Abstract
Background and Purpose
Nasal sensory nerves play an important role in symptoms associated with rhinitis triggered by environmental stimuli. Here, we propose that TRPV1 is pivotal in nasal sensory nerve activation and assess the potential of SB-705498 as an intranasal therapy for rhinitis.
Experimental Approach
The inhibitory effect of SB-705498 on capsaicin-induced currents in guinea pig trigeminal ganglion cells innervating nasal mucosa was investigated using patch clamp electrophysiology. A guinea pig model of rhinitis was developed using intranasal challenge of capsaicin and hypertonic saline to elicit nasal secretory parasympathetic reflex responses, quantified using MRI. The inhibitory effect of SB-705498, duration of action and potency comparing oral versus intranasal route of administration were examined.
Key Results
SB-705498 concentration-dependently inhibited capsaicin-induced currents in isolated trigeminal ganglion cells (pIC50 7.2). In vivo, capsaicin ipsilateral nasal challenge (0.03–1 mM) elicited concentration-dependent increases in contralateral intranasal fluid secretion. Ten per cent hypertonic saline initiated a similar response. Atropine inhibited responses to either challenge.
SB-705498 inhibited capsaicin-induced responses by ∼50% at 10 mg·kg−1 (oral), non-micronized 10 mg·mL−1 or 1 mg·mL−1 micronized SB-705498 (intranasal) suspension.
Ten milligram per millilitre intranasal SB-705498, dosed 24 h prior to capsaicin challenge produced a 52% reduction in secretory response. SB-705498 (10 mg·mL−1, intranasal) inhibited 10% hypertonic saline responses by 70%.
Conclusions and Implications
The paper reports the development of a guinea pig model of rhinitis. SB-705498 inhibits capsaicin-induced trigeminal currents and capsaicin-induced contralateral nasal secretions via oral and intranasal routes; efficacy was optimized using particle-reduced SB-705498. We propose that TRPV1 is pivotal in initiating symptoms of rhinitis.
Keywords: nasal sensory nerves, parasympathetic reflex, MRI, SB-705498, guinea pig, TRPV1 ion channel, capsaicin, hypertonic saline, atropine
Introduction
In the nose, sensory nerves in the nasal mucosa continuously monitor the composition, humidity and temperature of inhaled air and transmit signals to brain centres, which can elicit noxious sensations such as itching and pain, trigger defensive motor efferent responses (sneezing) and initiate parasympathetic reflex responses such as glandular and/or vascular secretion leading to rhinorrhoea and nasal congestion (Sarin et al., 2006). At the nerve terminals, local sensory axonal responses can also drive the release of pro-inflammatory neuropeptide mediators such as Substance P and calcitonin gene-related peptide from the nerve terminals, and this can contribute further to nasal symptoms through vascular engorgement and leak (Sarin et al., 2006). Nasal sensory nerves can be triggered by a variety of factors including weather changes, cold air, spicy food, airbourne pollution particulates, perfumes, household cleaning products and tobacco smoke (Shusterman, 2007).
Dysregulation of the normal nasal responses, such that normal innocuous stimuli trigger defensive reflexes (‘nasal hyper-responsiveness’), has been proposed to be a key mechanism in patients in non-allergic rhinitits (Sarin et al., 2006, Salib et al., 2008) and indeed may also be a driver of some of the symptoms of allergic rhinitis (e.g. itch) (O'Hanlon et al., 2007; 2008). In non-allergic rhinitis, desensitization of sensory nerves with capsaicin has been shown to provide symptom relief in patients for up to 9 months (Van Rijswijk et al., 2003) strongly implicating the sensory neurones in mediating symptoms. Furthermore, stimulation of nasal fibres by low-dose capsaicin leads to the development of symptoms including burning sensation, lacrimation, rhinorrhoea and nasal congestion (Geppetti et al., 2005; Sanico et al., 1997; 1998). The predominant target of capsaicin is the transient receptor potential cation channel (subfamily V, member 1) (TRPV1) ion channel expressed on sensory neurones (Caterina et al., 1997): TRPV1 is sensitive to many triggers including capsaicin, heat, protons (pH), eicosanoid derivatives, anandamide, and products of inflammation, such as histamine, prostaglandins and bradykinin and has been implicated in a variety of disorders including migraine, chronic and acute pain and bladder irritability, chronic arthritis, acute pancreatitis and diabetes (Yiangou et al., 2001; Gunthorpe et al., 2002; Geppetti et al., 2005; Goadsby, 2005; Appendino and Szallasi, 2006; Suri and Szallasi, 2008). In this report, we propose TRPV1 as a key modulator of nasal sensory nerve activation, and explore the potential use of the selective TRPV1 antagonist, SB-705498 (Rami et al., 2006, Gunthorpe et al., 2007), for the treatment of sensory nerve evoked symptoms of rhinitis. This paper reports the in vitro characterization of SB-705498 in guinea pig isolated trigeminal ganglion cell bodies from cells innervating nasal mucosa and the in vivo pharmacology of SB-705498 on capsaicin and hypertonic saline-induced intranasal fluid secretion in the guinea pig, as measured by MRI.
Materials and methods
All animal studies were ethically reviewed and carried out in accordance with Animals (Scientific Procedures) Act 1986 and the GSK Policy on the Care, Welfare and Treatment of Laboratory Animals.
SB-705498 was manufactured at GlaxoSmithKline. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Guinea pig sensitization compounds: Albumin (chicken egg white; Grade V, minimum 99% agarose gel electrophoresis), aluminium hydroxide hydrate and capsaicin were obtained from Sigma-Aldrich Inc. (St. Louis, MO, USA). Capsaicin was made up in 5% dimethylsulfoxide (DMSO; BDH Laboratory Supplies, Poole, England), 5% Tween 80® (Sigma-Aldrich Inc.) and 90% PBS (pH 7.4) for all other vehicles 0.9% NaCl was used. SB-705498 (TRPV1 antagonist) vehicle: 5% DMSO, 45% polyethylene glycol, 50% H2O. Particle reduced SB-705498 was achieved by micronization and made up as a suspension with sonication for 15 min.
Electrophysiology
Preparation of guinea pig trigeminal ganglion cell bodies
Cell bodies from the trigeminal ganglia were prepared using a modified protocol from Taylor-Clark et al. (2005). In brief, two male Dunkin Hartley guinea pigs were pretreated with atropine (1 mg·kg−1 i.p.) before being anaesthetized with isoflurane. DiI (25 μL of 2% DiI in DMSO) was instilled into the left nostril and the guinea pig was placed on its left side to recover. This procedure was repeated 24 h later, and the right nostril dosed. Guinea pigs were culled 2–6 weeks later, and an area of the trigeminal ganglia corresponding to the area enriched with nasally innervating cell bodies (Taylor-Clarke et al., 2005) was isolated, cut into small pieces and incubated with papain (8 mg in 4 mL Leibovitz's L-15 medium) for 40 min. The papain solution was removed and the tissue washed twice with Minimal Essential Medium supplemented with 5% foetal bovine serum, 5% horse serum, 100 units·mL−1 penicillin/100 μg·mL−1 streptomycin, 0.3% glucose and 1.6 μg·mL−1 insulin/1.6 μg·mL−1 transferrin/1.6 ng·mL−1 sodium selenite. Cell bodies were dissociated by trituration with fire-polished glass Pasteur pipettes of decreasing tip pore size and the resulting cell suspension filtered and centrifuged at 800 g for 3 min. The cells were then resuspended in a 200 μL medium and 25 μL of the cell suspension was applied to poly-D-lysine and laminin coated 12 mm glass coverslips. Cells were left to adhere to the coverslip for 2 h at 37°C before being flooded with medium.
Whole cell patch clamp electrophysiology
Each coverslip was broken into pieces and individual pieces were transferred to a recording chamber mounted on an inverted microscope (Nikon Eclipse 2000; Nikon UK Ltd., Kingston upon Thames, Surrey, UK) equipped with phase-contrast optics and fluorescence. Cells were continuously perfused with extracellular solution consisting of (in mM) NaCl (130), KCl (5), BaCl2 (2), MgCl2 (1), glucose (30), HEPES (25) pH adjusted to 7.3 with NaOH and 310–317 mOsm. Pipettes (1.5–4 MΩ) were filled with (in mM) CsCl (140), MgCl2 (4), EGTA (10), HEPES (10 mM), ATPdisodium salt (4), GTPsodium salt (0.3) and CaCl2 (0.1) pH adjusted to 7.3 with CsOH and 295 mOsm. Cells were voltage clamped at −70 mV via an Axoxlamp 200B amplifier. 10 mM stock aliquots of capsaicin in ethanol and a 10 mM stock solution of SB-705498 in DMSO were prepared and stored at −20°C. Drug applications were controlled by a fast application system (Biologic RSC200, time for solution exchange ∼30 ms) using a triple-barrel pipette assembly.
DiI –positive cells bodies were identified via punctuate fluorescence. Preliminary experiments demonstrated that repeated application of capsaicin to trigeminal cell bodies underwent significant tachyphylaxis. Macroscopic currents to 1 μM capsaicin, however, were stable over a 60 s period (Figure 2) enabling the effect of SB-705498 to be examined. Until stable, 1 μM capsaicin was applied (20 s) and then SB-705498 and capsaicin solutions were co applied for 20 s before switching back to 1 μM capsaicin. A single concentration of SB-705498 was tested on each trigeminal cell body and the data pooled.
Figure 2.
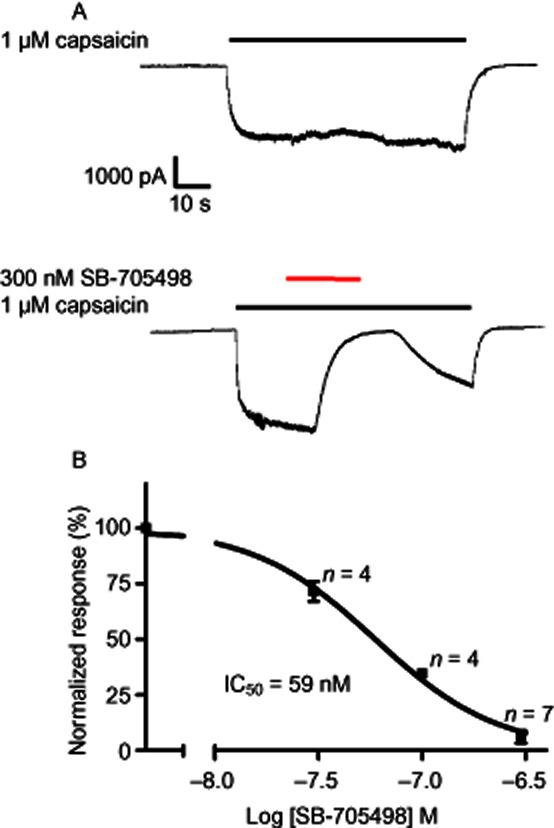
SB-705498 inhibits the capsaicin-induced current in nasally innervated guinea pig trigeminal cell bodies. (A) 1 μM capsaicin (black bar) produced an inward current that was stable over the 60 s application period. Application of 300 nM SB-705498 during the capsaicin response (red bar) produced almost complete inhibition that was rapidly reversed upon washout of SB-705498. (B) Pooled data generated from similar experiments to those described in A. Data were fitted with the Hill equation and gave an pIC50 estimate of 7.2 and a Hill coefficient of 1.47.
Data was graphically represented using Graphpad Prism (Version 5.0; GraphPad Software Inc., La Jolla, CA, USA) and a non-linear regression fitted using the built in equation log (inhibitor) versus normalized response – variable slope (also known as the Hill equation: Y = 100{1 + 10∧[(logIC50 − X) × HillSlope]}; where Y = normalized response and X = log [SB-705498]) to generate a pIC50 value and Hill coefficient.
Study details
In vivo guinea pig studies
Female Dunkin-Hartley guinea pigs were obtained from Harlan, UK at 180–200 g upon arrival. Animals were housed in groups of six in a temperature and humidity controlled environment, with a 12-h light : dark cycle. Food and water were available ad libitum. Animals were intranasally sensitized bilaterally, twice daily for 1 week with 25 μL of 20 ug·mL−1 ovalbumin + 180 mg·mL−1 aluminium hydroxide (excluding weekends). This was followed by once daily sensitization with ovalbumin (bilateral 25 μL, 5 mg·mL−1 solution) until the day of study (not including weekends).
Guinea pig weights ranged from 400 to 500 g on the day of study. Animals were anaesthetized with intraperitoneal urethane (1.5 g·kg−1) (99% minimum) (Sigma-Aldrich Inc.). Euthanasia: pentobarbitone sodium (Pentoject; Animal Care Ltd., York. UK).
Six to 12 animals per group were evaluated in each analysis (see individual studies for specific group sizes). All intranasal pretreatments of either atropine, SB-705498 or their respective vehicle controls were administered in 25 μL volumes to conscious guinea pigs. All animals were anaesthetized with urethane (1.5 g·kg−1) i.p. and MRI scanned to obtain baseline nasal images. Each animal was then removed from the scanner and capsaicin or hypertonic saline challenges (50 μL) were administered unilaterally to anesthetized guinea pigs as using a hand held Gilson pipette. Each animal was rescanned at 10 min post-challenge to measure contralateral fluid volumes and tissue changes. This time point was chosen following a time course study which indicated a robust, stable, maximal response was achieved at 10 min (data not shown).
Intranasal capsaicin or hypertonic saline challenges
The effect of 25 μL intranasal unilateral capsaicin doses of 0.03, 0.3 and 1 mM capsaicin or capsaicin vehicle (5% DMSO, 5% Tween 80, PBS) were investigated in dose response studies. In other studies a single 25 μL dose of 0.3 mM of capsaicin was used to elicit responses.
In studies investigating hypertonic saline-induced responses, 25 μL of either 0.9% (vehicle), or 10% saline was administered unilaterally.
Effect of intranasal atropine on responses to capsaicin or hypertonic saline challenge
Atropine (an anti-cholinergic inhibitor) was used to characterize parasympathetic reflexes. Atropine was applied as a 10 mg·mL−1 solution, 25 μL bilateral intranasal dose 60 minutes prior to either capsaicin or hypertonic saline challenge.
Effect of oral SB-705498 on capsaicin provoked nasal responses
Animals received an oral dose of 1 mL of 3, 10 or 30 mg·kg−1 SB705498 or vehicle 60 min prior to capsaicin challenge.
Effects of intranasal SB-705498 on capsaicin provoked nasal secretion
Animals received a 25 μL bilateral intranasal dose of either 1 mg·mL−1, 10 mg·mL−1 and 30 mg·mL−1 of SB-705498 or vehicle. SB-705498 was applied as a suspension using non-micronized material or micronized material. Pretreatment times prior to capsaicin challenge were either 1 h, or in duration of action studies, 24 h.
Effects of intranasal SB-705498 on hypertonic saline provoked nasal secretion
Animals received a 25 μL bilateral intranasal dose of either 10 mg·mL−1 of micronized SB-705498 or vehicle. Pretreatment times prior to 10% hypertonic saline challenge were either 1 h, or in the duration study, 24 h.
MRI methodology
Anaesthetized, free-breathing guinea pigs under respiratory monitoring were positioned supine within a 2 Tesla Bruker Medspec 96 cm S200 bore system using a 55 mm purpose-built coil in a 22 cm gradient insert. Physiological monitoring was carried out for the duration of the experiment (Small Animal Monitoring System obtained from SA Instruments), with the respiration rate (using a pneumatic pressure pad) being continuously measured. Following positioning of the animal in the centre of the magnet, and tuning and matching of the coil, initial pilot scans were carried out in each of the three planes; saggital, coronal and axial. Two scans were performed pre- and post-challenge; one scan sequence enhanced tissue and air space intensity [four averages, spin echo multi echo pulse sequence (RARE factor) 4, echo time (TE) 8.00 ms, Effective TE 32 ms, repetition time (TR) 1800 ms, field of view 5 × 5 cm, matrix 256 × 192, total scan time of approximately 3 min; Figure 1], while the second enhanced fluid intensity (four averages, RARE factor 16, TE 10.00 ms, effective TE 80 ms, TR 4681 ms, field of view 5 × 5 cm, matrix 256 × 192, total scan time of approximately 4 min; Figure 2). Acquiring baseline images allowed each animal to act as its own control and comparisons among animals to be made. The baseline status of each animal was also considered prior to any challenging. Animals that had significant background congestion associated with reduced airspace, because either fluid or tissue, would inevitably influence the animal's response to a challenge, and hence would be carefully assessed for inclusion into the final analysis. Animals with baseline fluid of anything above 5 μL and or tissue congestion of more than 50% were excluded from any analysis.
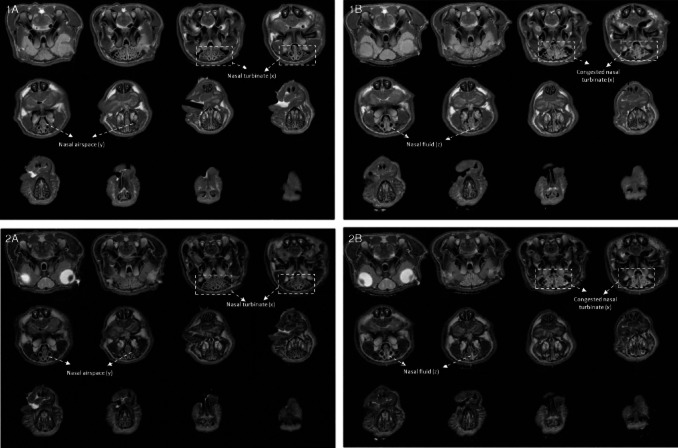
Figure 1.
Figures 1A (baseline ‘anatomical’ images) and 2A (Baseline ‘fluid’ images) illustrate typical baseline images of the nasal cavity region of the guinea pig using either a short or long TE spin echo sequence, respectively. Figure 1A and 2A produces good anatomical contrast allowing quantification of tissue (x) and airspace (y), whereas Figures 1B (10 min post unilateral capsaicin challenge) and 2B (10 min post unilateral capsaicin challenge) allow accurate quantification of fluid secretion into the nasal airspace. Following a capsaicin challenge, fluid (z) accumulation within the nasal airspace can be visualized in Figure 2B.
Image analysis
Using these two specific imaging sequences, nasal airspace and fluid volumes were quantified using Analyze 7.0 software (Mayo Clinic, Rochester, MN, USA). A contour encompassing the nasal passages was drawn and applied to each of the 13 slices of the image. The threshold value when applied to the contour enabled the quantification of absolute fluid (mm3) for each slice. A simple subtraction calculation was used to determine the change in contralateral nasal fluid volume between baseline and post-challenge time points (mm3).
Statistical analysis
Repeated measures anova was applied to the data. Comparisons between group means were made in the form of differences and were tested to 95% confidence intervals.
Results
Trigeminal ganglion cell electrophysiology
Sensory afferents supplying the nasal mucosa in the guinea pigs have their cell bodies located in the trigeminal ganglion and therefore it was of interest to investigate the potency and reversibility of SB-705498 on capsaicin-evoked responses in this population of isolated trigeminal ganglion cells. Fluorescent DiI tracer, applied intranasally in vivo allowed subsequent identification of the isolated ganglionic cell bodies with afferents terminating in the nasal mucosa. These fluorescently labelled cells were used in the patch clamp experiments. Figure 2A shows that 1 μM capsaicin generated an inward current that was stable over the 60 s application period. Application of 300 nM SB-705498 during the capsaicin response produced almost complete inhibition that was rapidly reversed upon washout. The potency of SB-705498 was evaluated in trigeminal ganglion cell bodies; a single concentration of SB-705498 was evaluated per cell and the data pooled for concentration–response analysis (multiple concentrations per cell were not possible because of run-down). The concentration response curve generated a pIC50 of 7.2 and Hill coefficient of 1.47.
In vivo MRI studies
Dose–response to capsaicin and hypertonic saline challenges and the effect of atropine pretreatment
Figure 3 shows the dose–response to an ipsilateral nasal capsaicin challenge in the guinea pig. Fluid secretions observed at 10 min post-capsaicin challenge on the contralateral nasal passage increased in a concentration-dependent manner from 0.03 to 1 mM. Significance (P < 0.05 compared with capsaicin vehicle challenge; n = 6) was achieved at 0.3 and 1 mM capsaicin (50 μL). No significant increases were seen with the vehicle challenge group. The 0.3 mM capsaicin response was shown to be completely inhibited by 10 mg·mL−1 atropine (Figure 4). All further studies characterizing the effect of SB-705498 used a capsaicin challenge concentration/volume of 0.3 mM/50 μL.
Figure 3.

Effect of capsaicin on contralateral fluid secretion. Baseline measurements = pre capsaicin challenge, 10 min data point = 10 min post-capsaicin ipsilateral challenge. *P < 0.05; compared with vehicle pretreatment group, n = 7/8 per group. anova, with Dunnett's follow-up analysis.
Figure 4.

Effect of intranasal atropine on capsaicin and hypertonic saline-induced nasal secretion. Baseline measurements = pre capsaicin challenge, 10 min data point = 10 min post 0.3 mM capsaicin ipsilateral challenge. **P < 0.001; compared with vehicle pretreatment group, n = 5/8 per group. anova, with Dunnett's follow-up analysis.
The stimulatory effect of hypertonic saline is also shown in Figure 4. Whereas 0.9% saline was ineffective in producing a contralateral fluid response (Figure 3), 10% saline induced a significant increase of approximately 26 mm3: this response was inhibited by atropine by over 70%.
Effect of oral administration of SB-705498 on capsaicin provoked nasal secretion
Capsaicin challenge elicited approximately 15 μL of fluid secretion in vehicle control animals. SB-705498 administered orally 1 h prior to challenge inhibited the contralateral response at all doses evaluated in a dose-dependent manner. At the lowest dose of 3 mg·kg−1 a non-significant reduction of ∼30% (P < 0.06) was seen whereas at 10 and 30 mg·kg−1 approximately 50% (P < 0.05) and 70% (P < 0.001) reductions were observed, respectively (Figure 5).
Figure 5.
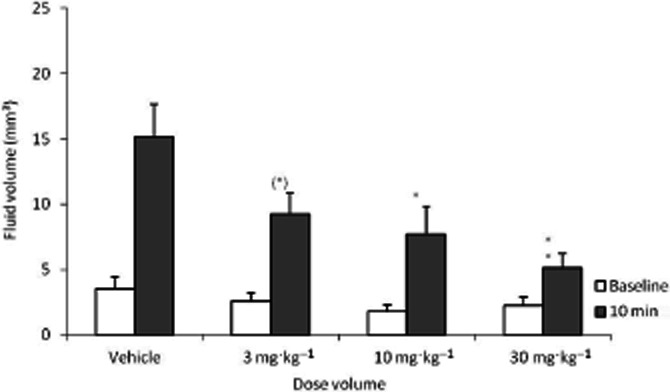
Effect of 1 h pretreatment of oral non-micronized SB-705498 on capsaicin provoked nasal secretion. Baseline measurements = pre capsaicin challenge, 10 min data point = 10 min post 0.3 mM capsaicin ipsilateral challenge. (*)P = 0.06; *P < 0.05; **P < 0.001 compared with vehicle pretreatment group, n = 10/12 per group. anova, with Dunnett's follow-up analysis.
Effects of topical intranasal administration of SB-705498 on capsaicin-induced nasal secretion
This experiment was conducted to determine whether SB-705498 is effective in inhibiting capsaicin-induced responses when delivered as an intranasal suspension directly to the nasal mucosa. Figure 6 shows that SB-705498 delivered topically in the nose inhibited fluid secretion in a concentration-dependent manner with approximately 50% inhibition (P < 0.005) obtained at 10 mg·mL−1 and 75% inhibition (P < 0.0001) at 30 mg·mL−1: approximately 35% reduction was observed at 1 mg·mL−1, but this was not significant (P < 0.07). It is interesting to note that the topical bilateral dose of 50 μL of a 10 mg·mL−1 suspension equates to a total dose of 0.5 mg of SB-705498 per animal. Equivalent inhibition via the oral route was obtained with a total dose of approximately 5 mg per animal when dosed orally (i.e., the 10 mg·kg−1 group), suggesting a 10-fold increase in potency is achieved through the topical route.
Figure 6.

Effect of 1 h pretreatment of intranasal non-micronized SB-705498 on capsaicin provoked nasal secretion. Baseline measurements = pre capsaicin challenge, 10 min data point = 10 min post 0.3 mM capsaicin ipsilateral challenge. (*)P < 0.07; *P < 0.005; ***P < 0.0001; compared with vehicle pretreatment group, n = 10/12 per group. anova, with Dunnett's follow-up analysis.
It was of interest to explore whether the intranasal potency of SB-705498 could be increased further by reducing the particle size of SB-705498 used in the intranasal suspension. The effect of micronized SB-705498 intranasal suspensions on capsaicin-induced responses is shown in Figure 7. At the lowest concentration of 1 mg·mL−1 (which given as a 50 μL bilateral dose equates to a 50 μg total dose per animal) a 50% inhibition of the capsaicin response was observed. This degree of inhibition was equivalent that seen at 10 mg·mL−1 of the non-micronized formulation. Similarly 75% inhibition was observed at 3 mg·mL−1 in the micronized formulation, which is comparable with the level of inhibition seen at 30 mg·mL−1 in the non-micronized formulation. These data suggest therefore that an additional 10-fold increase in potency can be achieved by particle reduction of SB-705498 used in the intranasal suspension.
Figure 7.

Effect of 1 h pretreatment of intranasal micronized SB-705498 on capsaicin provoked contralateral nasal fluid secretion. Baseline measurements = pre capsaicin challenge, 10 min data point = 10 min post 0.3 mM capsaicin ipsilateral challenge. **P < 0.0005 compared with vehicle pretreatment group, n = 10/12 per group. anova, with Dunnett's follow-up analysis.
A duration study was conducted to explore the inhibitory activity of SB-705498 at 24 h after intranasal dosing. Micronized SB-705498 (as a 10 mg·mL−1 suspension) was effective in inhibiting the capsaicin response by 52% (P < 0.001; Figure 8) Interestingly, non-micronized SB-705498 suspensions showed a trend to inhibition but this was not significant again suggesting particle reduction improves the in vivo efficacy of SB-705498.
Figure 8.
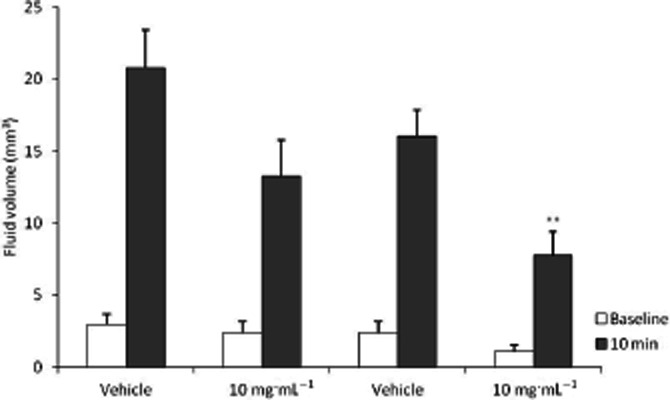
Effect of 24 h pretreatment of intranasal non-micronized and micronized SB-705498 on capsaicin provoked contralateral nasal fluid secretion. Baseline measurements = pre capsaicin challenge, 10 min data point = 10 min post 0.3 mM capsaicin ipsilateral challenge. **P < 0.001 compared with vehicle pretreatment group, n = 10/12 per group. anova, with Dunnett's follow-up analysis.
Effect of intranasal SB-705498 on the contralateral secretory response to 10% hypertonic saline challenge
In the clinic, hypertonic saline is used as a challenge agent to explore hyper-reactivity of nasal responses in patients with rhinitis and it has been suggested that this acts as a surrogate for cold dry air which is a recognized trigger in non-allergic rhinitic patients (Sanico et al., 1999). In rodent trigeminal ganglion cells it has been demonstrated that TRPV1 is acutely up-regulated following exposure to hypertonic media (Chen et al., 2009). It was of interest, therefore, to explore the pharmacology of the hypertonic saline challenge in the guinea pig model to assess whether the hypertonic saline response was sensitive to inhibition by SB-705498. Figure 9 illustrates the inhibitory effect of a micronized 10 mg·mL−1 suspension of SB-705498 on 10% hypertonic saline-induced responses. Ipsilateral challenge with 10% hypertonic saline produced significant increases in contralateral fluid (greater than 25 μL); micronized SB-705498 inhibited this secretory response by almost 70% (P < 0.005).
Figure 9.

Investigation into the contralateral effects of micronized SB-705498 pretreatment (–1 h) following an ipsilateral challenge with 10% hypertonic saline. Baseline measurements = Pre-hypertonic saline (HTS) challenge, 10 min data point = 10 min post HTS ipsilateral challenge. Pretreatment = micronized suspension of SB-705498 at 10 mg·mL−1. *P < 0.005 compared with vehicle pretreatment group, n = 6 per group. anova, with Dunnett's follow-up analysis.
Discussion and conclusions
The role of the TRPV1 in rhinitis was investigated in a novel preclinical guinea pig model. In the nose, stimulation of TRPV1 expressing nasal fibres elicits release of pro-inflammatory mediators, as well as producing reflex responses that contribute to rhinitic symptoms such as sneeze, rhinorrhoea, nasal itch and congestion. Blockade of the TRPV1 ion channel, which when activated elicits axonal depolarization leading to sensory neuronal firing, may therefore provide a novel approach for modulating symptoms of rhinitis. In order to validate this mechanism it was necessary to (i) confirm nasal sensory afferents functionally express TRPV1 and (ii) develop an animal model that would depict nasal symptoms that mimic clinical disease manifestation and be suitable for accurate quantitation of pharmacological effects.
To explore whether TRPV1 is functionally expressed in the target tissue (the nasal sensory neurone), we used a method employing DiI as an in vivo neuronal tracer to label cell bodies innervating the nasal mucosa in the guinea pig (Taylor-Clark et al., 2005). The population of fluorescently labelled cell bodies isolated from the trigeminal ganglion were targeted for whole-cell patch clamp electrophysiology and were shown to elicit inward currents in response to capsaicin that could be rapidly and reversibly inhibited by the TRPV1 selective antagonist, SB-705498. The potencies of both capsaicin and SB-705498 in the trigeminal cell bodies compare closely with values previously reported in cells recombinantly expressing guinea pig TRPV1 (Gunthorpe et al., 2007). These data thereby support functional expression of TRPV1 in nasally innervating sensory neurones.
Studies in guinea pigs where capsaicin was delivered topically to the nose has been shown to increase nasal airway resistance and secretions (Asakura et al., 1994), suggesting TRPV1 plays a role in mediating rhinitis symptoms. Similarly, hypertonic saline and cold dry air have also been shown to cause neurogenic inflammation in the human nose through activation of nociceptive receptors (Philip et al., 1993; Baraniuk et al., 1999), although in these studies, the TRPV1-dependency of the responses was not directly demonstrated. To date, techniques used in the studying of the enhancement of the neuronal component in rhinitis include rhinomanometry for nasal airway resistance and analysis of constituents and volumes of secreted fluid. Subjective reporting of symptoms has also been used as a measure of the response.
In this paper, we evaluated both capsaicin and hypertonic saline using MRI techniques, which allowed for highly accurate measurements of nasal secretion and airspace to be measured through the entire nasal cavity. We have developed a novel animal model depicting the parasympathetic neuronal reflex response of the guinea pig nose to intranasal ipsilateral challenge through absolute quantification of the fluid secretions in the contralateral nasal cavity. Challenge with 0.3 mM capsaicin produced a reproducible fluid response resulting in approximately 20 μL contralateral fluid secretion, and inhibition of the fluid response was achieved using topical atropine pretreatment demonstrating parasympathetic involvement. It is worth noting that in order to produce a robust model to support our investigations it was recognized early on that challenging naïve guinea pigs with capsaicin produced a high degree of variability as well as a reduced total response. In order to reduce this variability and enhance the total response to capsaicin, it was necessary to sensitize animals using ovalbumin/aluminium hydroxide, purely for producing a more robust model. This is the first demonstration in the guinea pig that nasal secretions in situ can be used to accurately quantify a parasympathetic reflex response elicited by a sensory neuronal trigger such as capsaicin. In addition, unlike more conventional methodologies such as filter paper absorbance and rhinomanometry, MRI has provided a tool that allows in vivo evaluation of nasal symptoms associated with both secretory and tissue with unprecedented accuracy that could be translated directly to the clinic.
The model presented herein describes a capsaicin-evoked reflex event which measures contralateral fluid secretion via MRI. The pharmacological profile of SB-705498 currently supports it as being a selective, potent TRPV1 antagonist (Gunthorpe et al., 2007) and therefore we are proposing the mechanism inhibiting the capsaicin-induced secretion is via suppression of TRPV1 activity of sensory nerves in the ipsilateral nostril. Further evidence for this mechanism could be explored in future studies by dosing SB-705498 unilaterally and exploring whether indeed the site of inhibitory action is the same as the site of capsaicin activation (i.e. at the sensory nerve site). Independent evaluation of the effect of SB-705498 applied on either the ipsilateral side or the contralateral side alone prior to ipslateral capsaicin challenge would better elucidate the site of action of topical SB-705498. Muscarinic receptors are known to mediate the parasympathetic reflex effects on nasal secretion (Kim and Baraniuk, 2007) and indeed, atropine was seen to inhibit the secretory response following capsaicin challenge. However, in vitro pharmacological profiling of SB-705498 conducted across a commercial binding receptor screening panel (conducted at CEREP, Celle l'Evescault, France) showed no appreciable interaction at the muscarinic receptors M1, M2, M3 and M4 (1 μM screening concentration yielded 11% or less inhibition of target radioligand binding; data unpublished).
In order to explore the TRPV1-dependency of the capsaicin-induced intranasal responses in the guinea pig rhinitis model, SB-705498 was dosed orally to guinea pigs at doses of 3, 10 and 30 mg·kg−1. This dose range and timing of the capsaicin challenge were chosen based on previous guinea pig pharmacology in a model of visceral pain (unpublished GSK data on file) and guinea pig pharmacokinetic data obtained following oral dosing (Rami et al., 2006). These studies have confirmed that SB-705498 has a profound inhibitory effect on the parasympathetic responses elicited following sensory nerve activation with the TRPV1 agonist, capsaicin and that the target can be engaged when the antagonist is released into the systemic circulation, following oral administration. More interestingly, the inhibitory effect can also be achieved after the TRPV1 antagonist is dosed topically, with a substantial reduction in total dose level required to elicit an equivalent response. The reduced ‘total body burden’ for SB-705498 after intranasal administration was approximately 10 times for a conventional suspension of the drug. A further order of magnitude could be gained by reducing the particle size of the suspended drug material. The comparison of the active doses of SB-705498 for different routes and formulations highlights the following: (i) considering the good oral bioavailability of SB-705498 in guinea pig (data not shown), it is highly unlikely the superior in vivo potency observed for the intranasal route is due to increased systemic exposure to the TRPV1 antagonist over the oral route through direct absorption into the bloodstream; (ii) the accessibility to the target/biophase in the nasal tissue is key to the in vivo efficacy and particle size reduced material showed enhanced efficacy because of the faster in situ dissolution, successfully competing with mucociliary clearance. The data suggest a reduction in dose to achieve a 50% inhibition from 5 mg per animal, when given by the oral route, to 50 μg per animal when micronized and delivered topically. Topical application in a clinical setting would provide a useful alternative to systemic treatments in part because of the established risk of hyperthermia in subjects treated with TRPV1 antagonists (Gavva et al., 2007; Krarup et al., 2011; Round et al., 2011). In preclinical species such as mouse, rat, dogs and monkeys moderate body temperature increases of 0.5–1.5°C have been reported where (Gavva et al., 2007): these are mostly transient in nature and in some instances subsequent acute exposure may show no perturbation in core temperature (Gavva et al., 2007). In this current study, topical administration of SB-705498 at 30 mg·mL−1 resulted in no measurable increase in core temperature as measured by a simple rectal probe technique (data not shown): A previous study with orally administered SB-705498 in guinea pig showed no effect on subcutaneous body temperature at 10 mg·kg−1 and up to 0.6 and 0.8°C degree increases at 30 and 100 mg·kg−1, respectively (unpublished data). From our limited studies it would appear therefore that reducing the systemic dose through targeting local administration in the nose can limit the risks associated with potential hyperthermia.
The effect of a low topical dose of a solution formulation of SB-705498 on capsaicin challenge were recently reported by Alenmyr et al. (2012) in patients with seasonal allergic rhinitis. Here, a dose approximating 0.2 mg, delivered by nasal lavage, inhibited the nasal pain and heat sensation elicited by a 5 mM capsaicin spray applied 2 min after the SB-705498. In contrast, this low dose was ineffective in reducing the sensation of nasal secretion. Duration of action of the effect of this SB-705498 formulation was not measured in this clinical study. To compare this study with the guinea pig study in this report, proportionally higher doses of SB-705498 were dosed at 1 h prior to capsaicin challenge and effects of the volume of nasal secretion were measured objectively via MRI.
One of the most appealing aspects of the investigation completed on SB-705498 is its extended duration of action in the MRI model after intranasal administration. A long lasting effect is a particularly challenging goal for the intranasal route, especially when the active compound is presented as a suspension, as the mucociliary clearance is a highly efficient mechanism that rapidly removes undissolved particles from the site of administration. Moreover, the electrophysiology data obtained at the TRPV1 receptor suggests a rapid on–off rate, consistent with previous recombinant cell assays (data not shown), thus suggesting the long duration of action cannot be explained in terms of favourable receptor kinetics. Therefore, a plausible explanation for the effects observed is that SB-705498 rapidly dissolves in the nasal fluid, readily penetrates into the nasal turbinate tissue (hypothesis supported by the high in vitro permeability measured for the compound in an Madin–Darbey canine kidney assay, data not shown), where it is retained for several hours by virtue of its lipophilicity/tissue affinity, which did not translate in the in vivo state. This long duration of action of SB-705498 seen in the nose of the guinea pig may therefore be in part associated with the clearance mechanisms required for a suspension that is mucocilliary and or absorption through the mucosal epithelium.
In addition to its inhibitory action on capsaicin-induced neuronal responses, SB-705498 was also effective in inhibiting fluid secretory responses to 10% hypertonic saline, demonstrating that TRPV1 plays a major role in hypertonic saline responses in vivo. Capsaicin-induced currents have previously been reported to be potentiated by changes in tonicity in rat isolated trigeminal ganglion cells, which was associated with increases in TRPV1 immunolocalization in the plasma membrane strongly implicating TRPV1 regulation by changes in osmolarity (Liu et al., 2007). Thus, the findings in guinea pig described herein further suggest a functional role for TRPV1 in mediating neuronal responses to changes in local tonicity such as occurring in nasal mucosa of cold dry air sensitive non-allergic rhinitics (Cruz et al., 2006). The clinical utility of a TRPV1 antagonist in patients with rhinitis is therefore of interest. Given the clinical finding that TRPV1 antagonists can cause hyperthermia and alternation in thermal sensation in skin in humans following oral dosing (Gavva et al., 2008; Krarup et al., 2011; Rowbotham et al., 2011), localizing the antagonist to the target site of the nasal mucosa and substantially reducing systemic exposure is of key importance in developing an effective and safe therapy for rhinitis. The guinea pig pharmacology presented here showed that topically applied, micronized SB-705498 substantially reduced the total dose required for inhibition of nasal secretory responses. In conclusion, we suggest that TRPV1 plays a key role in guinea pig nasal secretory responses to capsaicin and hypertonic saline.
Acknowledgments
The authors would like to recognize the contribution of Laboratory Animals Sciences, Dr Bob Murdoch and the former Neuronal and Gastrointestinal Centre of Excellence for Drug Discovery at GlaxoSmithKline for help and advice in completing these studies.
Glossary
- RARE factor
spin echo multi echo pulse sequence
- TE
echo time
- TR
repetition time
- TRPV1
transient receptor potential cation channel (subfamily V, member 1)
Conflict of interest
There is no conflict of interest of any of the authors.
References
- Alenmyr L, Greiff L, Andersson M, Sterner O, Zygmunt PM, Högestätt ED. Effect of mucosal TRPV1 inhibition in allergic rhinitis. Basic Clin Pharmacol Toxicol. 2012;110:264–268. doi: 10.1111/j.1742-7843.2011.00803.x. [DOI] [PubMed] [Google Scholar]
- Appendino G, Szallasi A. Clinically useful vanilloid receptor TRPV1 antagonists: just around the corner (or too early to tell)? Prog Med Chem. 2006;44:145–180. doi: 10.1016/S0079-6468(05)44404-5. [DOI] [PubMed] [Google Scholar]
- Asakura K, Narita S, Kojima T, Saito H, Kataura A. Changes in nasal airway resistance and secretory response in the guinea pig after nasal challenge with capsaicin and histamine. Eur Arch Otorhinolaryngol. 1994;251:224–228. doi: 10.1007/BF00628428. [DOI] [PubMed] [Google Scholar]
- Baraniuk J, Ali M, Yuta A, Tang S, Naranch K. Hypertonic saline nasal provocation stimulates nociceptive nerves, substance P release, and glandular mucous exocytosis in normal humans. Am J Respir Crit Care Med. 1999;160:655–662. doi: 10.1164/ajrccm.160.2.9805081. [DOI] [PubMed] [Google Scholar]
- Caterina M, Schumacher M, Tominaga M, Rosen T, Levine J, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu C, Liu L. Osmolality-induced tuning of action potentials in trigeminal ganglion neurons. Neurosci Lett. 2009;452:79–83. doi: 10.1016/j.neulet.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz A, Naclerio R, Proud D, Togias A. Epithelial shedding is associated with nasal reactions to cold, dry air. J Allergy Clin Immunol. 2006;117:1351–1358. doi: 10.1016/j.jaci.2006.01.054. [DOI] [PubMed] [Google Scholar]
- Gavva N, Bannon A, Surapaneni S, Hovland D, Jr, Lehto S, Gore A. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci. 2007;27:3366–3374. doi: 10.1523/JNEUROSCI.4833-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavva N, Treanor J, Garami A, Fang L, Surapaneni S, Akrami A, et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain. 2008;136:202–210. doi: 10.1016/j.pain.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Geppetti P, Capone J, Trevisani M, Nicoletti P, Zagli G, Tola M. CGRP and migraine: neurogenic inflammation revisited. J Headache Pain. 2005;6:61–70. doi: 10.1007/s10194-005-0153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppetti P, Fusco BM, Marabini S, Maggi CA, Fanciullacci M, Sicuteri F. Secretion, pain and sneezing induced by the application of capsaicin to the nasal mucosa in man. Br J Pharmacol. 1988;93:509–514. doi: 10.1111/j.1476-5381.1988.tb10305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goadsby P. Calcitonin gene-related peptide antagonists as treatments of migraine and other primary headaches. Drugs. 2005;65:2557–2567. doi: 10.2165/00003495-200565180-00002. [DOI] [PubMed] [Google Scholar]
- Gunthorpe MJ, Benham CD, Randall A, Davis JB. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci. 2002;23:183–191. doi: 10.1016/s0165-6147(02)01999-5. [DOI] [PubMed] [Google Scholar]
- Gunthorpe MJ, Hannan S, Smart D, Jerman J, Arpino S, Smith G, et al. Characterization of SB-705498, a potent and selective vanilloid receptor-1 (VR1/TRPV1) antagonist that inhibits the capsaicin-,acid-, and heat-mediated activation of the receptor. J Pharmacol Exp Ther. 2007;312:1183–1192. doi: 10.1124/jpet.106.116657. [DOI] [PubMed] [Google Scholar]
- Kim D, Baraniuk JN. Neural aspects of allergic rhinitis. Curr Opin Otolaryngol Head Neck Surg. 2007;15:268–723. doi: 10.1097/MOO.0b013e328259c372. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krarup A, Ny L, Astrand M, Bajor A, Hvid-Jensen F, Hansen M, et al. Randomised clinical trial: the efficacy of a transient receptor potential vanilloid 1 antagonist AZD1386 in human oesophageal pain. Aliment Pharmacol Ther. 2011;33:1113–1122. doi: 10.1111/j.1365-2036.2011.04629.x. [DOI] [PubMed] [Google Scholar]
- Liu L, Chen L, Liedtke W, Simon S. Changes in osmolality sensitise the response to capsaicin in trigeminal sensory neurones. J Neurophysiol. 2007;97:2001–2015. doi: 10.1152/jn.00887.2006. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanlon S, Min Keh S, Saleh H. The role of neuronal mechanisms in allergic rhinitis. Otorinolaringologia. 2008;58:17–30. [Google Scholar]
- O'Hanlon S, Facer P, Simpson K, Sandhu G, Saleh H, Anand P. Neuronal markers in allergic rhinitis: expression and correlation with sensory testing. Laryngoscope. 2007;117:1519–1527. doi: 10.1097/MLG.0b013e3180ca7846. [DOI] [PubMed] [Google Scholar]
- Philip G, Jankowski R, Baroody FM, Naclerio RM, Togias AG. Reflex activation of nasal secretion by unilateral inhalation of cold dry air. Am Rev Respir Dis. 1993;148(6 Pt 1):1616–1622. doi: 10.1164/ajrccm/148.6_Pt_1.1616. [DOI] [PubMed] [Google Scholar]
- Rami H, Thompson M, Stemp G, Fell S, Jerman J, Stevens AJ, et al. Discovery of SB-705498: a potent, selective and orally bioavailable TRPV1 antagonist suitable for clinical development. Bioorg Med Chem Lett. 2006;16:3287–3291. doi: 10.1016/j.bmcl.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Round P, Priestley A, Robinson J. An investigation of the safety and pharmacokinetics of the novel TRVP1 antagonist XEN-D0501 in healthy subjects. Br J Clin Pharmacol. 2011;72:921–931. doi: 10.1111/j.1365-2125.2011.04040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbotham M, Nothaft W, Duan W, Wang Y, Faltynek C, McGaraughty S, et al. Oral and cutaneous thermosensory profile of selective TRPV1 inhibition by ABT-102 in a randomisized healthy volunteer trial. Pain. 2011;152:1192–1200. doi: 10.1016/j.pain.2011.01.051. [DOI] [PubMed] [Google Scholar]
- Salib R, Narries P, Nair S, Howarth P. Mechanisms and mediators of nasal symptoms in non-allergic rhinitis. Clin Exp Allergy. 2008;38:393–404. doi: 10.1111/j.1365-2222.2007.02926.x. [DOI] [PubMed] [Google Scholar]
- Sanico A, Atsura S, Proud D, Togias A. Dose-dependent effects of capsaicin nasal challenge: in vivo evidence of human airway neurogenic inflammation. J Allergy Clin Immunol. 1997;100:632–641. doi: 10.1016/s0091-6749(97)70167-2. [DOI] [PubMed] [Google Scholar]
- Sanico A, Philip G, Proud D, Naclerio R, Togias A. Comparison of nasal mucosal responsiveness to neuronal stimulation in non-allergic and allergic rhinitis: effects of capsaicin nasal challenge. Clin Exp Allergy. 1998;28:92–100. doi: 10.1046/j.1365-2222.1998.00182.x. [DOI] [PubMed] [Google Scholar]
- Sanico A, Philip G, Lai G, Togias A. Hyperosmolar saline induces reflex nasal secretions, evincing neural hyperresponsiveness in allergic rhinitis. J Appl Physiol. 1999;86:1202–1210. doi: 10.1152/jappl.1999.86.4.1202. [DOI] [PubMed] [Google Scholar]
- Sarin S, Undem B, Sanico A, Togias A. The role of the nervous system in rhinitis. J Allergy Clin Immunol. 2006;118:999–1014. doi: 10.1016/j.jaci.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Shusterman D. Trigeminally-mediated health effects of air pollutants: sources of inter-individual variability. Hum Exp Toxicol. 2007;26:149–157. doi: 10.1177/0960327107070550. [DOI] [PubMed] [Google Scholar]
- Suri A, Szallasi A. The emerging role of TRPV1 in diabetes and obesity. Trends Pharmacol Sci. 2008;29:29–36. doi: 10.1016/j.tips.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark T, Kollarik M, MacGlashan D, Jr, Undem B. Nasal sensory nerve populations responding to histamine and capsaicin. J Allergy Clin Immunol. 2005;116:1282–1288. doi: 10.1016/j.jaci.2005.08.043. [DOI] [PubMed] [Google Scholar]
- Van Rijswijk J, Boeke E, Keizer J, Mulder P, Blom H, Fokkens W. Intranasal capsaicin reduces nasal hyperreactivity in idiopathic rhinitis: a double-blind randomized application regimen study. Allergy. 2003;58:754–761. doi: 10.1034/j.1398-9995.2003.00203.x. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Dyer N, Chan C, Knowles C, Williams N, et al. Vanilloid receptor 1 immunoreactivity in inflamed human bowel. Lancet. 2001;357:1338–1339. doi: 10.1016/s0140-6736(00)04503-7. [DOI] [PubMed] [Google Scholar]