Abstract
The mitochondrial DNA (mtDNA) polymerase γ (POLG) mutator mice provide the first experimental evidence that high levels of somatic mtDNA mutations can be functionally significant. Here we report that older homozygous, but not heterozygous, POLG mice show significant reductions in striatal dopaminergic terminals as well as deficits in motor function. However, resting oxygen consumption, heat production, mtDNA content and mitochondrial electron transport chain activities are significantly decreased at older ages in both homozygous and heterozygous mice. These results indicate that high levels of somatic mtDNA mutations can contribute to dopaminergic dysfunction and to behavioral and metabolic deficits.
Keywords: mtDNA polymerase γ, mtDNA mutation, Parkinson’s disease, dopamine, behavioral deficits, metabolic deficits
1. Introduction
The mitochondrial theory of aging proposes that the lifelong accumulation of mtDNA mutations leads to the aging process and to age-related neurodegenerative diseases (Larsson, 2010; Linnane et al., 1989). However, experimental evidence in support of this fundamental theory of aging has been limited until two groups reported that mtDNA mutator mice carrying a proofreading deficient version (D257A) of POLG accumulated very high levels of mtDNA mutations associated with a striking premature aging phenotype including hair loss, graying, kyphosis, early loss of fertility, dilated cardiac hypertrophy, and reduced lifespan (Kujoth et al., 2005; Trifunovic et al., 2005; Trifunovic et al., 2004).
In contrast to homozygous POLG mutator mice, no significant increase in age related pathology and gross behavioral abnormalities have thus far been detected in heterozygous mice, despite significantly increased mtDNA mutation levels compared to WT mice (Trifunovic et al., 2004; Vermulst et al., 2007). Remarkably, the mean lifespan of heterozygous mice seems to remain unaffected (Kujoth et al., 2005; Trifunovic et al., 2004; Vermulst et al., 2007), thus questioning role of somatic mtDNA mutation including point mutations or deletions as a primary driving force of aging. Nevertheless, because there have been no formal behavioral or metabolic assessments reported for the heterozygous POLG mutator mice, we cannot exclude the possibility that heterozygous mice have motor or bioenergetic deficits associated with increased levels of mtDNA mutations.
Emerging reports suggest that mitochondrial dysfunction plays a critical role in the pathogenesis of neurodegenerative diseases including PD (Clark et al., 2011). Evidence in support of this comes from accidental human exposures to 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP), which causes parkinsonism by inhibition of mitochondrial complex-I (Burns et al., 1985; Langston et al., 1983). Mitochondrial complex-I defects are also found in the substantia nigra (SN) (Hattori et al., 1991; Parker et al., 1989; Schapira et al., 1989) and platelets (Parker et al, 1989) of PD patients (Hattori et al., 1991; Parker et al., 1989; Schapira et al., 1989). In addition, several studies have indicated that dopaminergic neurons in the SN are lost during normal aging in mice (Tatton et al., 1991), monkeys (Emborg et al., 1998; Siddiqi et al., 1999) and humans (Cruz-Sanchez et al., 1995; Rudow et al., 2008; Severson et al., 1982). Although we (Cantuti-Castelvetri et al., 2005) and others (Bender et al., 2006; Kraytsberg et al., 2006) have documented high levels of somatic mtDNA mutations in SN neurons in the brains of elderly subjects, it is unknown if somatic mtDNA mutations contribute to this age-related loss of SN neurons.
Given that POLG mutator mice accumulate somatic mtDNA mutations and show features of premature aging, they therefore provide an in vivo model to investigate the potential role of somatic mtDNA mutations in age-related deficits. POLG mutations in humans have been associated with a variety of neurological symptoms, including levodopa-responsive parkinsonism with SN neuronal loss (Betts-Henderson et al., 2009; Hudson et al., 2007; Invernizzi et al., 2008; Luoma et al., 2004) (Hudson et al., 2007; Invernizzi et al., 2008; Luoma et al., 2004)and alpha-synuclein accumulation (Betts-Henderson et al., 2009). We therefore hypothesized that somatic mtDNA mutations and mitochondrial dysfunction play a causal role in aging-associated dopaminergic dysfunction as well as behavioral and metabolic deficits. To test this hypothesis, we performed histological, behavioral and metabolic assessments in the heterozygous and homozygous POLG mutator mice and in WT littermate controls.
2. Material and methods
2.1 Mouse strains and husbandry
The heterozygous (+/mut) mice with POLG knock-in mutation (D257A) were provided by Dr.Tomas A. Prolla (University of Wisconsin, Madison). Homozygous mutator mice (mut/mut) and WT littermates were generated from a colony derived from heterozygous mice and maintained at the Animal Research Facility at Beth Israel Deaconess Medical Center (BIDMC). All animal studies were approved by the Institutional Animal Care and Use Committee at BIDMC. Mice were housed in a 14/10 light/dark cycle under controlled temperature (22~25 °C) and given free access to food and water. Genotyping was performed as described previously (Kujoth et al., 2005). All experiments were conducted in accordance with protocols approved by Institutional Animal Care and Use Committee at Beth Israel Deaconess Medical Center.
2.2 Immunohistochemisty
Cryoprotected brains were cut on a freezing microtome to generate sections of 30 mm thickness that were analyzed for the density of TH-immunopositive terminals in the striatal area. These sections were immunostained using a primary mouse monoclonal antibody against TH (1:1000; Sigma, MO) and a M.O.M Biotinylated Anti-mouse IgG secondary antibody (1:250; Vector Labs, Burlingame, CA), according to the manufacturer's protocol. Striatal density of specific TH staining was determined using digital images taken by Axioskop microscope (Zeiss Thornwood, NY) with fixed exposure settings. Mean pixel density (MPD) was calculated after digitally inverting black vs. white so that high staining intensities correspond with higher MPDs. MPD for adjacent cortex of the same section was subtracted to adjust for background staining intensity and unintended variation in section thickness. The average MPD was determined from a minimum of 3 sections for each mouse. TH+ neurons in SN/ventral tegmental area (SN/VTA) were counted in every fifth section throughout the entire extent of the SN/VTA using unbiased stereological methods via Stereo Investigator software (MBF Biosciences, Inc, Williston, VT) (Inoue et al., 2007; Koprich et al., 2008). The midbrain sections were viewed at low power (×2.5 objective) to outline SN and VTA. TH+ Neurons were then counted based on their nuclei at high power (×100 objective) using the optical fractionator method with the following stereologic parameters: grid size, 120 × 120 µm; counting frame size, 50 × 50 µm; dissector height, 10 µm; guard zones, 2 µm.
2.3 Measurements of dopamine and its metabolites
Levels of striatal dopamine and dopamine metabolites, HVA and 3,4-dihydroxyphenylacetic acid (DOPAC), were measured in frozen striatal tissue via HPLC through the Neurochemistry Core Facility at Vanderbilt University Medical Center (http://www.vandyneurocores.org/). In order to minimize the impact of batch to batch variability, the data were normalized to WT mice at each time point.
2.4 Behavioral tests
2.4.1 Rotarod
The rotarod apparatus (Ugo Basile Biological Research Apparatus, Varese, Italy) was used to measure balance and motor coordination. Rotarod testing was performed as previously described (Clark et al., 2010; Masliah et al., 2000). To acclimate them to the apparatus, mice were trained on the rotarod accelerating from 2 to 40 rpm in 240 seconds (five trials per day for 4 consecutive days). The 5th day constituted the test day and the mice were tested on the same experimental paradigm. Each mouse received seven consecutive trials and the mean latency to fall was used in the analysis.
2.4.2 Locomotor activity
Locomotor activity was evaluated using the comprehensive lab animal monitoring system (CLAMS; Columbus Instruments, Columbus, OH) that allow automated, noninvasive data collection. Mice were housed singly and maintained at ~24°C under a 12:12-h light-dark cycle (light period 0800–2000) (Fulton et al., 2006; Kokkotou et al., 2005). Food and water were available ad libitum. Mice were acclimatized to monitoring cages for 48 h before recordings of physiological parameters. Consecutive photobeam breaks occurring in adjacent photobeams along the x- and y-axes were scored as an ambulatory movement. Cumulative ambulatory activity counts were recorded throughout the light and dark cycles. To test if a dopamine increase could improve ambulatory activity, after measuring the baseline activity in the CLAMS chambers for 3.5 hours, benserazide (20 mg/kg) was administered to a subset of mice by intraperitoneal (i.p.) injection, followed by an i.p. injection of L-dopa (80 mg/kg) or saline 20 minutes later. These mice were then monitored in the CLAMS chambers for the subsequent 15 hours. Benserazide, a DOPA decarboxylase inhibitor, is incapable of crossing the blood-brain-barrier and thus reduces the peripheral breakdown of L-dopa and enhances delivery of L-dopa into the brain.
2.5 Metabolic assessment
Metabolic and locomotor activities of mice were measured simultaneously using CLAMS. Metabolic rate was measured by indirect calorimetry using an open-circuit Oxymax system of the CLAMS. Mice were weighed prior to each trial. An air sample was passed through oxygen (O2) and carbon dioxide (CO2) sensors (Columbus Instruments) for determination of O2 and CO2 content. O2 consumption (VO2) was determined by measuring O2 concentration in air entering the chamber compared with air leaving the chamber. Carbon dioxide production (VCO2) was similarly monitored in real time. As another index of metabolic rate, heat production per animal was calculated using the equation heat = [3.815 + (1.232 × RER)] × VO2, where RER is the respiratory exchange ratio = VCO2/VO2 (Badman et al., 2009; Chen et al., 2000).
2.6 Complex IV (COX) Enzyme Activity
Mouse brain cortex homogenization, protein extraction and COX activity measurement were performed using the Complex IV Rodent Enzyme Activity Microplate Assay Kit (MitoSciences) according to the manufacturer’s directions. Briefly, tissue was homogenized using the TissueLyser LT with 5 mm stainless steel beads (Qiagen). Protein concentration was determined using the BCA Protein Assay kit (Pierce Chemical). 50 µg of proteins in 200 µl assay solution were loaded into each well of 96 well plates coated with COX specific antibodies, and incubated for 3 h at room temperature. The oxidative capacity of COX in the presence of cytochrome C was monitored by absorbance at 550nm for 2 hours by an Epoch Microplate Spectrophotometer (BioTek). Rates of oxidation were calculated by using absorbance values during the time when the decrease of OD values was linear.
2.7 Immunoblotting
Protein samples were prepared as for the COX enzyme activity assay. Proteins were separated on 15% SDS-polyacrylamide gels and then electrophoretically transferred to PVDF membranes. Membranes were then incubated in blocking buffer (5% nonfat dry milk, 0.1% Tween 20, and 1× TBS) for 1 hour at room temperature. Membranes were incubated overnight at 4°C with MitoProfile® Total OXPHOS Rodent WB Antibody Cocktail (MitoSciences) on a shaker. The next day, membranes were washed with TBS/0.1% Tween 20, and then incubated with goat anti-mouse secondary antibody conjugated to horseradish peroxidase (Santa Cruz). Membranes were washed, and signal was detected using LumiGOLD ECL Western Blotting Detection Kit (SignaGen). The same membranes were then stripped and reprobed with β-actin antibody (Santa Cruz) as a loading control. Using ImageJ for Windows, immunoblots were quantified by calculating the integrated optical density of each protein band on the film. The integrated density of each band of interest was normalized to the integrated density for the loading control of that band.
2.8 mtDNA copy number
For quantification of mtDNA copy number, real-time PCR analysis was performed with the NovaQUANT™ Mouse Mitochondrial to Nuclear DNA Ratio Kit (Novagen, 72621) according to the manufacturer’s instructions. DNA extractions were performed on frozen mouse striatum using a QIAamp DNA mini kit (Qiagen, 51304). A set of four optimized PCR primer pairs targeting two mitochondrial genes (trLEV and 12s RNA) and two nuclear genes (BECN1 and NEB) were pre-aliquoted in an Applied Biosystems MicroAmp® Fast Optical 96-well Reaction Plate. A fast real-time qPCR system (Applied Biosystems 7900HT) was used to measure the ratio of mtDNA to nuclear DNA, the relative mtDNA copy number, reflecting the relative mtDNA content per cell. DNA from mouse rho zero cells lacking mtDNA was used as a negative control. The results of the qPCR reactions were analyzed with 2 −ΔCT method and normalized to WT control.
2.9 Statistical analysis
All data are presented as mean ± SEM and analyzed by one-way ANOVA or student’s t-test. Post hoc tests were conducted using Newman-Keuls's multiple-comparison tests. GraphPad Prism 4.0 was used for all statistical analyses. A probability level of p < 0.05 was considered to be statistically significant for all statistical tests.
3. Results
3.1 Older POLG mut/mut mice show dopaminergic dysfunction
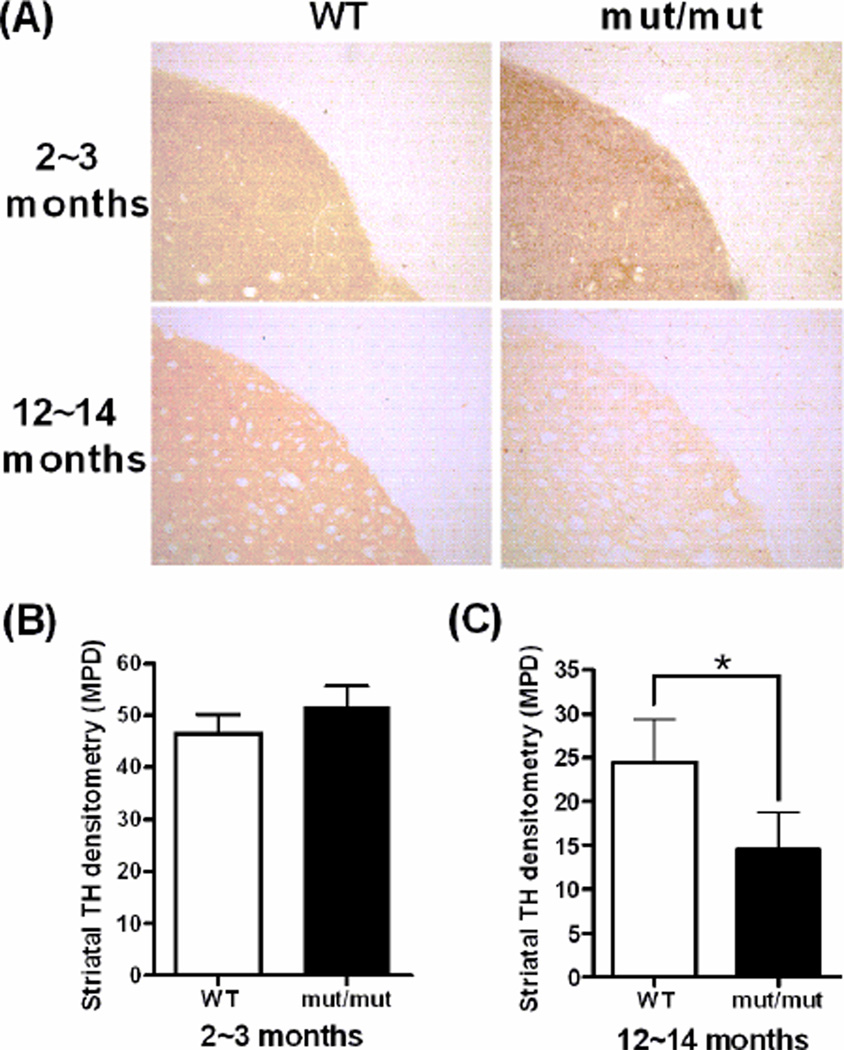
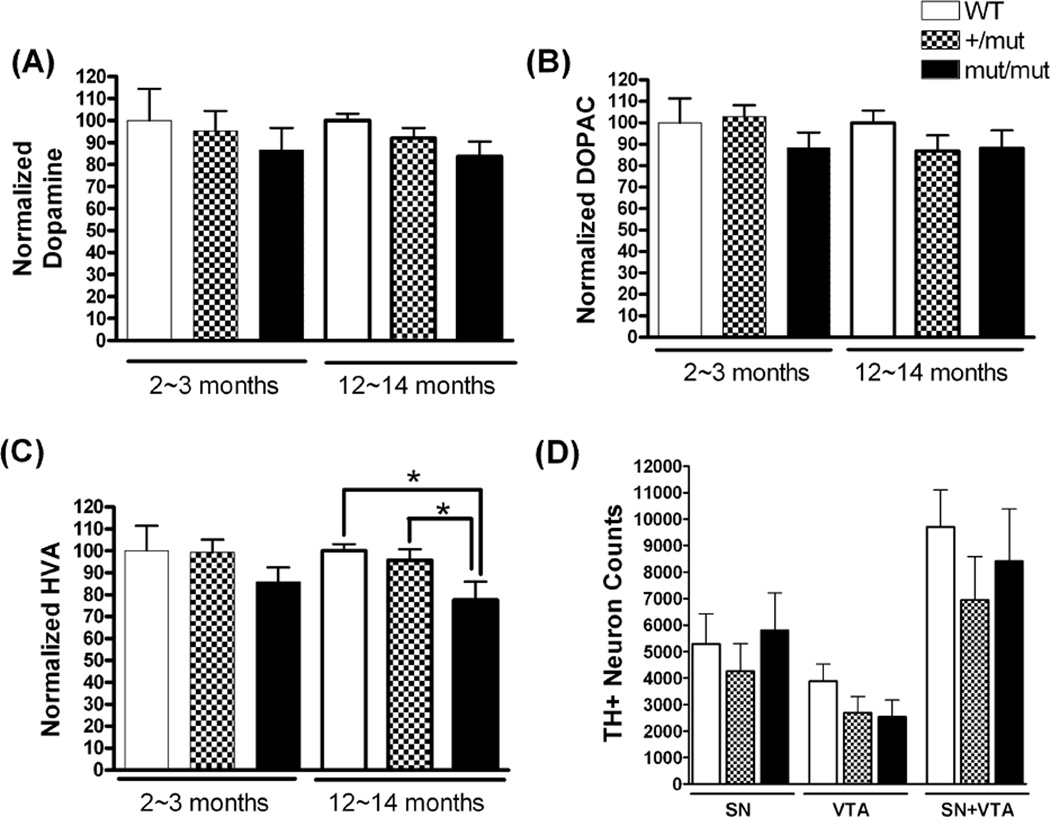
The integrity of dopaminergic SN neurons was assessed at 2~3 months and 12~14 months by measuring intensity of immunostaining for striatal TH, striatal dopamine and dopamine metabolites, and stereological counts of TH+ neurons in the SN and ventral tegmental area (VTA). At 2~3 months of age, there was no difference in striatal TH density between mut/mut and WT mice. However, at 12~14 months of age, mut/mut mice showed a mean decrease of 40.8% (95% CI 31.1~71.5%; p<0.01) in the striatal TH immunostaining intensity compared to WT littermate controls (Fig. 1). The levels of striatal dopamine and its metabolites, dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), were further assessed by HPLC (Fig. 2A, B and C). There was no significant difference in the levels of dopamine and its metabolites at 2~3 months of age between any groups of mice. At 12~14 months of age, mut/mut mice showed a significant decrease in HVA levels as compared to +/mut and WT mice. Although there was a trend towards reduced striatal dopamine and DOPAC in mut/mut and +/mut mice compared to WT controls, the difference was not statistically significant. Reduced TH immunostaining intensity and lower levels of HVA in the striatum could occur due to death of TH+ SN neurons, or due to loss of TH+ terminals without the death of those neurons. To address this issue, the numbers of TH+ neurons in the SN and VTA were counted by stereological methods (Fig. 2D). Numbers of TH+ SN and VTA neurons were similar among mut/mut, +/mut and WT mice, suggesting that loss of TH+ terminals without death of TH+ SN neurons accounts for the reduction of TH+ terminal density and HVA in the striatum.
Figure 1.
Densitometric analysis of the TH immunoreactivity in the striatum at 2~3 and 12~14 months of age. (A) Representative images of immunostaining of TH on striatal slides of WT and mut/mut mouse striatum. (B) Densitometric quantification indicated similar densities of TH-positive terminals in the striatum of mut/mut and WT mice at 2~3 months of age. (C) mut/mut mice showed a significant decrease in striatal TH terminal density compared to WT mice at 12~14 months of age. Data were analyzed using a 2-tailed Student’s t-test. *p < 0.01. n = 6 in each group.
Figure 2.
Levels of striatal dopamine and its metabolites, and stereological counts of TH-positive neurons. For dopamine (A) and DOPAC (B) levels, there were no significant differences among the three groups of mice at any age measured. There was a significant reduction in HVA (C) levels in mut/mut mice compared to +/mut or WT mice at 12~14 months of age. Data were analyzed using one-way ANOVA (F(2,17)=4.25; p = 0.03) followed by Newman-Keuls multiple-comparison test. *p < 0.05. n = 5~7 in each group. (D) Unbiased stereological counts of TH-positive neurons in the SN and VTA in mice at 12~14 months of age. There were no significant differences in TH+ neuronal numbers in the SN and/or VTA among mut/mut, +/mut and WT mice. Data were analyzed by oneway ANOVA. n = 3~5 in each group.
3.2 Older POLG mut/mut mice show impaired rotarod performance and locomotor activity
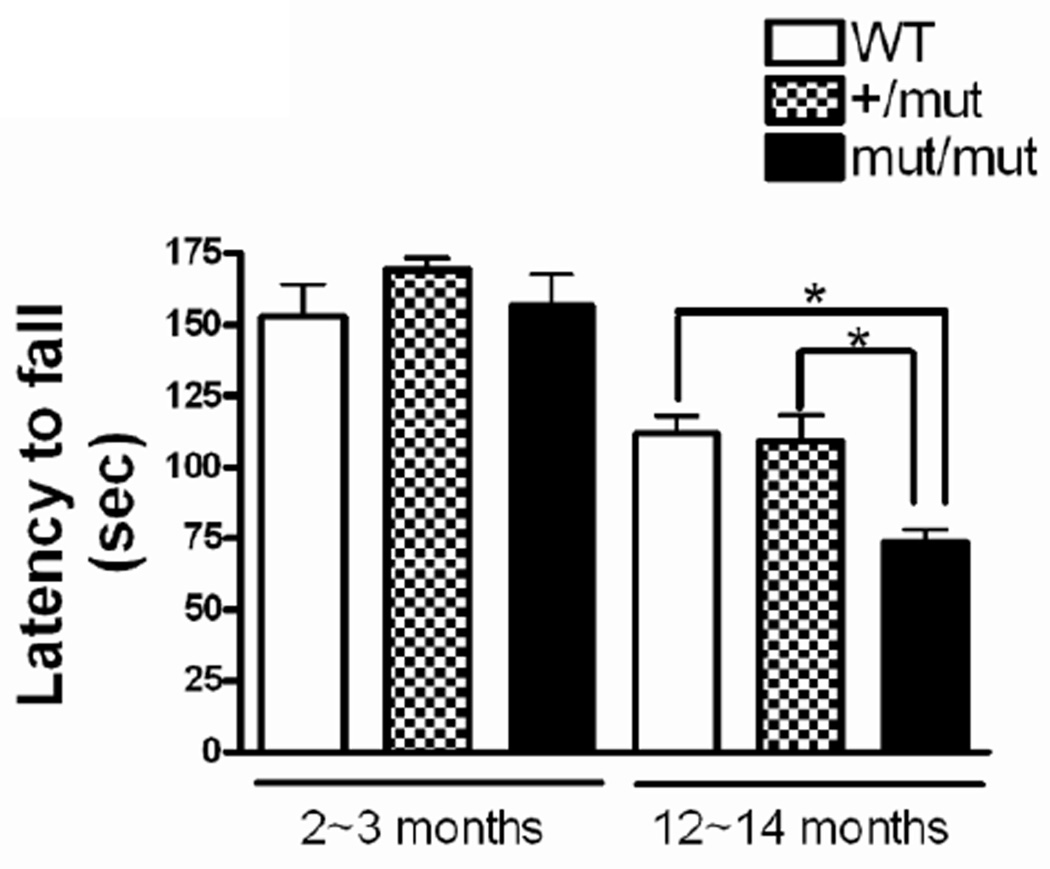
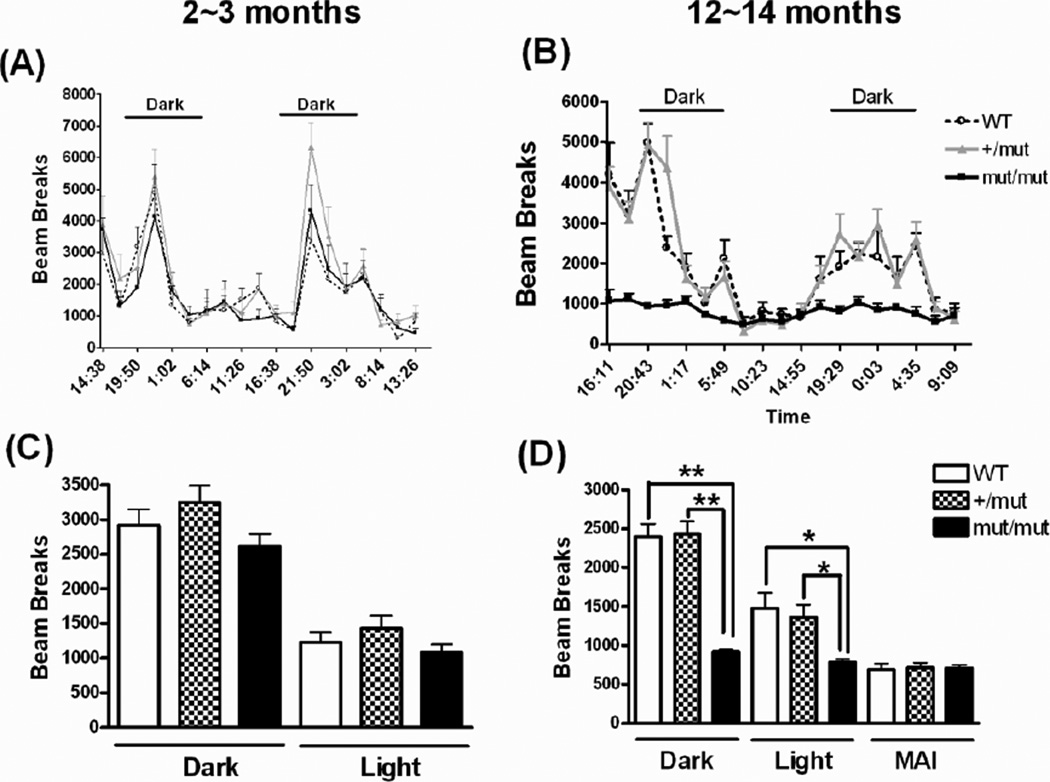
Rotarod was used to measure fore- and hindlimb motor coordination and balance at 2~3 and 12~14 months of age (Fig. 3). At 2~3 months of age, the mean latency to fall was not significantly different among the three groups of mice, 152.9 ± 11.1, 169.3 ± 4.1, 156.5 ± 11.1 s (Mean±SE) in WT, +/mut, and mut/mut mice, respectively. At 12~14 months of age, there was a decline in performance for all three groups of mice. Although the latency to fall in WT (111.7 ± 6.3 s) and +/mut mice (109.2 ± 9.0 s) were similar to each other, the mut/mut mice showed a significantly decreased latency to fall (73.6 ± 4.6 s). Further, locomotor activity was evaluated by consecutive photo-beam breaks occurring in adjacent beams using CLAMS under a 12:12 hour light:dark cycle. At 2~3 months of age, the locomotor activity was not significantly differed among the 3 groups of mice in either the dark or light cycles, although animals in all 3 groups exhibited greater locomotor activity during the dark cycles (Fig. 4A, C). At 12~14 months of age, heterozygous (+/mut) mice again showed no deficits in locomotor activity compared to WT mice. In contrast, at this age, mean activity levels of homozygous (mut/mut) mice were substantially lower (reduced by approximately 50%) compared to those seen in WT or +/mut mice in both dark and light cycles (Fig. 4B, D). There was no significant improvement in the locomotor activity when a subset of mut/mut and WT mice (n=2~4) received a single dose of 80 mg/kg L-dopa 20 minutes after an i.p. injection of 20 mg/kg benserazide, a peripheral dopa decarboxylase inhibitor (data not shown).
Figure 3.
Rotarod performance. Mice were placed on a rotating rod with increasing speed, from 2~40 rpm over 240 sec. The latency to fall off the rotarod within this time period was recorded. There was no difference in the latency to fall between any of the groups at 2~3 months of age, but mut/mut mice showed a significant decreased latency to fall as compared to either of the other two groups at 12~14 months of age. Data were analyzed using one-way ANOVA (F(2,31)=9.00; p = 0.008) followed by Newman-Keuls multiple-comparison test. *p < 0.01. n = 8~13 in each group.
Figure 4.
Locomotor activity measured by monitoring consecutive beam breaks in CLAMS at different ages. Average locomotor activity is plotted over a 44~48 hour cycle at 2~3 months (A, C) and at 12~14 months (B, D) of age. Bars in (A) and (B) indicate the dark cycles. MAI (D) refers to interval during the light cycle when all three groups of mice showed matched low levels of locomotor activity. Data were analyzed using oneway ANOVA (For dark cycle, F(2,105)=38.88, p < 0.0001; for light cycle, F(2,123)=6.18, p= 0.0028) followed by Newman-Keuls multiple-comparison test. *p < 0.01 and **p < 0.001. n = 5~13 in each group.
3.3 Metabolic deficits emerge in both heterozygous and homozygous POLG mice
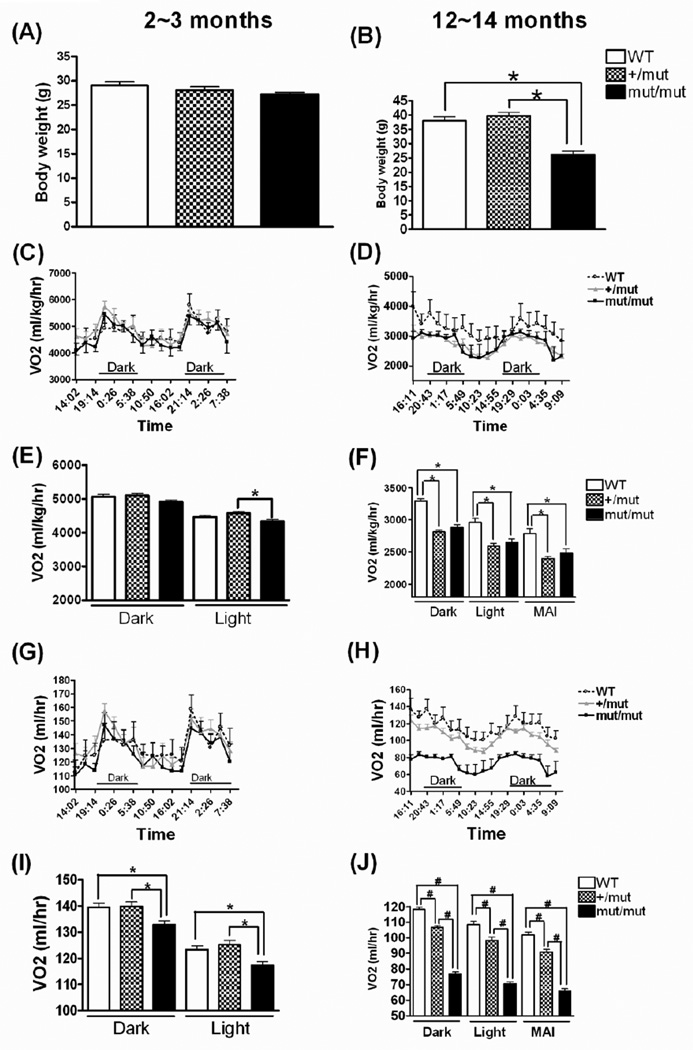
To minimize any effect of activity on our comparisons of metabolic activity between groups, we selected a specific period of time (10:23~14:55, representing a subset of the light cycle) when relatively low activity was observed in all 3 groups for comparing the metabolic parameters. There is no significant difference between any of the three groups in locomotor activity during this selected interval (Fig. 4D), hereafter denoted matched activity interval (MAI). The body weight didn’t differ across three groups at 2~3 months of age, but mut/mut mice had substantially reduced body weight (26.1 ± 1.4g) as compared to WT (38.0 ± 1.3g) or +/mut (39.8 ± 1.2g) at 12~14 months of age (Fig. 5A, B). To estimate the metabolic rate and substrate utilization, we measured VO2 and heat production from whole animals (Fig. 5, Fig. 6). After normalization to body weight, VO2 in both +/mut and mut/mut mice was significantly decreased compared to WT mice in either dark, light or MAI at 12~14 months of age (Fig. 5D, F). If analyzed on a per animal basis (without normalization to body weight), VO2 in mut/mut mice was significantly lower than in the other two groups in both dark and light cycles at both younger and older ages (Fig. 5G, H, I, J). This VO2 reduction (about 35% reduction as compared to WT mice during MAI at 12~14 months of age) is not solely accounted for by the lower body weight in the mut/mut mice because after normalized to weight, the difference still persists (about 10% decrease as compared to WT mice, p<0.001). Additionally, with body weight comparable to WT mice at 12~14 months of age, +/mut mice showed a significantly decreased VO2 compared to WT controls in either dark, light or MAI (Fig. 5 D, F, H, J). These differences were significant for VO2 normalized to body weight as well as for VO2 per animal.
Figure 5.
Body weight and O2 consumption measured by CLAMS at different ages. Body weight measured at 2~3 months of age (A) and at 12~14 months of age (B). Data were analyzed using one-way ANOVA (F(2,27)=36.10, p < 0.0001) followed by Newman-Keuls multiple-comparison test. *p < 0.001. n = 5~13 in each group. O2 consumption normalized to body weight is shown for a 44~48 hour cycle at 2~3 months of age (C) and at 12~14 months of age (D), with mean ± SE shown in (E) and (F), respectively. Data in (E) were analyzed using one-way ANOVA (F(2,87)=4.44, p=0.015) followed by Newman-Keuls multiple-comparison test. *p < 0.05. Because differences in locomotor activity between groups were identified at 12~14 months of age (see Fig. 6), data also are shown in (F) and (J) for interval of matched ambulatory activity (MAI) to allow comparison of VO2 between groups while avoiding a potential impact from differences in locomotor activity. Data in (F) were analyzed using one-way ANOVA (For dark cycle, F(2,126)=46.47, p < 0.0001; for light cycle, F(2,102)=13.88, p < 0.0001; for MAI, F(2,24)=10.51, p = 0.0005) followed by Newman-Keuls multiple-comparison test. *p < 0.01. O2 consumption also was analyzed per animal without normalization to body weight at 2~3 months of age (G, I) and at 12~14 months of age. (H, J) Data were analyzed using one-way ANOVA followed by Newman-Keuls multiple-comparison test. At 2~3 months: For the dark cycle, F(2,105)=5.64, p=0.0047; for the light cycle, F(2,87)=6.70, p = 0.002. At 12~14 months: For the dark cycle, F(2,126)=289.4, p < 0.0001; for the light cycle, F(2,102)=124.4, p < 0.0001; for MAI, F(2,24)=112.5, p < 0.0001. *p < 0.05 (I) or #p < 0.001 (J). n = 5~13 in each group.
Figure 6.
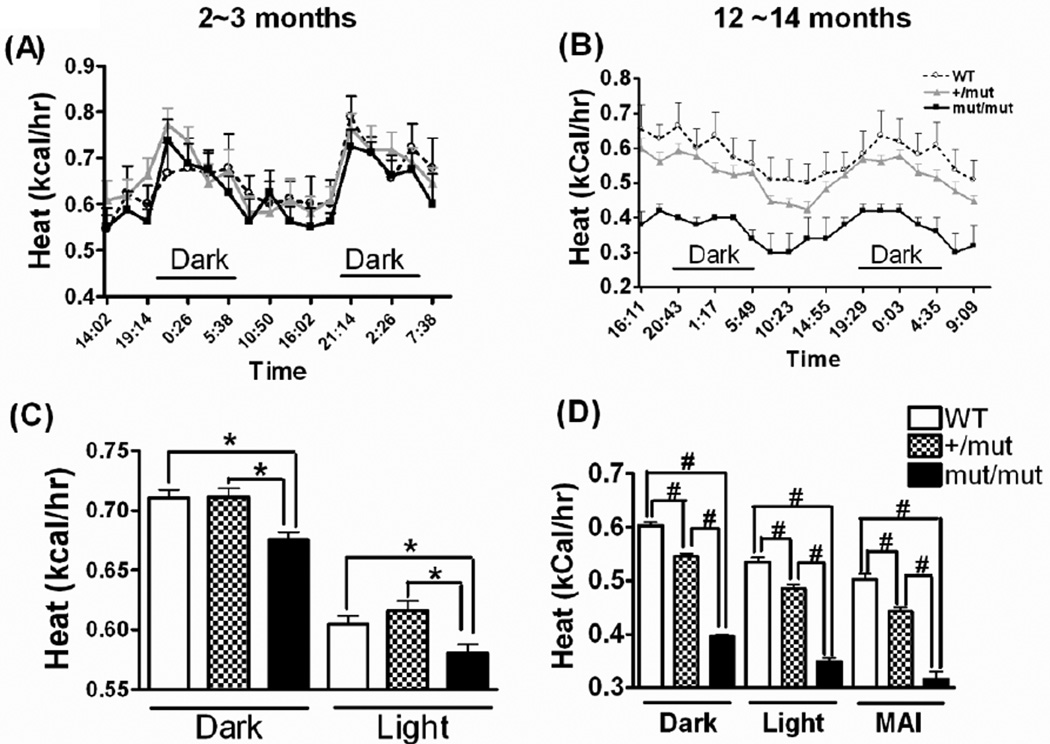
Heat production measured by CLAMS at different ages. Heat production is plotted over a 44~48 hour cycle at 2~3 months of age (A) and at 12~14 months of age (B), with mean ± SE indicated in (C) and (D), respectively. MAI (D) refers to interval during the light cycle when all three groups of mice showed matched low levels of locomotor activity. Data were analyzed using one-way ANOVA followed by Newman-Keuls multiple-comparison test. At 2~3 months of age, for the dark cycle, F(2,87)=8.55, p=0.0004; for light cycle, F(2,105)=6.10, p = 0.0031. At 12~14 months of age, for the dark cycle, F(2,105)=395.1, p<0.0001; for the light cycle, F(2,123)=162.7, p<0.0001; for MAI, F(2,24)=75.74, p<0.0001. *p < 0.05 (C) or #p < 0.001 (D). n = 5~13 in each group.
Energy released during metabolic processes is used to produce heat, perform work, or stored as chemical energy. This simple fact makes it possible to use heat production as an additional index of metabolic rate, especially when animal activities are low and similar across groups. The CLAMS apparatus calculates heat based on the respiratory exchange ratio (VCO2/VO2) and the observed VO2. At 2~3 months of age, WT and +/mut mice had similar mean heat productions (Kcal produced per hour) per animal in both the dark and light cycles, whereas mut/mut mice had significantly lower heat production than WT or +/mut mice (Fig. 6A, C). This significant difference persisted when animals were at 12~14 months of age, during the light/dark cycle as well as the MAI, when animals from all genotypes had similarly low locomotor activity, therefore the reduction in heat production is not accounted for by the lower locomotor activity in the mut/mut mice that was observed during the dark cycles. Additionally, +/mut mice, which had locomotor activity that was indistinguishable from WT (Fig. 4), also showed reduced heat production at 12~14 months compared to WT counterparts in the dark/light cycle and MAI, further indicating that the differences in heat production are not attributable to differences in locomotor activity (Fig. 6B, D).
3.4 Heterozygous and homozygous POLG mice both display decreased protein levels of Mitochondrial Complexes I and IV subunits and decreased COX activity
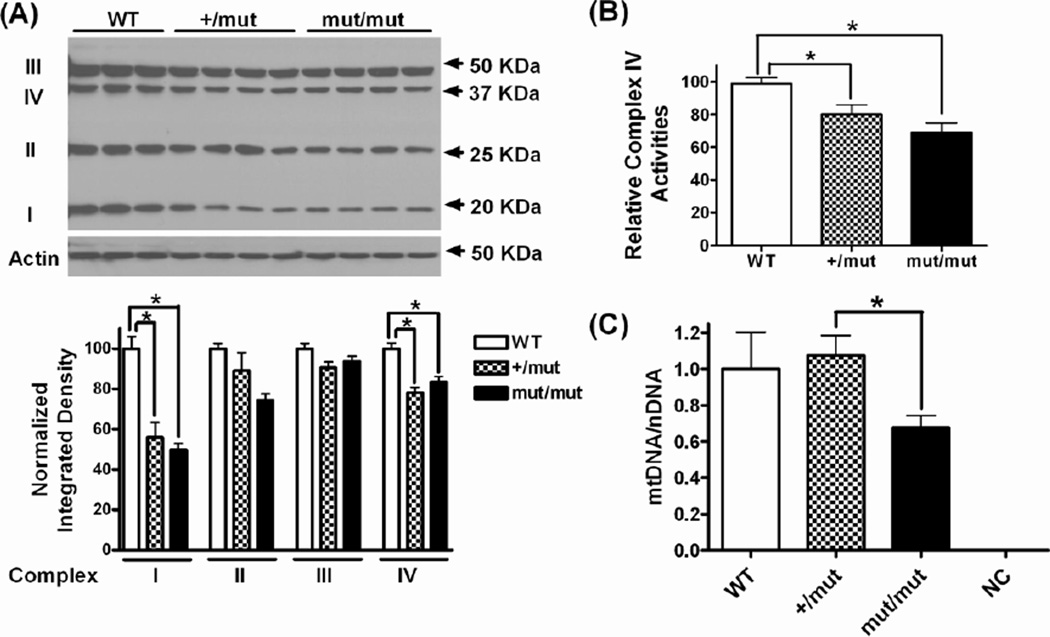
In order to confirm that the behavioral and metabolic deficits above were associated with mitochondrial dysfunction, we measured several protein subunits of mitochondrial electron transport chain (ETC) complexes, COX activity and mtDNA copy number in the brains of old mice (12~14 months of age). We found that both heterozygous (+/mut) and homozygous (mut/mut) mice had significantly decreased levels of several subunits of the mitochondrial ETC complexes I and IV relative to WT control mice (Fig. 7A). The levels of reduction were similar in +/mut and mut/mut mice (~40% reduction for complexes I and ~20% for complexes IV compared to WT). Though not statistically significant, there was also a trend toward lower levels of complexes II and III in +/mut and mut/mut mice. To determine if this reduction in mitochondrial ETC complexes was associated with impaired mitochondrial function in +/mut and mut/mut mice, we measured the activity of complexes IV (COX) in the mouse cerebral cortex (Fig. 7B). There was a significant decrease (20%~30% compared to WT) in COX activities in both +/mut and mut/mut mice, which is consistent with the reduction of complexes IV protein subunit. To determine if the development of behavioral and metabolic deficits in POLG mice is associated with mtDNA depletion, we measured the ratio of mtDNA to nuclear DNA (nDNA) as an index of mtDNA copy number in mouse striatum by quantitative real time PCR (qPCR). WT mice and +/mut mice had similar levels of mtDNA copy number. However, the mtDNA copy number in mut/mut mice was significantly lower than +/mut mice (p=0.035), although it was not significantly different from WT mice (p=0.205). The lack of statistical power may be related to the relatively small sample size (Figure 7C). When normalized to mtDNA copy number, COX activities in +/mut and mut/mut mice were not significantly different from that in WT mice (data not shown), suggesting that the reduction of measured COX activities was most likely due to a decrease of COX content.
Figure 7.
Mitochondrial complex subunits, COX activities and mtDNA content in the brain of 12~14 month old mice. (A) Top, Western blot showing the following subunits of the respiratory chain enzyme complexes: Complex I subunit NDUFB8 (I), Complex II subunit 30kDa (II), Complex III subunit Core 2 (III), and Complex IV subunit I (IV) in the cortex of mice at 12~14 months of age. Bottom, quantification of western blot showing protein levels normalized to actin. Data were analyzed using one-way ANOVA followed by Newman-Keuls multiple-comparison test. *p < 0.05. n = 3~4 in each group. (B) Relative complex IV activities in mouse cortex. The bars represent mean ± SEM from two separate experiments with 3~5 animal per group for each experiment. * p < 0.05 by unpaired student’s t-test (n = 6~7). (C) The mtDNA copy number in the striatum, measured as the ratio of mtDNA to nuclear DNA (mtDNA/nDNA) and normalized to WT mice. Mouse rho zero cells lacking mtDNA were used as a negative control (NC). * p < 0.05 by unpaired student’s t-test (n = 3).
4. Discussion
MtDNA mutations accumulate with aging in several tissues of various species, including in the brain in humans (Bender et al., 2006; Cantuti-Castelvetri et al., 2005; Corral-Debrinski et al., 1992; Kraytsberg et al., 2006). The possibility that high levels of somatic mtDNA mutations can play a causal role in mammalian aging is supported by the POLG mutator mouse model, which exhibits an accelerated aging phenotype (Kujoth et al., 2005; Safdar et al., 2011; Trifunovic et al., 2004). However, progeroid aging-related features were only observed in homozygous (mut/mut) mice, but not in heterozygous (+/mut) mice despite the fact that even the +/mut mice accumulate levels of mutations that are greater than those that found in normal aged WT mice (Vermulst et al., 2007). Based on these observations, it has been argued that the POLG mutator mouse model actually provides evidence against a role for somatic mtDNA mutations in normal aging (Khrapko et al., 2006; Vermulst et al., 2007). However, these conclusions were based in part on the lack of an overt phenotype in the +/mut mice. In this study, we have measured for the first time the behavioral and metabolic differences in heterozygous POLG mutator mice, and also explored the potential role of mtDNA mutations in the age-related changes in nigrostriatal dopaminergic function.
4.1 Dopaminergic system dysfunction
In humans, the loss of dopaminergic neurons during normal aging has been estimated to be about 4.7~9.8% per decade (Fearnley and Lees, 1991; Ma et al., 1999). A remarkable 36.2% loss of TH+ SN neurons was documented in a more recent study comparing older and younger control subjects (Rudow et al., 2008). Although the mechanisms contributing to this age-related loss of SN neurons are unknown, there is evidence that the age-related loss of TH+ neurons reflects neuronal death rather than loss of TH expression (Emborg et al., 1998). A more recent study demonstrated the potential role for mtDNA abnormalities by showing that dopaminergic neuron-specific expression of a mitochondria-targeted restriction enzyme to induce mtDNA depletion leads to a significant reduction in SN TH+ neurons, striatal TH and dopamine levels (Pickrell et al., 2011). Additionally, SN neurons in PD patients accumulated high levels of mtDNA deletions associated with respiratory chain deficiency (Bender et al., 2006). Levels mtDNA of point mutations reach particularly high levels in SN neurons at very early pathological stages of PD (Lin et al., 2012). POLG mutations result in the accumulation of multiple mtDNA deletions associated with parkinsonism in human (Luoma et al., 2004). Unexpectedly, although mut/mut mice showed a significant reduction (~41%) in striatal TH immunostaining intensity compared to WT mice at 12~14 months of age, we found no significant difference in TH+ neuron counts in the SN and/or VTA, suggesting a loss of TH+ striatal terminals, or TH expression within surviving terminals, rather than death of dopaminergic neurons. This reduction of striatal TH+ immunostaining intensity is consistent with the finding of mildly reduced striatal HVA, a dopamine metabolite. On the other hand, the observation that dopamine levels themselves were not significantly decreased despite the loss of TH+ terminals suggests that there may be compensatory changes in remaining striatal dopaminergic terminals. These data suggest a potential role for somatic mtDNA mutations in the age-related loss of integrity of nigral-striatal projections, but do not support a role for somatic mtDNA mutations in the age-related death of dopaminergic neurons.
4.2 Behavioral deficits
At 12~14 months of age, mut/mut mice showed significantly impaired rotarod performance and reduced voluntary locomotor activity compared with WT mice. These observations are consistent with a previous report indicating a decline in motor performance of mut/mut mice (Safdar et al., 2011). Locomotor activity is regulated in part by dopaminergic pathways (Kelly et al., 1975; Kokkotou et al., 2005). Although our experimental results indicated partial dysfunction of dopaminergic pathways in mut/mut mice, intraperitoneal injection of benserazide (20mg/kg) and L-dopa (80mg/kg) did not improve locomotor activity in these animals. It remains possible that the dose that we tested was insufficient to attenuate the dopaminergic deficits in these mice. However, striatal DA was not significantly reduced in mut/mut mice, whereas humans show substantial dopaminergic deficits before motor signs of parkinsonism emerge (Guttman et al., 1997; Pakkenberg et al., 1991). Thus, it is likely that factors other than DA deficiency account for the behavioral deficits. For example, loss of muscle mass (sarcopenia) occurs with aging in rodents (Wanagat et al., 2001) and humans (Lexell et al., 1988), and is a prominent feature in the skeletal muscle of the mut/mut POLG mice (Hiona et al., 2010; Kujoth et al., 2005; Safdar et al., 2011; Trifunovic et al., 2004). Moreover, the mut/mut mice develop a significant anemia at older ages (Chen et al., 2009; Kujoth et al., 2005), which may also have an impact on the locomotor activity. Unexpectedly, although +/mut mice bear an increased mtDNA mutational burden (Vermulst et al., 2007), their motor function measured by rotarod test and CLAMS is unimpaired, suggesting that the threshold at which mtDNA mutations becomes limiting for motor function is not reached in +/mut mice by 12~14 months of age, or that greater time is required for the impact of this mutation level to become apparent.
4.3 Metabolic deficits
A profound reduction in gene sets associated with mitochondrial function/biogenesis and in the content of ETC complexes has been found in mut/mut mice (Hiona et al., 2010; Safdar et al., 2011). These animals also display impaired mitochondrial bioenergetics associated with compromised state-3 respiration and lower ATP content (Hiona et al., 2010). Further, the accumulation of mtDNA point mutations may lead to a significant reduction in steady-state levels of complexes I, III, and IV in mut/mut mice (Edgar et al., 2009). These observations may contribute to the decreased O2 consumption and heat production that we now report in mut/mut mice. Altered body mass composition may play a role, as significant weight loss and reduced subcutaneous fat were clearly documented in mut/mut mice (Safdar et al., 2011; Trifunovic et al., 2004). However, weight loss itself does not fully account for the metabolic defects as the deficits remained significant after normalization to body mass, and were found in the +/mut mice at 12~14 months despite the fact that those mice did not display weight loss. Differences in locomotor activity also do not account for the observed metabolic defects as the differences were seen in the matched activity interval, during which mice of all genotypes displayed similarly low levels of activity. Furthermore, significant metabolic deficits in +/mut mice were found at 12~14 months of age despite the fact that these mice showed no deficits in locomotor activity. These data provide the first evidence that heterozygous (+/mut) mice, similar to mut/mut mice, suffer from mitochondrial dysfunction with decreased COX activity and ETC subunit levels, indicating a link between the observed phenotypes and mitochondrial dysfunction. Taken together, our data suggest that the accumulation of somatic mtDNA mutations in both heterozygous and homozygous POLG mutator mice leads to a significant reduction in metabolic rate independently of effects on weight or locomotor activity.
The finding of significant metabolic deficits in the heterozygous POLG mutator mice is particularly significant as a lack of a phenotype in those mice has been used as an argument against a role for somatic mtDNA mutations in normal aging (Khrapko et al., 2006; Vermulst et al., 2007). Then again, metabolic defects were not detected in the +/mut mice at 2~3 months of age despite the fact that those mice previously have been demonstrated to have high levels of somatic mtDNA mutations even at young ages (Vermulst et al., 2007). MtDNA mutations continue to accumulate with age in the POLG mutator mice, and so it is possible that the threshold for causing defects in metabolic activity is not reached until a later age for the +/mut mice. Alternatively, it may require time for the physiological impact of somatic mtDNA mutations at that level to manifest as metabolic defects. At this point, although the current and prior data on the POLG mutator mice clearly demonstrate that high levels of somatic mtDNA mutations can cause metabolic and behavioral deficits along with a premature aging phenotype, the role of somatic mtDNA mutations during normal aging and in age-related neurodegenerative diseases requires further investigation.
5. Conclusions
Our results suggest an experimental link between the accumulation of somatic mtDNA mutations and defects in motor and metabolic function in mammals. The finding of significant metabolic defects in heterozygous POLG mutator mice despite normal weight and behavioral function illustrates that detecting the impact of somatic mtDNA mutations depends on the manner of investigation. Although the role of somatic mtDNA mutations during normal aging and in neurodegenerative disorders remains uncertain, these data highlight the importance of further investigations to address these issues.
Acknowledgements
We thank Dr. Ippolita Cantuti-Castelvetri and Katharina Bilotti for helpful comments and suggestions. This study was supported by the National Institute on Aging (1R03AG035223-01; DKS) and the National Institute of Neurological Disorders and Stroke (1R21NS077758; DKS). None of the authors or co-authors have a financial interest that might be construed to influence the results or interpretation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ying Dai, Email: ydai@bidmc.harvard.edu.
Tomas Kiselak, Email: tomas.kiselak@gmail.com.
Joanne Clark, Email: jclark3@bidmc.harvard.edu.
Elizabeth Clore, Email: eclore@gmail.com.
Kangni Zheng, Email: kzheng@caregroup.harvard.edu.
Allen Cheng, Email: allen_cheng@hms.harvard.edu.
Gregory C. Kujoth, Email: gckujoth@wisc.edu.
Tomas A. Prolla, Email: taprolla@wisc.edu.
Eleftheria Maratos-Flier, Email: emaratos@bidmc.harvard.edu.
David K. Simon, Email: dsimon1@bidmc.harvard.edu.
References
- Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology. 2009;150:4931–4940. doi: 10.1210/en.2009-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- Betts-Henderson J, Jaros E, Krishnan KJ, Perry RH, Reeve AK, Schaefer AM, Taylor RW, Turnbull DM. Alpha-synuclein pathology and Parkinsonism associated with POLG1 mutations and multiple mitochondrial DNA deletions. Neuropathol Appl Neurobiol. 2009;35:120–124. doi: 10.1111/j.1365-2990.2008.00981.x. [DOI] [PubMed] [Google Scholar]
- Burns RS, LeWitt PA, Ebert MH, Pakkenberg H, Kopin IJ. The clinical syndrome of striatal dopamine deficiency. Parkinsonism induced by 1-methyl-4-phenyl- 1,2,3,6-tetrahydropyridine (MPTP) N Engl J Med. 1985;312:1418–1421. doi: 10.1056/NEJM198505303122203. [DOI] [PubMed] [Google Scholar]
- Cantuti-Castelvetri I, Lin MT, Zheng K, Keller-McGandy CE, Betensky RA, Johns DR, Beal MF, Standaert DG, Simon DK. Somatic mitochondrial DNA mutations in single neurons and glia. Neurobiol Aging. 2005;26:1343–1355. doi: 10.1016/j.neurobiolaging.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Chen AS, Metzger JM, Trumbauer ME, Guan XM, Yu H, Frazier EG, Marsh DJ, Forrest MJ, Gopal-Truter S, Fisher J, Camacho RE, Strack AM, Mellin TN, MacIntyre DE, Chen HY, Van der Ploeg LH. Role of the melanocortin- 4 receptor in metabolic rate and food intake in mice. Transgenic Res. 2000;9:145–154. doi: 10.1023/a:1008983615045. [DOI] [PubMed] [Google Scholar]
- Chen ML, Logan TD, Hochberg ML, Shelat SG, Yu X, Wilding GE, Tan W, Kujoth GC, Prolla TA, Selak MA, Kundu M, Carroll M, Thompson JE. Erythroid dysplasia, megaloblastic anemia, and impaired lymphopoiesis arising from mitochondrial dysfunction. Blood. 2009;114:4045–4053. doi: 10.1182/blood-2008-08-169474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J, Clore EL, Zheng K, Adame A, Masliah E, Simon DK. Oral N- acetyl-cysteine attenuates loss of dopaminergic terminals in alpha-synuclein overexpressing mice. PLoS One. 2010;5:e12333. doi: 10.1371/journal.pone.0012333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J, Dai Y, Simon DK. Do somatic mitochondrial DNA mutations contribute to Parkinson's disease? Parkinsons Dis. 2011 doi: 10.4061/2011/659694. 659694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, Beal MF, Wallace DC. Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat Genet. 1992;2:324–329. doi: 10.1038/ng1292-324. [DOI] [PubMed] [Google Scholar]
- Cruz-Sanchez FF, Cardozo A, Tolosa E. Neuronal changes in the substantia nigra with aging: a Golgi study. J Neuropathol Exp Neurol. 1995;54:74–81. doi: 10.1097/00005072-199501000-00009. [DOI] [PubMed] [Google Scholar]
- Edgar D, Shabalina I, Camara Y, Wredenberg A, Calvaruso MA, Nijtmans L, Nedergaard J, Cannon B, Larsson NG, Trifunovic A. Random point mutations with major effects on protein-coding genes are the driving force behind premature aging in mtDNA mutator mice. Cell Metab. 2009;10:131–138. doi: 10.1016/j.cmet.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Emborg ME, Ma SY, Mufson EJ, Levey AI, Taylor MD, Brown WD, Holden JE, Kordower JH. Age-related declines in nigral neuronal function correlate with motor impairments in rhesus monkeys. J Comp Neurol. 1998;401:253–265. [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Guttman M, Burkholder J, Kish SJ, Hussey D, Wilson A, DaSilva J, Houle S. [11C]RTI-32 PET studies of the dopamine transporter in early dopa-naive Parkinson's disease: implications for the symptomatic threshold. Neurology. 1997;48:1578–1583. doi: 10.1212/wnl.48.6.1578. [DOI] [PubMed] [Google Scholar]
- Hattori N, Tanaka M, Ozawa T, Mizuno Y. Immunohistochemical studies on complexes I, II, III, and IV of mitochondria in Parkinson's disease. Ann Neurol. 1991;30:563–571. doi: 10.1002/ana.410300409. [DOI] [PubMed] [Google Scholar]
- Hiona A, Sanz A, Kujoth GC, Pamplona R, Seo AY, Hofer T, Someya S, Miyakawa T, Nakayama C, Samhan-Arias AK, Servais S, Barger JL, Portero-Otin M, Tanokura M, Prolla TA, Leeuwenburgh C. Mitochondrial DNA mutations induce mitochondrial dysfunction, apoptosis and sarcopenia in skeletal muscle of mitochondrial DNA mutator mice. PLoS One. 2010;5:e11468. doi: 10.1371/journal.pone.0011468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson G, Schaefer AM, Taylor RW, Tiangyou W, Gibson A, Venables G, Griffiths P, Burn DJ, Turnbull DM, Chinnery PF. Mutation of the linker region of the polymerase gamma-1 (POLG1) gene associated with progressive external ophthalmoplegia and Parkinsonism. Arch Neurol. 2007;64:553–557. doi: 10.1001/archneur.64.4.553. [DOI] [PubMed] [Google Scholar]
- Inoue H, Lin L, Lee X, Shao Z, Mendes S, Snodgrass-Belt P, Sweigard H, Engber T, Pepinsky B, Yang L, Beal MF, Mi S, Isacson O. Inhibition of the leucine-rich repeat protein LINGO-1 enhances survival, structure, and function of dopaminergic neurons in Parkinson's disease models. Proc Natl Acad Sci U S A. 2007;104:14430–14435. doi: 10.1073/pnas.0700901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Invernizzi F, Varanese S, Thomas A, Carrara F, Onofrj M, Zeviani M. Two novel POLG1 mutations in a patient with progressive external ophthalmoplegia, levodopa-responsive pseudo-orthostatic tremor and parkinsonism. Neuromuscul Disord. 2008;18:460–464. doi: 10.1016/j.nmd.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Khrapko K, Kraytsberg Y, de Grey AD, Vijg J, Schon EA. Does premature aging of the mtDNA mutator mouse prove that mtDNA mutations are involved in natural aging? Aging Cell. 2006;5:279–282. doi: 10.1111/j.1474-9726.2006.00209.x. [DOI] [PubMed] [Google Scholar]
- Kokkotou E, Jeon JY, Wang X, Marino FE, Carlson M, Trombly DJ, Maratos-Flier E. Mice with MCH ablation resist diet-induced obesity through strain-specific mechanisms. Am J Physiol Regul Integr Comp Physiol. 2005;289:R117–R124. doi: 10.1152/ajpregu.00861.2004. [DOI] [PubMed] [Google Scholar]
- Koprich JB, Reske-Nielsen C, Mithal P, Isacson O. Neuroinflammation mediated by IL-1beta increases susceptibility of dopamine neurons to degeneration in an animal model of Parkinson's disease. J Neuroinflammation. 2008;5:8. doi: 10.1186/1742-2094-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet. 2006;38:518–520. doi: 10.1038/ng1778. [DOI] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Larsson NG. Somatic mitochondrial DNA mutations in mammalian aging. Annu Rev Biochem. 2010;79:683–706. doi: 10.1146/annurev-biochem-060408-093701. [DOI] [PubMed] [Google Scholar]
- Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- Lin MT, Cantuti-Castelvetri I, Zheng K, Jackson KE, Tan YB, Arzberger T, Lees AJ, Betensky RA, Beal MF, Simon DK. Somatic mitochondrial DNA mutations in early Parkinson and incidental Lewy body disease. Ann Neurol. 2012;71:850–854. doi: 10.1002/ana.23568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnane AW, Marzuki S, Ozawa T, Tanaka M. Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet. 1989;1:642–645. doi: 10.1016/s0140-6736(89)92145-4. [DOI] [PubMed] [Google Scholar]
- Luoma P, Melberg A, Rinne JO, Kaukonen JA, Nupponen NN, Chalmers RM, Oldfors A, Rautakorpi I, Peltonen L, Majamaa K, Somer H, Suomalainen A. Parkinsonism, premature menopause, and mitochondrial DNA polymerase gamma mutations: clinical and molecular genetic study. Lancet. 2004;364:875–882. doi: 10.1016/S0140-6736(04)16983-3. [DOI] [PubMed] [Google Scholar]
- Ma SY, Roytt M, Collan Y, Rinne JO. Unbiased morphometrical measurements show loss of pigmented nigral neurones with ageing. Neuropathol Appl Neurobiol. 1999;25:394–399. doi: 10.1046/j.1365-2990.1999.00202.x. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Moller A, Gundersen HJ, Mouritzen Dam A, Pakkenberg H. The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson's disease estimated with an unbiased stereological method. J Neurol Neurosurg Psychiatry. 1991;54:30–33. doi: 10.1136/jnnp.54.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker WD, Jr, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann Neurol. 1989;26:719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- Pickrell AM, Pinto M, Hida A, Moraes CT. Striatal dysfunctions associated with mitochondrial DNA damage in dopaminergic neurons in a mouse model of Parkinson's disease. J Neurosci. 2011;31:17649–17658. doi: 10.1523/JNEUROSCI.4871-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudow G, O'Brien R, Savonenko AV, Resnick SM, Zonderman AB, Pletnikova O, Marsh L, Dawson TM, Crain BJ, West MJ, Troncoso JC. Morphometry of the human substantia nigra in ageing and Parkinson's disease. Acta Neuropathol. 2008;115:461–470. doi: 10.1007/s00401-008-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdar A, Bourgeois JM, Ogborn DI, Little JP, Hettinga BP, Akhtar M, Thompson JE, Melov S, Mocellin NJ, Kujoth GC, Prolla TA, Tarnopolsky MA. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc Natl Acad Sci U S A. 2011;108:4135–4140. doi: 10.1073/pnas.1019581108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial complex I deficiency in Parkinson's disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- Severson JA, Marcusson J, Winblad B, Finch CE. Age-correlated loss of dopaminergic binding sites in human basal ganglia. J Neurochem. 1982;39:1623–1631. doi: 10.1111/j.1471-4159.1982.tb07996.x. [DOI] [PubMed] [Google Scholar]
- Siddiqi Z, Kemper TL, Killiany R. Age-related neuronal loss from the substantia nigra-pars compacta and ventral tegmental area of the rhesus monkey. J Neuropathol Exp Neurol. 1999;58:959–971. doi: 10.1097/00005072-199909000-00006. [DOI] [PubMed] [Google Scholar]
- Tatton WG, Greenwood CE, Verrier MC, Holland DP, Kwan MM, Biddle FE. Different rates of age-related loss for four murine monoaminergic neuronal populations. Neurobiol Aging. 1991;12:543–556. doi: 10.1016/0197-4580(91)90086-y. [DOI] [PubMed] [Google Scholar]
- Trifunovic A, Hansson A, Wredenberg A, Rovio AT, Dufour E, Khvorostov I, Spelbrink JN, Wibom R, Jacobs HT, Larsson NG. Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proc Natl Acad Sci U S A. 2005;102:17993–17998. doi: 10.1073/pnas.0508886102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- Vermulst M, Bielas JH, Kujoth GC, Ladiges WC, Rabinovitch PS, Prolla TA, Loeb LA. Mitochondrial point mutations do not limit the natural lifespan of mice. Nat Genet. 2007;39:540–543. doi: 10.1038/ng1988. [DOI] [PubMed] [Google Scholar]
- Wanagat J, Cao Z, Pathare P, Aiken JM. Mitochondrial DNA deletion mutations colocalize with segmental electron transport system abnormalities, muscle fiber atrophy, fiber splitting, and oxidative damage in sarcopenia. FASEB J. 2001;15:322–332. doi: 10.1096/fj.00-0320com. [DOI] [PubMed] [Google Scholar]