Abstract
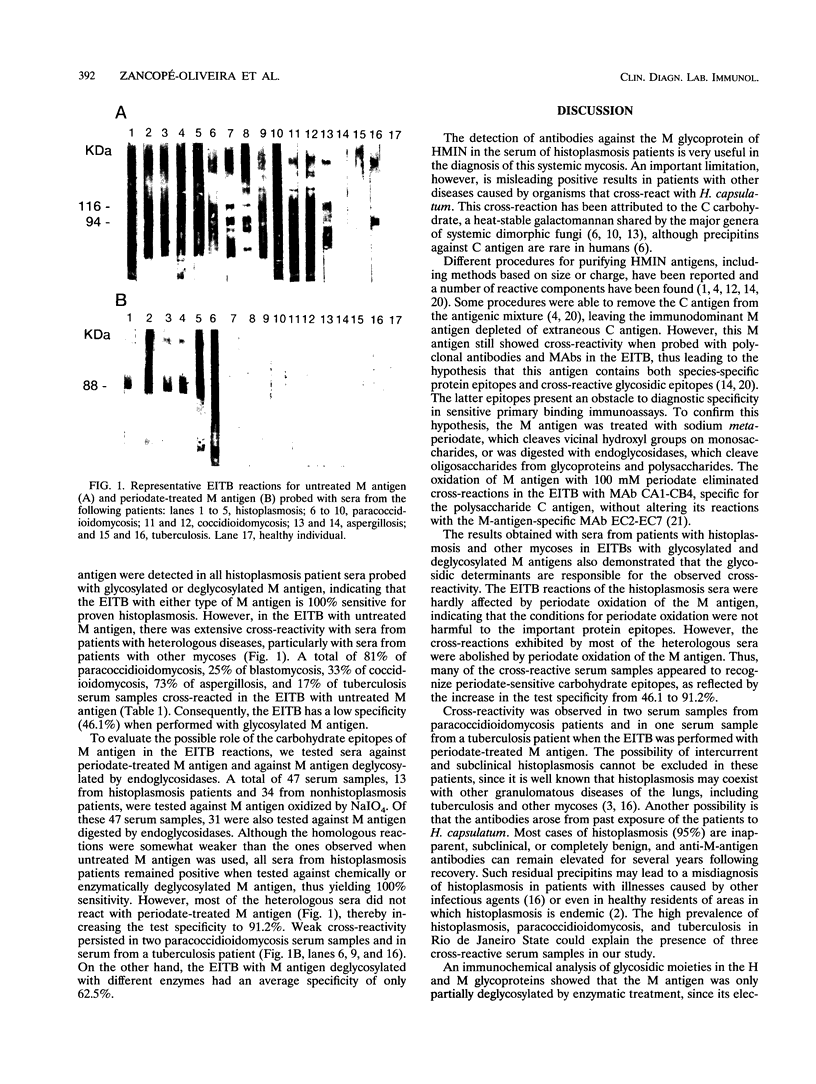
The enzyme-linked immunoelectrotransfer blot (EITB) method was evaluated as a suitable method for detecting antibodies against M antigen of Histoplasma capsulatum by use of both glycosylated and deglycosylated M protein of histoplasmin (HMIN). Sera from patients with histoplasmosis, paracoccidioidomycosis, blastomycosis, coccidioidomycosis, and aspergillosis were tested by the EITB with glycosylated M protein of HMIN. This assay demonstrated 100% sensitivity with histoplasmosis serum samples, all of which reacted with the 94-kDa glycoprotein (M antigen). Although the EITB is highly sensitive, it is not specific for histoplasmosis when glycosylated M protein is used as an antigen. A total of 81% of paracoccidioidomycosis, 25% of blastomycosis, 33% of coccidioidomycosis, 73% of aspergillosis, and 16% of tuberculosis serum samples cross-reacted with M protein of HMIN and yielded patterns indistinguishable from those obtained with histoplasmosis serum samples. The EITB reactions with both untreated M antigen and M antigen altered by periodate oxidation or by deglycosylation with endoglycosidases were compared. Cross-reactions with heterologous sera in the EITB could be attributed to periodate-sensitive carbohydrate epitopes, as reflected by the increase in the test specificity from 46.1 to 91.2% after periodate treatment of M protein. The EITB for the detection of antibodies to M antigen is a potential diagnostic test for histoplasmosis, provided that periodate-treated M protein is used as an antigen.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley G., Pine L., Reeves M. W., Moss C. W. Purification, composition, and serological characterization of histoplasmin-H and M antigens. Infect Immun. 1974 May;9(5):870–880. doi: 10.1128/iai.9.5.870-880.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra J. W. Histoplasmosis. Dermatol Clin. 1989 Apr;7(2):251–258. [PubMed] [Google Scholar]
- Gauto A. R., Cone L. A., Woodard D. R., Mahler R. J., Lynch R. D., Stoltzman D. H. Arterial infections due to Listeria monocytogenes: report of four cases and review of world literature. Clin Infect Dis. 1992 Jan;14(1):23–28. doi: 10.1093/clinids/14.1.23. [DOI] [PubMed] [Google Scholar]
- HEINER D. C. Diagnosis of histoplasmosis using precipitin reactions in agargel. Pediatrics. 1958 Oct;22(4 Pt 1):616–627. [PubMed] [Google Scholar]
- Kumar B. V., Medoff G., Kobayashi G. S., Sieling W. L. Cross-reacting human and rabbit antibodies to antigens of Histoplasma capsulatum, Candida albicans, and Saccharomyces cerevisiae. Infect Immun. 1985 Jun;48(3):806–812. doi: 10.1128/iai.48.3.806-812.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine L., Gross H., Malcolm G. B., George J. R., Gray S. B., Moss C. W. Procedures for the production and separation of H and M antigens in histoplasmin: chemical and serological properties of the isolated products. Mycopathologia. 1977 Oct 28;61(3):131–141. doi: 10.1007/BF00468007. [DOI] [PubMed] [Google Scholar]
- Reiss E., Knowles J. B., Bragg S. L., Kaufman L. Monoclonal antibodies against the M-protein and carbohydrate antigens of histoplasmin characterized by the enzyme-linked immunoelectrotransfer blot method. Infect Immun. 1986 Sep;53(3):540–546. doi: 10.1128/iai.53.3.540-546.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang V. C., Peralta J. M., Simons A. R. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 1983;92:377–391. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]
- Wheat J., French M. L., Kohler R. B., Zimmerman S. E., Smith W. R., Norton J. A., Eitzen H. E., Smith C. D., Slama T. G. The diagnostic laboratory tests for histoplasmosis: analysis of experience in a large urban outbreak. Ann Intern Med. 1982 Nov;97(5):680–685. doi: 10.7326/0003-4819-97-5-680. [DOI] [PubMed] [Google Scholar]
- Wheat L. J., Batteiger B. E., Sathapatayavongs B. Histoplasma capsulatum infections of the central nervous system. A clinical review. Medicine (Baltimore) 1990 Jul;69(4):244–260. doi: 10.1097/00005792-199007000-00006. [DOI] [PubMed] [Google Scholar]
- Wheat L. J., Connolly-Stringfield P., Blair R., Connolly K., Garringer T., Katz B. P. Histoplasmosis relapse in patients with AIDS: detection using Histoplasma capsulatum variety capsulatum antigen levels. Ann Intern Med. 1991 Dec 15;115(12):936–941. doi: 10.7326/0003-4819-115-12-936. [DOI] [PubMed] [Google Scholar]
- Wheat L. J. Diagnosis and management of histoplasmosis. Eur J Clin Microbiol Infect Dis. 1989 May;8(5):480–490. doi: 10.1007/BF01964063. [DOI] [PubMed] [Google Scholar]
- Zancopé-Oliveira R. M., Bragg S. L., Hurst S. F., Peralta J. M., Reiss E. Evaluation of cation exchange chromatography for the isolation of M glycoprotein from histoplasmin. J Med Vet Mycol. 1993;31(1):29–41. [PubMed] [Google Scholar]
- Zimmerman S. E., French M. L., Kleiman M. B., Wheat L. J. Evaluation of an enzyme-linked immunosorbent assay that uses ferrous metal beads for determination of antihistoplasmal immunoglobulins G and M. J Clin Microbiol. 1990 Jan;28(1):59–64. doi: 10.1128/jcm.28.1.59-64.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]