. doi: 10.3109/07357909309024830. [DOI] [PubMed] [Google Scholar]
- Stewart J. S., Hird V., Snook D., Sullivan M., Hooker G., Courtenay-Luck N., Sivolapenko G., Griffiths M., Myers M. J., Lambert H. E. Intraperitoneal radioimmunotherapy for ovarian cancer: pharmacokinetics, toxicity, and efficacy of I-131 labeled monoclonal antibodies. Int J Radiat Oncol Biol Phys. 1989 Feb;16(2):405–413. doi: 10.1016/0360-3016(89)90337-4. [DOI] [PubMed] [Google Scholar]
- Stewart J. S., Hird V., Snook D., Sullivan M., Myers M. J., Epenetos A. A. Intraperitoneal 131I- and 90Y-labelled monoclonal antibodies for ovarian cancer: pharmacokinetics and normal tissue dosimetry. Int J Cancer Suppl. 1988;3:71–76. [PubMed] [Google Scholar]
- Vadhan-Raj S., Cordon-Cardo C., Carswell E., Mintzer D., Dantis L., Duteau C., Templeton M. A., Oettgen H. F., Old L. J., Houghton A. N. Phase I trial of a mouse monoclonal antibody against GD3 ganglioside in patients with melanoma: induction of inflammatory responses at tumor sites. J Clin Oncol. 1988 Oct;6(10):1636–1648. doi: 10.1200/JCO.1988.6.10.1636. [DOI] [PubMed] [Google Scholar]
- Vaickus L., Foon K. A. Overview of monoclonal antibodies in the diagnosis and therapy of cancer. Cancer Invest. 1991;9(2):195–209. doi: 10.3109/07357909109044230. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Stone M., Amlot P., Fay J., May R., Till M., Newman J., Clark P., Collins R., Cunningham D. Phase I immunotoxin trial in patients with B-cell lymphoma. Cancer Res. 1991 Aug 1;51(15):4052–4058. [PubMed] [Google Scholar]
- Waldmann H., Hale G., Clark M., Cobbold S., Benjamin R., Friend P., Bindon C., Dyer M., Qin S. X., Bruggemann M. Monoclonal antibodies for immunosuppression. Prog Allergy. 1988;45:16–30. [PubMed] [Google Scholar]
- Wendling D., Wijdenes J., Racadot E., Morel-Fourrier B. Therapeutic use of monoclonal anti-CD4 antibody in rheumatoid arthritis. J Rheumatol. 1991 Mar;18(3):325–327. [PubMed] [Google Scholar]
- Winzelberg G. G., Grossman S. J., Rizk S., Joyce J. M., Hill J. B., Atkinson D. P., Sudina K., Anderson K., McElwain D., Jones A. M. Indium-111 monoclonal antibody B72.3 scintigraphy in colorectal cancer. Correlation with computed tomography, surgery, histopathology, immunohistology, and human immune response. Cancer. 1992 Apr 1;69(7):1656–1663. doi: 10.1002/1097-0142(19920401)69:7<1656::aid-cncr2820690704>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Yasunaga C., Cramer D. V., Cosenza C. A., Tuso P. J., Chapman F. A., Barnett M., Wu G. D., Putnam B. A., Makowka L. Effect of brequinar sodium on in vivo antibody production. Transplant Proc. 1993 Jun;25(3 Suppl 2):40–44. [PubMed] [Google Scholar]
- Zimmer A. M., Rosen S. T., Spies S. M., Goldman-Leikin R., Kazikiewicz J. M., Silverstein E. A., Kaplan E. H. Radioimmunotherapy of patients with cutaneous T-cell lymphoma using an iodine-131-labeled monoclonal antibody: analysis of retreatment following plasmapheresis. J Nucl Med. 1988 Feb;29(2):174–180. [PubMed] [Google Scholar]



