Abstract
Osteoporosis and atherosclerosis share common risk factors and the association of low bone mass with increased cardiovascular morbidity and mortality has been demonstrated in some studies. Nevertheless, most studies have been focused on women and only a few on individuals with type 2 diabetes mellitus (T2DM). The measurement of carotid intimal-medial thickness (CIMT) is able to detect early atherosclerotic changes and is a predictive marker of cardiovascular events. The aim of this study was to assess the CIMT and its relationship with bone mineral density (BMD) (in the femoral neck (FN) and lumbar spine (LS)) in men with T2DM. We conducted a cross-sectional study with 24 men with T2DM (aged 61 ± 6.4 years) and evaluated metabolic factors, bone densitometry values, and CIMT measured using B-mode Logic-E ultrasound machine. More than 5 years since the diagnosis of T2DM had passed in 75% of the patients, 41.6% were in statin use, mean body mass index (BMI) was 28.1 ± 3.4 kg/m2, abdominal circumference (AC) 97.8 ± 8.4 cm, systolic blood pressure (SBP) 143.8 ± 18.3 mmHg, diastolic blood pressure (DBP) 85.8 ± 12.3 mmHg, HbA1C 7.5% ± 1.3%, Triglycerides 141.7 ± 73 mg/dL, LDL-cholesterol 103.3 ± 35.9 mg/dL, HDL-cholesterol 41.6 ± 11.6 mg/dL. The patients were stratified into groups according to BMD. The group with normal BMD at FN had mean CIMT of 0.7 mm and the group with low bone mass (osteopenia or osteoporosis) had CIMT of 0.86 mm (P = 0.007). In addition, there were no significant differences between groups regarding age, duration of T2DM, BMI, AC, SBP, DBP, statin use, smoking, HbA1C, cholesterol, or triglycerides. Our data demonstrate a negative association between BMD at the FN and CIMT in type 2 diabetic men, which was unrelated to the traditional risk factors for atherosclerotic disease and degree of diabetes control.
Keywords: osteoporosis, diabetes, carotid artery intimal-medial thickness, carotid, osteoporosis
Introduction
The ageing population has a higher prevalence of diabetes mellitus, osteoporosis, and atherosclerosis, three of the major public health problems worldwide. The association between low bone mineral density (BMD) and increased cardiovascular morbidity and mortality has been suggested in some studies,1–6 conducted mainly in postmenopausal women.7–10 Few of them included men as well as patients who have type 2 diabetes mellitus (T2DM).11
The association between cardiovascular disease and calcium deposition was first described in the nineteenth century,12 but only in the late twentieth century were some elements linking atherosclerosis and low BMD discussed.13 These diseases share some common risk factors such as advanced age, estrogen deficiency, inflammation, and hyperhomocysteinemia.3–5 Additionally, some studies have suggested a close association between them.2,7,9
Measurement of the intimal-medial thickness in the carotid arteries with B-mode ultrasound is a noninvasive, sensitive, and reproducible technique for identifying and quantifying subclinical vascular disease and the risk of a wide range of cardiovascular diseases.14–20 It is, therefore, important to determine whether this occurs in the context of low BMD in men with longstanding T2DM, a population known to have a high risk of atherosclerotic vascular disease and a possible decrease in bone strength.21
The analysis of the association between low bone mass and atherosclerosis in male diabetic population is important because of the lack of studies on this subject. The study of this relationship helps to establish the need for further investigation on the presence of atherosclerotic involvement in elderly diabetic men with low bone mass and vice versa.
The aim of this study was to assess the carotid intimal-medial thickness (CIMT) and its relationship with BMD in men with T2DM.
Subjects and Methods
Study population
The patients were 24 men, aged from 60 to 80 years, with the diagnosis of T2DM according to the American Diabetes Association criteria.22 The study was approved by the Ethics in Research Committee of Agamenon Magalhães Hospital. The exclusion criteria were as follows: patients with type 1 diabetes, defined as patients diagnosed before the age of 20 or who have been hospitalized for diabetic ketoacidosis; and/or chronic use (past or present) of glucocorticoids. Laboratory or radiological evidence of one of the following: hyperthyroidism and hypothyroidism (patients with normal TSH levels were stabilized and included), hypoparathyroidism or hyperparathyroidism, rheumatoid arthritis, Paget’s disease of bone, bone disease such as osteomalacia or osteogenesis imperfecta, advanced scoliosis or extensive lumbar fusion, malabsorption syndrome, and/or malignant neoplasm in the last five years (except basal cell carcinoma).
After informed consent was obtained, the patients answered a specific questionnaire and underwent a complete physical examination. Blood was collected after an overnight fast for biochemical measurements.
The ultrasound study of carotid arteries was performed with subjects in the supine position using the B-mode Logic-E ultrasound machine (GE Medical Systems). The CIMT was measured at three different sites on the left and right sides. We calculated the average of both sides and then the bilateral mean, using a total of six measurements per patient which were performed by a single radiologist (EG).
Serum chemistries were processed in a dry chemistry auto-analyzer (Vitros system, Johnson & Johnson), and hemoglobin A1c concentrations were determined by immunoturbidimetric assay (Cobas 501, Roche Diagnostic).
The bone densitometry was performed by dual X-ray absorptiometry of the lumbar spine (LS) at L1 to L4 and femoral neck (FN) (GE Healthcare Lunar Prodigy Advance—Lunar Corporation, Madison, Wisconsin) and was used to measure the t-score to stratify patients according to the presence of osteoporosis (defined as a t-score less than −2.5) and osteopenia (defined as a t-score between −1 and −2.5), according to the World Health Organization criteria.23
Statistical analysis
Analyses were performed separately by taking into account the extent of BMD of FN or LS compared to CIMT. At each site, patients were divided into two groups, the first comprising patients who had normal BMD (t-score > −1), the second group comprising those with osteopenia or osteoporosis. Correlations were also performed between BMD sites in normal and osteopenia or osteoporosis patients. The differences between all the risk factors studied was obtained using the Student t test for quantitative variables and the chi square test in the case of qualitative variables. The correlation measurements were made using the Pearson correlation test. A confidence interval of 95% was used. The statistical analyses were performed using the SPSS Statistics (Version 17.0). The significance was considered for a P value less than 0.05.
Results
The baseline characteristics of the studied patients are presented in Table 1. No significant differences were found in age, time since the diagnosis of diabetes, smoking habits, alcohol intake, blood pressure, BMI, AC, HbA1c, and serum lipids between the groups with normal and abnormal BMD results both at LS and FN (Tables 2 and 3). The prevalence of osteopenia and osteoporosis at the FN was 45.8% and 16.6%, respectively, while the corresponding figures for the LS were 62.5% and 8.3%, respectively.
Table 1.
General characteristics of the study patients.
| Variable | Mean or % | SD |
|---|---|---|
| Age (years) | 61 | 6.4 |
| Mean CIMT (mm) | 0.80 | 0.14 |
| BMD FN (g/cm2) | 0.866 | 0.116 |
| BMD FN (t-score) | −1.50 | 0.92 |
| BMD LS (g/cm2) | 1.069 | 0.126 |
| BMD LS (t-score) | 1.30 | 1.00 |
| Diagnosis of diabetes > 5 years | 75% | |
| Use of statins | 41.6% | |
| Smoking | 4.1% | |
| Alcohol intake | 8.3% | |
| Total cholesterol (mg/dL) | 176.1 | 44.2 |
| Triglycerides (mg/dL) | 141.7 | 73.0 |
| LDL (mg/dL) | 103.3 | 35.9 |
| HDL (mg/dL) | 41.6 | 11.6 |
| HbA1C (%) | 7.5 | 1.3 |
| BMI kg/m2 | 28.1 | 3.4 |
| Abdominal circumfrence (cm) | 97.8 | 8.4 |
| SBP (mmHg) | 143.8 | 18.3 |
| DBP (mmHg) | 85.8 | 12.3 |
Table 2.
Characteristics of patients according to femoral neck BMD.
| Variable | Normal BMD | Abnormal BMD | P | ||
|---|---|---|---|---|---|
|
|
|
||||
| Mean or % | SD | Mean or % | SD | ||
| Age (years) | 59.5 | 6.89 | 62.06 | 6.27 | 0.370 |
| Diagnosis of diabetes > 5 years | 66.7% | 80% | 0.465 | ||
| Use of statins | 55.6% | 33.3% | 0.285 | ||
| Smoking | 0% | 6.7% | 0.429 | ||
| Alcohol intake | 22.2% | 0% | 0.057 | ||
| Total cholesterol (mg/dL) | 179.4 | 25.8 | 174.9 | 50.4 | 0.854 |
| Triglycerides (mg/dL) | 145.8 | 62.70 | 140.3 | 78.5 | 0.891 |
| LDL (mg/dL) | 103.8 | 16.2 | 103.2 | 41.7 | 0.977 |
| HDL (mg/dL) | 46.4 | 19.32 | 39.9 | 7.7 | 0.298 |
| HbA1C (%) | 7.67 | 1.76 | 7.17 | 0.86 | 0.431 |
| BMI (kg/m2) | 27.78 | 3.63 | 28.31 | 3.20 | 0.727 |
| Abdominal circumference (cm) | 97.11 | 9.76 | 98.45 | 7.33 | 0.722 |
| SBP (mmHg) | 140.55 | 13.79 | 146.00 | 19.75 | 0.480 |
| DBP (mmHg) | 86.11 | 10.24 | 85.64 | 13.56 | 0.930 |
Table 3.
Characteristics of patients according to lumbar spine BMD.
| Variable | Normal BMD | Abnormal BMD | P | ||
|---|---|---|---|---|---|
|
|
|
||||
| Mean or % | SD | Mean or % | SD | ||
| Age (years) | 60.0 | 9.05 | 61.58 | 5.31 | 0.597 |
| Diagnosis of diabetes > 5 years | 85.7% | 70.6% | 0.437 | ||
| Use of statins | 28.6% | 47.1% | 0.404 | ||
| Smoking | 0% | 5.9% | 0.512 | ||
| Alcohol intake | 0% | 11.8% | 0.343 | ||
| Total cholesterol (mg/dL) | 170.00 | 59.43 | 177.40 | 43.15 | 0.800 |
| Triglycerides (mg/dL) | 152.50 | 56.53 | 138.93 | 78.29 | 0.752 |
| LDL (mg/dL) | 100.25 | 51.23 | 104.28 | 32.89 | 0.850 |
| HDL (mg/dL) | 35.25 | 4.85 | 43.33 | 12.39 | 0.226 |
| HbA1C (%) | 7.22 | 0.73 | 7.59 | 1.48 | 0.643 |
| BMI (kg/m2) | 27.09 | 3.48 | 28.62 | 3.20 | 0.331 |
| Abdominal circumference (cm) | 98.78 | 8.93 | 97.42 | 8.21 | 0.732 |
| SBP (mmHg) | 142.8 | 13.80 | 144.31 | 19.32 | 0.860 |
| DBP (mmHg) | 89.28 | 7.31 | 84.31 | 13.63 | 0.377 |
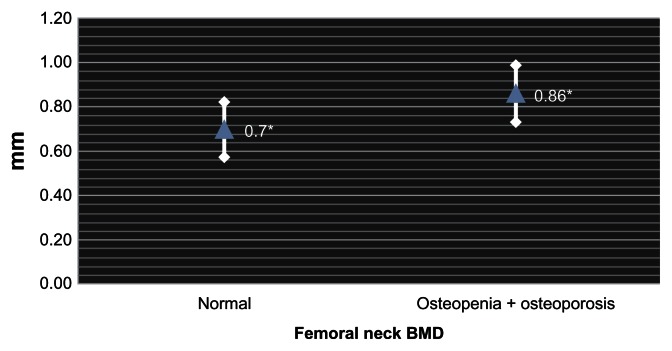
When stratified according to the FN BMD, nine patients had BMD within the normal range with mean CIMT of 0.7 mm (SD ± 0.12), while 15 patients met the criteria for osteopenia or osteoporosis with a mean carotid IMT of 0.86 mm (SD ± 0.13), statistical significance being observed in the variance between groups (P = 0.007) (Fig. 1). When comparing patients with normal BMD to those with osteopenia in the FN, we found a mean CIMT of 0.70 mm (SD ± 0.12) and 0.86 mm (SD ± 0.13), respectively, attaining statistical significance (P = 0.012). When comparing patients with normal BMD in FN patients with those with osteoporosis, we obtained a mean CIMT of 0.84 mm (SD ± 0.10) in the latter and 0.70 mm (SD ± 0.12) in the former (P = 0.081).
Figure 1.
Carotid intimal-medial thickness according to the mean BMD of the femoral neck.
Note: *P = 0.007.
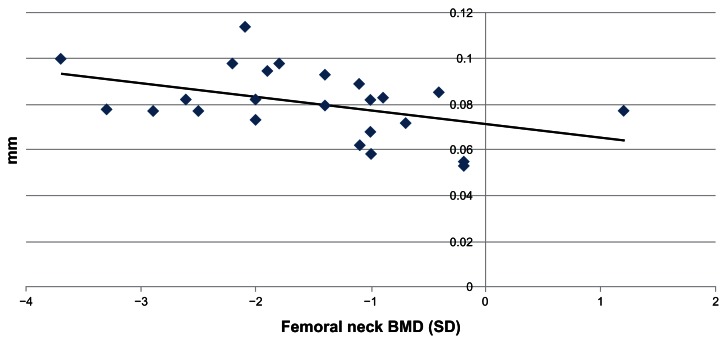
Regarding LSBMD, 7 (29%) patients had a normal result, while 17 patients (71%) were found to have osteopenia or osteoporosis. The mean CIMT was 0.81 mm (SD ± 0.21) in the normal group and 0.80 mm (SD ± 0.12) in the group with low BMD (Fig. 2); P = 0.913. The Pearson correlation analysis was performed between the FN BMD and the CIMT, resulting in a negative correlation (r = −0.449); P = 0.028, while the same analysis between the LS BMD and the carotid IMT yielded had no statistical significance (r = 0.059); P = 0.783.
Figure 2.
Correlation between bone mineral density in the femoral neck and carotid intimal-medial thickness.
Note: r = −0.449, P = 0.028.
Discussion
Our data showed a significant correlation between decreases in BMD and CIMT in men with T2DM, which was unrelated to traditional risk factors such as blood glucose control, blood pressure, or serum lipids concentrations. Atherosclerosis and osteoporosis may share common risk factors such as advanced age, estrogen deficiency, inflammation, hyperhomocysteinemia and activation of RANKL/OPG system. One study24 suggested that some polymorphisms in vitamin D receptor gene could be a common point between the BMD variation and CIMT. Another study has found an association with the RANK (receptor activator of nuclear factor kappa B)/RANK-Ligand/OPG (Osteoprotegerin) and vascular pathology due its activation in the case of high bone remodeling states.25,26
One study suggested a unified theory involving the cholesterol metabolites (isoprenoids) as the cause of atherosclerosis, peripheral vascular disease, coronary disease, and age-related diseases such as osteoporosis and T2DM.27
The measurement of BMD at the FN may be more accurate in old people when compared to that of the spine, because of a lack of accuracy in systematic measurements, due to spine elements irregularities, which may lead to false-positive findings. Because of this, the World Health Organization, when developing the absolute risk score for osteoporotic fractures, did not recommended the LS measurement.28 In the diabetic population, especially those with longstanding disease, it is expected to find a higher frequency of arterial calcification which, occurring in the aorta, could directly interfere with bone density acquisition at LS, also causing false-positive results. Likewise, we found considerable less bone loss at LS in comparison with FN in our diabetic male patients.
One of the proposed mechanisms to explain the increased risk of osteoporotic fracture in the diabetic population is the accumulation of advanced glycation end-products which is thought to influence collagen synthesis and structure.21,29 In fact, although with a relative higher BMD, type 2 diabetic patients tend to have an increased risk of fracture, which may be related to pentosidine levels.21,30 Advanced glycation end-products are also considered, particularly in the diabetic population, a known step for modifications of LDL particle and in a more direct way for changes in the arterial wall, thus contributing to the atherosclerosis process.31
Finally, we have recently demonstrated that men with longstanding T2DM also have significant iliac artery calcification as FN BMD decreases, which is not related to traditional risk factors for cardiovascular disease nor the presence of advanced renal insufficiency.32
The findings of this study are consistent with those of other studies previously conducted in postmenopausal women and suggest that both diseases are clearly present in the elderly diabetic population.
In conclusion, we found a significant association between an increased thickness of carotid intimal-medial complex and low bone density in patients with T2DM, irrespective of other cardiovascular risk factors. This indicates that these individuals, in addition to their risk of cardiovascular disease, have a concomitant increased risk of developing osteoporosis.
Footnotes
Author Contributions
Conceived and designed the experiments: MAPC, FB. Analyzed the data: MAPC, EB. Wrote the first draft of the manuscript: MAPC, JMCMA, ETAMG, GV. Contributed to the writing of the manuscript: MAPC, EB, JMCMA, ETAMG, GV, FB. Agree with manuscript results and conclusions: MAPC, EB, JMCMA, ETAMG, GV, FB. Jointly developed the structure and arguments for the paper: MAPC, EB, JMCMA, ETAMG, GV, FB. Made critical revisions and approved final version: MAPC, EB, JMCMA, ETAMG, GV, FB. All authors reviewed and approved of the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
Funding
Author(s) disclose no funding sources.
References
- 1.Anagnostis P, Karagiannis A, Kakafika AI, Tziomalos K, Athyros VG, Mikhailidis DP. Atherosclerosis and osteoporosis: age-dependent degenerative processes or related entities? Osteoporos Int. 2009 Feb;20(2):197–207. doi: 10.1007/s00198-008-0648-5. [DOI] [PubMed] [Google Scholar]
- 2.Bagger YZ, Rasmussen HB, Alexandersen P, et al. Links between cardiovascular disease and osteoporosis in postmenopausal women: serum lipids or atherosclerosis per se? Osteoporos Int. 2007;168(4):505–12. doi: 10.1007/s00198-006-0255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldini V, Mastropasqua M, Francucci CM, et al. Cardiovascular disease and osteoporosis. J Endocrinol Invest. 2005;28(Suppl 10):69–72. [PubMed] [Google Scholar]
- 4.Browner WAS, Lui LY, Cummings SR. Associations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly women. J Clin Endocrinol Metab. 2001;86(2):631–7. doi: 10.1210/jcem.86.2.7192. [DOI] [PubMed] [Google Scholar]
- 5.McFarlane SI, Muniyappa R, Shin JJ, Bahtiyar G, Sowers JR. Osteoporosis and cardiovascular disease: brittle bones and boned arteries, is there a link? Endocrine. 2004;23(1):1–10. doi: 10.1385/ENDO:23:1:01. [DOI] [PubMed] [Google Scholar]
- 6.Gupta G, Aronow WS. Atherosclerotic vascular disease may be associated with osteoporosis or osteopenia in postmenopausal women: a preliminary study. Arch Gerontol Geriatr. 2006;43(2):285–8. doi: 10.1016/j.archger.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Hyder JA, Allison MA, Wong N, et al. Association of coronary artery and aortic calcium with lumbar bone density: the MESA Abdominal Aortic Calcium Study. Am J Epidemiol. 2009;169(2):186–94. doi: 10.1093/aje/kwn303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanko LB, Christiansen C, Cox DA, Geiger MJ, McNabb MA, Cummings SR. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res. 2005;20(11):1912–20. doi: 10.1359/JBMR.050711. [DOI] [PubMed] [Google Scholar]
- 9.Uyama O, Yoshimoto Y, Yamamoto Y, Kawai A. Bone changes and carotid atherosclerosis in postmenopausal women. Stroke. 1997;28(9):1730–2. doi: 10.1161/01.str.28.9.1730. [DOI] [PubMed] [Google Scholar]
- 10.Tamaki J, Iki M, Hirano Y, et al. Low bone mass is associated with carotid atherosclerosis in postmenopausal women: the Japanese Population-based Osteoporosis (JPOS) Cohort Study. Osteoporos Int. 2009;20(1):53–60. doi: 10.1007/s00198-008-0633-z. [DOI] [PubMed] [Google Scholar]
- 11.Kitamura A, Iso H, Imano H, et al. Carotid intima-media thickness and plaque characteristics as a risk factor for stroke in Japanese elderly men. Stroke. 2004;35(12):2788–94. doi: 10.1161/01.STR.0000147723.52033.9e. [DOI] [PubMed] [Google Scholar]
- 12.Virchow R. Lecture XVI-atheromatous affection of arteries. Nutr Rev. 1858;47(1):23–5. doi: 10.1111/j.1753-4887.1989.tb02747.x. [DOI] [PubMed] [Google Scholar]
- 13.Laroche M. Arteriosclerosis and osteoporosis. Press Med. 1996;25(2):52–4. [PubMed] [Google Scholar]
- 14.Belcaro G, Nicolaides AN, Ramaswami G, et al. Carotid and femoral ultrasound morphology screening and cardiovascular events in low risk subjects: The 10-year follow-up study (the COFFEES-CAVE study) Atherosclerosis. 2001;156(2):379–87. doi: 10.1016/s0021-9150(00)00665-1. [DOI] [PubMed] [Google Scholar]
- 15.Chambless LE, Heiss G, Folsom AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities. Am J Epidemiol. 1997;146(6):483–94. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 16.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults: Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340(1):14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 17.Chambless LE, Folsom AR, Clegg LX, et al. Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 2000;151(5):478–87. doi: 10.1093/oxfordjournals.aje.a010233. [DOI] [PubMed] [Google Scholar]
- 18.Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS) Stroke. 2006;37(1):87–92. doi: 10.1161/01.STR.0000196964.24024.ea. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115(4):459–67. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 20.Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography. J Am Soc Echocardiogr. 2008;21(2):93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz A, Garnero P, Hillier TE, et al. Pentosidine and increased fracture risk in older adults with type 2 diabetes. J Clin Endocrinol Metab. 2009;94(7):2380–6. doi: 10.1210/jc.2008-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olson DE, Rhee MK, Herrick K, Ziemer D, Twombly JG, Phillips LS. Screening for diabetes and pre-diabetes with proposed A1C-based diagnostic criteria. Diabetes Care. 2010;33(10):2184–9. doi: 10.2337/dc10-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanis JA, Gluer CC. An update on the diagnosis and assessment of osteoporosis with densitometry. Osteoporos Int. 2000;11(3):191–202. doi: 10.1007/s001980050281. [DOI] [PubMed] [Google Scholar]
- 24.Kammerer CM, Dualan AA, Samollow PB, et al. Bone mineral density, carotid artery intimal medial thickness, and the vitamin D receptor BsmI polymorphism in Mexican American women. Calcif Tissue Int. 2004;75(4):292–8. doi: 10.1007/s00223-004-0215-9. [DOI] [PubMed] [Google Scholar]
- 25.Collin-Osdoby P. The regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ Res. 2004;95(11):1046–57. doi: 10.1161/01.RES.0000149165.99974.12. [DOI] [PubMed] [Google Scholar]
- 26.Pennisi P, Signorelli SS, Riccobene S, et al. Low bone density and abnormal bone turnover in patients with atherosclerosis of peripheral vessels. Osteoporos Int. 2004;15(5):389–95. doi: 10.1007/s00198-003-1550-9. [DOI] [PubMed] [Google Scholar]
- 27.Omoigui S. Cholesterol synthesis is the trigger and isoprenoid dependent interleukin-6 mediated inflammation is the common causative factor and therapeutic target for atherosclerotic vascular disease and age-related disorders including osteoporosis and type 2 diabetes. Med Hypothesis. 2005;65(3):559–69. doi: 10.1016/j.mehy.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Kanis JA, McCloskey EV, Johansson H, et al. Case finding for the management of osteoporosis with FRAX—assessment and intervention thresholds for the UK. Osteoporos Int. 2008;19(10):1395–408. doi: 10.1007/s00198-008-0712-1. [DOI] [PubMed] [Google Scholar]
- 29.Lindsey JB, Cipollone F, Abdullah SM, McGuire DK. Receptor for advanced glycation end-products (RAGE) and soluble RAGE (sRAGE): cardiovascular implications. Diab Vasc Dis Res. 2009;6(1):7–14. doi: 10.3132/dvdr.2009.002. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto M, Yamaguchi T, Yamauchi M, Yano S, Sugimoto T. Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab. 2008;93(3):1013–9. doi: 10.1210/jc.2007-1270. [DOI] [PubMed] [Google Scholar]
- 31.Dan Q, Wong R, Chung SK, Chung SS, Lam KS. Interaction between the polyol pathway and non-enzymatic glycation on aortic smooth muscle cell migration and monocyte adhesion. Life Sci. 2044;76(4):445–59. doi: 10.1016/j.lfs.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Bandeira E, Neves AP, Costa C, Bandeira F. Association between vascular calcification and osteoporosis in men with type 2 diabetes. J Clin Densitom. 2012;15(1):55–60. doi: 10.1016/j.jocd.2011.07.002. [DOI] [PubMed] [Google Scholar]