Abstract
Background and Purpose
Pitolisant, a histamine H3 receptor inverse agonist/antagonist is currently under Phase III clinical trials for treatment of excessive daytime sleepiness namely in narcoleptic patients. Its drug abuse potential was investigated using in vivo models in rodents and monkeys and compared with those of Modafinil, a psychostimulant currently used in the same indications.
Experimental Approach
Effects of Pitolisant on dopamine release in the nucleus accumbens, on spontaneous and cocaine-induced locomotion, locomotor sensitization were monitored. It was also tested in three standard drug abuse tests i.e. conditioned place preference in rats, self-administration in monkeys and cocaine discrimination in mice as well as in a physical dependence model.
Key Results
Pitolisant did not elicit any significant changes in dopaminergic indices in rat nucleus accumbens whereas Modafinil increased dopamine release. In rodents, Pitolisant was without any effect on locomotion and reduced the cocaine-induced hyperlocomotion. In addition, no locomotor sensitization and no conditioned hyperlocomotion were evidenced with this compound in rats whereas significant effects were elicited by Modafinil. Finally, Pitolisant was devoid of any significant effects in the three standard drug abuse tests (including self-administration in monkeys) and in the physical dependence model.
Conclusions and Implications
No potential drug abuse liability for Pitolisant was evidenced in various in vivo rodent and primate models, whereas the same does not seem so clear in the case of Modafinil.
Keywords: histamine, H3 receptor, Pitolisant, drug abuse, sensitization, self-administration, discrimination, conditioned place preference, rodents, monkeys
Introduction
Histamine H3 autoreceptors control histamine synthesis and release from tuberomamillary neurons, a brain system involved in the control of wakefulness, attention, learning and other cognitive functions (Schwartz et al., 1991; Haas and Panula, 2003; Lin et al., 2011). Therefore the use of H3 receptor inverse agonists, a class of compounds reversing the high constitutive activity of the native receptors (Morisset et al., 2000), appears as a useful therapeutic approach to enhance wakefulness in states of excessive daytime sleepiness such as narcolepsy, obstructive sleep apnoea or Parkinson's disease (Lazewska and Kiec-Kononowicz, 2010; Kuhne et al., 2011; Leurs et al., 2011).
The first compound of this class to be introduced in the clinics, PitolisantINN (formerly named tiprolisant, BF2.649, [1-{3-[3-(4-chlorophenyl)propoxy]propyl}piperidine, hydrochloride]), a potent and highly selective non-imidazole histamine H3-receptor inverse agonist (Ligneau et al., 2007b), constitutes a promising tool for the treatment of narcolepsy as shown in an animal model of this pathology, the orexin−/− mouse as well as in clinical trials (Lin et al., 2008, Schwartz, 2011). Dopamine-releasing agents currently used to fight against daytime somnolence in narcolepsy comprise amphetamine derivatives, sodium oxybate and Modafinil which both suffer, to a variable degree, from abuse liability. In the case of Modafinil, the abuse potential seems limited but its dopamine-releasing effect in rodent and human striatum (Dopheide et al., 2007; Volkow et al., 2009) and some discriminative and/or reinforcing properties (Gold and Balster, 1996, Andersen et al., 2010; Newman et al., 2010; Paterson et al., 2010) have been detected and these observations, together with other side effects, have recently led to a restricted use recommendation by the European Medicines Agency (2011).
The abuse potential of this novel drug class of H3-receptor ligands does not appear to have been thoroughly investigated so far. The aim of the present study was to assess such potential of Pitolisant, on a variety of animal models, namely in comparison with Modafinil.
Methods
Animals and drugs
Animals were housed in group under a 12-h light/dark cycle (lights on at 7:00 a.m.) in a temperature 21 ± 2°C and 45 ± 15% humidity-controlled environment with free access to food and water, except when noted below. Experiments were conducted in accordance with European ethical standards (86/609-EEC) and the French National Committee (87/848) for the Care and Use of Laboratory Animals. Cocaine self-administration and discrimination tests were conducted under corresponding NIH Guidelines in an Association for Assessment and Accreditation of Laboratory Animal Care International approved laboratory with protocols approved by Virginia Commonwealth's University's Institutional Animal Care and Use Committee. Male Wistar rats (220–300 g) were from Janvier (Le Genest Saint Isle, France). Male C57BL/6J mice and Swiss-Webster mice were from Charles River (L'Arbresle, France) and Harlan (Indianapolis, IN, USA) respectively. Pitolisant (BF2.649 hydrochloride salt) or Modafinil were from Bioprojet (Paris, France). Cocaine-HCl was from NIDA (Rockville, MD, USA) or from Sigma-Aldrich (Saint Quentin Fallavier, France) which provided also nicotine and morphine. Except when indicated, drug doses were expressed as free base. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Effect of Pitolisant on catecholamines in rat nucleus accumbens
Rats received vehicle (methylcellulose 1%, p.o.), Pitolisant (10 mg·kg−1, p.o.) or d-amphetamine (2.5 mg·kg−1, i.p. in saline). Ninety minutes later, they were killed by decapitation and nucleus accumbens were dissected out, weighed, frozen in liquid nitrogen and stored at −80°C. Tissues were homogenized in 1 mL of a 0.4 N perchloric acid/2.7 mM EDTA solution. After centrifugation (8000 rpm, 20 min, 4°C), supernatants were analysed by HPLC coupled to electrochemical detection according to Ligneau et al. (2007a). Tissue concentrations of dopamine (DA), dihydroxyphenyl acetic acid (DOPAC) and homovanillic acid (HVA) were determined and the corresponding ratios (DOPAC/DA, HVA/DA) were calculated.
Effect of Pitolisant on extracellular dopamine in rat nucleus accumbens
Anaesthetized rats (chloral hydrate 400 mg·kg−1, i.p.) were positioned in a Kopf stereotaxic frame. A guide cannula (CMA/12 Microdialysis, Phymep, Paris, France) was implanted into the nucleus accumbens (AP, +1.2 from bregma; ML, +0.18; DV, −5.8 mm from dura) according to the atlas of Paxinos and Watson (1986) and secured with dental cement and anchor screws into the skull. Rats were single-housed for postoperative recovery at least for 5 days. Then, microdialysis experiments were performed as described previously (Ligneau et al., 2007b) using a CMA/12 microdialysis probe (2-mm length) measuring the effects on extracellular dopamine of saline, Pitolisant (10 mg·kg−1 in saline) or Modafinil (120 mg·kg−1 in cyclodextrin 30%) given by i.p. route.
Effect of Pitolisant and Modafinil (single or repeated administration) on locomotor activity in rats
Apparatus
Rats were tested in black wooden open fields (76 × 76 × 45 cm height) located in a dimly lit room. A video-tracking system (Ethovision XT4.1, Noldus, Wageningen, Netherlands) allowed behavioural analyses based on centre-point detection.
Experimental procedure
For single administration (between 9:00 and 12:00 a.m.), rats received i.p. vehicle (sterile water), Pitolisant (3 and 10 mg·kg−1), cocaine (10 mg·kg−1) or Modafinil (32, 64 and 128 mg·kg−1 in cyclodextrin 30%) 30 min before their introduction in the open field. Locomotor activity was recorded continuously for 60 min by 5-min intervals.
For repeated administrations, rats received i.p. vehicle, Pitolisant (10 mg·kg−1) or Modafinil (64 mg·kg−1) immediately before their introduction in the open field. Each rat was subjected to four exploration sessions in an open field (always the same for each rat) between 1:00 and 5:00 p.m. on 4 successive days. Locomotor activity was recorded continuously for 40 min. Then, on the fifth and 16th day (i.e. after a 10-day wash-out period), rats received again vehicle or drugs (Pitolisant or Modafinil) and some rats, previously vehicle-treated over the 4 days, received Pitolisant or Modafinil before the actimetry session. In addition, these rats received vehicle and were introduced 72 h after the fifth session in the open field and their locomotion was recorded over 40 min for detection of any cue-induced locomotor activity. Another group of rats received Modafinil in their home cage for 5 days, approximately 5 h after locomotor sessions, to reduce the potential association between drug treatment and environment, and was tested in parallel.
Effect of Pitolisant on spontaneous and cocaine-induced locomotor activity in mice
C57BL/6J mice (24–26 g) received between 9:00 a.m and 1:00 p.m. vehicle (NaCl 0.9%) or Pitolisant (5 mg·kg−1, i.p.) and were introduced individually in an infrared detection actimeter (Imetronic, Pessac, France) with individual boxes (20.5 × 11.0 × 20.0 cm height) to measure horizontal movements. Counts for locomotor activity were incremented each time the animal moved from one-half part of the cage to the other and recorded continuously for 30 min (5-min intervals) before the administration of vehicle (NaCl 0.9%) or cocaine (10 mg·kg−1, s.c.). Mouse locomotion was further recorded over 90 min.
Effect of Pitolisant or Modafinil on the conditioned place preference in rats
Conditioned place preference consisted of a conditioning phase with rats receiving the drug in a distinctive environment and vehicle in another, and an expression phase in which drug-free animals’ preference of the environment previously paired with the test compound is evaluated (Tzschentke, 1998; Duarte et al., 2003; Le Foll et al., 2005). Effects of Pitolisant, cocaine, nicotine and Modafinil administered alone during conditioning were studied as follows:
Rats were daily handled, weighed and habituated to drug administration with a s.c. saline injection daily for 1 week. For nicotine place conditioning experiments, rats were also given nicotine (0.12 mg·kg−1) three times during the habituation week to avoid aversion usually elicited by first nicotine administrations. Drugs were prepared in NaCl 0.9% and administered s.c. except Modafinil which was in cyclodextrin 30% and administered i.p. Nicotine tartrate salt was dissolved in NaCl 0.9% and pH adjusted to 7.4 with 0.1 N NaOH.
Apparatus
The experimental device described above using a one compartment apparatus in an unbiased experimental design was used: the floor of each open field was covered with removable quadrants made of one of two textures (wire mesh or rough Plexiglas) chosen after preliminary studies indicating that naïve rats exhibited no unconditioned preference for one of them. A masking noise was applied.
Experimental procedure (two phases)
Conditioning
Each rat was subjected to eight 30-min conditioning sessions using always the same open field whose four floor quadrants were of identical texture. There was two sessions per day, 4 h apart, over 1 week. On days 1 to 4, drugs or saline were administered immediately before afternoon sessions, paired with one floor texture. Saline was injected just before morning sessions, paired with the other texture. Drug texture pairings were counterbalanced so that for half of the rats the drug was associated with the wire mesh floor, and for the other half with the Plexiglas floor.
Testing
On day 5, rats were subjected to a single 20-min test session without any injection. The open-field floor was covered diagonally by two quadrants of the drug-paired texture and two quadrants of the saline-paired texture. Times spent on each texture were recorded.
Effect of Pitolisant in self-administration tests in Rhesus monkeys
Subjects
Four adult male rhesus monkeys weighing 14.5 kg (M2188), 12.6 kg (M1344), 8.8 kg (M1375) and 11.8 kg (M1385) were individually housed in ventilated cubicles (1 × 1 × 1 m) and either restrained by a stainless steel harness and tether system (M1375 and M1385) or by nylon mesh harness and tether system (M1288 and M1344; Lomir Biomedicals, Notre-Dame-de-l'Île Perrot, Québec, Canada). Water was freely available and chow (Laboratory Fiber-Plus® Monkey-Diet, Purina, Saint Louis, MO, USA) and fruits were provided in an amount to maintain a constant body weight. Silicone rubber (Cole-Parmer Instrument Company, Vernon Hills, IL, USA) or Micro-Renathane (Braintree Scientific, Braintree, MA, USA) catheters were implanted into the internal and external jugular, femoral or brachial veins under pentobarbital-phencyclidine anaesthesia as described previously (Beardsley et al., 1990). Catheters exited from the midscapular region and were connected to a stopcock valve which, in turn, was connected to a peristaltic pump at one channel and to a syringe pump at another channel. When peristaltic pumps were activated during experimental sessions they delivered 10-s, 1-mL sterile intravenous infusions. When syringe pumps were activated between experimental sessions they delivered 0.9% saline at a rate of 1 mL·h−1 to maximize the longevity of catheter patency. Periodically, catheter patency was tested and positively inferred when a rapid loss of muscular control occurred following an acute infusion of ∼4 mg·kg−1 methohexital or of ketamine-HCl. If patency was lost in a catheter it was removed, the monkey given a minimum of a 2-week absence from testing, and an alternate vein was then recatheterized.
Apparatus
Animals were housed in semi-airtight fibreglass chambers (1 × 1 × 1 m) with a transparent Plexiglas front door. The air supply for the cubicles was exhausted through an air filtration system. Two response levers were located on the front door. Above each lever were three stimulus lights, two amber-coloured lights on either side of a white light. Scheduling of infusions and collection of data were controlled by a computer located in an adjacent room (Med-PC, Med Associates, Saint Albans, VT, USA).
Procedure
Monkeys were either trained to self-administer 0.03 mg·kg−1 per infusion (M1288, M1344 and M1385) or 0.01 mg·kg−1 per infusion (M1375) cocaine hydrochloride during daily (7 days·week−1) 1-h experimental sessions which commenced at approximately 10:00 a.m. Monkey M1375 was trained at a reduced cocaine dose relative to the other monkeys because his preliminary baseline level of 0.03 mg·kg−1 per infusion cocaine was lower than the other monkeys. The amber stimulus lights above the left lever were illuminated at the beginning of each session. Completion of every 50 presses of the left-side lever (fixed ratio 50, FR50) resulted in a 10-s, 1-mL delivery of the available infusate. During infusions, the amber lights were extinguished and the white centre light was illuminated above the left-side lever. At the end of each infusion, the amber lights were re-illuminated and the white light extinguished. Lever presses during infusions were recorded but were not applied towards the fixed ratio contingency. Presses of the right lever did not have scheduled contingencies during test sessions but were recorded.
After lever pressing maintained by cocaine had stabilized for at least three consecutive sessions, substitution tests with other infusates could be conducted. Stability of cocaine-maintained responding was assumed when the number of obtained infusions during the first and third sessions were not solely the highest and lowest number for those sessions and during which the number of each session's infusions did not deviate from the mean number by more than 20% (typically, these deviations were much less). Between testing each dose, the subjects were returned to cocaine baseline for at least three sessions and until response rates were again stable.
Substitution tests with saline were conducted immediately prior to tests with Pitolisant. Substitution tests with saline served as a negative control. Each dose of Pitolisant was substituted for four consecutive sessions. If any dose of Pitolisant appeared to serve as a positive reinforcer during regular testing (see below), saline was retested at the end of all other tests in order to re-establish control values considering months had elapsed since the beginning of testing to its end.
All doses of cocaine and Pitolisant (doses expressed in salt) were prepared in a 0.9% sterile saline and delivered in 1.0 mL volume in 10-s infusions. Drug solutions were filtered (0.2 μm Acrodisc Filters with HT Tuffryn Membrane, Pall Corporation, East Hills, NY, USA) to insure sterility.
All substitution test data from the last three sessions of each 4-day substitution were used in the analyses. Data from the first days of substitution were excluded because they represent the monkeys’ initial experience with each test substance and are considered not indicative of typical performances. Infusion rates during these initial sessions reflect a transition between rates of cocaine-maintained responding and responding under the test condition. A test dose was considered to serve as a reinforcer and be self-administered when the average number of infusions during the last three sessions of substitution at FR50 exceeded the average number of saline infusions obtained and their ranges did not overlap.
Additionally, analysis of group infusions were conducted by comparing infusions obtained by all monkeys during the last three sessions of testing at each dose of Pitolisant, cocaine and saline with a two-way repeated measures anova with repeated measures on both ‘Drug Condition’ and ‘Test day’ and with Dunnett's post-tests comparing individual Drug Conditions against the saline condition. Statistics were conducted using Prism 6 software (GraphPad Software, San Diego, CA, USA) and comparisons were considered statistically significant if P < 0.05.
Tests with Pitolisant in cocaine discrimination in mice
Subjects
Eleven, experimentally naive, adult male Swiss-Webster mice were housed individually on a 12-h/12-h light/dark cycle, and their weights maintained at approximately 30–40 g by post-session supplemental feedings (4–6 g).
Apparatus
Standard, light- and sound-attenuated mouse operant conditioning chambers were used (ENV-307A, Med Associates). Each chamber was equipped with two response levers separated by a trough into which a 0.01 mL dipper cup could be presented. A house light was centred at the top of the front panel and three cue lights were located above each lever. Control of lights, dipper presentations and recording of lever presses were computer controlled (Med-PC IV, Med Associates).
Procedure
Mice were trained to discriminate 10 mg·kg−1 i.p. cocaine from saline reinforced according to a FR20 schedule with sweetened milk delivery during daily (Monday–Friday) 15-min experimental sessions performed during the light phase using procedures described previously (Kolhatkar et al., 2004).
Initially, saline and doses of cocaine (0.3–17 mg·kg−1) were tested followed by tests with Pitolisant (1–30 mg·kg−1) and its vehicle given 20 min before test sessions.
All doses of cocaine and Pitolisant (doses expressed in salt) were prepared in a 0.9% sterile saline and administered i.p. to mice in a volume of 10 mL·kg−1. Drug solutions were filtered as described above to insure sterility.
The percentage of responses on the cocaine designated lever (%CLR) was calculated for each mouse during test sessions by dividing the number of lever presses emitted upon the cocaine-associated lever by the total number of lever presses emitted on both levers and then this quotient was multiplied by 100. Additionally, rates of responding were calculated for each mouse by dividing the total number of responses emitted on both levers by 900 s. Means of individual cocaine-lever responding percentages and responses per second were then calculated. If a mouse failed to emit a total of 20 lever presses (sufficient to obtain one milk delivery) then its data were excluded from calculations of mean %CLR (to minimize disproportionate contributions to %CLR by near-zero response rate performances), but were included for mean response rate calculations. Separate, ordinary, one-way ANOVAs were conducted on %CLR for cocaine and its vehicle, as well as Pitolisant and its vehicle. If the ANOVAs were significant, individual groups were compared with their respective vehicle condition with Dunnett's post-tests. ED50 values (± 95% CI) in mg·kg−1 body weight were calculated for suppression of response rates using a sigmoidal dose-response curve fitting procedure with variable slope. A log transformation on dose was used. Statistics and calculations of ED50 values were conducted using Prism 6 software and comparisons were considered statistically significant if P < 0.05.
Withdrawal symptoms after Pitolisant chronic administration in rats
Rats received twice daily (at ∼9:00 a.m. then ∼4:00 p.m.) for 11 days, vehicle (NaCl 0.9% i.p.) or Pitolisant (10 mg·kg−1, i.p.) or morphine. Escalating doses of morphine were given subcutaneously twice a day (b.i.d.) starting from 5 mg·kg−1 and increasing to 40 mg·kg−1 on the seventh day according to the following sequence (5, 10, 15, 20, 25, 30 and 40). The dose was maintained at 40 mg·kg−1 b.i.d. for 4 days. Animals were weighed, observed 24 and 48 h following the last treatment with measurement of body temperature and evaluation of withdrawal symptoms according to the Gellert-Holtzman scale (1978) by a blind observer.
Results
Effect of Pitolisant or Modafinil on dopamine in rat nucleus accumbens
Extracellular levels of dopamine in the core of the nucleus accumbens were increased after Modafinil administration (+ 96% of basal levels 90 min after administration) while no effects were observed after Pitolisant administration. The area under the curve value (calculated over 150 min post-administration) of Modafinil group was significantly enhanced by 67% (P < 0.05) whereas Pitolisant was without any significant effect (Figure 1). These results were confirmed by measures of indices of dopamine metabolism in rat nucleus accumbens following Pitolisant, Modafinil or d-amphetamine treatment. Indeed, DOPAC/DA ratios in nucleus accumbens were reduced significantly by d-amphetamine (2.5 mg·kg-1) and Modafinil (64 mg·kg-1) with respective effects of −40%, P < 0.001 and −22%, P < 0.01 versus control (basal DOPAC/DA ratio 0.27 ± 0.02) whereas Pitolisant was without any effect (+1%, P > 0.05 vs. control). These reductions elicited by d-amphetamine and Modafinil resulted mainly from the decrease of 54% (P < 0.001) and 31% (P < 0.05) in DOPAC levels, respectively as compared with controls whereas dopamine levels were not significantly modified by the two drugs (data not shown). Moreover, HVA levels in nucleus accumbens were reduced following d-amphetamine and Modafinil dosing (−50%, P < 0.01 and −41%, P < 0.01, respectively) without any effect elicited by Pitolisant.
Figure 1.
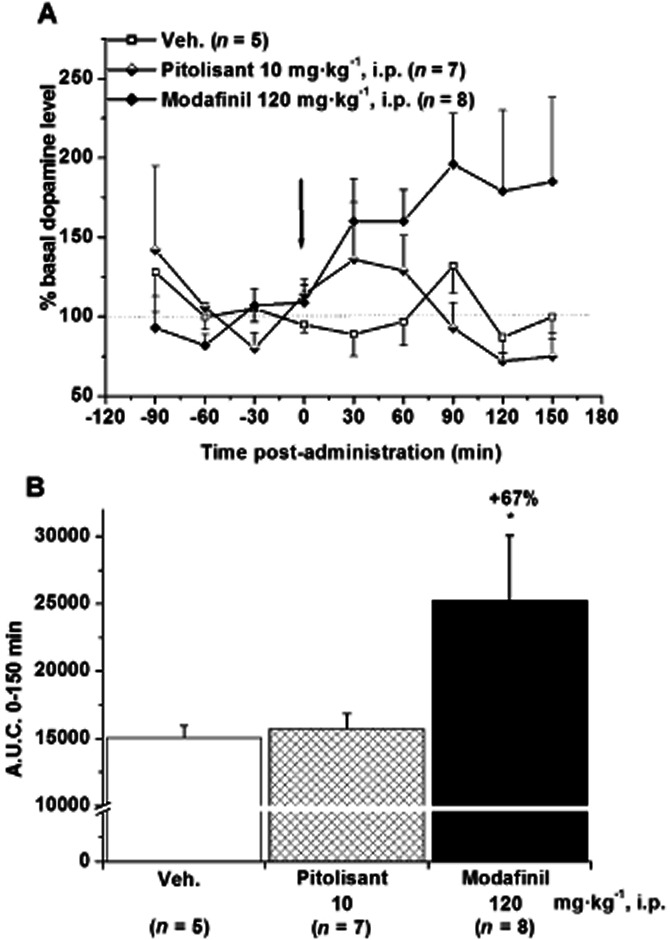
Effect of Pitolisant (10 mg·kg−1, i.p.) or Modafinil (120 mg·kg−1, i.p.) on dopamine levels in microdialysates of rat nucleus accumbens core. Data are expressed as percentage of the baseline value (A) and in the corresponding AUC over 150 min post administration (B), calculated as the mean amount in the last three samples preceding the drug challenge. Mean ± SEM of n rats. anova followed by a PLSD Fisher test: F(2,17) = 5.883, P = 0.0114 and ⋆P < 0.05 versus control.
Effect of Pitolisant and Modafinil on spontaneous locomotor activity in rats
In single administration, Pitolisant (3 and 10 mg·kg−1) was without any significant effect on locomotion. In similar conditions, cocaine (10 mg·kg−1) elicited a doubling of locomotion (+110% vs. control, P < 0.001) and Modafinil (32, 64 and 128 mg·kg−1) increased in a dose-dependent manner this parameter (+34% P > 0.05, +113% P < 0.001 and +247% P < 0.001 vs. control, respectively) (Figure 2).
Figure 2.
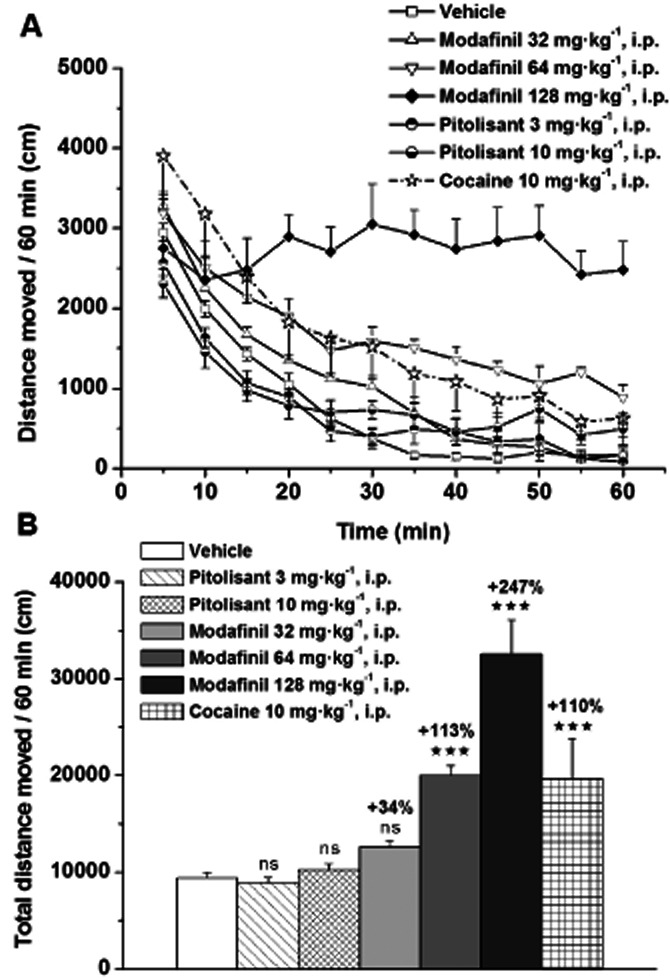
Effects of Pitolisant, Modafinil or cocaine on spontaneous locomotor activity in male Wistar rats. Rats received vehicle or drugs i.p. 30 min before their introduction in the open field. Time-course changes on locomotion (A) and total distance moved (B) are presented. Mean ± SEM of four to 11 rats. anova followed by a PLSD Fisher test: F(6,37) = 17.87, P < 0.0001 with ns P > 0.05, ⋆⋆⋆P < 0.001 versus vehicle.
Repeated administration of Pitolisant 10 mg·kg−1 did not modify locomotor activity (Figure 3) whereas Modafinil (64 mg·kg−1) progressively increased locomotor activity over the 5 consecutive testing days (from +61% P < 0.01 to +259% P < 0.001 vs. control). On the fifth session locomotor activity of Modafinil chronically treated rats was enhanced by 80% (P < 0.001) compared with the first treatment. This sensitization persisted after a 10-day washout period with a Modafinil-induced response on day 16 still increased by 72% (P < 0.001) as compared with initial response (Figure 3). Interestingly, when introduced without any drug treatment in the open-field for measurement of the locomotor activity 72 h after the fifth session, rats previously chronically Modafinil-treated elicited a locomotion significantly higher to the activity recorded in corresponding vehicle animals (+46%, P < 0.01), reflecting a phenomenon of conditioned locomotion for Modafinil (Figure 4). In addition, rats treated in their home cage with Modafinil ∼5 h after the five locomotor recording sessions, did not elicit any differences in their locomotor activity as compared with controls during the five sessions (not shown) and no conditioned hyperlocomotion in the cue-session 72 h after the fifth session (Figure 4).
Figure 3.
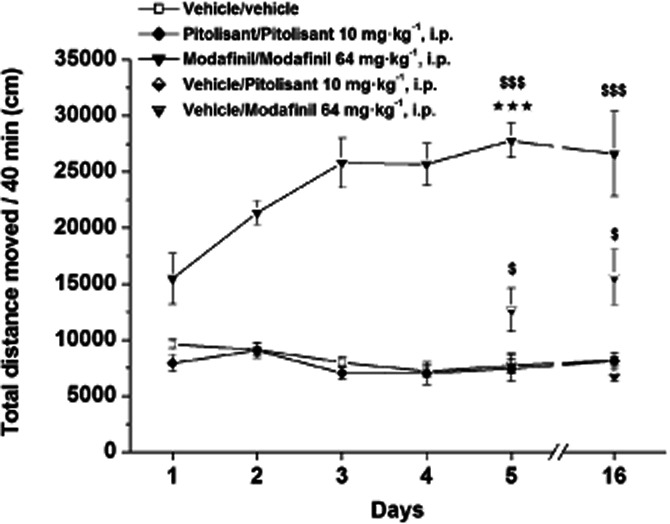
Effects of acute or repeated administrations of Pitolisant (10 mg·kg−1, i.p.) or Modafinil (64 mg·kg−1, i.p.) on locomotor activity in male Wistar rats. Rats received daily for 4 days vehicle or Pitolisant or Modafinil. Then, on day 5 and on day 16 (i.e. after a 10-day washout period), they received either vehicle (vehicle/vehicle) or Pitolisant (Pitolisant/Pitolisant) or Modafinil (Modafinil/Modafinil) and some animals vehicle-treated for 4 days received either Pitolisant (vehicle/Pitolisant) or Modafinil (vehicle/Modafinil). Mean ± SEM of six rats. anova followed by a PLSD Fisher test at day 5: F(4,25) = 44.202, P < 0.0001 and at day 16: F(4, 25) = 16.25, P < 0.0001 with $P < 0.05, $$$P < 0.001 versus corresponding vehicle/vehicle. Student's paired t-test: ⋆⋆⋆P < 0.001 versus corresponding group on day 1.
Figure 4.
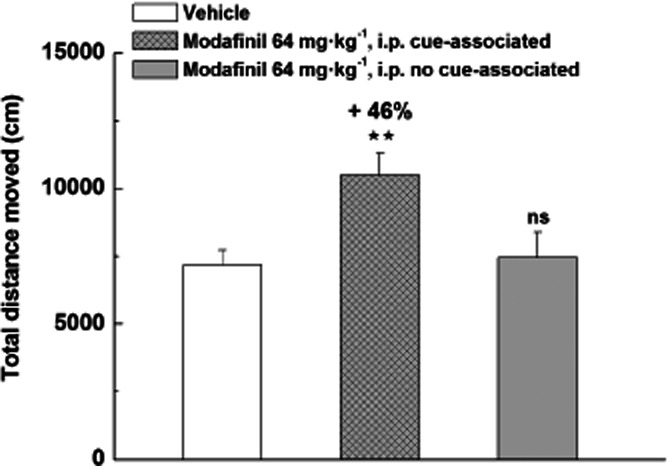
Conditioned hyperlocomotion elicited by Modafinil in the cue-associated environment 72 h after five locomotor recording sessions in male Wistar rats. Rats received daily for 5 days vehicle or Modafinil directly before recording each locomotor session. One group of rats received Modafinil in their home cage 5 h after locomotor sessions. Then, on day 8 (i.e. 72 h after the last session), they received vehicle and were introduced in the open field for recording of their locomotor activity in a ‘cue session’. Mean ± SEM of eight to 14 rats. anova followed by a PLSD Fisher test: F(2,33) = 6.344, P = 0.0047 with ns P > 0.05, ⋆⋆P < 0.01 versus vehicle.
Effect of Pitolisant on cocaine-induced hyperlocomotion in C57BL/6J mice
Pitolisant (5 mg·kg−1) reduced by 48% (P = 0.0162) the cocaine-induced hyperlocomotion in mice, whereas Pitolisant alone did not modify spontaneous locomotor activity (Figure 5).
Figure 5.
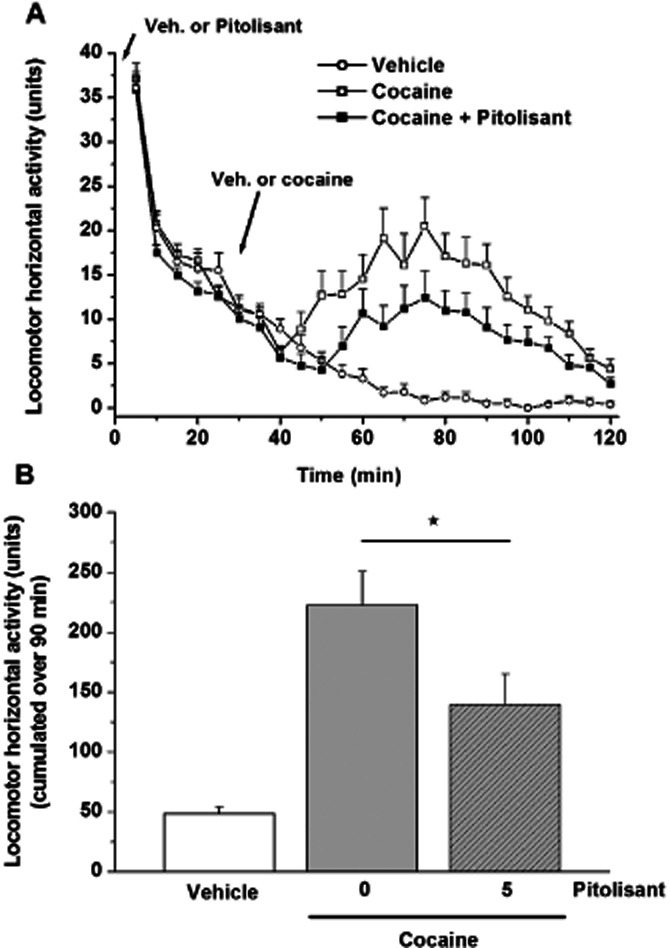
Effect of a Pitolisant (5 mg·kg−1, i.p.) pretreatment on the time course (A) and cumulated horizontal locomotor activity over 90 min (B) of vehicle or cocaine-treated (10 mg·kg−1, s.c.) mice. Mean ± SEM of 23 to 42 mice. Statistical comparison of cumulated locomotor activity over the 90-min period was performed by anova followed by a PLSD Fisher test: F(2,103) = 9.625, P = 0.0001 with ⋆P < 0.05 versus cocaine group.
Effect of Pitolisant on conditioned place preference in rats
Over the 20-min test session, control rats spent 488 ± 40 s on the vehicle-paired texture. Rats receiving cocaine (2 mg·kg−1) or nicotine (0.12 mg·kg−1) during the conditioning phase spent more time on the paired texture than controls (739 ± 66 s or 684 ± 42 s, P < 0.05 or P < 0.01 vs. controls, respectively), indicating that cocaine or nicotine supported conditioned place preference. Rats given Pitolisant (10 mg·kg−1) during the conditioning phase stayed 502 ± 94 s on the paired texture, a value not statistically different from that of controls, indicating that Pitolisant did not support place preference (Figure 6). In similar conditions, Modafinil (10 mg·kg−1) was without any effect whereas some aversive effects appeared at higher doses (32 and 64 mg·kg−1) with significant reductions by 45 and 41% of the time spent on the paired texture as compared with controls (306 ± 76 s, P < 0.05 and 330 ± 82 s, P < 0.05 vs. 555 ± 49 s, respectively) (Figure 6).
Figure 6.
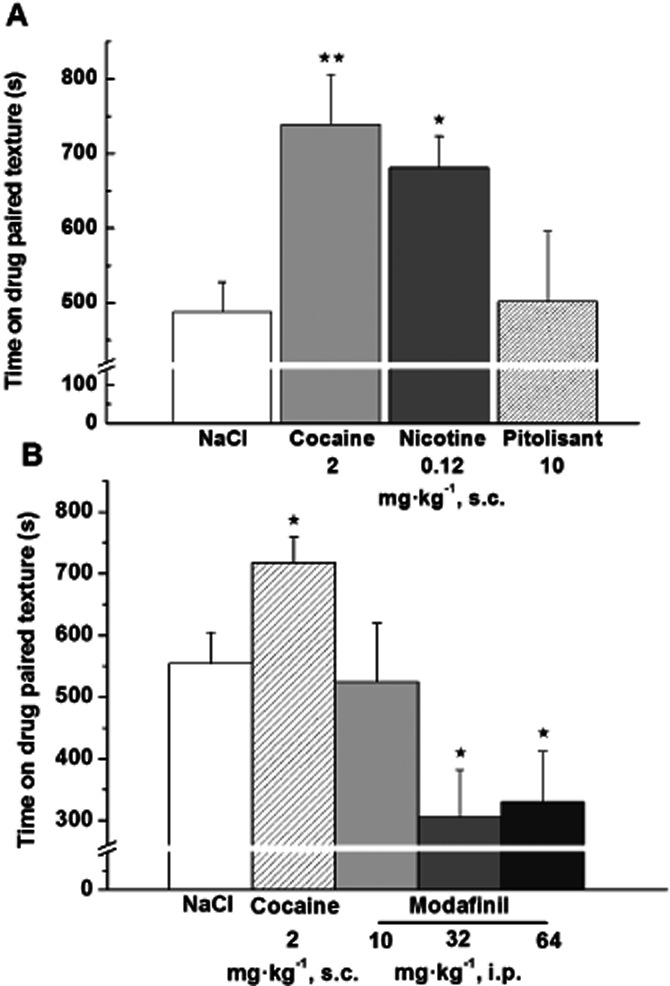
Effects of Pitolisant, Modafinil, cocaine or nicotine on the acquisition of place preference in male Wistar rats. Time spent during the 20-min test session on the floor texture previously paired with (A) s.c. saline, cocaine 2 mg·kg−1, nicotine 0.12 mg·kg−1 or Pitolisant 10 mg·kg−1 or (B) s.c. saline, cocaine (2 mg·kg−1, s.c.) or Modafinil (10, 32 or 64 mg·kg−1, i.p.) was measured. Animals were drug-free during the test session. Histogram represents the mean ± SEM (A 6 to 14 values or B 8 to 40 values). anova followed by a PLSD Fisher test: A F(3,32) = 4.255, P = 0.0123 and ⋆P < 0.05, ⋆⋆P < 0.01 time spent on the cocaine or nicotine-paired texture versus time spent by control group on saline-paired texture or B F(4,98) = 6.195, P = 0.0002 and ⋆P < 0.05 time spent on the cocaine or Modafinil-paired texture versus time spent by control group on saline-paired texture.
Ability of Pitolisant to maintain self-administration in rhesus monkeys
Figure 7 (upper panel) shows the mean number of infusions (± SEM) obtained of saline, cocaine and of Pitolisant for the monkey group as a function of dose. The repeated measures anova indicated that there was a significant effect of drug condition [F(6,18) = 6.079; P = 0.0013]. Dunnett's post-tests indicated that only the cocaine condition significantly differed from the saline condition (q = 3.574, d.f. = 18, P < 0.05) with more mean infusions of cocaine occurring than of saline (25.7 ± 2.32 vs. 11.50 ± 1.18, respectively).
Figure 7.
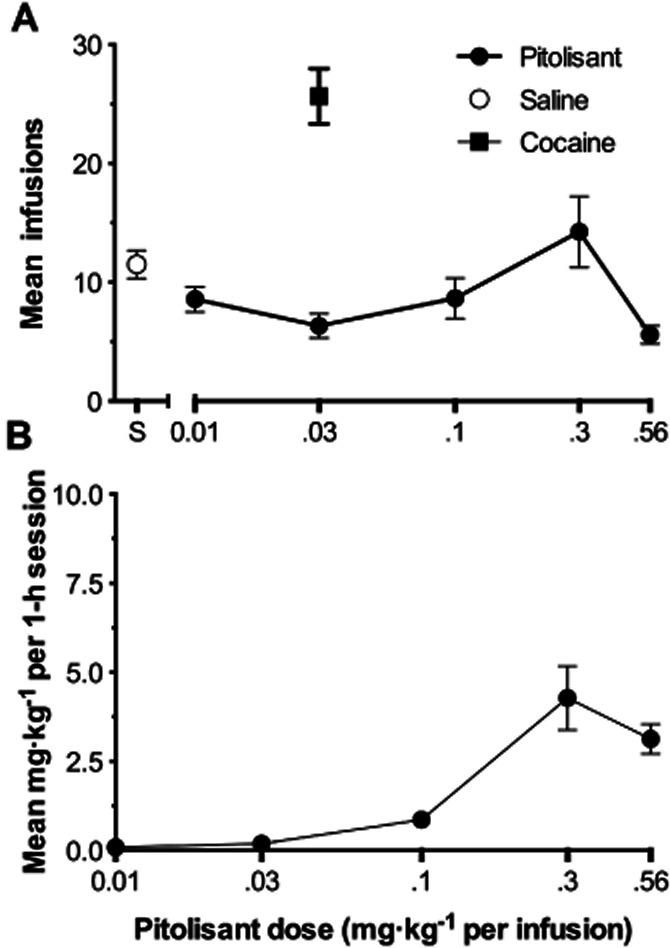
(A) Group mean infusions of Pitolisant (filled circles), saline (empty circles) and of cocaine (filled squares). Cocaine control data point includes the results of all monkeys regardless of cocaine training dose. Each data point represents the mean of 12 values (four monkeys × three test days each). Bars through the symbols represent the SEM. (B) Group mean mg·kg−1 intake of Pitolisant during the 1-h test sessions. Other details as in the Panel A.
For all four monkeys, mean cocaine infusions exceeded those of saline and their ranges did not overlap indicating that it was serving as a reinforcer for all monkeys (data not shown). When Pitolisant was substituted from 0.01 to 0.56 mg·kg−1 for the cocaine training dose, it maintained greater mean numbers of infusions than saline, and their ranges did not overlap, at 0.3 mg·kg−1 for monkeys M1288 and M1344. For none of the other monkeys and at none of the other conditions did infusions of Pitolisant exceed the ranges of saline. When saline was retested at the end of studies for monkeys M1288 and M1344, its range of infusions overlapped with those 0.3 mg·kg−1 of Pitolisant obtained under FR50 conditions.
Peak mean cumulative Pitolisant intake (mg·kg−1 per 1-h session) for the group was 4.3 ± 0.90 mg·kg−1 at 0.3 mg·kg−1 (Figure 7, lower panel), and this level was exceeded in three of the four monkeys. The greatest cumulative intake of Pitolisant for any monkey during a 1-h test session occurred for M1344 during its fourth test session at the unit test dose of 0.3 mg·kg−1 at which time he obtained 9.6 mg·kg−1 of Pitolisant.
Effect of Pitolisant on discrimination test in mice
In mice trained to discriminate 10 mg·kg−1 cocaine from saline for producing percent cocaine-lever (%CLR) selection, saline administered at 10 or 20 min pre-session as a control occasioned near-zero levels of %CLR with group mean response rates of 1.36 ± 0.17 or 1.37 ± 0.24 lever presses·s−1, respectively (Figure 8). When cocaine was tested, levels of %CLR were near-zero at 0.3 mg·kg−1, the lowest dose tested, and progressively increased until at the 10 mg·kg−1 training dose and greater complete (i.e. ≥ 80%) %CLR occurred. When Pitolisant was tested from 1 to 30 mg·kg−1, low (always <22%) levels of cocaine-lever selection occurred indicating that it failed to occasion the cocaine stimulus. The anova conducted with cocaine and its vehicle was significant [F(5,59) = 79.73; P < 0.0001]. Dunnett's post-tests indicated that the 3 mg·kg−1 (q = 4.85, d.f. = 59, P < 0.001), 10 mg·kg−1 (q = 13.06, d.f. = 59, P < 0.0001) and the 17 mg·kg−1 (q = 12.73 d.f. = 59, P < 0.0001) cocaine doses evoked significantly greater %CLR than did saline control. The anova conducted with Pitolisant and its vehicle was non-significant [F(4,47) = 2.312; P = 0.0714].
Figure 8.
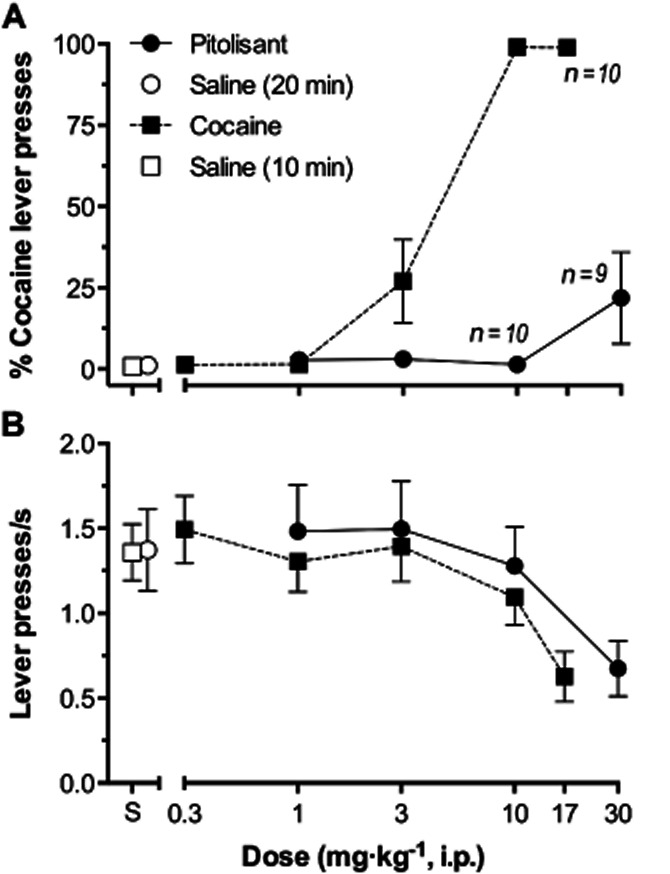
(A) Mean percent cocaine lever presses as a function of Pitolisant or cocaine dose. Each symbol represents the ratio of mean number of lever presses emitted upon the cocaine-designated lever divided by the total presses upon both levers, multiplied by 100. Bars through symbols indicate SEM. Filled circles and squares indicate results with Pitolisant and cocaine respectively. Empty circles and squares indicate results of saline tests when administered 20 and 10 min pre-session, corresponding to the administration of Pitolisant and cocaine, respectively. n = 11 except at 10 and 30 mg·kg−1 of Pitolisant where n = 10 and 9, respectively, and at 17 mg·kg−1 cocaine where n = 10. The Ns were less than 11 at these doses because some mice failed to meet response rate criteria and their data were excluded (see text). ED50 mg·kg−1 (± 95% CI): cocaine, 3.8 mg·kg−1 (2.6–5.4); Pitolisant, not determinable (see text). (B) Mean number of lever presses emitted per second as a function of Pitolisant, or cocaine dose. Each symbol represents the mean number of lever presses emitted per second at either lever during the 900-s test sessions. n = 11 at all test doses. Other details as described for upper panel. ED50 mg·kg−1 (± 95% CI): cocaine, 15.85 mg·kg−1 (10.00–19.95); Pitolisant, 25.12 mg·kg−1 (12.59–50.12).
As the doses of cocaine and Pitolisant were increased, response rates decreased. The ED50's (± CI) for reducing response rates, relative to control rates, were 15.85 mg·kg−1 (10.00–19.95) and 25.12 mg·kg−1 (12.59–50.11) for cocaine and Pitolisant, respectively.
Effect of Pitolisant on drug withdrawal symptoms in rats
Whereas Pitolisant did not modify body weight and apparent behaviour during the 11-day period of treatment, escalating doses of morphine elicited a dramatic reduction (−66%, P < 0.05 vs. controls on day 11) of body weight gain and its usual behavioural effects (i.e. transient excitatory effects followed by prostration). No effects on body weight, body temperature and behaviour were evidenced 24 and 48 h after deprivation of Pitolisant when compared with controls. In morphine-treated rats deprived for 48 h, body weight decreased by 12 g versus a gain of 16 g in controls as compared with last treatment day. Whereas temperature did not change 24 h post Pitolisant withdrawal as compared with controls, it decreased by 0.5°C (P < 0.001) 24 h post-morphine withdrawal (38.6 ± 0.1, 38.1 ± 0.1 and 38.7 ± 0.1°C for control, morphine and Pitolisant-treated rats, respectively). This effect vanished 48 h post withdrawal. Usual withdrawal symptoms (mainly abnormal posture and gaits, increased grooming and irritability, neglected fur and more sporadically diarrhoea, wet dog shakes) were observed in rats deprived of morphine 24 h (data not shown) and 48 h post withdrawal (Figure 9).
Figure 9.
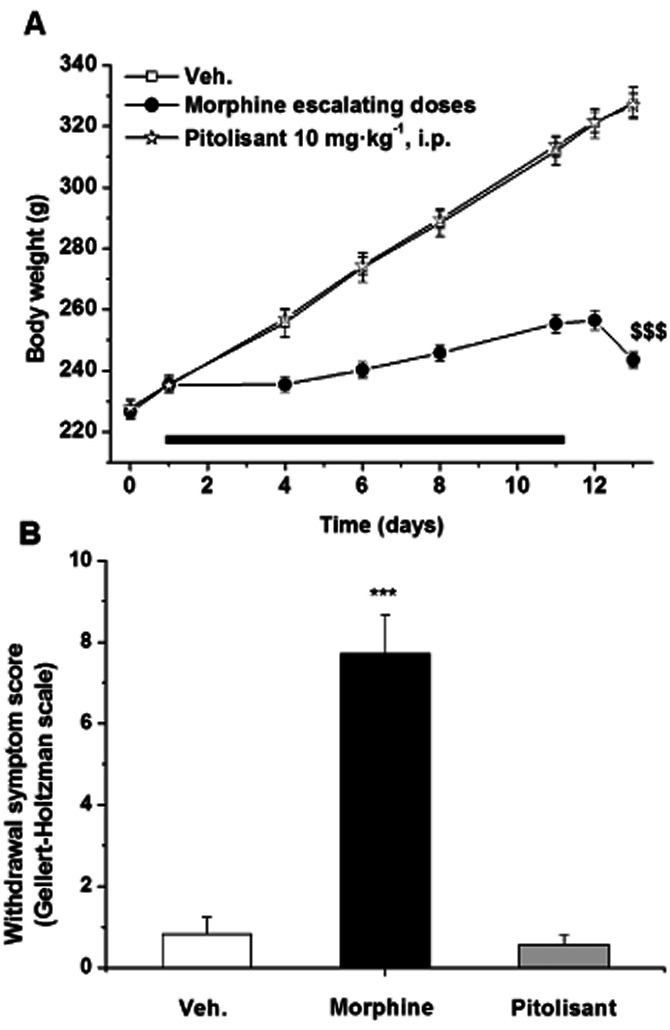
Effect of Pitolisant and morphine chronic treatment on body weight (A) and withdrawal symptoms 48 h following last administration (B). Rats received b.i.d. vehicle, Pitolisant (10 mg·kg−1, i.p.) or daily escalating doses of morphine (5 to 40 mg·kg−1, s.c.) for 11 days. Withdrawal symptoms were scored according to the Gellert-Holtzman scale. Mean ± SEM of 11–12 rats. Statistics (A) Student's paired t-test: $$$P < 0.001 versus body weight at day 11 in morphine-treated rats, (B) anova followed by a PLSD Fisher test: F(2,32) = 45.990, P < 0.0001 and ⋆⋆⋆P < 0.001 versus vehicle.
Discussion
Potent wake-promoting agents of the amphetamine class present, among other drawbacks, a clear propensity to induce drug abuse in humans and psychomotor activation in rodents, both effects that many studies show as being related to their propensity to release dopamine in the nucleus accumbens (Di Chiara et al., 2004). Pitolisant, in spite of its marked wake-promoting actions in animals (Ligneau et al., 2007b) as well as in humans (Lin et al., 2008, Schwartz 2011), clearly contrasts with these psychostimulants prone to addiction liability as shown hereafter on a large panel on most relevant in vivo models for drug abuse risk evaluation in rodents and monkeys.
In rodents, whatever the administration route (p.o., i.p. or s.c.), Pitolisant was tested at doses ensuring a drug plasma exposure eliciting a maximal effect via histamine H3 receptors on the tele-methylhistamine brain level, a reliable index of central histamine turnover (Ligneau et al. 2007a, b). Drug plasma levels in monkeys during the testing period were similar to those recorded in rodents in the range of the active doses and slightly higher than those in human at therapeutic dosages (Schwartz, 2011). Experiments were performed in males as no sex-linked differences were evidenced in pharmacokinetic parameters in rodents and monkeys as well as in the efficacy of Pitolisant in enhancing tele-methylhistamine brain levels in mice (data not shown). In some of these in vivo tests, Pitolisant was compared with Modafinil, a pro-waking drug acting by a distinct mechanism, which was tested at doses already reported (Gold and Balster, 1996; Simon et al., 1996; Lin et al., 2008).
First, Pitolisant fails to release dopamine in the striatal complex, including the nucleus accumbens, a brain region critical for the rewarding effect of drug of abuse, as shown here by analysis of in vivo microdialysates and tissue dopamine metabolites. This is somewhat paradoxical in view of the demonstrated expression of H3 receptors on dopamine neurons in substantia nigra and ventral tegmental area (Pollard et al., 1993; Goodchild et al., 1999; Pillot et al., 2002) and the H3 receptor mediated inhibition of striatal dopamine release in vitro (Schlicker et al., 1993); also Pitolisant enhances dopamine release from the rat frontal cortex (Ligneau et al., 2007b). Similar paradoxical findings were also reported for other, chemically unrelated, H3 receptor inverse agonists (Fox et al., 2005; Medhurst et al., 2007). Altogether, they suggest that H3 receptors might be diversely activated in a tonic manner on several classes of dopamine neurons in relation with either differences in local endogenous histamine release or in the constitutive activity of H3 receptors these neurons express. In agreement, inverse agonist activity of compounds has been shown as a prerequisite for their histamine-releasing activity, implying that autoreceptors are constitutively active (Morisset et al., 2000). As a corollary, H3 receptors on several other classes of neurons might not be constitutively active, possibly as a consequence of their lower densities than on histamine neurons or differences in G-protein coupling.
Consistent with its lack of effect on striatal dopamine, Pitolisant failed to induce any locomotor activation at any single dose or upon repeated administration and, even, reduced the psychomotor activation elicited by cocaine (Figure 5) or amphetamine (Ligneau et al., 2007a). Again, similar findings were reported with other H3 receptor antagonists/inverse agonists belonging to various chemical classes (Clapham and Kilpatrick, 1994, Morisset et al., 2000; Komater et al., 2003; Barbier et al., 2004; Esbenshade et al., 2005; Fox et al., 2005), indicating that the absence of accumbal dopamine release, psychomotor activation and behavioural sensitization differentiates the whole drug class from that of psychomotor stimulants of the amphetamine/cocaine type.
In contrast, Modafinil elicited a dose-dependent locomotor activation of an amplitude similar to that of cocaine (Figure 2) in agreement with previous reports (Simon et al., 1996; Zolkowska et al., 2009; Paterson et al., 2010); this response progressively increased under repeated administration (Figure 3, see also Paterson et al., 2010; Wuo-Silva et al., 2011), an effect presumably reflecting progressive conditioned cue association with the environment (Figure 4). Both behavioural properties, shared with cocaine and amphetamines, can be regarded as consequences of a primary effect on dopamine reuptake resulting in enhanced extracellular dopamine level in the striatal complex, particularly in nucleus accumbens, that was shown here in mice and rats as also reported by others (Ferraro et al., 1996; Zolkowska et al., 2009; Loland et al., 2012), including in humans (Volkow et al., 2009). Indeed, among other biological properties, Modafinil displays DAT-inhibitory properties (Zolkowska et al., 2009; Andersen et al., 2010).
Pitolisant was then tested on a series of animal models predictive for drug abuse liability, particularly of psychostimulants in humans, that all led to negative responses.
In agreement, Pitolisant failed to induce conditioned place preference in a rat model in which the positive effects of cocaine and nicotine, two dopamine-releasing drugs, were clearly revealed (Figure 6). The lack of reinforcing efficacy on a similar non-operant rodent model was previously reported for thioperamide, another H3 receptor inverse agonist (Brabant et al., 2005). Furthermore, methamphetamine place preference was not modified in H3 receptor knock-out (KO) mice (Okuda et al., 2009) and that of cocaine was unchanged in histidine decarboxylase KO mice (Brabant et al., 2007) as compared with wild-type mice, suggesting a lack of participation of brain histaminergic systems in these models. Interestingly, however, two H3 receptor inverse agonists inhibited alcohol place preference in mice (Nuutinen et al., 2011b), and H3 receptor KO mice failed to develop place preference to alcohol (Nuutinen et al., 2011a). Taken together with the reduction of the ethanol-induced locomotion by the prototypic H3-receptor inverse agonist, ciproxifan in C57BL/6J mice (Nuutinen et al., 2010) as wells as in H3 receptor KO mice (Nuutinen et al., 2011a) and with the reduction of alcohol intake, preference and self-administration in the rat by JNJ-39220675, another H3-receptor inverse agonist, (Galici et al., 2011), it suggests some potential for alcoholism treatment of this class of drugs. However, this is dampened by opposite results (e.g. increase of the alcohol-induced locomotion in DBA/2J mice from Nuutinen et al., 2011b) indicating probably a more complex situation depending tests and test conditions (species, strain …).
In monkeys trained to self-administer cocaine, group mean infusions of Pitolisant and of its vehicle control were found to be non-significantly different from one another following the anova analysis indicating a general lack of reinforcing efficacy, and by inference, a negligible abuse liability. There were two test conditions with individual monkeys (monkeys M1288 and M1344 at 0.3 mg·kg−1) out of twenty in total (four monkeys × five doses each) in which mean numbers of Pitolisant infusions obtained exceeded those of saline and their ranges didn't overlap. However, when saline was retested at the end of the study, numbers of vehicle infusions did overlap or exceeded all doses of Pitolisant in these two monkeys, indicating that its sufficiency to be identified as a reinforcer was labile and dependent upon the referent vehicle control condition.
In mice trained to discriminate cocaine from vehicle, Pitolisant was found to be incapable of occasioning cocaine-like discriminative stimulus effects. Others have reported that the histamine H3 antagonists, thioperamide and clobenpropit, also failed to occasion the discriminative stimulus psychomotor stimulant effects of methamphetamine (Munzar et al., 2004). It is important to note that a sufficiently broad enough range of Pitolisant doses had been tested to include those with behavioural activity, for high doses suppressed overall response rates. In previous studies (Munzar et al., 1998; 2004; Mori et al., 2002), thioperamide was reported to enhance the discriminative stimulus effects of cocaine, amphetamine and methamphetamine in the rat, but due to the cytochrome P450 inhibitory properties of this imidazole derivative, interpretations of these effects are difficult in the absence of information regarding potential drug interactions on this enzyme system.
Finally, no overt physical or behavioural withdrawal symptom could be detected at the end of a chronic treatment with Pitolisant but it should be mentioned that psychostimulants also fail to do so in rodents, to a certain degree in contrast with humans.
From the present studies, and of other published reports, the situation seems less clear in the case of Modafinil. First, in our rat conditioned place preference model Modafinil exerted a clearly aversive response that contrasts with the no less clear cue-induced hyperlocomotor response to this drug's repeated administration, a difference which may reflect a progressive dissolution of its aversive effect. In addition, responses to Modafinil during conditioned place preference tests seem to vary according to species and/or test designs. For example, whereas Nguyen et al. (2011), Wuo-Silva et al. (2011) and Shuman et al. (2012) observed place preference in mice, Deroche-Gamonet et al. (2002) and Tahsili-Fahadan et al. (2010) failed to do so in rats. In this latter species, Bernardi et al. (2009) also did not observe place preference but report that Modafinil induces a long-lasting reinstatement of extinguished cocaine place preference.
Clear species-related differences were also reported for Modafinil in intravenous self-administration models; whereas in rats it was inactive (Deroche-Gamonet et al., 2002) and, even, depressed the methamphetamine-primed reinstatement (Reichel and See 2010; 2012), in monkeys, Modafinil had reinforcing properties (Gold and Balster, 1996) and reinstated a cocaine-maintained response (Andersen et al., 2010).
To reconcile these various data, it might be hypothesized that Modafinil displays positive effects on these two animal models of drug abuse liability, in agreement with its DAT-inhibiting and accumbal dopamine release properties, but they are obscured in rats due to aversive properties that could be associated with another of the multiple targets of this drug.
In conclusion, no evidence of potential drug abuse liability could be observed in the case of Pitolisant, whereas the same does not seem so clear in the case of Modafinil.
Glossary
- Amph
amphetamine
- b.i.d
twice a day
- DA
dopamine
- DOPAC
dihydroxyphenyl acetic acid
- FR
fixed ratio
- FFR
first fixed ratio
- HVA
homovanillic acid
- KO
knock-out
- veh
vehicle
- %CLR
percentage of responses on cocaine lever
Conflict of interest
S.B., X.L., D.P. and M.U. are employees of Bioprojet. J.M.L. is CEO, co-founder and shareholder of Bioprojet. J.C.S. is scientific director, co-founder and shareholder of Bioprojet. P.M.B. conducted the mouse drug discrimination and monkey self-administration tests under contract by Bioprojet, which terminated before the preparation of this manuscript, and for which he was not compensated.
References
- Andersen ML, Kessler E, Murnane KS, McClung JC, Tufik S, Howell LL. Dopamine transporter-related effects of modafinil in rhesus monkeys. Psychopharmacology (Berlin) 2010;210:439–448. doi: 10.1007/s00213-010-1839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier AJ, Berridge C, Dugovic C, Laposky AD, Wilson SJ, Boggs J, et al. Acute wake-promoting actions of JNJ-5207852, a novel, diamine-based H3 antagonist. Br J Pharmacol. 2004;143:649–661. doi: 10.1038/sj.bjp.0705964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Hayes BA, Balster RL. The self-administration of MK-801 can depend upon drug-reinforcement history, and its discriminative stimulus properties are phencyclidine-like in rhesus monkeys. J Pharmacol Exp Ther. 1990;252:953–959. [PubMed] [Google Scholar]
- Bernardi RE, Lewis JR, Lattal KM, Berger SP. Modafinil reinstates a cocaine conditioned place preference following extinction in rats. Behav Brain Res. 2009;204:250–253. doi: 10.1016/j.bbr.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabant C, Charlier Y, Quertemont E, Tirelli E. The H3 antagonist thioperamide reveals conditioned preference for a context associated with an inactive small dose of cocaine in C57BL/6J mice. Behav Brain Res. 2005;160:161–168. doi: 10.1016/j.bbr.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Brabant C, Quertemont E, Anaclet C, Lin JS, Ohtsu H, Tirelli E. The psychostimulant and rewarding effects of cocaine in histidine decarboxylase knockout mice do not support the hypothesis of an inhibitory function of histamine on reward. Psychopharmacology (Berlin) 2007;190:251–263. doi: 10.1007/s00213-006-0603-0. [DOI] [PubMed] [Google Scholar]
- Clapham J, Kilpatrick GJ. Thioperamide, the selective histamine H3 receptor antagonist, attenuates stimulant-induced locomotor activity in the mouse. Eur J Pharmacol. 1994;259:107–114. doi: 10.1016/0014-2999(94)90498-7. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Darnaudéry M, Bruins-Slot L, Piat F, Le Moal M, Piazza PV. Study of the addictive potential of modafinil in naive and cocaine-experienced rats. Psychopharmacology (Berlin) 2002;161:387–395. doi: 10.1007/s00213-002-1080-8. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, et al. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47:227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Dopheide MM, Morgan RE, Rodvelt KR, Schachtman TR, Miller DK. Modafinil evokes striatal [3H]dopamine release and alters the subjective properties of stimulants. Eur J Pharmacol. 2007;568:112–123. doi: 10.1016/j.ejphar.2007.03.044. [DOI] [PubMed] [Google Scholar]
- Duarte C, Lefebvre C, Chaperon F, Hamon M, Thiébot MH. Effects of a dopamine D3 receptor ligand, BP 897, on acquisition and expression of food-, morphine-, and cocaine-induced conditioned place preference, and food-seeking behavior in rats. Neuropsychopharmacology. 2003;28:1903–1915. doi: 10.1038/sj.npp.1300276. [DOI] [PubMed] [Google Scholar]
- Esbenshade TA, Fox GB, Krueger KM, Miller TR, Kang CH, Denny LI, et al. Pharmacological properties of ABT-239 [4-(2-{2-[(2R)-2-Methylpyrrolidinyl]ethyl}-benzofuran-5-yl)benzonitrile]: I. Potent and selective histamine H3 receptor antagonist with drug-like properties. J Pharmacol Exp Ther. 2005;313:165–175. doi: 10.1124/jpet.104.078303. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency EMA/725532/2010 rev. 2011. Questions and answers on the review of medicines containing modafinil. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Modafinil_31/WC500099177.pdf (accessed 3/26/2013)
- Ferraro L, Tanganelli S, O'Connor WT, Antonelli T, Rambert F, Fuxe K. The vigilance promoting drug modafinil increases dopamine release in the rat nucleus accumbens via the involvement of a local GABAergic mechanism. Eur J Pharmacol. 1996;306:33–39. doi: 10.1016/0014-2999(96)00182-3. [DOI] [PubMed] [Google Scholar]
- Fox GB, Esbenshade TA, Pan JB, Radek RJ, Krueger KM, Yao BB, et al. Pharmacological properties of ABT-239 [4-(2-{2-[(2R)-2-Methylpyrrolidinyl]ethyl}-benzofuran-5-yl)benzonitrile]: II. Neurophysiological characterization and broad preclinical efficacy in cognition and schizophrenia of a potent and selective histamine H3 receptor antagonist. J Pharmacol Exp Ther. 2005;313:176–190. doi: 10.1124/jpet.104.078402. [DOI] [PubMed] [Google Scholar]
- Galici R, Rezvani AH, Aluisio L, Lord B, Levin ED, Fraser I, et al. JNJ-39220675, a novel selective histamine H3 receptor antagonist, reduces the abuse-related effects of alcohol in rats. Psychopharmacology (Berlin) 2011;214:829–841. doi: 10.1007/s00213-010-2092-4. [DOI] [PubMed] [Google Scholar]
- Gellert VF, Holtzman SG. Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions. J Pharmacol Exp Ther. 1978;205:536–546. [PubMed] [Google Scholar]
- Gold LH, Balster RL. Evaluation of the cocaine-like discriminative stimulus effects and reinforcing effects of modafinil. Psychopharmacology (Berlin) 1996;126:286–292. doi: 10.1007/BF02247379. [DOI] [PubMed] [Google Scholar]
- Goodchild RE, Court JA, Hobson I, Piggott MA, Perry RH, Ince P, et al. Distribution of histamine H3-receptor binding in the normal human basal ganglia: comparison with Huntington's and Parkinson's disease cases. Eur J Neurosci. 1999;11:449–456. doi: 10.1046/j.1460-9568.1999.00453.x. [DOI] [PubMed] [Google Scholar]
- Haas H, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci. 2003;4:121–130. doi: 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolhatkar R, Cook CD, Ghorai SK, Deschamps J, Beardsley PM, Reith ME, et al. Further structurally constrained analogues of cis-(6-benzhydrylpiperidin-3-yl)benzylamine with elucidation of bioactive conformation: discovery of 1,4-diazabicyclo[3.3.1]nonane derivatives and evaluation of their biological properties for the monoamine transporters. J Med Chem. 2004;47:5101–5113. doi: 10.1021/jm049796t. [DOI] [PubMed] [Google Scholar]
- Komater VA, Browman KE, Curzon P, Hancock AA, Decker MW, Fox GB. H3 receptor blockade by thioperamide enhances cognition in rats without inducing locomotor sensitization. Psychopharmacology (Berlin) 2003;167:363–372. doi: 10.1007/s00213-003-1431-0. [DOI] [PubMed] [Google Scholar]
- Kuhne S, Wijtmans M, Lim HD, Leurs R, de Esch IJ. Several down, a few to go: histamine H3 receptor ligands making the final push towards the market? Expert Opin Investig Drugs. 2011;20:1629–1648. doi: 10.1517/13543784.2011.625010. [DOI] [PubMed] [Google Scholar]
- Lazewska D, Kiec-Kononowicz K. Recent advances in histamine H3 receptor antagonists/inverse agonists. Expert Opin Ther Pat. 2010;20:1147–1169. doi: 10.1517/13543776.2010.509346. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Sokoloff P, Stark H, Goldberg SR. Dopamine D3 receptor ligands block nicotine-induced conditioned place preferences through a mechanism that does not involve discriminative-stimulus or antidepressant-like effects. Neuropsychopharmacology. 2005;30:720–730. doi: 10.1038/sj.npp.1300622. [DOI] [PubMed] [Google Scholar]
- Leurs R, Vischer HF, Wijtmans M, de Esch IJ. En route to new blockbuster anti-histamines: surveying the offspring of the expanding histamine receptor family. Trends Pharmacol Sci. 2011;32:250–257. doi: 10.1016/j.tips.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Ligneau X, Landais L, Perrin D, Piriou J, Uguen M, Denis E, et al. Brain histamine and schizophrenia: potential therapeutic applications of H3-receptor inverse agonists studied with BF2.649. Biochem Pharmacol. 2007a;73:1215–1224. doi: 10.1016/j.bcp.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Ligneau X, Perrin D, Landais L, Camelin JC, Calmels TP, Berrebi-Bertrand I, et al. BF2.649 [1-{3-[3-(4-Chlorophenyl)propoxy]propyl}piperidine, hydrochloride], a nonimidazole inverse agonist/antagonist at the human histamine H3 receptor: preclinical pharmacology. J Pharmacol Exp Ther. 2007b;320:365–375. doi: 10.1124/jpet.106.111039. [DOI] [PubMed] [Google Scholar]
- Lin JS, Dauvilliers Y, Arnulf I, Bastuji H, Anaclet C, Parmentier R, et al. An inverse agonist of the histamine H(3) receptor improves wakefulness in narcolepsy: studies in orexin−/− mice and patients. Neurobiol Dis. 2008;30:74–83. doi: 10.1016/j.nbd.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Lin JS, Sergeeva OA, Haas HL. Histamine H3 receptors and sleep-wake regulation. J Pharmacol Exp Ther. 2011;336:17–23. doi: 10.1124/jpet.110.170134. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Mereu M, Okunola OM, Cao J, Prisinzano TE, Mazier S, et al. R-modafinil (armodafinil): a unique dopamine uptake inhibitor and potential medication for psychostimulant abuse. Biol Psychiatry. 2012;72:405–413. doi: 10.1016/j.biopsych.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhurst AD, Atkins AR, Beresford IJ, Brackenborough K, Briggs MA, Calver AR, et al. GSK189254, a novel H3 receptor antagonist that binds to histamine H3 receptors in Alzheimer's disease brain and improves cognitive performance in preclinical models. J Pharmacol Exp Ther. 2007;321:1032–1045. doi: 10.1124/jpet.107.120311. [DOI] [PubMed] [Google Scholar]
- Mori T, Narita M, Onodera K, Suzuki T. Modulation of the discriminative stimulus effects of cocaine and methamphetamine by the histaminergic system. Nihon Shinkei Seishin Yakurigaku Zasshi. 2002;22:73–78. [PubMed] [Google Scholar]
- Morisset S, Rouleau A, Ligneau X, Gbahou F, Tardivel-Lacombe J, Stark H, et al. High constitutive activity of native H3 receptors regulates histamine neurons in brain. Nature. 2000;408:860–864. doi: 10.1038/35048583. [DOI] [PubMed] [Google Scholar]
- Munzar P, Nosál R, Goldberg SR. Potentiation of the discriminative-stimulus effects of methamphetamine by the histamine H3 receptor antagonist thioperamide in rats. Eur J Pharmacol. 1998;363:93–101. doi: 10.1016/s0014-2999(98)00789-4. [DOI] [PubMed] [Google Scholar]
- Munzar P, Tanda G, Justinova Z, Goldberg SR. Histamine H3 receptor antagonists potentiate methamphetamine self-administration and methamphetamine-induced accumbal dopamine release. Neuropsychopharmacology. 2004;29:705–717. doi: 10.1038/sj.npp.1300380. [DOI] [PubMed] [Google Scholar]
- Newman JL, Negus SS, Lozama A, Prisinzano TE, Mello NK. Behavioral evaluation of modafinil and the abuse-related effects of cocaine in rhesus monkeys. Exp Clin Psychopharmacol. 2010;18:395–408. doi: 10.1037/a0021042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TL, Tian YH, You IJ, Lee SY, Jang CG. Modafinil-induced conditioned place preference via dopaminergic system in mice. Synapse. 2011;65:733–741. doi: 10.1002/syn.20892. [DOI] [PubMed] [Google Scholar]
- Nuutinen S, Karlstedt K, Aitta-Aho T, Korpi ER, Panula P. Histamine H3 receptor-dependent mechanisms regulate ethanol stimulation and conditioned place preference in mice. Psychopharmacology. 2010;208:75–86. doi: 10.1007/s00213-009-1710-5. [DOI] [PubMed] [Google Scholar]
- Nuutinen S, Lintunen M, Vanhanen J, Ojala T, Rozov S, Panula P. Evidence for the role of histamine H3 receptor in alcohol consumption and alcohol reward in mice. Neuropsychopharmacology. 2011a;36:2030–2040. doi: 10.1038/npp.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuutinen S, Vanhanen J, Pigni MC, Panula P. Effects of histamine H3 receptor ligands on the rewarding, stimulant and motor-impairing effects of ethanol in DBA/2J mice. Neuropharmacology. 2011b;60:1193–1199. doi: 10.1016/j.neuropharm.2010.10.027. [DOI] [PubMed] [Google Scholar]
- Okuda T, Zhang D, Shao H, Okamura N, Takino N, Iwamura T, et al. Methamphetamine- and 3,4-methylenedioxymethamphetamine-induced behavioral changes in histamine H3-receptor knockout mice. J Pharmacol Sci. 2009;111:167–174. doi: 10.1254/jphs.09024fp. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Fedolak A, Olivier B, Hanania T, Ghavami A, Caldarone B. Psychostimulant-like discriminative stimulus and locomotor sensitization properties of the wake-promoting agent modafinil in rodents. Pharmacol Biochem Behav. 2010;95:449–456. doi: 10.1016/j.pbb.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 1986. [Google Scholar]
- Pillot C, Heron A, Cochois V, Tardivel-Lacombe J, Ligneau X, Schwartz JC, et al. A detailed mapping of the histamine H(3) receptor and its gene transcripts in rat brain. Neuroscience. 2002;14:173–193. doi: 10.1016/s0306-4522(02)00135-5. [DOI] [PubMed] [Google Scholar]
- Pollard H, Moreau J, Arrang JM, Schwartz JC. A detailed autoradiographic mapping of histamine H3 receptors in rat brain areas. Neuroscience. 1993;52:169–189. doi: 10.1016/0306-4522(93)90191-h. [DOI] [PubMed] [Google Scholar]
- Reichel CM, See RE. Modafinil effects on reinstatement of methamphetamine seeking in a rat model of relapse. Psychopharmacology (Berlin) 2010;210:337–346. doi: 10.1007/s00213-010-1828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, See RE. Chronic modafinil effects on drug-seeking following methamphetamine self-administration in rats. Int J Neuropsychopharmacol. 2012;15:919–929. doi: 10.1017/S1461145711000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicker E, Fink K, Detzner M, Göthert M. Histamine inhibits dopamine release in the mouse striatum via presynaptic H3 receptors. J Neural Transm Gen Sect. 1993;93:1–10. doi: 10.1007/BF01244933. [DOI] [PubMed] [Google Scholar]
- Schwartz JC. The histamine H3 receptor: from discovery to clinical trials with pitolisant. Br J Pharmacol. 2011;163:713–721. doi: 10.1111/j.1476-5381.2011.01286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JC, Arrang JM, Garbarg M, Pollard H, Ruat M. Histaminergic transmission in the mammalian brain. Physiol Rev. 1991;71:1–51. doi: 10.1152/physrev.1991.71.1.1. [DOI] [PubMed] [Google Scholar]
- Shuman T, Cai DJ, Sage JR, Anagnostaras SG. Interactions between modafinil and cocaine during the induction of conditioned place preference and locomotor sensitization in mice: implications for addiction. Behav Brain Res. 2012;235:105–112. doi: 10.1016/j.bbr.2012.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon P, Hémet C, Costentin J. Analysis of stimulant locomotor effects of modafinil in various strains of mice and rats. Fundam Clin Pharmacol. 1996;10:431–435. doi: 10.1111/j.1472-8206.1996.tb00597.x. [DOI] [PubMed] [Google Scholar]
- Tahsili-Fahadan P, Carr GV, Harris GC, Aston-Jones G. Modafinil blocks reinstatement of extinguished opiate-seeking in rats: mediation by a glutamate mechanism. Neuropsychopharmacology. 2010;35:2203–2210. doi: 10.1038/npp.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;6:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, et al. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA. 2009;301:1148–1154. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuo-Silva R, Fukushiro DF, Borçoi AR, Fernandes HA, Procópio-Souza R, Hollais AW, et al. Addictive potential of modafinil and cross-sensitization with cocaine: a pre-clinical study. Addict Biol. 2011;16:565–579. doi: 10.1111/j.1369-1600.2011.00341.x. [DOI] [PubMed] [Google Scholar]
- Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, et al. Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharmacol Exp Ther. 2009;329:738–746. doi: 10.1124/jpet.108.146142. [DOI] [PMC free article] [PubMed] [Google Scholar]


