Abstract
Background and Purpose
Retinopathy, as a common complication of diabetes, is a leading cause of reduced visual acuity and acquired blindness in the adult population. The aim of present study was to investigate the therapeutic effect of hydrogen sulfide on streptozotocin (STZ)-induced diabetic retinopathy in rats.
Experimental Approach
Rats were injected with a single i.p. injection of STZ (60 mg·kg−1) to induce diabetic retinopathy. Two weeks later, the rats were treated with NaHS (i.p. injection of 0.1 mL·kg−1·d−1 of 0.28 mol·L−1 NaHS, a donor of H2S) for 14 weeks.
Key Results
Treatment with H2S had no significant effect on blood glucose in STZ-induced diabetic rats. Treatment with exogenous H2S enhanced H2S levels in both plasma and retinas of STZ-induced diabetic rats. Treatment with H2S in STZ-treated rats improved the retinal neuronal dysfunction marked by enhanced amplitudes of b-waves and oscillatory potentials and expression of synaptophysin and brain-derived neurotrophic factor, alleviated retinal vascular abnormalities marked by reduced retinal vascular permeability and acellular capillary formation, decreased vitreous VEGF content, down-regulated expressions of HIF-1α and VEGFR2, and enhanced occludin expression, and attenuated retinal thickening and suppressed expression of extracellular matrix molecules including laminin β1 and collagen IVα3 expression in retinas of STZ-induced diabetic rats. Treatment with H2S in retinas of STZ-induced diabetic rats abated oxidative stress, alleviated mitochondrial dysfunction, suppressed NF-κB activation and attenuated inflammation.
Conclusions and Implications
Treatment with H2S alleviates STZ-induced diabetic retinopathy in rats possibly through abating oxidative stress and suppressing inflammation.
Keywords: hydrogen sulfide, streptozotocin, diabetic retinopathy, oxidative stress, inflammation
Introduction
Diabetic retinopathy (DR), as a prevalent complication of diabetes mellitus (DM), is a leading cause of reduced visual acuity and acquired blindness in working-age adult population in both developed and developing nations (Congdon et al., 2003). The early clinical events of DR in patients and animals with DM include retinal glial dysfunction, neurodegeneration and vascular dysfunction (Kern and Barber, 2008; Ali and El-Remessy, 2009). Although laser therapy has shown partial therapeutic effect on DR, the current treatments for DR are far from satisfactory. As the prevalence of DR progressively rises throughout the world, there is a need for searching more specific therapeutic strategy.
Despite its long-standing reputation as a foul smelling and toxic gas, hydrogen sulfide (H2S) is the most recent addition to the endogenous gasotransmitter family. Recently, accumulating reports revealed that H2S played beneficial roles in several diseases, including hypertension (Zheng et al., 2011), atherosclerosis (Bełtowski et al., 2010) and ischaemia-reperfusion injuries (Salloum et al., 2012). Lower levels of H2S were observed in the blood of diabetes patients and streptozotocin (STZ)-treated rats (Jain et al., 2010). Various studies showed an important role of H2S deficiency in the pathogenesis of diabetic endothelial dysfunction, diabetic nephropathy and cardiomyopathy (Lefer, 2008; Szabo, 2012), which suggested supplement of H2S in diabetes could be a novel strategy for complications of diabetes. In addition, treatment with H2S has been shown protective effect in retina against injuries induced by light (Mikami et al., 2011) and ischaemia/reperfusion (Osborne et al., 2010; Biermann et al., 2011).
Based on these findings, the aim of current study was to examine the effect of treatment with H2S on STZ-induced DR in rats and investigate the underlying mechanism.
Methods
Ethical statement
All the animals used in this work received humane care in compliance with institutional animal care guidelines and were approved by the Local Institutional Committee. All the surgical and experimental procedures were in accordance with institutional animal care guidelines. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Materials and animals
All other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise noted. Sprague–Dawley (SD) rats (male, 2 months old, 200–250 g) were purchased from Vital-Aiver Animal Ltd (Beijing, China) and housed 2 per cage in a room under controlled temperature (23–25°C), humidity (50%) and lighting (12 h light/dark cycle), with food and water provided ad libitum.
Animal model
SD rats were injected with a single i.p. injection of STZ (60 mg·kg−1) in 10 mM citrate buffer. Control non-diabetic rats received injections of an equal volume of citrate buffer only. Glucose concentrations were measured in blood samples obtained from the tail vein with a commercial blood glucose analyser. After 48 h of STZ injection, only STZ-treated rats with blood glucose concentration higher than 15 mmol·L−1 were considered as diabetic and included in this study. Glycaemia and body weight were recorded on the day of the experiment.
Measurement of blood-retinal barrier (BRB) breakdown
Retinal vascular permeability was assessed using Evans blue dye extravasation technique as previously described (Kusari et al., 2007). Briefly, Evans blue was dissolved in normal saline at 45 mg·mL−1. The animal was deeply anaesthetized, and the right jugular vein and iliac artery were cannulated, and Evans blue solution (45 mg·kg−1 in saline) was injected through the jugular vein. After 2 min of Evans blue injection, blood was withdrawn from the iliac artery and then every 30 min for 120 min. After 120 min of Evans blue injection, the chest cavity was opened and the animal was perfused through the left ventricle with a solution of citrate buffer (0.05 M) for 2 min to wash out intravascular dye. Immediately after perfusion, the eyes were enucleated. Evans blue in the blood samples and retinas was detected as described previously (Qaum et al., 2001). BRB permeability was determined by the calculation, BRB permeability = (retinal Evan blue in micrograms/retina dry weight in grams)/(time-averaged plasma Evans blue in micrograms/plasma volume in microlitres × circulation time in hours), and was expressed as microlitres plasma/gram retina dry weight per hour.
Measurement of retinal thickness
Retinal thickness was calculated by using Image-Pro Plus 4.5 (IPP4.5; Media Cybernetics, Silver Spring, MD, USA) on 6 μM haematoxylin and eosin-stained sections as previously described (Martin et al., 2004). Digital photographs were taken using 20 × objectives from the nasal and temporal sides (about 300 mm from the optic nerve) in each of the contralateral and ipsilateral retinae. In each photo, width from the inner limiting membrane to the tips of the photoreceptor outer segments represented the thickness of the whole retina.
Retinal leukostasis measurement
One day after implanting a jugular vein catheter, retinal leukostasis measurements were performed as the method described (Abiko et al., 2003).
Measurement of H2S content in the plasma and retina tissue
Measurement of H2S levels in plasma or retinas of rats was performed by using ELIT Ion Analyzer (ELIT 9801; Electro Analytic Instruments Ltd, London, UK) as previously described (Geng et al., 2007).
Electroretinography
Retinal neuronal functions were tested with electroretinography (ERG) (EP-1000 System; Tomey, Nagoya, Japan) as previously described (Zhang et al., 2011).
The b-wave amplitude was tested from the trough of the a-wave to the peak of the b-wave, which was defined by the International Society for Clinical Electrophysiology of Vision (Marmor et al., 2004).
Oscillatory potentials (OPs) are six wavelets in the electroretinogram that present on the rising phase of the b-wave (Tzekov and Arden, 1999). The magnitude of the OPs was determined as the sum of the three major amplitudes.
Measurement of acellular capillaries
The number of pericytes and acellular capillaries was evaluated by using previously described quantitative methods (Zheng et al., 2009). Sample preparations were set onto polylysine-coated glass slides and stored at −20°C until used for periodic acid Schiff and haematoxylin staining.
Analysis of vitreous VEGF
For analysis of vitreous VEGF concentrations, vitreous fluid was pooled from both eyes of each rat, and the concentration of VEGF protein in the vitreous fluid was measured with an elisa kit (R&D Systems Inc., Minneapolis, MN, USA).
Measurement of retinal malondialdehyde (MDA)
Retinal homogenates were used for the determination of MDA using a kit (Cayman, Ann Arbor, MI, USA). Final results were normalized to total protein determined by the Bradford method with a protein assay reagent kit (Bio-Rad, Hercules, CA, USA).
Retinal O2− and OONO− production
Retinal O2− and OONO− production was detected according to the method described by Elks et al. (2009).
Quantitative real-time PCR analysis
Total RNA was extracted from retinas or cultured cells by using TRIzol (Life Technologies Inc., Gaithersburg, MD, USA) according to the manufacturer's protocol. First-strand cDNA was prepared from total RNA by using SuperScript First-Strand Synthesis Kit (Invitrogen, Carlsbad, CA, USA). The sequences of primers are listed in Supporting Information Table S1. Real-time PCR analysis was performed with a QuantiTectTM SYBR® Green PCR (Tiangen, Shanghai, China) according to the manufacturer's instructions. The highly specific measurement of mRNA was carried out for cystathionine γ-lyase (CSE), cystathionine β-synthase (CBS), 3-mercaptopyruvate sulfurtransferase (3-MST), IL-1β, VEGFR2 (receptor 2 for VEGF; receptor nomenclature follows Alexander et al., 2011), synaptophysin, brain-derived neurotrophic factor (BDNF), zona occluden-1 (ZO-1), occludin, fibronectin, laminin β1, collagen IVα3, intercellular adhesion molecule 1 (ICAM-1), inducible NOS (iNOS), COX-2 and GAPDH using the LightCycler system (Bio-Rad, Carlsbad, CA, USA). Each sample was run and analysed in duplicate. CSE, CBS, 3-MST, IL-1β, VEGFR2, synaptophysin, BDNF, occludin, fibronectin, laminin β1, collagen IVα3, ICAM-1, iNOS and COX-2 mRNA levels were adjusted as the values relative to GAPDH, which was used as the endogenous control to ensure equal starting amounts of cDNA. The control group was used as the calibrator with a given value of 1, and the other groups were compared with this calibrator.
Western blotting analysis
The protein concentration of retinal homogenates was determined with BSA as a standard by a Bradford assay. Equal amount of protein preparations (20 μg in 10 μL buffer) was run on SDS-polyacrylamide gels, electrotransferred to polyvinylidine difluoride membranes, and blotted with a primary antibody against synaptophysin, BDNF, fibronectin, laminin β1, collagen IVα3, HO-1, p47phox, NOX2, IκBα and NF-κB p65 (all of these antibodies were obtained from Santa Cruz Biotechnology, Inc., Sta. Cruz, CA, USA) overnight at 4°C using slow rocking. Then, they were blotted with HRP-conjugated secondary antibody (1:5000, Sigma) and HRP-conjugated monoclonal antibody against β-actin (1:10 000, Sigma). Immunoreactive bands were detected by a chemiluminescent reaction (ECL kit; Amersham Pharmacia, CA, USA). The results were calculated as the mean ratio of the target protein density to the β-actin density. The control group was used as the calibrator with a given value of 100%, and the other groups were compared with this calibrator.
Determination of retinal nitrite/nitrate (NOx) content
The levels of NOx, the stable end products of NO, in retina were measured using a Total Nitrite/Nitrate Assay kit (Dojindo, Kumamoto, Japan), which employed the Griess method. Retinal NOx concentration was calculated as nmol·mg−1 of protein.
Measurement of prostaglandin E2 (PGE2) in retina
PGE2 in retinal homogenates was measured by elisa using a commercial kit (Cayman Chemical, Ann Arbor, MI, USA). Final results were normalized to protein concentration.
Measurement of superoxide dismutase (SOD) activity in retina
Retinal SOD activity was measured using an SOD-525™ reagent kit (OXIS International, Foster, CA, USA). Final results were normalized to protein concentration.
Measurement of mitochondrial function in retina
Mitochondria were isolated by differential centrifugation of retinal homogenates according to the method described previously (Mariappan et al., 2007).
Rates of ATP formation were determined with commercially available kit (BioVision, Mountain View, CA, USA).
Mitochondrial reactive oxygen species (ROS) production was determined by lucigenin chemiluminescence. The results were normalized to protein concentration.
Measurement of mitochondrial swelling was done according to the method described previously (Mariappan et al., 2007). The absorbance was measured at 540 nm.
The activities of nicotinamide-adenine dinucleotide cytochrome c reductase (NCCR; marker for electron coupling capacity between complexes I and III) or succinate cytochrome c reductase (SCCR; marker for electron coupling capacity between complexes II and III) were determined by using a thermostatically regulated Thermo-Spectronic spectrophotometer (Fisher Scientific, Pittsburgh, PA, USA) (Maher et al., 2007).
Cell culture and treatment
Retinal Müller (glial) cells (rMC-1 cell line) were cultured and passaged in DMEM medium containing 5 mM glucose and 10% FBS. Rat retinal endothelial cells (RRECs) were isolated and grown as previously described (Antonetti and Wolpert, 2003).
NF-κB luciferase assay
NF-κB activity was determined using the NF-κB luciferase assay. Cells were seeded on 24-well culture plates at 2 × 104 cells/well. Cells were incubated for 1 h with a total of 170 ng plasmids (85 ng NF-κB-dependent luciferase reporter and 85 ng pcDNA3–β-gal), 1 μL Tfx-50 reagent (Promega, Madison, WI, USA) and 200 μL serum-free RPMI. In all, 800 μL RPMI containing FBS was then added, and incubation continued. After 24 h of incubation, cells were treated with indicated chemicals for 1 h. Luciferase activity was measured using a luciferase assay system and normalized against β-galactosidase activity.
Study design
Experiment 1
SD rats were randomly divided into three groups and treated as follows: (i) control group (control); (ii) STZ-induced diabetic group (DM); and (iii) STZ-induced diabetic group treated with H2S (DM+ H2S). Two weeks after diabetes induction, rats were treated with NaHS by i.p. injection of 0.1 mL·kg−1·d−1 of 0.28 mol·L−1 NaHS for 14 weeks. All end points were determined on the last day of treatment.
Experiment 2
The rMC-1 or RREC was cultured in DMEM containing high glucose (HG; 25 mM) for 24 h. Cells treated in low glucose (5 mM, plus 20 mM mannitol) served as control. Sodium hydrogen sulfide (1 mM) was treated as H2S donor. BAY-11-7082 (5 μM) was treated as a NF-κB-specific inhibitor.
Statistical analysis
All data are expressed as mean ± SD. Comparison among groups was analysed using a two-way anova followed by Bonferroni t-test. P < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS 11.0.0 software (SPSS Inc., Chicago, IL, USA).
Results
Effect of treatment with exogenous H2S on H2S levels in plasma and retinas of STZ-treated rats
As shown in Table 1, plasma glucose concentration was significantly higher in STZ-diabetic rats at the time of killing when compared with age-matched control rats. Body weight was significantly lower in STZ-diabetic rats compared with controls. Treatment with exogenous H2S had no significant effect on plasma glucose and body weight in STZ-diabetic rats.
Table 1.
Effect of treatment with H2S on body weight and glycaemia
| Control | DM | DM + H2S | |
|---|---|---|---|
| Body weight (g) | 412 ± 43 | 361 ± 32a | 367 ± 36a |
| Plasma glucose (mM) | 5.3 ± 0.72 | 26.9 ± 4.3a | 25.8 ± 5.5a |
Values are means ± SD. n = 35–38 in each group.
P < 0.05 versus control group.
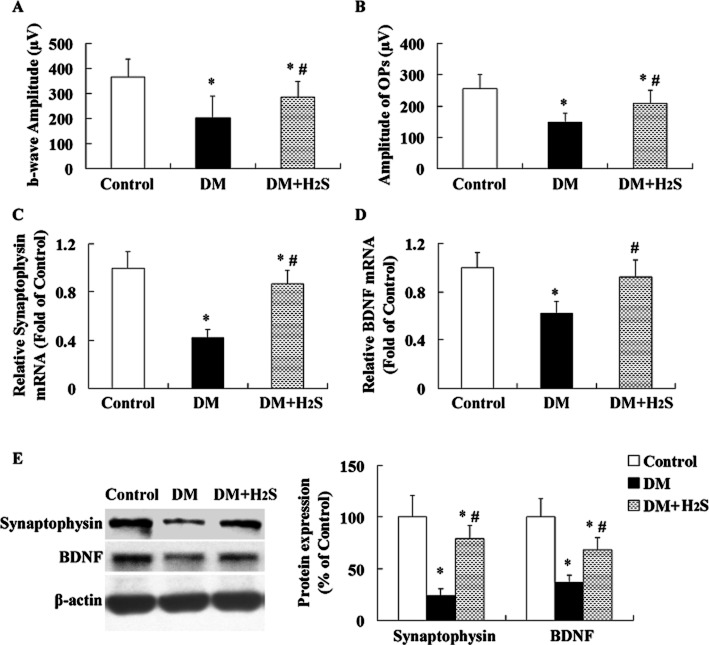
In the STZ-induced diabetic rats, H2S levels in both plasma ( Figure 1A) and retinas (Figure 1B) were lower than that in control rats. CSE and 3-MST mRNA expression (Figure 1D, E) were lower than that in control rats. CBS mRNA expression was similar between two groups (Figure 1C).
Figure 1.
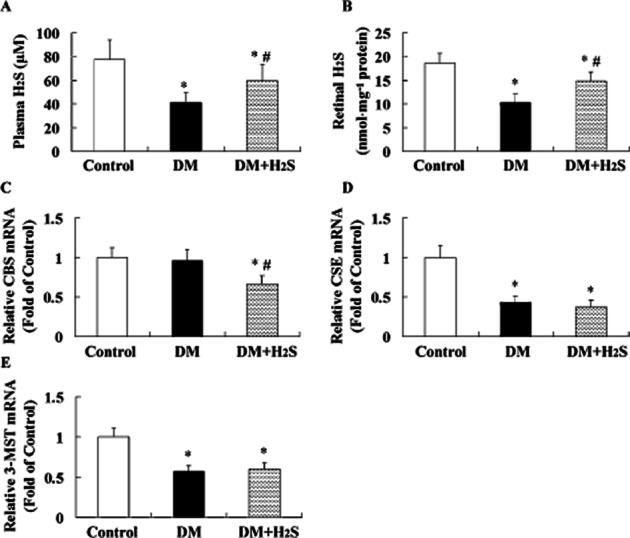
Effect of treatment with exogenous H2S on H2S levels in plasma and retinas of STZ-treated rats. Measurement of H2S levels in plasma (A) or retinas (B) of rats was performed by using ELIT Ion Analyzer. Retinal CBS (C), CSE (D) and 3-MST (E) mRNA expressions were evaluated with quantitative real-time PCR method. Values are means ± SD. n = 7 in each group; *P < 0.05 versus control group; #P < 0.05 versus DM group.
Treatment with exogenous H2S enhanced H2S levels in both plasma and retinas and decreased retinal CBS mRNA expression in STZ-treated rats, but had no significant effect on CSE and 3-MST mRNA expression.
Effect of treatment with H2S on neuronal dysfunction in STZ-treated rats
The amplitudes of b-waves (Figure 2A) and OPs (Figure 2B) were two indices of neuronal function. The amplitudes of b-waves and OPs were significantly lower in retinas of STZ-induced diabetic rats 16 weeks after diabetes onset when compared with the controls. In addition, the mRNA and protein levels of synaptophysin (Figure 2C, E) and BDNF (Figure 2D, E) were lower than that in control groups.
Figure 2.
Effect of treatment with H2S on neuronal dysfunction in STZ-treated rats. Amplitude of b-waves (A) and OPs (B) were examined with ERG. Retinal synaptophysin (C, E) and BDNF (D, E) mRNA and protein expressions were evaluated with quantitative real-time PCR method and Western blotting analysis respectively. Values are means ± SD. n = 7 in each group; *P < 0.05 versus control group; #P < 0.05 versus DM group.
Treatment with H2S in STZ-treated rats enhanced the amplitudes of b-waves and OPs and increased mRNA and protein expression of synaptophysin and BDNF.
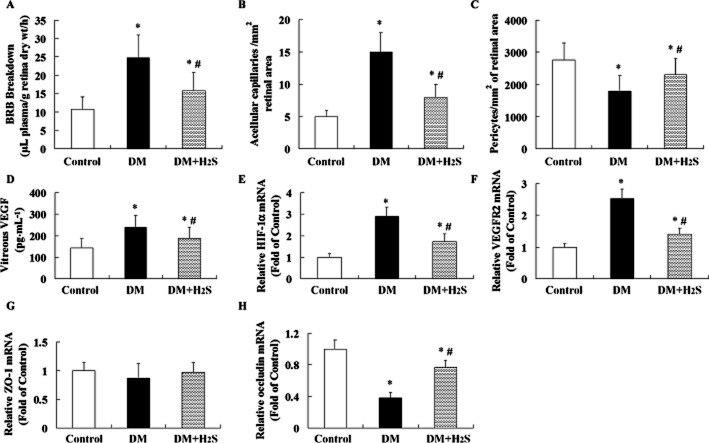
Effect of treatment with H2S on retinal vascular abnormalities in STZ-treated rats
An enhancement of retinal vascular permeability (Figure 3A), numbers of acellular capillary (Figure 3B), vitreous VEGF (Figure 3D), mRNA levels of HIF-1α (Figure 3E) and VEGFR2 (Figure 3F), and reduction of numbers of pericytes (Figure 3C) and mRNA levels of occludin (Figure 3H) were observed in retinas of STZ-induced diabetic rats. ZO-1 mRNA (Figure 3G) lever was similar between groups.
Figure 3.
Effect of treatment with H2S on retinal vascular abnormalities in STZ-treated rats. BRB breakdown (A) was measured using Evans blue dye. Acellular capillary (B) and pericytes (C) in the retinal vessels were observed after staining with periodic acid Schiff and haematoxylin. Vitreous VEGF (D) content was determined with elisa method. The mRNA levels of HIF-1α (E), VEGFR2 (F), ZO-1 (G) and occludin (H) were determined using quantitative real-time PCR method. Values are means ± SD. n = 7 in each group; *P < 0.05 versus control group; #P < 0.05 versus DM group.
Treatment with H2S attenuated retinal vascular leakage; reduced acellular capillary formation, vitreous VEGF content, and mRNA expression of HIF-1α and VEGFR2; enhanced occludin mRNA expression; and increased numbers of pericytes in retinas of STZ-treated rats.
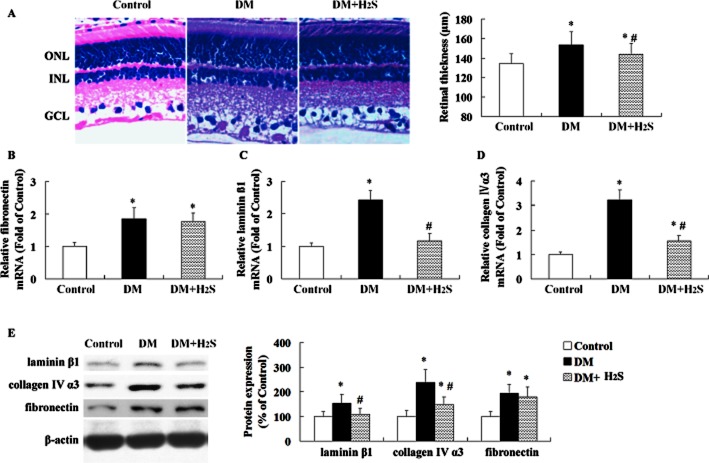
Effect of treatment with H2S on retinal thickening and gene expression of extracellular matrix (ECM) in STZ-treated rats
The retinal thickness (Figure 4A) and mRNA and protein levels of fibronectin (Figure 4B, E), laminin β1 (Figure 4C, E), and collagen IVα3 (Figure 4D, E) increased significantly in STZ-induced diabetic rats 16 weeks after diabetes onset compared with the controls.
Figure 4.
Effect of treatment with H2S on retinal thickening and extracellular matrix in STZ-treated rats. Retinal thickness (A) was evaluated on haematoxylin and eosin-stained sections. The mRNA and protein levels of fibronectin (B, E), laminin β1 (C, E) and collagen IVα3 (D, E) were determined by quantitative real-time PCR method and Western blotting analysis respectively. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Values are means ± SD. n = 7 in each group; *P < 0.05 versus control group; #P < 0.05 versus DM group.
Treatment with H2S prevented retinal thickening and reduced mRNA and protein expression of laminin β1 and collagen IVα3 in retinas of STZ-treated rats, but had no significant effect on fibronectin expression.
Effect of treatment with H2S on oxidative stress in retina of STZ-treated rats
A significant enhancement of formation of MDA (Figure 5A), O2− (Figure 5B) and OONO− (Figure 5C) was observed in retinas of STZ-induced diabetic rats. SOD activity (Figure 5D) was lower and protein expression of p47phox and NOX2 (Figure 5E) was higher in retinas of STZ-treated rats than that in control rats.
Figure 5.
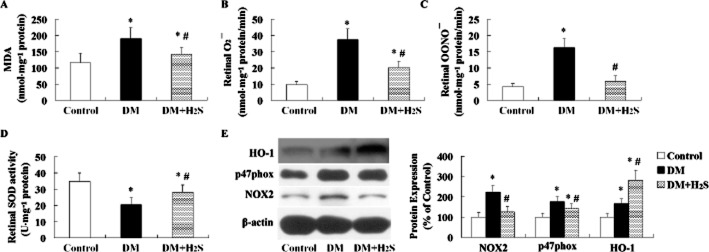
Effect of treatment with H2S on oxidative stress in retinas of STZ-treated rats. Retinal MDA content (A) was determined using a kit. Retinal formation of ROS including O2− (B) and OONO− (C) was detected. Retinal SOD activity (D) was measured using an SOD-525™ reagent kit. The protein expression (E) including HO-1, p47phox and NOX2 was determined with Western blotting analysis. O2−, superoxide anion; OONO−, peroxynitrite; HO-1, haem oxygenase 1; NOX2, NADPH oxidase 2. Values are means ± SD. n = 7 in each group; *P < 0.05 versus control group; #P < 0.05 versus DM group.
Treatment with H2S in retinas of STZ-treated rats reduced formation of MDA, O2− and OONO−; enhanced SOD activity; and decreased protein expression of p47phox and NOX2.
HO-1 expression was found up-regulated in retinas of STZ-induced diabetic rats (Figure 5E). When treated with H2S, its expression was further enhanced.
Effect of treatment with H2S on mitochondrial function in retina of STZ-treated rats
Mitochondrial ATP formation (Figure 6A) was lower and ROS formation (Figure 6B) was higher in retinas of STZ-treated rats than that in control rats. Activities of NCCR (Figure 6C) and SCCR (Figure 6D) in mitochondria isolated from retinas of STZ-treated rats were lower than that in the controls. In addition, increased mitochondrial swelling (Figure 6E) was observed in retinas of STZ-treated rats.
Figure 6.
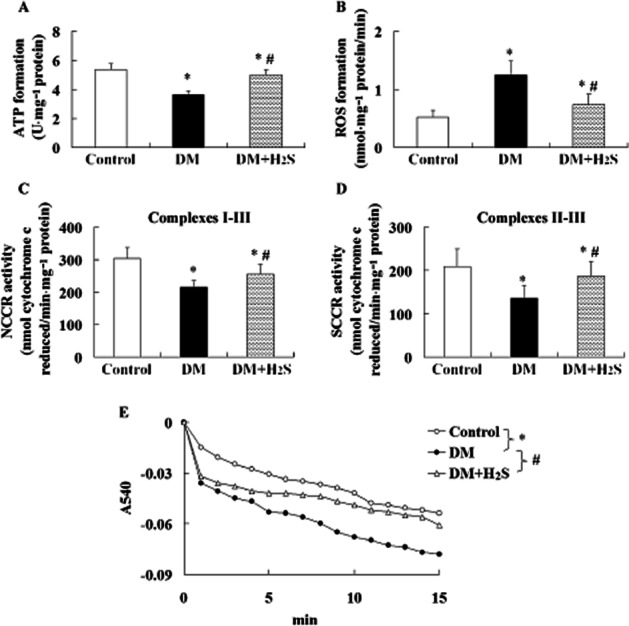
Effect of treatment with H2S on mitochondrial function in retinas of STZ-treated rats. Graphs showed ATP formation (A), ROS formation (B), activities of NCCR (C) and SCCR (D), and optical densities for mitochondrial swelling assay (E) in isolated mitochondria of retina. Values are means ± SD. n = 7 in each group; *P < 0.05 versus control group; #P < 0.05 versus DM group.
Treatment with H2S in STZ-treated rats alleviated mitochondrial dysfunction through enhancing ATP formation, reducing ROS formation, increasing activities of NCCR and SCCR, and reducing mitochondrial swelling in retina.
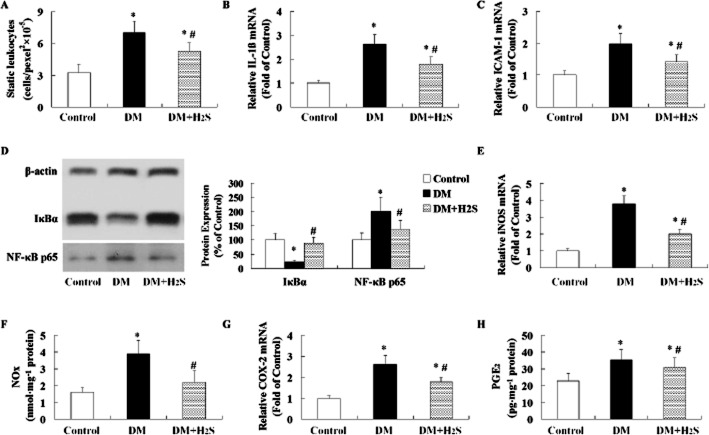
Effect of treatment with H2S on inflammation in retina of STZ-treated rats
In STZ-induced diabetic rats, an enhancement of retinal leukostasis (Figure 7A), retinal mRNA expression of cytokines of IL-1β (Figure 7B), ICAM-1 (Figure 7C), iNOS (Figure 7E), and COX2 (Figure 7G), and contents of NOx (Figure 7F) and PGE2 (Figure 7H) was observed. In STZ-induced diabetic rats, retinal NF-κB signalling was activated marked by decreased IκBα expression and increased NF-κB p65 expression (Figure 7D).
Figure 7.
Effect of treatment with H2S on inflammation in retinas of STZ-treated rats. Retinal leukostasis measurement (A) was performed using acridine orange leukocyte fluorography. The mRNA levels of IL-1β (B), ICAM-1 (C), iNOS (E) and COX-2 (G) were determined using quantitative real-time PCR method. The protein expression (D) including IκBα and NF-κB p65 was determined by Western blotting analysis. The levels of NOx (F) in retina were measured using a Total Nitrite/Nitrate Assay kit. The levels of PGE2 (H) in retina were measured with elisa method. Values are means ± SD. n = 7 in each group; *P < 0.05 versus control group; #P < 0.05 versus DM group.
Treatment with H2S in STZ-treated rats attenuated inflammation through reducing leukostasis, mRNA expressions of IL1β, ICAM-1, iNOS and COX2, and contents of NOx and PGE2, and suppressing NF-κB signalling.
Effect of treatment with NaHS (a donor of H2S) and BAY-11-7082 (one NF-κB inhibitor) on high-glucose-induced NF-κB activation and inflammation in cultured rMC-1 and RREC
When cultured rMC-1 and RREC were treated with HG, NF-κB activity (Figure 8A) and mRNA levels (Figure 8B, C) of IL-1β, ICAM-1, iNOS and COX-2 were increased. Treatment with BAY-11-7082 reduced NF-κB activity and mRNA levels of IL-1β, ICAM-1, iNOS and COX-2. Treatment with NaHS reduced NF-κB activity and mRNA levels of IL-1β, ICAM-1, iNOS and COX-2. Our results indicated that treatment with NaHS suppressed high-glucose-induced NF-κB activation, which in turn suppressed formation of IL-1β, ICAM-1, iNOS and COX-2.
Figure 8.
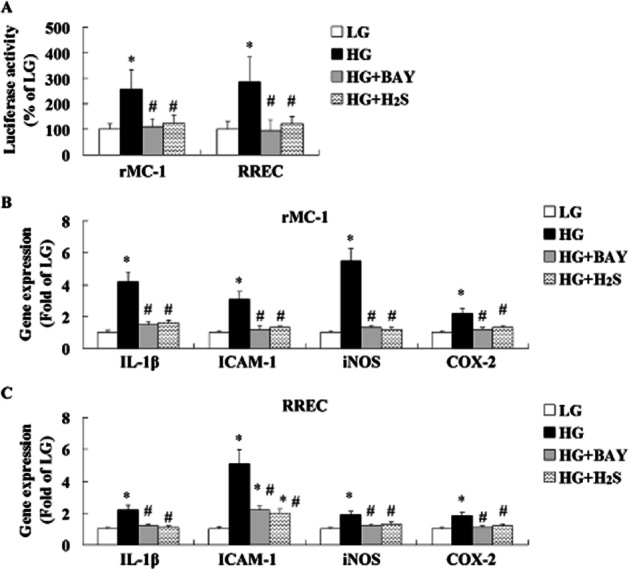
Effect of treatment with H2S and BAY-11-7082 (a specific NF-κB inhibitor) on high-glucose-induced inflammation in cultured rMC-1 and RREC. NF-κB activity (A) and mRNA levels of IL-1β, ICAM-1, iNOS and COX-2 in cultured rMC-1 (B) and RREC (C) were determined by luciferase assay and real-time PCR method respectively. BAY, BAY-11-7082. The in vitro experiments were performed at least three times and each experiment was performed with replicates. Values are means ± SD. *P < 0.05 versus control group; #P < 0.05 versus DM group.
Discussion
The role of H2S in diabetes is dual. On the one hand, experimental evidence is summarized implicating H2S overproduction as a causative factor in the pathogenesis of beta cell death in diabetes (Ali et al., 2007; Yang et al., 2007). Exogenous H2S administration instantly increased blood glucose, decreased plasma insulin and deteriorated glucose tolerance in mice (Yang et al., 2011). On the other hand, experimental evidence is presented supporting the role of H2S deficiency in the pathogenesis of diabetic endothelial dysfunction, diabetic nephropathy and cardiomyopathy (Szabo, 2012).
In this work, both circulating and retinal H2S levels in STZ-induced diabetic rats were lower than that in sham-operated rats, which was similar with previous studies (Brancaleone et al., 2008; Jain et al., 2010; Whiteman et al., 2010). CSE and 3-MST, as key enzymes involved in the production of H2S (Wang, 2002), were down-regulated in retinas of STZ-induced diabetic rats, which might contribute to the reduction of H2S formation in retinas of STZ-induced diabetic rats. In this work, replacement therapy with NaHS (a donor of H2S) restored the H2S levels in both plasma and retinas of STZ-induced diabetic rats partly. But treatment with exogenous H2S reduced CBS expression, and did not restore the expression of CSE and 3-MST in retinas of STZ-induced diabetic rats. Our result indicated that H2S itself could regulate expression of H2S-producing enzymes and there might be a negative feedback regulation in CBS/H2S pathway.
In this work, treatment with exogenous H2S had no significant effect on plasma glucose levels, indicating that the effect of exogenous H2S on pancreatic beta cells in STZ-treated rats could be ignored because they had been damaged by STZ.
Diabetes can damage neurons and vascular tissues within the retina. Studies in which objective electrophysiological tests, such as the electroretinogram (ERG) (Shirao and Kawasaki, 1998) and the visual-evoked potential (Parisi and Uccioli, 2001), were used have shown neuronal damage before evidence of vascular change in diabetic eyes. OPs were abnormal in early diabetes in both human patients and experimental animals, and reflected the diabetic neurogenerative changes (Hancock and Kraft, 2004; Kizawa et al., 2006), which also was observed in this work. In addition, we found the expressions of synaptophysin and BDNF was reduced in retinas of STZ-induced diabetic rats. Lack of synaptophysin induced a decrease in synaptic vesicles and disturbed neurotransmitter release and synaptic network activity (Spiwoks-Becker et al., 2001). BDNF regulated neurotransmitter release and neuronal activity (Binder and Scharfman, 2004). In this work, treatment with exogenous H2S prevented diabetic neurodegeneration and enhanced expressions of synaptophysin and BDNF in retinas.
DR is also characterized by vascular abnormality including breakdown of BRB, formation of acellular capillaries, and abnormal neovascularization (Frank, 2004), leading to the increased permeability of blood vessels, resulting in diabetic macular oedema. Our results showed a reduction in BRB permeability and acellular capillaries following treatment with exogenous H2S in retinas of STZ-induced diabetic rats, which could be explained by the concomitant reduction in vitreous VEGF content and gene expression of HIF-1α, VEGFR2, and increased expression of occludin. Occludin is responsible for the direct cell-to-cell attachment in the tight junction barrier (Matter and Balda, 1999) and is a crucial determinant of tight junction permeability properties in endothelial cells (Hirase et al., 1997). VEGF is a hypoxia-induced angiogenic factor and activated by HIF-1α in diabetic retina (Lin et al., 2011). HIF-1α/VEGF/VEGFR2 signalling is a major vasopermeability factor that has emerged as a key mediator of BRB breakdown and neovascularization in DM (Qaum et al., 2001). VEGF knockout mice exhibited significantly reduced depletion of tight junction proteins, number of acellular capillaries, and vascular leakage compared with wild-type mice when diabetes was induced by STZ injection (Wang et al., 2010).
In addition, treatment with exogenous H2S suppressed gene expression of ECM molecules including laminin β1 and collagen IVα3. It was reported that combined down-regulation of mRNA levels of the ECM components collagen type IV and laminin not only prevented retinal basement membrane thickening but also reduced vascular leakage in the retinas of STZ-induced diabetic rats (Oshitari et al., 2006). Therefore, down-regulation of laminin β1 and collagen IVα3 might contribute to the protective effect of H2S on STZ-induced DR.
To investigate the underlying mechanism of protective effect of H2S, we first focused on oxidative stress in retina. ROSs generated by HG are considered a causal link between elevated glucose and the pathways of development of diabetic complications. Oxidative stress plays an important role in the neurogeneration (Sasaki et al., 2010; Krügel et al., 2011) and vascular abnormality (Zheng et al., 2010) in diabetic retina. In this work, treatment with H2S not only functioned as a direct scavenger of ROS, but also influenced some important enzymes associated with oxidative stress. Mitochondria and NOX are two major sources of ROS, and it was reported that mitochondria was impaired (Santos and Kowluru, 2011) and NOX was up-regulated (Al-Shabrawey et al., 2008) in diabetic retina, which was also observed in this work. Here, treatment with H2S not only improved mitochondrial function and reduced mitochondrial ROS formation, but also suppressed NOX2 and gp47phox expression. In addition, we found that treatment with H2S preserved antioxidant enzyme SOD and enhanced HO-1 activity, which were demonstrated to be beneficial for DR (Kanwar et al., 2007; Fan et al., 2012).
Apart from oxidative stress, inflammation is thought to be another important mediator in the development of DR (Kern, 2007). In this work, treatment with H2S reduced leukostasis, mRNA expressions of cytokines of IL1β, ICAM-1, iNOS, and COX2, and contents of NOx and PGE2. The anti-inflammatory effect of H2S might be secondary to its anti-oxidative effect. Excess ROSs activate the redox-sensitive transcription factor NF-κB, resulting in enhancement of its expression and activity (Janssen-Heininger et al., 2000). Increased expression and activity of NF-κB induce gene transcription of ICAM-1 (Miyamoto et al., 1999), IL-1β (Kowluru and Odenbach, 2004), iNOS (Du et al., 2004) and COX-2 (Du et al., 2004) to increase their production. Our in vitro studies revealed that treatment with H2S suppressed high-glucose-induced NF-κB activation, which in turn suppressed formation of these pro-inflammatory cytokines. Therefore, retinal NF-κB activation in STZ-induced diabetic rats was inhibited by treatment with H2S, which might interpret the anti-inflammatory property of H2S, at least in part.
Finally, one limitation in the present study should be noted. The underlying mechanism of modulation of H2S on enzymes or cytokines involved in the oxidative stress and inflammation remained unclear. Mikami et al. (2011) demonstrated that H2S suppressed Ca2+ influx by activating vacuolar type H+-ATPase in retina. Ca2+ acts as a second messenger involved in a broad spectrum of intracellular signalling pathways. Whether H2S modulated those enzymes by regulation of Ca2+ influx in retina of STZ-treated rats required further investigation.
In conclusion, the present study demonstrated, for the first time, treatment with NaHS, a donor of H2S, attenuated STZ-induced retinopathy, possibly through abating oxidative stress and suppressing inflammation.
Glossary
- 3-MST
3-mercaptopyruvate sulfurtransferase
- BDNF
brain-derived neurotrophic factor
- BRB
blood-retinal barrier
- CBS
cystathionine β-synthase
- CSE
cystathionine γ-lyase
- DM
diabetes mellitus
- ECM
extracellular matrix
- ERG
electroretinography
- HIF-1α
hypoxia-inducible factor 1α
- HO-1
haem oxygenase 1
- ICAM-1
intercellular adhesion molecule 1
- iNOS
inducible NOS
- MDA
malondialdehyde
- NCCR
nicotinamide-adenine dinucleotide cytochrome c reductase
- NOx
nitrite and nitrate
- OPs
oscillatory potentials
- PGE2
prostaglandin E2
- ROS
reactive oxygen species
- SCCR
succinate cytochrome c reductase
- SD
Sprague–Dawley
- SOD
superoxide dismutase
- STZ
streptozotocin
- VEGFR2
VEGF receptor 2
- ZO-1
zona occluden-1
Conflicts of interest
None to declare.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Table S1 Sequences of oligonucleotides used as primers.
References
- Abiko T, Abiko A, Clermont AC, Shoelson B, Horio N, Takahashi J, et al. Characterization of retinal leukostasis and hemodynamics in insulin resistance and diabetes. Diabetes. 2003;52:829–837. doi: 10.2337/diabetes.52.3.829. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MY, Whiteman M, Low CM, Moore PK. Hydrogen sulphide reduces insulin secretion from HIT-T15 cells by a KATP channel-dependent pathway. J Endocrinol. 2007;195:105–112. doi: 10.1677/JOE-07-0184. [DOI] [PubMed] [Google Scholar]
- Ali TK, El-Remessy AB. Diabetic retinopathy: current management and experimental therapeutic targets. Pharmacotherapy. 2009;29:182–192. doi: 10.1592/phco.29.2.182. [DOI] [PubMed] [Google Scholar]
- Al-Shabrawey M, Bartoli M, El-Remessy AB, Ma G, Matragoon S, Lemtalsi T, et al. Role of NADPH oxidase and Stat3 in statin-mediated protection against diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49:3231–3238. doi: 10.1167/iovs.08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonetti DA, Wolpert EB. Isolation and characterization of retinal endothelial cells. Methods Mol Med. 2003;89:365–374. doi: 10.1385/1-59259-419-0:365. [DOI] [PubMed] [Google Scholar]
- Bełtowski J, Jamroz-Wiśniewska A, Tokarzewska D. Hydrogen sulfide and its modulation in arterial hypertension and atherosclerosis. Cardiovasc Hematol Agents Med Chem. 2010;8:173–186. doi: 10.2174/187152510792481207. [DOI] [PubMed] [Google Scholar]
- Biermann J, Lagrèze WA, Schallner N, Schwer CI, Goebel U. Inhalative preconditioning with hydrogen sulfide attenuated apoptosis after retinal ischemia/reperfusion injury. Mol Vis. 2011;17:1275–1286. [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancaleone V, Roviezzo F, Vellecco V, De Gruttola L, Bucci M, Cirino G. Biosynthesis of H2S is impaired in non-obese diabetic (NOD) mice. Br J Pharmacol. 2008;155:673–680. doi: 10.1038/bjp.2008.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA. 2003;290:2057–2060. doi: 10.1001/jama.290.15.2057. [DOI] [PubMed] [Google Scholar]
- Du Y, Sarthy V, Kern T. Interaction between NO and COX pathways in retinal cells exposed to elevated glucose and retina of diabetic rats. Am J Physiol. 2004;287:R735–R741. doi: 10.1152/ajpregu.00080.2003. [DOI] [PubMed] [Google Scholar]
- Elks CM, Mariappan N, Haque M, Guggilam A, Majid DS, Francis J. Chronic NF-kB blockade reduces cytosolic and mitochondrial oxidative stress and attenuates renal injury and hypertension in SHR. Am J Physiol Renal Physiol. 2009;296:F298–F305. doi: 10.1152/ajprenal.90628.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Xu G, Jiang T, Qin Y. Pharmacologic induction of heme oxygenase-1 plays a protective role in diabetic retinopathy in rats. Invest Ophthalmol Vis Sci. 2012;53:6541–6556. doi: 10.1167/iovs.11-9241. [DOI] [PubMed] [Google Scholar]
- Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350:48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- Geng B, Cui Y, Zhao J, Yu F, Zhu Y, Xu G, et al. Hydrogen sulfide downregulates the aortic L-arginine/nitric oxide pathway in rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1608–R1618. doi: 10.1152/ajpregu.00207.2006. [DOI] [PubMed] [Google Scholar]
- Hancock HA, Kraft TW. Oscillatory potential analysis and ERGs of normal and diabetic rats. Invest Ophthalmol Vis Sci. 2004;45:1002–1008. doi: 10.1167/iovs.03-1080. [DOI] [PubMed] [Google Scholar]
- Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, et al. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci. 1997;110:1603–1613. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- Jain SK, Bull R, Rains JL, Bass PF, Levine SN, Reddy S, et al. Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxid Redox Signal. 2010;12:1333–1337. doi: 10.1089/ars.2009.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen-Heininger YMW, Poynter ME, Baeuerle PA. Recent advances towards understanding redox mechanisms in the activation of nuclear factor κB. Free Radic Biol Med. 2000;28:1317–1327. doi: 10.1016/s0891-5849(00)00218-5. [DOI] [PubMed] [Google Scholar]
- Kanwar M, Chan PS, Kern TS, Kowluru RA. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest Ophthalmol Vis Sci. 2007;48:3805–3811. doi: 10.1167/iovs.06-1280. [DOI] [PubMed] [Google Scholar]
- Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007;2007:95103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern TS, Barber AJ. Retinal ganglion cells in diabetes. J Physiol. 2008;586:4401–4408. doi: 10.1113/jphysiol.2008.156695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizawa J, Machida S, Kobayashi T, Gotoh Y, Kurosaka D. Changes of oscillatory potentials and photopic negative response in patients with early diabetic retinopathy. Jpn J Ophthalmol. 2006;50:367–373. doi: 10.1007/s10384-006-0326-0. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Odenbach S. Role of interleukin-1beta in the pathogenesis of diabetic retinopathy. Br J Ophthalmol. 2004;88:1343–1347. doi: 10.1136/bjo.2003.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krügel K, Wurm A, Pannicke T, Hollborn M, Karl A, Wiedemann P, et al. Involvement of oxidative stress and mitochondrial dysfunction in the osmotic swelling of retinal glial cells from diabetic rats. Exp Eye Res. 2011;92:87–93. doi: 10.1016/j.exer.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Kusari J, Zhou S, Padillo E, Clarke KG, Gil DW. Effect of memantine on neuroretinal function and retinal vascular changes of streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci. 2007;48:5152–5159. doi: 10.1167/iovs.07-0427. [DOI] [PubMed] [Google Scholar]
- Lefer DJ. Potential importance of alterations in hydrogen sulphide (H2S) bioavailability in diabetes. Br J Pharmacol. 2008;155:617–619. doi: 10.1038/bjp.2008.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Chen Y, Jin J, Hu Y, Zhou KK, Zhu M, et al. Ischaemia-induced retinal neovascularisation and diabetic retinopathy in mice with conditional knockout of hypoxia-inducible factor-1 in retinal Müller cells. Diabetologia. 2011;54:1554–1566. doi: 10.1007/s00125-011-2081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P, Salgado KF, Zivin JA, Lapchak PA. A novel approach to screening for new neuroprotective compounds for the treatment of stroke. Brain Res. 2007;1173:117–125. doi: 10.1016/j.brainres.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariappan N, Soorappan RN, Haque M, Sriramula S, Francis J. TNF-α-induced mitochondrial oxidative stress and cardiac dysfunction: restoration by superoxide dismutase mimetic Tempol. Am J Physiol Heart Circ Physiol. 2007;293:H2726–H2737. doi: 10.1152/ajpheart.00376.2007. [DOI] [PubMed] [Google Scholar]
- Marmor MF, Holder GE, Seeliger MW, Yamamoto S. Standard for clinical electroretinography (2004 update) Doc Ophthalmol. 2004;108:107–114. doi: 10.1023/b:doop.0000036793.44912.45. [DOI] [PubMed] [Google Scholar]
- Martin PM, Roon P, Van-Ells TK, Ganapathy V, Smith SB. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci. 2004;45:3330–3336. doi: 10.1167/iovs.04-0247. [DOI] [PubMed] [Google Scholar]
- Matter K, Balda MS. Occludin and the functions of tight junctions. Int Rev Cytol. 1999;186:117–146. doi: 10.1016/s0074-7696(08)61052-9. [DOI] [PubMed] [Google Scholar]
- Mikami Y, Shibuya N, Kimura Y, Nagahara N, Yamada M, Kimura H. Hydrogen sulfide protects the retina from light-induced degeneration by the modulation of Ca2+ influx. J Biol Chem. 2011;286:39379–39386. doi: 10.1074/jbc.M111.298208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, Khosrof S, Bursell SE, Rohan R, Murata T, Clermont AC, et al. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A. 1999;96:10836–10841. doi: 10.1073/pnas.96.19.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne NN, Ji D, Abdul Majid AS, Fawcett RJ, Sparatore A, Del Soldato P. ACS67, a hydrogen sulfide-releasing derivative of latanoprost acid, attenuates retinal ischemia and oxidative stress to RGC-5 cells in culture. Invest Ophthalmol Vis Sci. 2010;51:284–294. doi: 10.1167/iovs.09-3999. [DOI] [PubMed] [Google Scholar]
- Oshitari T, Polewski P, Chadda M, Li AF, Sato T, Roy S. Effect of combined antisense oligonucleotides against high-glucose- and diabetes-induced overexpression of extracellular matrix components and increased vascular permeability. Diabetes. 2006;55:86–92. [PubMed] [Google Scholar]
- Parisi V, Uccioli L. Visual electrophysiological responses in persons with type 1 diabetes. Diabetes Metab Res Rev. 2001;17:12–18. doi: 10.1002/dmrr.177. [DOI] [PubMed] [Google Scholar]
- Qaum T, Xu Q, Joussen AM, Clemens MW, Qin W, Miyamoto K, et al. VEGF-initiated blood–retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001;42:2408–2413. [PubMed] [Google Scholar]
- Salloum FN, Das A, Samidurai A, Hoke NN, Chau VQ, Ockaili RA, et al. Cinaciguat, a novel activator of soluble guanylate cyclase, protects against ischemia/reperfusion injury: role of hydrogen sulfide. Am J Physiol Heart Circ Physiol. 2012;302:H1347–H1354. doi: 10.1152/ajpheart.00544.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JM, Kowluru RA. Role of mitochondria biogenesis in the metabolic memory associated with the continued progression of diabetic retinopathy and its regulation by lipoic acid. Invest Ophthalmol Vis Sci. 2011;52:8791–8798. doi: 10.1167/iovs.11-8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Ozawa Y, Kurihara T, Kubota S, Yuki K, Noda K, et al. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia. 2010;53:971–979. doi: 10.1007/s00125-009-1655-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirao Y, Kawasaki K. Electrical responses from diabetic retina. Prog Retin Eye Res. 1998;17:59–76. doi: 10.1016/s1350-9462(97)00005-0. [DOI] [PubMed] [Google Scholar]
- Spiwoks-Becker I, Vollrath L, Seeliger MW, Jaissle G, Eshkind LG, Leube RE. Synaptic vesicle alterations in rod photoreceptors of synaptophysin-deficient mice. Neuroscience. 2001;107:127–142. doi: 10.1016/s0306-4522(01)00345-1. [DOI] [PubMed] [Google Scholar]
- Szabo C. Roles of hydrogen sulfide in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal. 2012;17:68–80. doi: 10.1089/ars.2011.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzekov R, Arden GB. The electroretinogram in diabetic retinopathy. Surv Ophthalmol. 1999;44:53–60. doi: 10.1016/s0039-6257(99)00063-6. [DOI] [PubMed] [Google Scholar]
- Wang J, Xu X, Elliott MH, Zhu M, Le YZ. Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010;59:2297–2305. doi: 10.2337/db09-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Gooding KM, Whatmore JL, Ball CI, Mawson D, Skinner K, et al. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulphide. Diabetologia. 2010;53:1722–1726. doi: 10.1007/s00125-010-1761-5. [DOI] [PubMed] [Google Scholar]
- Yang G, Yang W, Wu L, Wang R. H2S, endoplasmic reticulum stress, and apoptosis of insulin-secreting beta cells. J Biol Chem. 2007;282:16567–16576. doi: 10.1074/jbc.M700605200. [DOI] [PubMed] [Google Scholar]
- Yang G, Tang G, Zhang L, Wu L, Wang R. The pathogenic role of cystathionine γ-lyase/hydrogen sulfide in streptozotocin-induced diabetes in mice. Am J Pathol. 2011;179:869–879. doi: 10.1016/j.ajpath.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang J, Wang Q, Lei X, Chu Q, Xu GT, et al. Intravitreal injection of exendin-4 analogue protects retinal cells in early diabetic rats. Invest Ophthalmol Vis Sci. 2011;52:278–285. doi: 10.1167/iovs.09-4727. [DOI] [PubMed] [Google Scholar]
- Zheng M, Zeng Q, Shi XQ, Zhao J, Tang CS, Sun NL, et al. Erythrocytic or serum hydrogen sulfide association with hypertension development in untreated essential hypertension. Chin Med J (Engl) 2011;124:3693–3701. [PubMed] [Google Scholar]
- Zheng Z, Chen H, Ke G, Fan Y, Zou H, Sun X, et al. Protective effect of perindopril on diabetic retinopathy is associated with decreased vascular endothelial growth factor-to-pigment epithelium-derived factor ratio: involvement of a mitochondria-reactive oxygen species pathway. Diabetes. 2009;58:954–964. doi: 10.2337/db07-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Chen H, Wang H, Ke B, Zheng B, Li Q, et al. Improvement of retinal vascular injury in diabetic rats by statins is associated with the inhibition of mitochondrial reactive oxygen species pathway mediated by peroxisome proliferator-activated receptor gamma coactivator 1alpha. Diabetes. 2010;59:2315–2325. doi: 10.2337/db10-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.