Abstract
Inflammatory processes play both regenerative and destructive roles in multiple sclerosis, stroke, CNS trauma, amyotrophic lateral sclerosis and aging-related neurodegenerative diseases such as Alzheimer's, Parkinson's and Huntington's. Endogenous defence mechanisms against these pathologies include those that are directly neuroprotective, and those that modulate the expression of inflammatory mediators in microglia, astrocytes, and invading inflammatory cells. While a number of mechanisms and molecules have been identified that can directly promote neuronal survival, less is known about how the brain protects itself from harmful inflammation, and further, how it co-opts the healing function of the immune system to promote CNS repair. The two closely related neuroprotective peptides, vasoactive intestinal peptide (VIP) and pituitary adenylyl cyclase-activating peptide (PACAP), which are up-regulated in neurons and immune cells after injury and/or inflammation, are known to protect neurons, but also exert powerful in vivo immunomodulatory actions, which are primarily anti-inflammatory. These peptide actions are mediated by high-affinity receptors expressed not only on neurons, but also astrocytes, microglia and peripheral inflammatory cells. Well-established immunomodulatory actions of these peptides are to inhibit macrophage and microglia production and release of inflammatory mediators such as TNF-α and IFN-γ, and polarization of T-cell responses away from Th1 and Th17, and towards a Th2 phenotype. More recent studies have revealed that these peptides can also promote the production of both natural and inducible subsets of regulatory T-cells. The neuroprotective and immunomodulatory actions of VIP and PACAP suggest that receptors for these peptides may be therapeutic targets for neurodegenerative and neuroinflammatory diseases and other forms of CNS injury.
Keywords: multiple sclerosis, experimental autoimmune encephalomyelitis, EAE, VIP, PACAP, neuropeptides, inflammation, neurodegeneration, injury
Introduction
The brain was once thought to be excluded from immune surveillance and other inflammatory processes except under a few specific pathological conditions such as CNS infection and multiple sclerosis (MS). Indeed foreign antigens are quite limited in their ability to elicit robust T-cell or humoral responses in the CNS due to a relative lack of lymphoid drainage and limited expression of major histocompatibility complex molecules. Moreover, neither strong innate nor amplified adaptive inflammatory responses are generally observed in the brain due to the presence of tight endothelial junctions and astrocyte endfeet that provide a barrier against influx of inflammatory cells. A long-standing view in neuroscience that the brain is a highly immunoprivileged organ, as opposed to one that is relatively immune-privileged, however, impeded progress in our understanding of fundamental mechanisms by which the brain attempts to protect and repair itself in common pathologies Fortunately, this view is changing with newly identified genetic linkages of immune-related genes with neurological diseases and the realization that perhaps all neurodegenerative diseases and even certain mental health disorders such as autism are often accompanied by neuroinflammation and peripheral markers of inflammation. Attesting to this change of thinking was the dedication in 2009 of an entire issue of Neuron to neural-immune interactions, and issues of Nature Reviews Immunology and Nature Neuroscience in 2009 and 2012, respectively, with a major focus on the topic of neuroimmunology. The following review is intended to describe how two neuropeptides, vasoactive intestinal peptide (VIP) and pituitary adenylyl cyclase-activating peptide (PACAP), may act in neurological diseases as neuroprotective and immunomodulatory factors.
Significance of inflammation in the nervous system
It has been presumed that the process of neurotransmission in the brain might be too delicate to withstand the robust release of reactive oxygen species during active inflammation. While this may be true, an estimated 10–20% of brain cells are microglia, the resident macrophages (MΦ) of the CNS. These cells, along with astrocytes, can be activated under various pathological conditions to produce a host of molecules, some of which are proinflammatory, while others are anti-inflammatory, neuroprotective or regenerative. In addition, it is now clear that perivascular MΦ are abundantly associated with the leptomeningeal blood vessels on the surface of the brain and major penetrating blood vessels. Moreover, in normal animals, T lymphocytes constantly flux in and out of the brain parenchyma. Whether or not these T-cells function to scan for foreign or altered antigens or if they are involved in tolerance or protection remains to be firmly established. In any case, the presence of myeloid and lymphocytic cells within or near the brain parenchyma implies that at least some degree of immune cell activity may occur in the CNS, most likely to deter infection, provide tolerance and/or to abet healing after injury. Finally, it must be considered that the normal physical and molecular barriers to immune cell flux can be severely disrupted in stroke, CNS trauma and autoimmune diseases such as MS, and in some cases are overwhelmed by infectious agents. It thus seems likely that additional mechanisms need to be deployable by the nervous system to modulate excessive CNS inflammation, prevent damage and promote healing after stroke, trauma and perhaps neurodegeneration. As discussed below, neuropeptides such as VIP and PACAP are strongly up-regulated in injury and inflammation and function in these capacities.
VIP, PACAP, ligands and receptors: general biological functions and signalling mechanisms
VIP was discovered in 1970 as a 28-amino acid polypeptide in intestinal extracts capable of inducing system vasodilation, and later found to be present in myenteric and submucosal gastrointestinal neurons, but also in specific populations of neurons of the central, autonomic and sensory nervous systems. PACAP was discovered almost two decades later as a 38-amino acid hypothalamic neuropeptide (and a carboxy-terminal truncated 27-amino acid form) 70% identical to VIP that potently induced cAMP levels in pituitary cells (Miyata et al., 1989). Although widely regarded as neuropeptides that meditate or modulate diverse processes in the mammalian brain such as circadian rhythms and stress responses (reviewed in Vaudry et al., 2009), considerable evidence implicate PACAP and VIP as neuroprotective and regeneration factors in the diseased and injured brain (reviewed in Brenneman, 2007; Dejda et al., 2008; Reglodi et al., 2011; Seaborn et al., 2011). Moreover, systemic administration of these peptides is reported by several groups to be efficacious in animal models of CNS pathologies such as stroke (Chen et al., 2006; Ohtaki et al., 2006; Stetler et al., 2010), MS (Kato et al., 2004; Gonzalez-Rey et al., 2006) and Parkinson's disease (Reglodi et al., 2004; Wang et al., 2008).
Three heterotrimeric GPCRs mediate the actions of VIP and PACAP, officially named by IUPHAR as PAC1, VPAC1 and VPAC2 (Harmar et al., 1998), each with a unique expression pattern. Of these, PAC1 binds only PACAP with high affinity, whereas VPAC1 and VPAC2 can bind avidly either VIP or PACAP (Harmar et al., 2012; Figure 1). The genes encoding PACAP and VIP were recently given the gene names ADCYAP1 and VIP, respectively, and the receptors ADCYAP1R1, VIPR1, VIPR2. Unfortunately, the receptor gene nomenclature may be incorrectly interpreted to imply that the receptors encoding by VIPR1 and VIPR2 function mainly as VIP receptors. This may explain why recent papers reporting the linkage of VIPR2 to schizophrenia (Levinson et al., 2011; Vacic et al., 2011) discussed only VIP as the relevant ligand, giving no mention to the fact that the receptor encoded by VIPR2 binds PACAP with an affinity equal to or higher than VIP. It is thus important to recognize that VIPR1 and VIPR2 encode physiological receptors for either VIP or PACAP, depending on the specific neuropeptide released from nearby cells or axon terminals.
Figure 1.
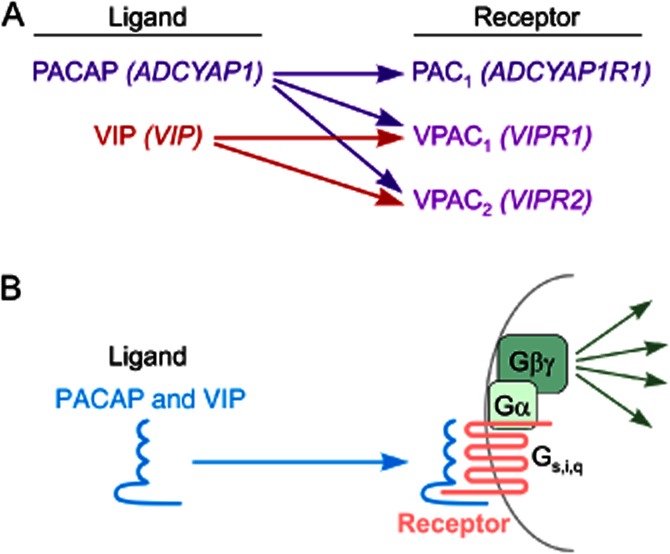
VIP/PACAP ligand/receptor interactions. (A) IUPHAR and gene names are indicated in standard text and italics respectively. PAC1 receptors are highly selective for PACAP and are generally only responsive to PACAP. VPAC1 and VPAC2, on the other hand, serve as physiological receptors for either PACAP or VIP, depending on the specific neuropeptide released from nearby cells or axon terminals. (B) General interaction of PACAP and VIP with their cell surface receptors. Each of the receptors is a seven-transmembrane GPCR coupled primarily via Gs to adenylate cyclase. Other pathways can be activated via βγ subunits, and by alternative coupling of the receptors to other G-proteins such as Gi and Gq (see text).
Each of these receptors is coupled primarily to Gs, and activates adenylyl cyclase and PKA, but other pathways are often activated or inhibited in some cells in parallel or downstream of cAMP, including pathways involving exchange proteins activated by cAMP (Ster et al., 2007), NO (Murthy et al., 1993), PLC (Spengler et al., 1993), phosphatidylinositol 3-kinase (Straub and Sharp, 1996), src (Koh, 1991), MAPK (Barrie et al., 1997; Villalba et al., 1997; Lelievre et al., 1998), Jak/STAT and NF-κB (Delgado and Ganea, 1999; 2000).
Up-regulation of the VIP/PACAP ligand/receptor signalling system in neurons after injury and during inflammation
Considerable effort has been made to understand how the VIP/PACAP ligand/receptor signalling system responds in various injury models and the mechanisms involved. A number of laboratories have utilized peripheral nerve injury models, which have the advantage that they can be carefully controlled and mechanistically dissected. Those studies have repeatedly demonstrated strong up-regulation in PACAP and/or VIP in injured neurons, including motor (Zhou et al., 1999), sympathetic (Mohney et al., 1994; Moller et al., 1997) and sensory neurons (Zhang et al., 1995). PACAP gene expression was also shown to be up-regulated in sensory neurons following target inflammation (Zhang et al., 1998) and both VIP and PACAP mRNA were induced in motor neurons following application of an inflammatory stimulus directly to the nerve (Armstrong et al., 2004). Mechanisms responsible for the up-regulation of VIP and PACAP in sympathetic neurons have been investigated in detail (reviewed in Zigmond, 2011), and found to involve both loss of target-derived factors and an induction in expression of cytokines in the gp130 family (such as leukaemia inhibitory factor), a pathway shown by others to regulate VIP gene transcription via STAT proteins and cytokine-responsive elements on the VIP gene (Symes et al., 1995; Jones et al., 2000). Using a different approach, it was shown that the induction of PACAP gene expression in facial motor neurons after axotomy was blocked in SCID mice (which lack lymphocytes), but maintained in SCID mice pre-infused with wild-type (WT) splenocytes (Armstrong et al., 2003), providing evidence that inflammatory cells per se are required in this injury model for the induction of neuropeptide expression after injury.
Other investigations have examined changes in PACAP and PAC1 receptor expression in cortical and hippocampal neurons after CNS injury. For example, PACAP gene transcripts were found to be significantly induced in pyramidal neurons in layers II-III of the cortex after focal ischaemic injury (Stumm et al., 2007). Other studies indicated that gene expression for PAC1 receptors was increased and VPAC2 receptors decreased in the mouse hippocampus in response to ischaemia induced by bilateral common carotid artery occlusion (Nakamachi et al., 2012b). In a model of moderate traumatic injury to rat brain cortex, PACAP gene expression was found to be induced in the ipsilateral cortex, especially in the perifocal region, and also within the dentate gyrus of the hippocampus (Skoglosa et al., 1999). In a model of cortical focal cold injury, VPAC2 receptor immunoreactivity was up-regulated in reactive astrocytes, whereas VIP became expressed in microglia (Nishimoto et al., 2011). Finally, gene expression for both PACAP and PAC1 were induced in the spinal cord after moderate compression injury (Tsuchikawa et al., 2012). Immunohistochemistry colocalization studies demonstrated that PACAP was induced in motor neurons, whereas PAC1 was induced in both motor neurons and astrocytes. The changes in expression of these ligands and receptors after different forms of injury suggest that PACAP and VIP signalling systems might be involved in attempts to minimize the impact of injury and/or to promote recovery. Proposed actions of these peptides and potential mechanisms involved are described in the following sections.
Direct actions of VIP and PACAP on neuronal survival, axon integrity and axonal growth
VIP and PACAP are pleiotropic growth factors, affecting proliferation, differentiation, survival and maturation of multiple neural and non-neural cell types. These actions have been extensively reviewed elsewhere (Waschek, 1995; Waschek et al., 2000; Waschek, 2002; Brenneman, 2007; Falluel-Morel et al., 2007; Dejda et al., 2008; Nakamachi et al., 2011; Reglodi et al., 2011; Seaborn et al., 2011; Shioda and Gozes, 2011; Nakamachi et al., 2012a) so the discussion here will be limited to points considered salient for this review and new findings that suggest novel mechanisms of protection. Perhaps the most well-studied and best understood growth factor-like action of these peptides is the PACAP inhibition of neuronal apoptosis. In this regard, PACAP has been reported to promote the survival of numerous neuronal cell types in culture, including cortical, dopaminergic, motor, olfactory and cerebellar granule neurons, neural stem/progenitors and PC12 pheochromocytoma cells (reviewed in Waschek, 2002; Dejda et al., 2008; Seaborn et al., 2011). Most mechanistic work has been performed on cerebellar granule neurons and supports a model whereby PACAP acts in these cells on PAC1 receptors to trigger both non-canonical and canonical cAMP/PKA pathways to induce of Bcl-2 gene expression (Dejda et al., 2008; Falluel-Morel et al., 2008). PAC1 signalling in these cells is also reported to trigger a more rapid, but as yet undefined pathway that results in inhibition of caspase 3 activity but does not require intervening protein synthesis (Falluel-Morel et al., 2004).
Loss of axons and/or dendrites independent of (or at least preceding) neuron loss appears to be involved in most neurodegenerative diseases and certain neuropsychiatric afflictions such as schizophrenia. Thus, a potentially important goal is to identify mechanisms normally employed by the brain to minimize axonal and dendritic loss. Furthermore, regeneration of axons occurs concurrently with axon degeneration in MS and other neurodegenerative diseases, suggesting that mechanisms that promote axonal regeneration can also be targeted in these pathologies. In these regards, other well-described, albeit less-understood actions of PACAP and VIP include their abilities to promote the integrity and growth of axons (reviewed in Waschek, 2002; Nakamachi et al., 2011). We addressed the in vivo relevance of PACAP action on axonal growth a few years ago, demonstrating that PACAP-deficient (knockout, KO) mice exhibited delayed axonal regeneration in a facial nerve crush model (Armstrong et al., 2008), while others have provided evidence that PACAP administration in vivo can attenuate axon degeneration in CNS injury models (Chen and Tzeng, 2005; Tamas et al., 2006). In vitro studies implicate PAC1 receptors, PKA and MAPK as mediating the effects of PACAP to enhance neurite outgrowth (Lu and DiCicco-Bloom, 1997; Guirland et al., 2003; Monaghan et al., 2008; Emery and Eiden, 2012), although downstream effectors remain relatively ill defined. Very recently, using primary hippocampal neurons cultures, it was reported that PACAP promoted neuritogenesis in association with enhanced mitochondria membrane potential, and increased the expression of peroxisome proliferator-activated receptor γ co-activator 1α (PGC1α), a master transcriptional co-regulator of mitochondria biogenesis and activation (Kambe and Miyata, 2012). While PGC1α activity is posttranslationally induced during high-energy demand (high AMP/ATP ratio) via the AMP-activated protein kinase, a lesser known mode of PGC1α regulation occurs at the level of gene expression. This operates in the context of plasticity and/or following external cellular signals such as β-adrenergic stimulation, and apparently mediated by cAMP-responsive element-binding protein (Puigserver et al., 1998; Handschin et al., 2003; Puigserver and Spiegelman, 2003; Cui et al., 2006; St-Pierre et al., 2006; Cheng et al., 2010; Hondares et al., 2011). Thus, PACAP-mediated induction of mitochondria biosynthesis might allow increased ATP synthesis during axonal growth. Alternatively, a PGC1α-mediated PACAP up-regulation of mitochondria biogenesis might also serve to maintain axon integrity during inflammation and/or oxidative stress by replacing mitochondria that have been damaged and removed by mitophagy.
Another potentially relevant finding linking PACAP to mitochondrial biology came from a study in which intracerebroventricular PACAP administration inhibited oxidative DNA stress and promoted survival in CA1 hippocampal neurons in a rat global ischaemia model (Stetler et al., 2010). The pertinent finding was that this occurred in association with induced expression of apurinic/apyrimidinic endonuclease (APE1), a DNA repair enzyme that localizes to mitochondria in response to oxidative stress. APE1 knockdown in vivo abrogated the neuroprotective action of PACAP, a finding confirmed in vitro on cultured neurons. Thus, a hitherto unrecognized core function of PACAP/PAC1 signalling in stressed neurons might be to maintain a healthy pool of mitochondria within axons and neural cell bodies, thereby providing mechanisms to protect neurons and axons from degeneration and to promote axonal regrowth.
Neuropeptide regulation of reactive gliosis
Reactive gliosis is a collective term that refers to the heterogeneous responses of astrocytes and microglia to acute and chronic brain injury, and is observable at some level in all neurodegenerative diseases. In acute injury, both microglia and astrocytes change morphology and release growth factors, cytokines, chemokines and other immunomodulators. Several of these alterations in glia cellular and molecular phenotype persist in chronic injury and neurodegenerative diseases. Although commonly viewed as harmful due to its association with proinflammatory molecules and scar tissue production, reactive gliosis has probably evolved as an important defensive reaction that attempts to minimize injury and promote repair. For example, several recent studies in genetically modified mice have indicated that interference with astrogliosis can worsen the course of CNS injury (Okada et al., 2006; Herrmann et al., 2008; Nobuta et al., 2012), and it is clear that microgliosis plays a role in repair processes (reviewed in Carson et al., 2006; Ransohoff, 2007).
Functional VIP and PACAP receptors are present on both astrocytes and microglia in culture, raising the possibility that VIP and PACAP provide some of their neuroprotective and regenerating actions via these glial cell types. In this regard, a number of studies have shown that VIP and PACAP can potential regulate the proliferation and morphology of astrocytes in culture and induce the release of cytokines and growth and survival factors (reviewed in Dejda et al., 2005; Nakamachi et al., 2011). In vitro and in vivo studies also support that VIP and PACAP can modulate microglia activity by altering the production of cytokines, chemokines and other molecules (Kim et al., 2000; Wainwright et al., 2008). The latter is not at all surprising give the abundant data indicating that these peptides are potent modulators of MΦ function (see below).
VIP and PACAP action on neural stem cell fate
Subsequent to CNS insults and other pathological conditions, neural stem cells, which are normally relatively quiescent, actively proliferate, migrate to lesion sites and differentiate in an attempt to salvage or repair damaged circuits. Several studies have examined the actions of VIP and PACAP on the fate of neural progenitors and stem cells in vitro (extensively reviewed in Nakamachi et al., 2011). These have demonstrated diverse PAC1, VPAC1 and VPAC2-mediated actions on proliferation, survival and neuronal and glial differentiation, and underscore the importance of the source and developmental stage of stem/progenitors, and the experimental conditions used for study. One recent investigation addressed receptor-mediated actions on neural stem cells in vivo, showing that VPAC2-deficient mice exhibited a specific reduction in nestin-positive precursors in the dentate subgranular stem cell niche, as well as a reduction in the number of newly generated and surviving neurons in the dentate gyrus (Zaben et al., 2009). So far, no studies have examined PACAP action on stem cell fate in neuroinflammatory and other forms of CNS injury or pathology.
The role of VIP and PACAP in the regulation of systemic inflammation
VIP immunoreactivity is present in the innervation of lymph nodes, thymus and other lymphoid tissue (Bellinger et al., 1997), leading to the suggestion that neuronal sources of VIP might be used to regulate immune cell activity that is initiated, amplified or otherwise regulated in these lymphoid tissues. Interestingly, VIP and PACAP are also expressed in several subpopulations of immune cells (Gomariz et al., 1993; Gaytan et al., 1994; Leceta et al., 1996; Abad et al., 2002), providing potential non-neuronal sources of these peptides to regulate peripheral immune responses. The importance of an immune source of VIP during inflammation was suggested by the fact that a variety of inflammatory stimuli were shown to induce VIP production and release from cultures of lymph nodes, spleen and thymus (Martinez et al., 1999), and from purified Th2-differentiated CD4+ Th lymphocytes (Vassiliou et al., 2001). Finally, a bone marrow chimera approach was recently used to show that expression of VIP in radiosensitive haematopoietic cells was required to constrain Th polarization in a viral infection model (Li et al., 2011). Although Th polarization occurs mainly if not exclusively in the periphery, the latter finding raises the general possibility that VIP (or PACAP) produced in immune cells, including microglia (Nishimoto et al., 2011), are involved in modifying local inflammation or degeneration in the CNS
Nearly all immune cell types express one or more VIP and PACAP receptor subtypes. VPAC1 and VPAC2 receptors have been implicated in most of the immunomodulatory actions of PACAP and VIP (reviewed in Delgado et al., 2004a), although PAC1 receptors are expressed in MΦ, where they appear to have functional significance in innate (Martinez et al., 2002), and perhaps adaptive immunity (Delgado et al., 1999b,c). MΦ express constitutively VPAC1 and PAC1 receptors, and when exposed to inflammatory stimulus express VPAC2 (Delgado et al., 1999b). VIP and PACAP actions on various immune cell types in culture has undergone considerable investigation (extensively reviewed in Delgado et al., 2004a; Yadav and Goetzl, 2008), and only certain critical points will be highlighted here. VIP and PACAP were repeatedly shown to inhibit the LPS-induced production of proinflammatory cytokines such as TNF-α, IL-6 and several chemokines in MΦ cultures, and to increase the synthesis and release of IL-10 and the IL-1 receptor antagonist (IL-1Ra; Delgado et al., 1999a; Delgado and Ganea, 2001). VIP and PACAP also appear to play an important role in regulating T-cell biology. Both neuropeptides act on MΦ and dendritic cells to modify the expression of costimulatory molecules B7.1 and B7.2, with a resultant stimulatory activity on Th cells (Delgado et al., 2000; 2004b). CD4+ T-cells also express VPAC1, and are induced to express VPAC2 during differentiation to the Th2 phenotype. Evidence suggest that VIP can promote a positive Th2 to Th1 balance by acting directly and specifically on Th2-differentiated cells to increase their proliferation, survival and chemotaxis (reviewed in Delgado et al., 2004a). More recent experiments indicate that exogenously administered VIP and PACAP may regulate the production or expansion of regulatory T-cells (Tregs) Fernandez-Martin et al., 2006 and see below). Other well-described immunomodulatory actions mediated by VIP and PACAP receptors include regulation of chemotaxis and modulation of matrix metalloproteinases (reviewed in Delgado et al., 2004a). The intracellular signalling mechanisms by which VIP and PACAP regulate various aspects of immune cell physiology are cell type and context dependent, and involves cAMP-dependent and cAMP-independent pathways, which impinge on the classical PKA, NF-kB and STAT signalling pathways (see Delgado et al., 2004a for a more detailed discussion).
Dissection of VIP and PACAP actions in the experimental autoimmune encephalomyelitis (EAE) model of MS
Like that observed in other inflammatory diseases (Tornwall et al., 1994; Belai et al., 1997; Boyer et al., 2007; Juarranz et al., 2008), patients with MS have reported alterations in components of the VIP/PACAP signalling system. For example, patients with MS reportedly have decreased levels of VIP in their cerebral spinal fluid (Andersen et al., 1984; Sharpless et al., 1984), aberrant regulation of VPAC2 receptors in lymphocytes when stimulated in vitro, and a distinct DNA footprinting pattern in the promoter region of the VPAC2 gene (Sun et al., 2006). These studies imply that VPAC receptor signalling may be altered either as a part of the pathology of MS or as an attempt to control inflammation. We and other have used the EAE model to study the potential significance of VIP and PACAP signalling in MS. In addition to being a widely used model for MS research, EAE provides an excellent experimental system to investigate specific interactions of the immune system with the brain as well as tolerance mechanisms that operate to prevent autoimmune diseases. This is possible because EAE can be reproducibly induced with defined CNS antigens, employs physiologically relevant processes that regulate flux of peripheral immune cells into the brain and involves well-characterized innate and adaptive immune mechanisms that promote inflammatory disease and normally prevent autoimmunity. Briefly, the classical EAE model is induced by immunization with a myelin peptide fragment such as MOG35–55 (amino acids 35–55 of myelin oligodendrocyte glycoprotein). This results in the generation of autoreactive T-cells, which subsequently invade the CNS to initiate EAE, eventually culminating in myelin destruction.
The first use of this model to study the immunomodulatory actions of VIP or PACAP was reported almost 10 years ago (Kato et al., 2004). In that study, PACAP was administrated i.p. to C57BL/6 mice every other day after immunization with MOG35–55. PACAP administration was found to ameliorate both the clinical and pathological manifestations of EAE. Splenocyte cultures from these mice exhibited significantly reduced MOG-specific IFN-γ production. In more extensive work by another group focusing on the therapeutic action of VIP, the clinical and pathological scores in chronic MOG35–55-induced EAE in C57BL/6 mice were dramatically reduced by a 3 day VIP treatment either during the induction or after the onset of disease (Fernandez-Martin et al., 2006; Gonzalez-Rey et al., 2006). Three-day treatment with either VIP or PACAP was also found to ameliorate EAE in a relapsing-remitting model, with a blockade of symptoms lasting 60 days. This was associated with decreased spinal cord levels of several proinflammatory cytokines, chemokines and chemokine receptors, and increased levels of the anti-inflammatory cytokines IL-10, IL-1Ra and TGF-β, the latter occurring despite a fourfold reduction in the number of inflammatory cells in the spinal cord. Lymph nodes from VIP-treated mice showed reduced antigen-induced proliferation, and lower and higher productions of Th1 and Th2 cytokines respectively. Finally, in a relapsing-remitting EAE model, the administration of VIP resulted in the expansion of Tregs (CD4+CD25+Foxp3+) in the periphery and the nervous system. These Tregs were reported to suppress T-cell activation to a greater extent than Tregs from untreated mice (Fernandez-Martin et al., 2006).
These rather profound anti-inflammatory actions of administered VIP and PACAP in these models raised the question of whether or not the endogenously produced peptides played protective roles in inflammation. We thus characterized the EAE phenotype of PACAP- and VIP-deficient (KO) mice (C57BL/6) using the MOG35–55 model (Tan et al., 2009; Abad et al., 2010). Clinical disease in PACAP KO mice was much more severe than in WT mice, involving four limb paralyses and requiring euthanization in 30% of cases. The increased sensitivity was accompanied by enhanced mRNA expression of Th1 and Th17 cytokines in the spinal cord, but down-regulation of Th2 cytokines. Ex vivo antigen-rechallenge assays indicated that PACAP KO mice exhibited increased T-cell proliferation in response to antigen, with a more pronounced induction of Th1 and Th17 cytokines. Moreover, the relative abundance of CD4+CD25+FoxP3+ Tregs in lymph nodes and levels of FoxP3 mRNA in the spinal cord were reduced. In addition to demonstrating that endogenous PACAP protects against EAE by controlling cytokine responses, the results suggested that PACAP might function as one of the few known intrinsic regulators of Tregs. The type of Tregs affected in this model are natural Treg (nTregs), as opposed to inducible Tregs (iTregs, also called adaptive Tregs), which are mainly generated in models of more chronic inflammation. To this point, it has been clearly demonstrated in C57BL/6 mice that the induction of Tregs during MOG35–55-induced EAE comes about exclusively by the expansion of the nTreg population, and not to the de novo production of iTregs from naïve T-cells (Korn et al., 2007). Thus, the impairment in Treg expansion in PACAP KO mice in this model appears to involve primarily nTregs.
In contrast to the hyper-inflammatory phenotype of PACAP-deficient animals, VIP KO mice exhibited a paradoxical, nearly complete resistance to MOG35–55-induced EAE (Abad et al., 2010). The EAE resistance in VIP KO mice was reversed by pre-administering VIP for 2 weeks prior to EAE induction, demonstrating that this unexpected phenotype was indeed due to specific loss of VIP. Despite the absence of disease in VIP KO mice, immune cells isolated from the lymph nodes of VIP KO mice responded robustly to MOG in vitro, and induced EAE when transferred to WT recipient mice. Thus, VIP KO mice developed an aggressive T-cell response to MOG immunization, but clinical disease in these mice was blocked at a step downstream from immunization. In agreement, MOG-specific T-cells generated in wild-type mice could induce EAE when transferred into WT but not VIP KO recipients. Remarkably, inflammatory cells accumulated in the CNS in MOG-treated VIP KO mice, but seemed to be ‘trapped’ in the meningeal space and rarely invaded the parenchyma. This suggested that trans-migration of immune cells into the CNS parenchyma may be impaired in VIP KO mice, and demonstrated that VIP is somehow required for the EAE process to be fully manifested (Abad and Waschek, 2011). While the finding raises important questions for future study, the presence of this resistance has so far precluded an investigation in EAE of how endogenous VIP might modulate Treg production and other aspects of neuroinflammation.
Our more recent experiments have focused on mechanisms to explain the reduction of Treg abundance in PACAP KO mice. Initially, we focused on potentially defective mechanisms regulating the expansion of FoxP3+ Tregs of PACAP KO mice by FACS using Ki67 as a mitotic marker, and Annexin V and 7-AAD to detect apoptotic cells. These studies, performed at the peak of disease (day 14) and during recovery (day 20) revealed that both proliferation and survival rates of FoxP3+ Tregs in the lymph nodes were increased in WT mice during the course of EAE, but that these increases were markedly blunted in PACAP KO mice. In the CNS, a defect was observed in proliferation only. We also examined the proliferation rate of committed FoxP3+ natural Tregs in their site of origin, the thymus, at the peak of disease. As in the lymph nodes and CNS, EAE enhanced the proliferation of FoxP3+ Tregs in the thymus in WT mice, but this was significantly blunted in PACAP KO mice. These results revealed that PACAP is critically required to expand and maintain populations of committed FoxP3+Tregs in the thymus as well as extrathymic sites.
A speculative model for VIP and PACAP involvement in the regulation of peripheral inflammation
The above analyses demonstrate that loss of VIP and PACAP critically affects important aspects of inflammation and tolerance during EAE, but provide little detail with respect to exactly where, when and how the endogenous neuropeptides act. In fact, relatively little is known with respect to how neurons in the CNS respond to and control local CNS inflammation. On the other hand, considerable mechanistic information is available to explain how the brain controls systemic inflammation by way of innervation of lymph nodes, spleen, thymus and bone marrow, where the activation and expansion of immune cells to a large extent occurs. Much has come from investigation of the so-called inflammatory reflex, in which inflammation in the periphery is sensed in the brain stem and hypothalamus, then transmitted to the periphery via the autonomic nervous system, in particular the sympathetic nervous system (SNS; Figure 2). Historically, a neural circuit coined ‘the cholinergic anti-inflammatory pathway’, mediated prototypically by the vagus nerve, was shown to have important roles in regulating the immune response in several experimental disease models, including sepsis, haemorrhagic shock, pancreatitis and post-operative ileus (reviewed in Rosas-Ballina and Tracey, 2009; Tracey, 2009; Thayer and Sternberg, 2010). However, despite an abundance of supporting functional data, the concept of this circuit was considered highly controversial because few investigators could provide solid evidence for cholinergic innervation of the spleen, lymph nodes or other immune organs. The conundrum seems to have been resolved to some extent in recent years with data supporting a model whereby vagal stimulation of the celiac or other sympathetic ganglia results in release of noradrenaline (NA) in lymphoid organs such as the spleen. NA then acts on MΦ in these organs to release ACh, which in turn acts in an autocrine or paracrine manner on immune cells that express α7 nicotinic receptors to alter their function (Rosas-Ballina and Tracey, 2009; Tracey, 2009; Thayer and Sternberg, 2010). When viewed separately, the concept of SNS control of immune responses, although still controversial, appears more straightforward (reviewed in Nance and Sanders, 2007; Bellinger et al., 2008). For example, it is well documented that peripheral lymphoid organs, including spleen, lymph nodes, thymus and probably bone marrow, receive abundant innervation from sympathetic ganglia, with nerve terminals residing adjacent to immune and stromal cells.
Figure 2.
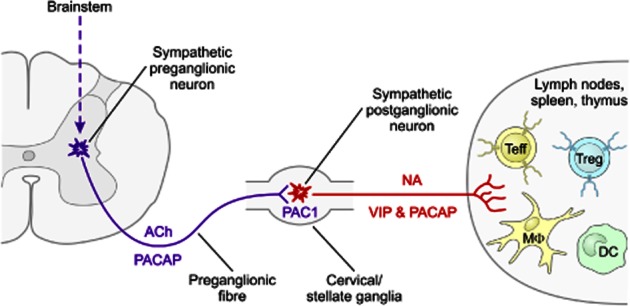
Potential neural circuitry by which PACAP and VIP modulate peripheral immune cell activity during inflammatory stress. Neurons in the brainstem and hypothalamus (not shown) sense inflammation and activate preganglionic sympathetic neurons in the spinal cord IML column. PACAP is expressed with ACh in the preganglionic neurons in thoracic spinal cord IML neurons. When released during stress or inflammation, PACAP acts via PAC1 receptors expressed on sympathetic neurons in cervical/stellate ganglia to alter NA synthesis and/or activity, and to increase the production of VIP or PACAP. The latter neuropeptides are released along with NA in peripheral immune sites such as the thymus, lymph nodes and spleen. VIP or PACAP may then act directly with VPAC1, VPAC2 or PAC1 receptors on immune cells, or indirectly via action on stromal cells expressing these receptors. Teff, T effector cell (Th1, Th2 and Th17); DC, dendritic cells.
The role of the SNS in controlling inflammation has been examined primarily by peripheral administration of 6-hydoxydopamine, which does not appreciably penetrate the blood–brain barrier, and thus selectively ablates sympathetic neurons in the periphery. Most of these studies, as well as studies in which immune cells were treated with NA receptor analogues in vitro, suggested that NA acts primarily via β-2 adrenoreceptors, and mainly inhibits innate inflammatory responses, and either promotes or inhibits adaptive immunity (Nance and Sanders, 2007; Bellinger et al., 2008). Unfortunately, very limited illuminating data are available so far which address this problem at the molecular level using genetically engineered mice. For example, a comprehensive analysis of the immune phenotype of β-2 adrenoceptor-deficient mice reported essentially normal immune responses (Sanders et al., 2003). On the other hand, dopamine β-hydroxylase-deficient mice were found to exhibit diminished Th1 responses to pathogen challenge (Sanders et al., 2003), providing evidence that SNS actions might subserve, rather than inhibit, inflammation in this context. Overall, the studies using sympathectomy, genetically engineered mice, and in vitro assays have generally not examined the role of other signalling molecules, such as neuropeptides, that are released by sympathetic neurons or their presynaptic innervation.
VIP and PACAP are expressed in this circuitry, and are known to regulate sympathetic function in other contexts. In this regard, PACAP is expressed in the Ach-expressing preganglionic neurons of the sympathetic ganglia, including those that innervate the thymus and cervical lymph nodes (Beaudet et al., 1998; Pettersson et al., 2004; Figure 2). Retrograde tracing with pseudorabies virus and other anatomical methods demonstrate that neurons in the superior cervical ganglia (SCG), which innervate the thymus and cervical lymph nodes, receive their presynaptic innervation from the thoracic intermediolateral (IML) neurons in the spinal cord. At least two groups have shown that IML neurons abundantly express PACAP and PACAP mRNA (Beaudet et al., 1998; Pettersson et al., 2004), and furthermore, more than half of the IML preganglionic neurons that project to the SCG express PACAP gene transcripts (Beaudet et al., 1998). PACAP and PACAP gene transcripts are also expressed in locations of higher order neurons that regulate sympathetic outflow to the lymphoid organs. There is good evidence that PACAP generally functions in sympathetic neurons to alter tone and/or activity. For example, PACAP has been shown to increase electrical activity in sympathetic nerves in anaesthetized rats (Tanida et al., 2010), and to induce catecholamine release and tyrosine hydroxylase gene expression in cultured SCG (May and Braas, 1995). Moreover, PACAP KO mice exhibit a thermogenesis defect in the first 2 weeks of life that is linked to a deficit in brown fat metabolism, a process regulated by sympathetic tone (Gray et al., 2002). In this respect, levels of NA and two enzymes involved in fat metabolism regulated by NA were significantly reduced in these mice. In a metabolic stress model (insulin challenge), hypoglycaemia was more profound and longer lasting in PACAP KO than WT mice, and was associated with impaired long-term secretion of epinephrine, a lack of induction of tyrosine hydroxylase activity in the adrenal medulla and a depletion of adrenomedullary epinephrine stores. Finally, an additional mode of PACAP action is suggested by the fact that PACAP induces its own gene expression as well as that of VIP in cultured sympathetic neurons (Braas et al., 2007) via action on PACAP-selective PAC1 receptors (the only PACAP receptor on sympathetic neurons; Girard et al., 2004; Braas et al., 2007). Accordingly, PACAP from preganglionic neurons might trigger the release of VIP or PACAP in postsynaptic neurons, which then may act directly or indirectly on immune cells within peripheral lymphoid organs such as the thymus and lymph nodes. The fact that most immune cells express VIP and PACAP receptors suggests that postsynaptic release of VIP and/or PACAP in the target organ might mediate the effects of presynaptic PACAP independent of its actions on NA release and/or activity. Alternatively, as previously discussed, PACAP might be available from other relevant sources such as T lymphocytes (Gaytan et al., 1994; Abad et al., 2002). Future study employing cell-specific gene targeting and other approaches will be necessary to test various aspects of this model.
Acknowledgments
The author is funded by the National Multiple Sclerosis Society Grants RG3928, RG4859 and National Institutes of Health Grants HD04612, HD068686, NS070580 and MH098506.
Glossary
- APE1
apurinic/apyrimidinic endonuclease
- IL-1Ra
IL-1 receptor antagonist
- IML
intermediolateral
- KO
knockout
- MOG35-55
myelin oligodendrocyte glycoprotein, amino acids 35-55
- MS
multiple sclerosis
- MΦ
macrophage
- NA
noradrenaline
- PACAP
pituitary adenylyl cyclase-activating peptide
- PGC1α
peroxisome proliferator-activated receptor γ co-activator 1α
- SCG
superior cervical ganglia
- SNS
sympathetic nervous system
- Treg
regulatory T-cell
- VIP
vasoactive intestinal peptide
Conflicts of interest
None declared.
References
- Abad C, Waschek JA. Immunomodulatory roles of VIP and PACAP in models of multiple sclerosis. Curr Pharm Des. 2011;17:1025–1035. doi: 10.2174/138161211795589364. [DOI] [PubMed] [Google Scholar]
- Abad C, Martinez C, Leceta J, Juarranz MG, Delgado M, Gomariz RP. Pituitary adenylate-cyclase-activating polypeptide expression in the immune system. Neuroimmunomodulation. 2002;10:177–186. doi: 10.1159/000067180. [DOI] [PubMed] [Google Scholar]
- Abad C, Tan YV, Lopez R, Nobuta H, Dong H, Phan P, et al. Vasoactive intestinal peptide loss leads to impaired CNS parenchymal T-cell infiltration and resistance to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2010;107:19555–19560. doi: 10.1073/pnas.1007622107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O, Fahrenkrug J, Wikkelso C, Johansson BB. VIP in cerebrospinal fluid of patients with multiple sclerosis. Peptides. 1984;5:435–437. doi: 10.1016/0196-9781(84)90249-3. [DOI] [PubMed] [Google Scholar]
- Armstrong BD, Hu Z, Abad C, Yamamoto M, Rodriguez WI, Cheng J, et al. Lymphocyte regulation of neuropeptide gene expression after neuronal injury. J Neurosci Res. 2003;74:240–247. doi: 10.1002/jnr.10750. [DOI] [PubMed] [Google Scholar]
- Armstrong BD, Hu Z, Abad C, Yamamoto M, Rodriguez WI, Cheng J, et al. Induction of neuropeptide gene expression and blockade of retrograde transport in facial motor neurons following local peripheral nerve inflammation in severe combined immunodeficiency and BALB/C mice. Neuroscience. 2004;129:93–99. doi: 10.1016/j.neuroscience.2004.06.085. [DOI] [PubMed] [Google Scholar]
- Armstrong BD, Abad C, Chhith S, Cheung-Lau G, Hajji OE, Nobuta H, et al. Impaired nerve regeneration and enhanced neuroinflammatory response in mice lacking pituitary adenylyl cyclase activating peptide. Neuroscience. 2008;151:63–73. doi: 10.1016/j.neuroscience.2007.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrie AP, Clohessy AM, Buensuceso CS, Rogers MV, Allen JM. Pituitary adenylyl cyclase-activating peptide stimulates extracellular signal-regulated kinase 1 or 2 (ERK1/2) activity in a Ras-independent, mitogen-activated protein Kinase/ERK kinase 1 or 2-dependent manner in PC12 cells. J Biol Chem. 1997;272:19666–19671. doi: 10.1074/jbc.272.32.19666. [DOI] [PubMed] [Google Scholar]
- Beaudet MM, Braas KM, May V. Pituitary adenylate cyclase activating polypeptide (PACAP) expression in sympathetic preganglionic projection neurons to the superior cervical ganglion. J Neurobiol. 1998;36:325–336. [PubMed] [Google Scholar]
- Belai A, Boulos PB, Robson T, Burnstock G. Neurochemical coding in the small intestine of patients with Crohn's disease. Gut. 1997;40:767–774. doi: 10.1136/gut.40.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DL, Lorton D, Horn L, Brouxhon S, Felten SY, Felten DL. Vasoactive intestinal polypeptide (VIP) innervation of rat spleen, thymus, and lymph nodes. Peptides. 1997;18:1139–1149. doi: 10.1016/s0196-9781(97)00075-2. [DOI] [PubMed] [Google Scholar]
- Bellinger DL, Millar BA, Perez S, Carter J, Wood C, ThyagaRajan S, et al. Sympathetic modulation of immunity: relevance to disease. Cell Immunol. 2008;252:27–56. doi: 10.1016/j.cellimm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer L, Sidpra D, Jevon G, Buchan AM, Jacobson K. Differential responses of VIPergic and nitrergic neurons in paediatric patients with Crohn's disease. Auton Neurosci. 2007;134:106–114. doi: 10.1016/j.autneu.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Braas KM, Schutz KC, Bond JP, Vizzard MA, Girard BM, May V. Microarray analyses of pituitary adenylate cyclase activating polypeptide (PACAP)-regulated gene targets in sympathetic neurons. Peptides. 2007;28:1856–1870. doi: 10.1016/j.peptides.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman DE. Neuroprotection: a comparative view of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Peptides. 2007;28:1720–1726. doi: 10.1016/j.peptides.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WH, Tzeng SF. Pituitary adenylate cyclase-activating polypeptide prevents cell death in the spinal cord with traumatic injury. Neurosci Lett. 2005;384:117–121. doi: 10.1016/j.neulet.2005.04.070. [DOI] [PubMed] [Google Scholar]
- Chen Y, Samal B, Hamelink CR, Xiang CC, Chen Y, Chen M, et al. Neuroprotection by endogenous and exogenous PACAP following stroke. Regul Pept. 2006;137:4–19. doi: 10.1016/j.regpep.2006.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Hou Y, Mattson MP. Mitochondria and neuroplasticity. ASN Neuro. 2010;2:e00045. doi: 10.1042/AN20100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Dejda A, Sokolowska P, Nowak JZ. Neuroprotective potential of three neuropeptides PACAP, VIP and PHI. Pharmacol Rep. 2005;57:307–320. [PubMed] [Google Scholar]
- Dejda A, Jolivel V, Bourgault S, Seaborn T, Fournier A, Vaudry H, et al. Inhibitory effect of PACAP on caspase activity in neuronal apoptosis: a better understanding towards therapeutic applications in neurodegenerative diseases. J Mol Neurosci. 2008;36:26–37. doi: 10.1007/s12031-008-9087-1. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit interleukin-12 transcription by regulating nuclear factor kappaB and Ets activation. J Biol Chem. 1999;274:31930–31940. doi: 10.1074/jbc.274.45.31930. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Inhibition of IFN-gamma-induced janus kinase-1-STAT1 activation in macrophages by vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. J Immunol. 2000;165:3051–3057. doi: 10.4049/jimmunol.165.6.3051. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Inhibition of endotoxin-induced macrophage chemokine production by vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide in vitro and in vivo. J Immunol. 2001;167:966–975. doi: 10.4049/jimmunol.167.2.966. [DOI] [PubMed] [Google Scholar]
- Delgado M, Munoz-Elias EJ, Gomariz RP, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide enhance IL-10 production by murine macrophages: in vitro and in vivo studies. J Immunol. 1999a;162:1707–1716. [PubMed] [Google Scholar]
- Delgado M, Munoz-Elias EJ, Gomariz RP, Ganea D. VIP and PACAP inhibit IL-12 production in LPS-stimulated macrophages. Subsequent effect on IFNgamma synthesis by T cells. J Neuroimmunol. 1999b;96:167–181. doi: 10.1016/s0165-5728(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Delgado M, Sun W, Leceta J, Ganea D. VIP and PACAP differentially regulate the costimulatory activity of resting and activated macrophages through the modulation of B7.1 and B7.2 expression. J Immunol. 1999c;163:4213–4223. [PubMed] [Google Scholar]
- Delgado M, Gomariz RP, Martinez C, Abad C, Leceta J. Anti-inflammatory properties of the type 1 and type 2 vasoactive intestinal peptide receptors: role in lethal endotoxic shock. Eur J Immunol. 2000;30:3236–3246. doi: 10.1002/1521-4141(200011)30:11<3236::AID-IMMU3236>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev. 2004a;56:249–290. doi: 10.1124/pr.56.2.7. [DOI] [PubMed] [Google Scholar]
- Delgado M, Reduta A, Sharma V, Ganea D. VIP/PACAP oppositely affects immature and mature dendritic cell expression of CD80/CD86 and the stimulatory activity for CD4(+) T cells. J Leukoc Biol. 2004b;75:1122–1130. doi: 10.1189/jlb.1203626. [DOI] [PubMed] [Google Scholar]
- Emery AC, Eiden LE. Signaling through the neuropeptide GPCR PAC1 induces neuritogenesis via a single linear cAMP- and ERK-dependent pathway using a novel cAMP sensor. FASEB J. 2012;26:3199–3211. doi: 10.1096/fj.11-203042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falluel-Morel A, Aubert N, Vaudry D, Basille M, Fontaine M, Fournier A, et al. Opposite regulation of the mitochondrial apoptotic pathway by C2-ceramide and PACAP through a MAP-kinase-dependent mechanism in cerebellar granule cells. J Neurochem. 2004;91:1231–1243. doi: 10.1111/j.1471-4159.2004.02810.x. [DOI] [PubMed] [Google Scholar]
- Falluel-Morel A, Chafai M, Vaudry D, Basille M, Cazillis M, Aubert N, et al. The neuropeptide pituitary adenylate cyclase-activating polypeptide exerts anti-apoptotic and differentiating effects during neurogenesis: focus on cerebellar granule neurones and embryonic stem cells. J Neuroendocrinol. 2007;19:321–327. doi: 10.1111/j.1365-2826.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- Falluel-Morel A, Aubert N, Vaudry D, Desfeux A, Allais A, Burel D, et al. Interactions of PACAP and ceramides in the control of granule cell apoptosis during cerebellar development. J Mol Neurosci. 2008;36:8–15. doi: 10.1007/s12031-008-9111-5. [DOI] [PubMed] [Google Scholar]
- Fernandez-Martin A, Gonzalez-Rey E, Chorny A, Ganea D, Delgado M. Vasoactive intestinal peptide induces regulatory T cells during experimental autoimmune encephalomyelitis. Eur J Immunol. 2006;36:318–326. doi: 10.1002/eji.200535430. [DOI] [PubMed] [Google Scholar]
- Gaytan F, Martinez-Fuentes AJ, Garcia-Navarro F, Vaudry H, Aguilar E. Pituitary adenylate cyclase-activating peptide (PACAP) immunolocalization in lymphoid tissues of the rat. Cell Tissue Res. 1994;276:223–227. doi: 10.1007/BF00306107. [DOI] [PubMed] [Google Scholar]
- Girard BM, Keller ET, Schutz KC, May V, Braas KM. Pituitary adenylate cyclase activating polypeptide and PAC1 receptor signaling increase Homer 1a expression in central and peripheral neurons. Regul Pept. 2004;123:107–116. doi: 10.1016/j.regpep.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Gomariz RP, Delgado M, Naranjo JR, Mellstrom B, Tormo A, Mata F, et al. VIP gene expression in rat thymus and spleen. Brain Behav Immun. 1993;7:271–278. doi: 10.1006/brbi.1993.1027. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Fernandez-Martin A, Chorny A, Martin J, Pozo D, Ganea D, et al. Therapeutic effect of vasoactive intestinal peptide on experimental autoimmune encephalomyelitis: down-regulation of inflammatory and autoimmune responses. Am J Pathol. 2006;168:1179–1188. doi: 10.2353/ajpath.2006.051081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SL, Yamaguchi N, Vencova P, Sherwood NM. Temperature-sensitive phenotype in mice lacking pituitary adenylate cyclase-activating polypeptide. Endocrinology. 2002;143:3946–3954. doi: 10.1210/en.2002-220401. [DOI] [PubMed] [Google Scholar]
- Guirland C, Buck KB, Gibney JA, DiCicco-Bloom E, Zheng JQ. Direct cAMP signaling through G-protein-coupled receptors mediates growth cone attraction induced by pituitary adenylate cyclase-activating polypeptide. J Neurosci. 2003;23:2274–2283. doi: 10.1523/JNEUROSCI.23-06-02274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Rhee J, Lin J, Tarr PT, Spiegelman BM. An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle. Proc Natl Acad Sci U S A. 2003;100:7111–7116. doi: 10.1073/pnas.1232352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, et al. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev. 1998;50:265–270. [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, Fahrenkrug J, Gozes I, Laburthe M, May V, Pisegna JR, et al. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br J Pharmacol. 2012;166:4–17. doi: 10.1111/j.1476-5381.2012.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, et al. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondares E, Rosell M, Diaz-Delfin J, Olmos Y, Monsalve M, Iglesias R, et al. Peroxisome proliferator-activated receptor alpha (PPARalpha) induces PPARgamma coactivator 1alpha (PGC-1alpha) gene expression and contributes to thermogenic activation of brown fat: involvement of PRDM16. J Biol Chem. 2011;286:43112–43122. doi: 10.1074/jbc.M111.252775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EA, Conover J, Symes AJ. Identification of a novel gp130-responsive site in the vasoactive intestinal peptide cytokine response element. J Biol Chem. 2000;275:36013–36020. doi: 10.1074/jbc.M007373200. [DOI] [PubMed] [Google Scholar]
- Juarranz Y, Gutierrez-Canas I, Santiago B, Carrion M, Pablos JL, Gomariz RP. Differential expression of vasoactive intestinal peptide and its functional receptors in human osteoarthritic and rheumatoid synovial fibroblasts. Arthritis Rheum. 2008;58:1086–1095. doi: 10.1002/art.23403. [DOI] [PubMed] [Google Scholar]
- Kambe Y, Miyata A. Role of mitochondrial activation in PACAP dependent neurite outgrowth. J Mol Neurosci. 2012;48:550–557. doi: 10.1007/s12031-012-9754-0. [DOI] [PubMed] [Google Scholar]
- Kato H, Ito A, Kawanokuchi J, Jin S, Mizuno T, Ojika K, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) ameliorates experimental autoimmune encephalomyelitis by suppressing the functions of antigen presenting cells. Mult Scler. 2004;10:651–659. doi: 10.1191/1352458504ms1096oa. [DOI] [PubMed] [Google Scholar]
- Kim WK, Kan Y, Ganea D, Hart RP, Gozes I, Jonakait GM. Vasoactive intestinal peptide and pituitary adenylyl cyclase-activating polypeptide inhibit tumor necrosis factor-alpha production in injured spinal cord and in activated microglia via a cAMP-dependent pathway. J Neurosci. 2000;20:3622–3630. doi: 10.1523/JNEUROSCI.20-10-03622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SW. Signal transduction through the vasoactive intestinal peptide receptor stimulates phosphorylation of the tyrosine kinase pp60c-src. Biochem Biophys Res Commun. 1991;174:452–458. doi: 10.1016/0006-291x(91)91437-h. [DOI] [PubMed] [Google Scholar]
- Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leceta J, Martinez C, Delgado M, Garrido E, Gomariz RP. Expression of vasoactive intestinal peptide in lymphocytes: a possible endogenous role in the regulation of the immune system. Adv Neuroimmunol. 1996;6:29–36. doi: 10.1016/s0960-5428(96)00001-0. [DOI] [PubMed] [Google Scholar]
- Lelievre V, Pineau N, Du J, Wen CH, Nguyen T, Janet T, et al. Differential effects of peptide histidine isoleucine (PHI) and related peptides on stimulation and suppression of neuroblastoma cell proliferation. A novel VIP-independent action of PHI via MAP kinase. J Biol Chem. 1998;273:19685–19690. doi: 10.1074/jbc.273.31.19685. [DOI] [PubMed] [Google Scholar]
- Levinson DF, Duan J, Oh S, Wang K, Sanders AR, Shi J, et al. Copy number variants in schizophrenia: confirmation of five previous findings and new evidence for 3q29 microdeletions and VIPR2 duplications. Am J Psychiatry. 2011;168:302–316. doi: 10.1176/appi.ajp.2010.10060876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JM, Southerland L, Hossain MS, Giver CR, Wang Y, Darlak K, et al. Absence of vasoactive intestinal peptide expression in hematopoietic cells enhances Th1 polarization and antiviral immunity in mice. J Immunol. 2011;187:1057–1065. doi: 10.4049/jimmunol.1100686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu N, DiCicco-Bloom E. Pituitary adenylate cyclase-activating polypeptide is an autocrine inhibitor of mitosis in cultured cortical precursor cells. Proc Natl Acad Sci U S A. 1997;94:3357–3362. doi: 10.1073/pnas.94.7.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez C, Delgado M, Abad C, Gomariz RP, Ganea D, Leceta J. Regulation of VIP production and secretion by murine lymphocytes. J Neuroimmunol. 1999;93:126–138. doi: 10.1016/s0165-5728(98)00216-1. [DOI] [PubMed] [Google Scholar]
- Martinez C, Abad C, Delgado M, Arranz A, Juarranz MG, Rodriguez-Henche N, et al. Anti-inflammatory role in septic shock of pituitary adenylate cyclase-activating polypeptide receptor. Proc Natl Acad Sci U S A. 2002;99:1053–1058. doi: 10.1073/pnas.012367999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May V, Braas KM. Pituitary adenylate cyclase-activating polypeptide (PACAP) regulation of sympathetic neuron neuropeptide Y and catecholamine expression. J Neurochem. 1995;65:978–987. doi: 10.1046/j.1471-4159.1995.65030978.x. [DOI] [PubMed] [Google Scholar]
- Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, et al. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- Mohney RP, Siegel RE, Zigmond RE. Galanin and vasoactive intestinal peptide messenger RNAs increase following axotomy of adult sympathetic neurons. J Neurobiol. 1994;25:108–118. doi: 10.1002/neu.480250203. [DOI] [PubMed] [Google Scholar]
- Moller K, Reimer M, Ekblad E, Hannibal J, Fahrenkrug J, Kanje M, et al. The effects of axotomy and preganglionic denervation on the expression of pituitary adenylate cyclase activating peptide (PACAP), galanin and PACAP type 1 receptors in the rat superior cervical ganglion. Brain Res. 1997;775:166–182. doi: 10.1016/s0006-8993(97)00923-2. [DOI] [PubMed] [Google Scholar]
- Monaghan TK, Mackenzie CJ, Plevin R, Lutz EM. PACAP-38 induces neuronal differentiation of human SH-SY5Y neuroblastoma cells via cAMP-mediated activation of ERK and p38 MAP kinases. J Neurochem. 2008;104:74–88. doi: 10.1111/j.1471-4159.2007.05018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy KS, Zhang KM, Jin JG, Grider JR, Makhlouf GM. VIP-mediated G protein-coupled Ca2+ influx activates a constitutive NOS in dispersed gastric muscle cells. Am J Physiol. 1993;265(4 Pt 1):G660–G671. doi: 10.1152/ajpgi.1993.265.4.G660. [DOI] [PubMed] [Google Scholar]
- Nakamachi T, Farkas J, Watanabe J, Ohtaki H, Dohi K, Arata S, et al. Role of PACAP in neural stem/progenitor cell and astrocyte – from neural development to neural repair. Curr Pharm Des. 2011;17:973–984. doi: 10.2174/138161211795589346. [DOI] [PubMed] [Google Scholar]
- Nakamachi T, Matkovits A, Seki T, Shioda S. Distribution and protective function of pituitary adenylate cyclase-activating polypeptide in the retina. Front Endocrinol. 2012a;3:1–10. doi: 10.3389/fendo.2012.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamachi T, Tsuchida M, Kagami N, Yofu S, Wada Y, Hori M, et al. IL-6 and PACAP receptor expression and localization after global brain ischemia in mice. J Mol Neurosci. 2012b;48:518–525. doi: 10.1007/s12031-012-9819-0. [DOI] [PubMed] [Google Scholar]
- Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav Immun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto M, Miyakawa H, Wada K, Furuta A. Activation of the VIP/VPAC2 system induces reactive astrocytosis associated with increased expression of glutamate transporters. Brain Res. 2011;1383:43–53. doi: 10.1016/j.brainres.2011.01.082. [DOI] [PubMed] [Google Scholar]
- Nobuta H, Ghiani CA, Paez PM, Spreuer V, Dong H, Korsak RA, et al. STAT3-Mediated astrogliosis protects myelin development in neonatal brain injury. Ann Neurol. 2012;72:750–765. doi: 10.1002/ana.23670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtaki H, Nakamachi T, Dohi K, Aizawa Y, Takaki A, Hodoyama K, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) decreases ischemic neuronal cell death in association with IL-6. Proc Natl Acad Sci U S A. 2006;103:7488–7493. doi: 10.1073/pnas.0600375103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med. 2006;12:829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- Pettersson LM, Heine T, Verge VM, Sundler F, Danielsen N. PACAP mRNA is expressed in rat spinal cord neurons. J Comp Neurol. 2004;471:85–96. doi: 10.1002/cne.20015. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM. Microgliosis: the questions shape the answers. Nat Neurosci. 2007;10:1507–1509. doi: 10.1038/nn1207-1507. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Lubics A, Tamas A, Szalontay L, Lengvari I. Pituitary adenylate cyclase activating polypeptide protects dopaminergic neurons and improves behavioral deficits in a rat model of Parkinson's disease. Behav Brain Res. 2004;151:303–312. doi: 10.1016/j.bbr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Kiss P, Lubics A, Tamas A. Review on the protective effects of PACAP in models of neurodegenerative diseases in vitro and in vivo. Curr Pharm Des. 2011;17:962–972. doi: 10.2174/138161211795589355. [DOI] [PubMed] [Google Scholar]
- Rosas-Ballina M, Tracey KJ. The neurology of the immune system: neural reflexes regulate immunity. Neuron. 2009;64:28–32. doi: 10.1016/j.neuron.2009.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders VM, Kasprowicz DJ, Swanson-Mungerson MA, Podojil JR, Kohm AP. Adaptive immunity in mice lacking the beta(2)-adrenergic receptor. Brain Behav Immun. 2003;17:55–67. doi: 10.1016/s0889-1591(02)00056-9. [DOI] [PubMed] [Google Scholar]
- Seaborn T, Masmoudi-Kouli O, Fournier A, Vaudry H, Vaudry D. Protective effects of pituitary adenylate cyclase-activating polypeptide (PACAP) against apoptosis. Curr Pharm Des. 2011;17:204–214. doi: 10.2174/138161211795049679. [DOI] [PubMed] [Google Scholar]
- Sharpless NS, Thal LJ, Perlow MJ, Tabaddor K, Waltz JM, Shapiro KN, et al. Vasoactive intestinal peptide in cerebrospinal fluid. Peptides. 1984;5:429–433. doi: 10.1016/0196-9781(84)90248-1. [DOI] [PubMed] [Google Scholar]
- Shioda S, Gozes I. VIP and PACAP: novel approaches to brain functions and neuroprotection. Curr Pharm Des. 2011;17:961. doi: 10.2174/138161211795589391. [DOI] [PubMed] [Google Scholar]
- Skoglosa Y, Lewen A, Takei N, Hillered L, Lindholm D. Regulation of pituitary adenylate cyclase activating polypeptide and its receptor type 1 after traumatic brain injury: comparison with brain-derived neurotrophic factor and the induction of neuronal cell death. Neuroscience. 1999;90:235–247. doi: 10.1016/s0306-4522(98)00414-x. [DOI] [PubMed] [Google Scholar]
- Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, et al. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- Ster J, De Bock F, Guerineau NC, Janossy A, Barrere-Lemaire S, Bos JL, et al. Exchange protein activated by cAMP (EPAC) mediates cAMP activation of p38 MAPK and modulation of Ca2+-dependent K+ channels in cerebellar neurons. Proc Natl Acad Sci U S A. 2007;104:2519–2524. doi: 10.1073/pnas.0611031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler RA, Gao Y, Zukin RS, Vosler PS, Zhang L, Zhang F, et al. Apurinic/apyrimidinic endonuclease APE1 is required for PACAP-induced neuroprotection against global cerebral ischemia. Proc Natl Acad Sci U S A. 2010;107:3204–3209. doi: 10.1073/pnas.1000030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Straub SG, Sharp GW. A wortmannin-sensitive signal transduction pathway is involved in the stimulation of insulin release by vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide. J Biol Chem. 1996;271:1660–1668. doi: 10.1074/jbc.271.3.1660. [DOI] [PubMed] [Google Scholar]
- Stumm R, Kolodziej A, Prinz V, Endres M, Wu DF, Hollt V. Pituitary adenylate cyclase-activating polypeptide is up-regulated in cortical pyramidal cells after focal ischemia and protects neurons from mild hypoxic/ischemic damage. J Neurochem. 2007;103:1666–1681. doi: 10.1111/j.1471-4159.2007.04895.x. [DOI] [PubMed] [Google Scholar]
- Sun W, Hong J, Zang YC, Liu X, Zhang JZ. Altered expression of vasoactive intestinal peptide receptors in T lymphocytes and aberrant Th1 immunity in multiple sclerosis. Int Immunol. 2006;18:1691–1700. doi: 10.1093/intimm/dxl103. [DOI] [PubMed] [Google Scholar]
- Symes AJ, Corpus L, Fink JS. Differences in nuclear signaling by leukemia inhibitory factor and interferon-gamma: the role of STAT proteins in regulating vasoactive intestinal peptide gene expression. J Neurochem. 1995;65:1926–1933. doi: 10.1046/j.1471-4159.1995.65051926.x. [DOI] [PubMed] [Google Scholar]
- Tamas A, Zsombok A, Farkas O, Reglodi D, Pal J, Buki A, et al. Postinjury administration of pituitary adenylate cyclase activating polypeptide (PACAP) attenuates traumatically induced axonal injury in rats. J Neurotrauma. 2006;23:686–695. doi: 10.1089/neu.2006.23.686. [DOI] [PubMed] [Google Scholar]
- Tan YV, Abad C, Lopez R, Dong H, Liu S, Lee A, et al. Pituitary adenylyl cyclase-activating polypeptide is an intrinsic regulator of Treg abundance and protects against experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2009;106:2012–2017. doi: 10.1073/pnas.0812257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida M, Shintani N, Morita Y, Tsukiyama N, Hatanaka M, Hashimoto H, et al. Regulation of autonomic nerve activities by central pituitary adenylate cyclase-activating polypeptide. Regul Pept. 2010;161:73–80. doi: 10.1016/j.regpep.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Sternberg EM. Neural aspects of immunomodulation: focus on the vagus nerve. Brain Behav Immun. 2010;24:1223–1228. doi: 10.1016/j.bbi.2010.07.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornwall J, Uusitalo H, Hukkanen M, Sorsa T, Konttinen YT. Distribution of vasoactive intestinal peptide (VIP) and its binding sites in labial salivary glands in Sjogren's syndrome and in normal controls. Clin Exp Rheumatol. 1994;12:287–292. [PubMed] [Google Scholar]
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchikawa D, Nakamachi T, Tsuchida M, Wada Y, Hori M, Farkas J, et al. Neuroprotective effect of endogenous pituitary adenylate cyclase-activating polypeptide on spinal cord injury. J Mol Neurosci. 2012;48:508–517. doi: 10.1007/s12031-012-9817-2. [DOI] [PubMed] [Google Scholar]
- Vacic V, McCarthy S, Malhotra D, Murray F, Chou HH, Peoples A, et al. Duplications of the neuropeptide receptor gene VIPR2 confer significant risk for schizophrenia. Nature. 2011;471:499–503. doi: 10.1038/nature09884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassiliou E, Jiang X, Delgado M, Ganea D. TH2 lymphocytes secrete functional VIP upon antigen stimulation. Arch Physiol Biochem. 2001;109:365–368. doi: 10.1076/apab.109.4.365.4245. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- Villalba M, Bockaert J, Journot L. Pituitary adenylate cyclase-activating polypeptide (PACAP-38) protects cerebellar granule neurons from apoptosis by activating the mitogen-activated protein kinase (MAP kinase) pathway. J Neurosci. 1997;17:83–90. doi: 10.1523/JNEUROSCI.17-01-00083.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright DA, Xin J, Sanders VM, Jones KJ. Differential actions of pituitary adenylyl cyclase-activating polypeptide and interferon gamma on Th2- and Th1-associated chemokine expression in cultured murine microglia. J Neurodegener Regen. 2008;1:31–34. [PMC free article] [PubMed] [Google Scholar]
- Wang G, Pan J, Tan YY, Sun XK, Zhang YF, Zhou HY, et al. Neuroprotective effects of PACAP27 in mice model of Parkinson's disease involved in the modulation of K(ATP) subunits and D2 receptors in the striatum. Neuropeptides. 2008;42:267–276. doi: 10.1016/j.npep.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Waschek JA. Vasoactive intestinal peptide: an important trophic factor and developmental regulator? Dev Neurosci. 1995;17:1–7. doi: 10.1159/000111268. [DOI] [PubMed] [Google Scholar]
- Waschek JA. Multiple actions of pituitary adenylyl cyclase activating peptide in nervous system development and regeneration. Dev Neurosci. 2002;24:14–23. doi: 10.1159/000064942. [DOI] [PubMed] [Google Scholar]
- Waschek JA, Dicicco-Bloom EM, Lelievre V, Zhou X, Hu Z. PACAP action in nervous system development, regeneration, and neuroblastoma cell proliferation. Ann N Y Acad Sci. 2000;921:129–136. doi: 10.1111/j.1749-6632.2000.tb06959.x. [DOI] [PubMed] [Google Scholar]
- Yadav M, Goetzl EJ. Vasoactive intestinal peptide-mediated Th17 differentiation: an expanding spectrum of vasoactive intestinal peptide effects in immunity and autoimmunity. Ann N Y Acad Sci. 2008;1144:83–89. doi: 10.1196/annals.1418.020. [DOI] [PubMed] [Google Scholar]
- Zaben M, Sheward WJ, Shtaya A, Abbosh C, Harmar AJ, Pringle AK, et al. The neurotransmitter VIP expands the pool of symmetrically dividing postnatal dentate gyrus precursors via VPAC2 receptors or directs them toward a neuronal fate via VPAC1 receptors. Stem Cells. 2009;27:2539–2551. doi: 10.1002/stem.184. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Shi TJ, Ji RR, Zhang YZ, Sundler F, Hannibal J, et al. Expression of pituitary adenylate cyclase-activating polypeptide in dorsal root ganglia following axotomy: time course and coexistence. Brain Res. 1995;705:149–158. doi: 10.1016/0006-8993(95)01150-1. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Danielsen N, Sundler F, Mulder H. Pituitary adenylate cyclase-activating peptide is upregulated in sensory neurons by inflammation. Neuroreport. 1998;9:2833–2836. doi: 10.1097/00001756-199808240-00027. [DOI] [PubMed] [Google Scholar]
- Zhou X, Rodriguez WI, Casillas RA, Ma V, Tam J, Hu Z, et al. Axotomy-induced changes in pituitary adenylate cyclase activating polypeptide (PACAP) and PACAP receptor gene expression in the adult rat facial motor nucleus. J Neurosci Res. 1999;57:953–961. [PubMed] [Google Scholar]
- Zigmond RE. gp130 cytokines are positive signals triggering changes in gene expression and axon outgrowth in peripheral neurons following injury. Front Mol Neurosci. 2011;4:1–18. doi: 10.3389/fnmol.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]


