Abstract
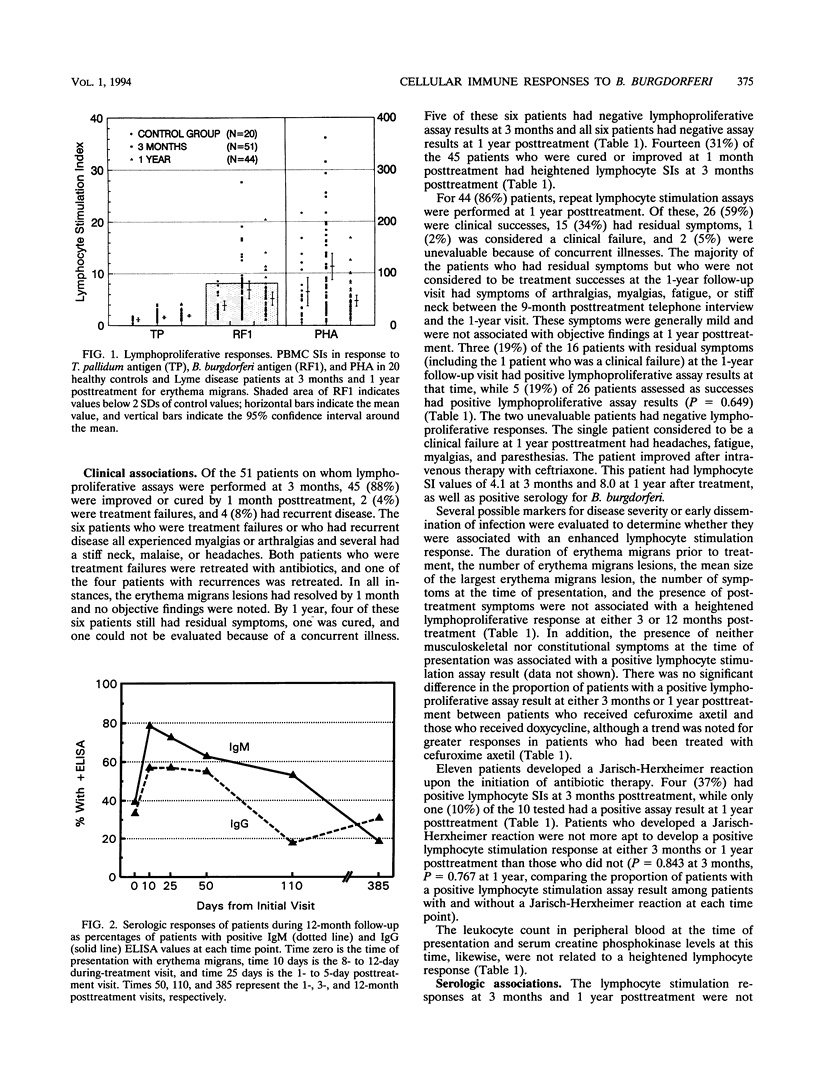
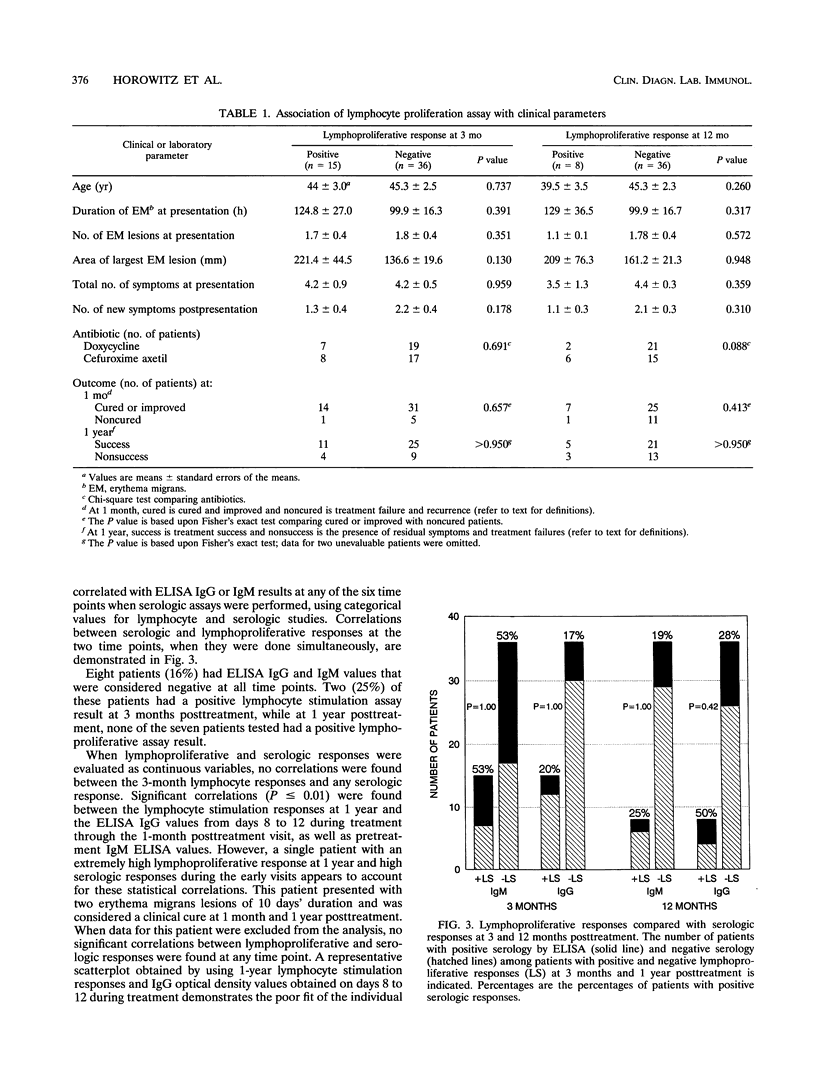
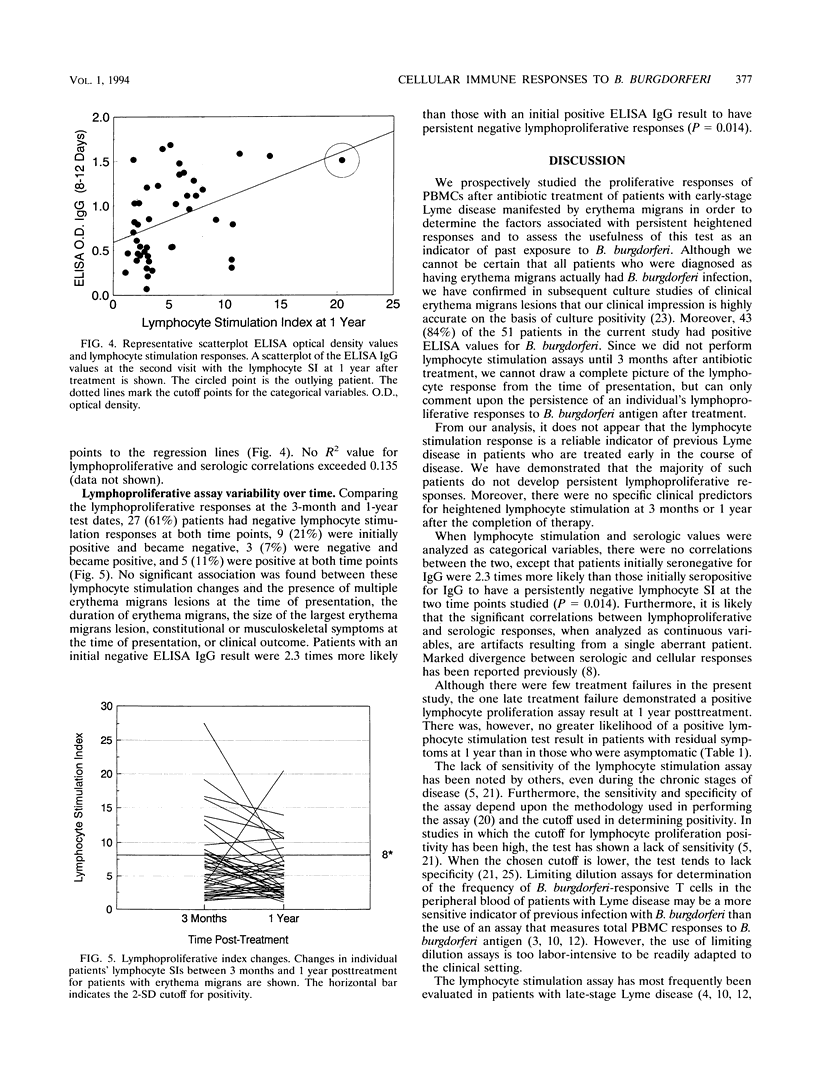
Fifty-one patients with erythema migrans were followed up prospectively with serial clinical evaluations, serologic determinations for antiborrelial antibodies, and lymphocyte stimulation responses to Borrelia burgdorferi antigens to determine (i) the factors associated with sustained cellular immune responses and (ii) whether lymphocyte stimulation is a good indicator of prior exposure to B. burgdorferi in patients treated early after erythema migrans. Positive lymphocyte stimulation responses ( > 2 standard deviations above normal control values) were found in 15 (29%) of 51 patients 3 months after treatment for erythema migrans and in 8 (18%) of 44 patients 1 year posttreatment. Heightened lymphocyte responses were not associated with the number or duration of erythema migrans lesions prior to treatment, the mean size of the largest erythema migrans lesion, or the number of symptoms at the time of presentation. The development of Jarisch-Herxheimer reaction, choice of antibiotic, and clinical outcome also were not associated with a positive lymphoproliferation assay result. Changes in the lymphocyte stimulation indices between the two time points assessed (3 months and 1 year posttreatment) also did not correlate with the above variables. When serologic results and lymphoproliferative responses were evaluated as categorical or continuous variables, there were no correlations between values. One year after treatment for early Lyme disease, lymphocyte reactivity is not a good indicator of prior infection with B. burgdorferi.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berardi V. P., Weeks K. E., Steere A. C. Serodiagnosis of early Lyme disease: analysis of IgM and IgG antibody responses by using an antibody-capture enzyme immunoassay. J Infect Dis. 1988 Oct;158(4):754–760. doi: 10.1093/infdis/158.4.754. [DOI] [PubMed] [Google Scholar]
- Dattwyler R. J., Thomas J. A., Benach J. L., Golightly M. G. Cellular immune response in Lyme disease: the response to mitogens, live Borrelia burgdorferi, NK cell function and lymphocyte subsets. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):151–159. doi: 10.1016/s0176-6724(86)80118-3. [DOI] [PubMed] [Google Scholar]
- Dattwyler R. J., Volkman D. J., Halperin J. J., Luft B. J., Thomas J., Golightly M. G. Specific immune responses in Lyme borreliosis. Characterization of T cell and B cell responses to Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:93–102. doi: 10.1111/j.1749-6632.1988.tb31842.x. [DOI] [PubMed] [Google Scholar]
- Dattwyler R. J., Volkman D. J., Luft B. J., Halperin J. J., Thomas J., Golightly M. G. Seronegative Lyme disease. Dissociation of specific T- and B-lymphocyte responses to Borrelia burgdorferi. N Engl J Med. 1988 Dec 1;319(22):1441–1446. doi: 10.1056/NEJM198812013192203. [DOI] [PubMed] [Google Scholar]
- Dressler F., Yoshinari N. H., Steere A. C. The T-cell proliferative assay in the diagnosis of Lyme disease. Ann Intern Med. 1991 Oct 1;115(7):533–539. doi: 10.7326/0003-4819-115-7-533. [DOI] [PubMed] [Google Scholar]
- Krause A., Brade V., Schoerner C., Solbach W., Kalden J. R., Burmester G. R. T cell proliferation induced by Borrelia burgdorferi in patients with Lyme borreliosis. Autologous serum required for optimum stimulation. Arthritis Rheum. 1991 Apr;34(4):393–402. doi: 10.1002/art.1780340404. [DOI] [PubMed] [Google Scholar]
- Krause A., Burmester G. R., Rensing A., Schoerner C., Schaible U. E., Simon M. M., Herzer P., Kramer M. D., Wallich R. Cellular immune reactivity to recombinant OspA and flagellin from Borrelia burgdorferi in patients with Lyme borreliosis. Complexity of humoral and cellular immune responses. J Clin Invest. 1992 Sep;90(3):1077–1084. doi: 10.1172/JCI115923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger S. W., Krauss E. Serologic tests for Lyme disease. Interlaboratory variability. Arch Intern Med. 1990 Apr;150(4):761–763. [PubMed] [Google Scholar]
- Martin R., Ortlauf J., Sticht-Groh V., Bogdahn U., Goldmann S. F., Mertens H. G. Borrelia burgdorferi--specific and autoreactive T-cell lines from cerebrospinal fluid in Lyme radiculomyelitis. Ann Neurol. 1988 Oct;24(4):509–516. doi: 10.1002/ana.410240406. [DOI] [PubMed] [Google Scholar]
- Nadelman R. B., Luger S. W., Frank E., Wisniewski M., Collins J. J., Wormser G. P. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Ann Intern Med. 1992 Aug 15;117(4):273–280. doi: 10.7326/0003-4819-117-4-273. [DOI] [PubMed] [Google Scholar]
- Neumann A., Schlesier M., Schneider H., Vogt A., Peter H. H. Frequencies of Borrelia burgdorferi-reactive T lymphocytes in Lyme arthritis. Rheumatol Int. 1989;9(3-5):237–241. doi: 10.1007/BF00271888. [DOI] [PubMed] [Google Scholar]
- Pachner A. R., Steere A. C., Sigal L. H., Johnson C. J. Antigen-specific proliferation of CSF lymphocytes in Lyme disease. Neurology. 1985 Nov;35(11):1642–1644. doi: 10.1212/wnl.35.11.1642. [DOI] [PubMed] [Google Scholar]
- Pavia C. S., Bittker S., Cooper D. Immune response to the Lyme spirochete Borrelia burgdorferi affected by ethanol consumption. Immunopharmacology. 1991 Nov-Dec;22(3):165–173. doi: 10.1016/0162-3109(91)90041-v. [DOI] [PubMed] [Google Scholar]
- Pavia C. S., Niederbuhl C. J. Adoptive transfer of anti-syphilis immunity with lymphocytes from Treponema pallidum-infected guinea pigs. J Immunol. 1985 Oct;135(4):2829–2834. [PubMed] [Google Scholar]
- Sigal L. H., Steere A. C., Freeman D. H., Dwyer J. M. Proliferative responses of mononuclear cells in Lyme disease. Reactivity to Borrelia burgdorferi antigens is greater in joint fluid than in blood. Arthritis Rheum. 1986 Jun;29(6):761–769. doi: 10.1002/art.1780290609. [DOI] [PubMed] [Google Scholar]
- Stiernstedt G., Dattwyler R., Duray P. H., Hansen K., Jirous J., Johnson R. C., Karlsson M., Preac-Mursic V., Schwan T. G. Diagnostic tests in Lyme borreliosis. Scand J Infect Dis Suppl. 1991;77:136–142. [PubMed] [Google Scholar]
- Wallach F. R., Murray H. W. Lymphocyte proliferation assay in Lyme disease. J Infect Dis. 1992 Oct;166(4):938–939. doi: 10.1093/infdis/166.4.938-a. [DOI] [PubMed] [Google Scholar]
- Weyand C. M., Goronzy J. J. Immune responses to Borrelia burgdorferi in patients with reactive arthritis. Arthritis Rheum. 1989 Sep;32(9):1057–1064. doi: 10.1002/anr.1780320902. [DOI] [PubMed] [Google Scholar]
- Wormser G. P., Forseter G., Cooper D., Nowakowski J., Nadelman R. B., Horowitz H., Schwartz I., Bowen S. L., Campbell G. L., Goldberg N. S. Use of a novel technique of cutaneous lavage for diagnosis of Lyme disease associated with erythema migrans. JAMA. 1992 Sep 9;268(10):1311–1313. [PubMed] [Google Scholar]
- Yoshinari N. H., Reinhardt B. N., Steere A. C. T cell responses to polypeptide fractions of Borrelia burgdorferi in patients with Lyme arthritis. Arthritis Rheum. 1991 Jun;34(6):707–713. doi: 10.1002/art.1780340611. [DOI] [PubMed] [Google Scholar]
- Zoschke D. C., Skemp A. A., Defosse D. L. Lymphoproliferative responses to Borrelia burgdorferi in Lyme disease. Ann Intern Med. 1991 Feb 15;114(4):285–289. doi: 10.7326/0003-4819-114-4-285. [DOI] [PubMed] [Google Scholar]



