Abstract
During development, a properly coordinated expression of Hox genes, within their different genomic clusters is critical for patterning the body plans of many animals with a bilateral symmetry. The fascinating correspondence between the topological organization of Hox clusters and their transcriptional activation in space and time has served as a paradigm for understanding the relationships between genome structure and function. Here, we review some recent observations, which revealed highly dynamic changes in the structure of chromatin at Hox clusters, in parallel with their activation during embryonic development. We discuss the relevance of these findings for our understanding of large-scale gene regulation.
Keywords: embryonic patterning, collinearity, chromatin architecture, long-range regulation, nuclear organization
1. Introduction
Understanding the relationship between the structural organization of genomes, on the one hand, and their transcription during development, ageing and pathogenesis, on the other hand, is a major challenge of the post genomic-sequencing era. In recent years, considerable efforts have been devoted to the identification of functional elements within non-coding genomic intervals, as well as to the mapping of chromatin structure and spatial organization of chromosomes in the nuclear space [1–3]. These large-scale approaches tentatively define global relationships between the chromatin structure observed at a particular genomic locus, the three-dimensional organization of this locus and the associated gene activity. For instance, both local chromatin post-translational modifications as well as long-range physical interactions are used to identify candidate transcriptional regulatory elements and their target genes, genome-wide.
The emerging picture suggests that genes playing particularly important roles during embryonic development generally display a highly intricate regulatory organization locally, which involves a large number of control elements. Such elements are usually dispersed within large flanking regions which can be several hundreds of kilobases long and they establish complex patterns of contacts with their target genes. In a broader genomic context, active and inactive gene loci seem to occupy distinct nuclear spaces, which may relate to specialized nuclear compartments such as transcription factories [4–6]. These novel approaches are nevertheless largely descriptive and the future integration of these large datasets within a functional framework, for example by combining them with genetic approaches, will be critical to firmly establish the physiological relevance of these structural parameters.
The coordinated transcription of Hox genes within their respective genomic clusters has been a paradigm to study these questions, ever since high-throughput technologies have been available. This is mostly due to the structure of these loci, which display one of the highest concentrations of genes in the genome (in average ten genes within 120 kb) and their enigmatic transcriptional regulation, in response to both local and long-range strategies. Because these genes have a tightly constrained topological organization, they often have been (and still are) used as examples to illustrate the relationships between genome structure and function. Here, we review recent progress in deciphering the potential mechanisms, which may link chromatin organization to the transcriptional control of Hox genes in embryo.
2. Structural and functional organization of Hox clusters
Hox genes encode homeodomain transcription factors critical for the proper establishment of regional identities along the main body axis of bilaterian animals. In many species, they are grouped into genomic clusters, which share a conserved structural organization, indicating that a clustered set of genes was probably present in the ancestor of all extant bilateria. This ancestral cluster was split in the Drosophila lineage, where eight Hox genes are distributed in the Antennapedia (Ant-C) and Bithorax (BX-C) complexes; in contrast, the two rounds of whole-genome duplications that accompanied the emergence of vertebrates led to the presence of four paralogous Hox clusters in this group, termed HoxA to HoxD, where a total of 39 genes can be scored in mammals (see [7] for references).
This peculiar genomic organization is closely associated with a regulatory process referred to as ‘collinearity’, i.e. tight correspondence that exists between the order of Hox genes within each cluster, on the one hand, and the succession of their expression territories along the anterior–posterior embryonic axis, on the other hand. This property, referred to as spatial collinearity, was originally proposed by studying the genetics of the Drosophila BX-C [8]. It was subsequently extended to vertebrates [9–11], which indicated that animals apparently displaying highly divergent morphologies nevertheless rely on the same genetic systems to pattern their body plans. Part of this spatial collinear distribution of Hox expression domain is caused by a temporal sequence in their transcriptional activation, which reflects their genomic order, along with the extension of the embryonic axis (‘temporal collinearity’ [12]). A tight control of Hox genes transcription, in both space and time, is thus critical for proper development, because variations in the expected HOX proteins combinations at any given anterior–posterior level in the embryo usually lead to alterations in axial patterning [13,14].
Because of this capacity to initiate a coordinated transcriptional response of this series of genes involved in patterning processes, these collinear properties were co-opted several times in the course of vertebrate evolution, along with the emergence of structures displaying some kind of axial specification, such as the gut or the appendages. In such cases, specific Hox gene clusters were recruited, usually via the evolution of global regulation involving remote control elements, leading to the global transcription of several Hox genes at once ([15]; see below). The mechanistic relationship(s) between Hox genes clustering and collinear regulation, however, is still elusive. For example, in some animal species, Hox genes display this peculiar expression patterns along the embryonic axis, even though the clustered organization has been lost ([16]; see [7] for a discussion of this issue). Furthermore, Hox genes often, yet not always, recapitulate some aspects of their genuine expression specificities in the developing trunk, when isolated from their endogenous cluster and integrated at random genomic locations as transgenes, indicating that transcription units carry some of the requested regulatory information [17,18].
Despite these observations, targeted modifications of Hox clusters have shown that changing the relative position of a gene within its cluster has a critical impact on several aspects of its transcriptional regulation, such as the timing of expression along the main axis, as well as transcript distribution along secondary axes, such as the growing limbs [18–22]. Therefore, global regulatory influences act over Hox clusters as a whole, on the top of more local, gene-specific proximal controls. A transition in chromatin structure, from an initially repressed state to a configuration progressively open for transcription, was proposed to accompany this coordinated activation early on [23]. We discuss below recent observations, which suggest that both local histone modifications and changes in the higher-order organization of chromatin are involved in the transcriptional control of Hox clusters.
3. Epigenetic control by Polycomb and Trithorax complexes
The epigenetic regulation of Hox gene clusters seems to rely, mostly, on the activities of protein complexes encoded by Polycomb (PcG) and Trithorax (TrxG) group genes. During early Drosophila embryogenesis, maternally supplied transcription factors define the spatial patterns of Hox genes' activity [24]. These patterns are maintained at later developmental stages through the action of PcG genes, required for stable Hox gene repression. TrxG genes, on the other hand, counteract PcG silencing and keep Hox genes expressed in the appropriate domains [25].
PcG and TrxG gene products are found in multi-protein complexes, which mediate the post-translational modification of histone tails and thus affect chromatin structure. PcG-mediated silencing relies on the combined actions of the Polycomb repressive complexes 1 and 2 (PRC1 and PRC2). PRC2 tri-methylates lysine 27 of histone H3 tail (H3K27), a mark that is tightly associated with gene repression [26–29]. PRC1 is recruited to H3K27me3 [26,29–31] and contains the Ring1B E3 ubiquitin ligase that triggers the ubiquitylation of H2A at lysine 119 [32,33]. By contrast, TrxG complexes are responsible for the tri-methylation of histone H3 tail at lysine 4 (H3K4me3), a chromatin mark associated with transcriptional activation [34].
In mouse and human cultured cells, H3K27me3 and H3K4me3 predominantly decorate silent or active promoters, respectively [35–38]. Surprisingly, a significant number of silent loci, including Hox clusters, display both H3K4 and H3K27 tri-methylation in pluripotent embryonic stem (ES) cells, a chromatin signature referred to as ‘bivalent domains’ [39,40]. Differentiation of ES cells leads to a resolution of these bivalency into either active or repressive chromatin marks, suggesting that such bivalent domains might label genes that are kept silent, but ‘poised’ for rapid activation upon lineage commitment. Such plasticity is at odds with the classical view of PcG and TrxG function as mediators of a stable memory of epigenetic states [41], but is supported by the identification of H3K27 lysine de-methylases recruited to gene promoters upon transcriptional activation [42–45].
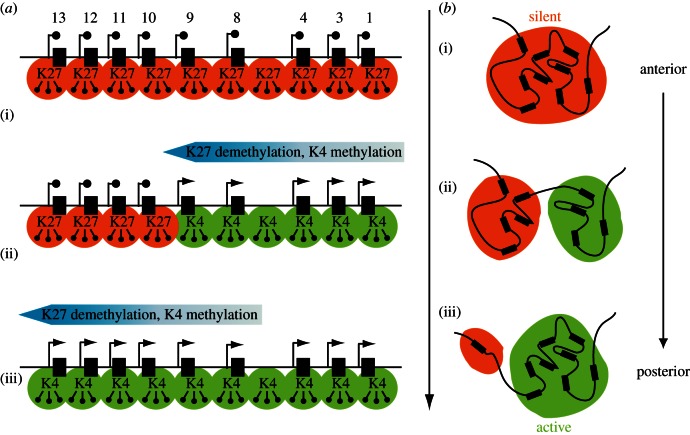
Similar to their Drosophila orthologues, PcG and trxG genes are required for vertebrate embryonic development, and mutations in these genes lead to a deregulation of Hox genes, amongst other defects (reviewed in [46,47]). Recently, a progressive loss of H3K27me3, concomitantly to a gain of H3K4me3, was observed together with the sequential activation of Hoxd genes during the extension of the main body axis in the mouse in vivo ([48]; figure 1a). Transcriptional activation of Hoxd genes thus occurs within the region of transition between these two epigenetic states, in a window that shifts along with time, from one extremity of the cluster to the other. The analysis of embryos carrying an engineered split HoxD cluster further revealed that clustering is not necessary for the initial deposition of H3K27me3, yet it is required for a fully coordinated transition in histone modifications [48].
Figure 1.
Collinearity during trunk extension and chromatin dynamics at Hox clusters. Expression of Hox gene along the anterior-to-posterior (AP) embryonic axis is collinear with gene order within the cluster. (a) During axial extension, the sequential onset of Hox gene transcriptional activation is accompanied by a transition in histone modifications over the gene cluster. In ES cells (i), the whole cluster is labelled with H3K27me3 (orange), a mark associated with Polycomb-mediated silencing. In the developing embryo, this mark is progressively erased and replaced by H3K4me3 (green), concomitantly with gene activation. (b) Active and silent Hox loci segregate into distinct spatial compartments along the AP axis. In embryonic tissues where the whole cluster is repressed, such as the forebrain (i), Hox clusters form a compact three-dimensional structure. In regions where subsets of Hox genes are expressed (anterior trunk, ii), active and silent genes segregate in distinct compartments, labelled with either H3K27me3 (silent compartment) or H3K4me3 (active compartment). In posterior embryonic regions (iii), most genes are transcribed and participate in the active compartment.
The mechanisms recruiting PcG proteins to their target loci are not fully understood. In Drosophila, relatively short sequences termed Polycomb response elements (PREs) seem to be both necessary and sufficient for PcG recruitment and gene silencing, although these sites cannot be defined by a consensus DNA sequence [25]. The situation is more complex in vertebrates, where classical PREs—as defined in flies—remain to be identified. For instance, an element from the human HOXD cluster was recently reported to bind PcG proteins and to induce repression of a reporter gene [49]. However, the deletion of the murine orthologous sequence did not cause any dramatic change in Hoxd gene regulation [50], suggesting that it is not critical for PcG recruitment or, alternatively, that it is part of a robust mechanism with high compensatory capacities. Interestingly, CG-rich sequences, which are particularly abundant within Hox clusters, were proposed as a hallmark of PcG target promoters, at least in ES cells [51,52]. Silencing at these particular loci might thus involve the cooperative activity of multiple DNA elements.
4. Higher-order chromatin organization
The coordinated control of these dynamic epigenetic states might be facilitated by the spatial compartmentalization of Hox clusters. Changes in higher-order chromatin organization of Hox clusters were first observed by microscopy approaches such as fluorescent in situ hybridization (FISH), which allow the visualization of specific loci within the nucleus. Differentiation of ES cells after retinoic acid treatment leads to a decompaction of the HoxB cluster that parallels its global transcriptional activation [53]. Furthermore, Hox clusters are also less compact in those embryonic territories where Hox genes are probably active, than in silent regions [54]. However, the resolution of this approach did not allow the stepwise, collinear transition to be documented.
Also, owing to its limited resolution, FISH cannot currently provide a precise mapping of the three-dimensional organization of Hox loci, in either their active or inactive states. The development of the chromosome conformation capture (3C) technique, which provides an estimation of the average frequency of specific DNA–DNA ‘contacts’ (or proximity, see below) within a cell population, has greatly helped to overcome this limitation. While early 3C analyses only addressed interactions between a limited number of sequences, variant approaches such as 4C (circular 3C), 5C (3C carbon copy) or Hi-C generate rather unbiased datasets, where the mapping of all sequences contacting a locus of interest, or the analysis of mutual interactions between a large number of pre-determined sites—or even within an entire genome, can be produced [55]. Using these approaches, changes in the three-dimensional organization of Hox clusters were detected upon cell differentiation in culture or between cell lines derived from various regions of the body [56–58].
In dissected murine embryonic tissues, this configuration is tightly associated with the transcriptional activity of the gene clusters. In tissues were Hox genes are silent, such as the developing forebrain, Hox gene clusters form distinct spatial structures, as defined by widespread interactions between the various gene loci, within each cluster. In contrast, in regions where distinct subsets of Hox genes are transcribed, active and repressed genes segregate into distinct compartments, labelled by different chromatin marks [59,60]. In anterior regions of the main embryonic axis, expressed Hox genes appear to cluster together within a H3K4me3 decorated domain, whereas silent genes form a distinct compartment marked with H3K27me3. In more posterior areas, most Hox genes are found in the active compartment, suggesting a dynamic reorganization of the three-dimensional micro-architecture of these clusters ([59]; figure 1b).
This spatial separation between the active and silent parts of Hox clusters might in part reflect the PcG-mediated compaction of repressed loci, as shown in vitro [61]. Interestingly, the loss of either Eed (a protein member of PRC2) or Ring1B (a protein member of PRC1) leads to the decompaction of Hox clusters in ES cells [62]. In Drosophila, Polycomb targets segregate into discrete nuclear foci termed Polycomb bodies [63]. Despite a genomic distance of nearly 10 megabases (Mb), the Ant-C and BX-C complexes contact each other in a PcG dependent manner, in embryonic regions where both loci are silent [64].
Whether these patterns of contacts participate in the mechanism controlling Hox gene collinearity or, instead, merely reflect a general tendency of different chromatin segments to segregate into distinct nuclear domains depending on their modifications, remains to be established. However, the presence of separate spatial domains, as seen by using 4C, makes a biochemical artefact induced by cross-linking unlikely. In such a case, chromatin domains labelled either by H3K27me3 or by H3K4me3 would indeed be expected to cross-link together. Instead, a physical separation between active and repressed Hox subsets might participate in a tighter control of the sequential activation of Hox clusters, by isolating posterior genes from early/anterior activating influences.
5. Long-range control and regulatory archipelagos
The type of collinear regulation described above follows a mechanism acting purely in cis, and is shared by all paralogous Hox clusters. Therefore, it may represent an ancestral mechanism at work in those animals that activate their Hox genes in a precise time sequence (see above). In addition, in the vertebrate lineage, specific Hox clusters have evolved additional patterning functions [23,65,66], which required the emergence of novel regulatory modalities. These novel regulatory specificities often rely upon enhancer elements located outside the gene clusters, at a distance, likely to prevent deleterious effects of evolving additional regulatory elements within the gene clusters themselves, where the cis-regulatory sequences necessary to implement the ancestral collinear regulation along the anterior–posterior axis are mostly located [67].
Such potent enhancers have indeed been described and can act over large distances. They sometimes also affect unrelated, bystander transcription units, thus defining the concept of genomic ‘regulatory landscapes’ [68]. The emergence of these long-range controls, acting on several neighbouring Hox genes in a coordinated manner may have provided a selective pressure for the gene clusters to consolidate their structural organization in vertebrates [7].
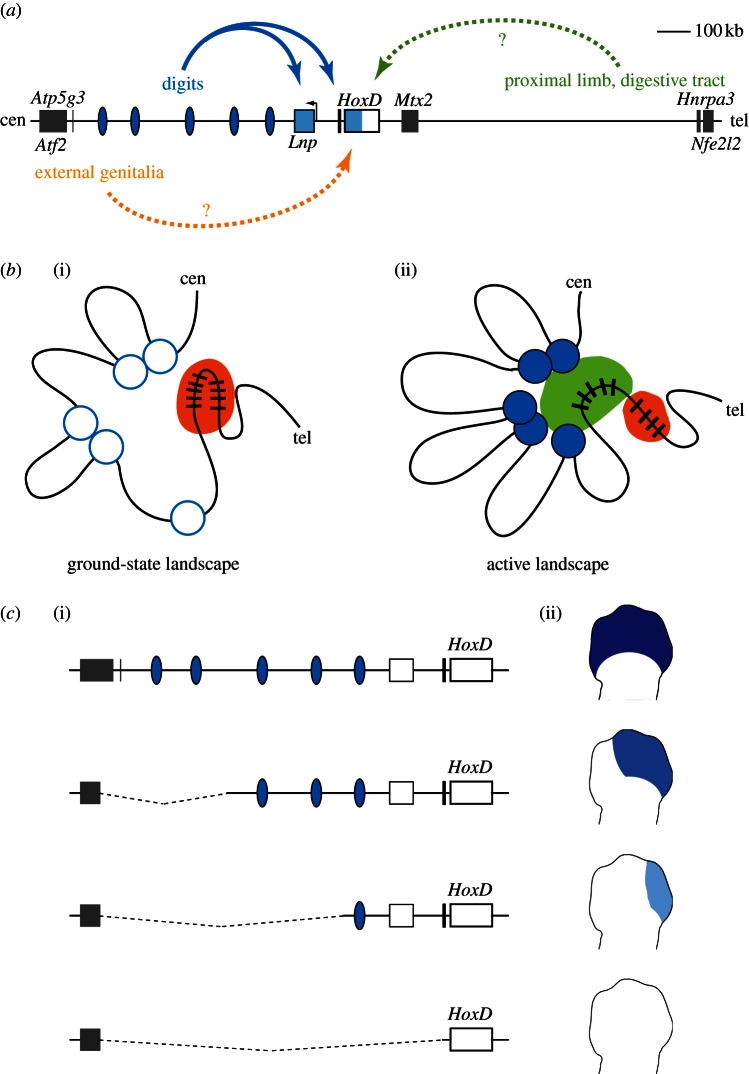
The transcription of Hoxd genes in developing limbs is the clearest example of such kind of acquired distal regulations around Hox gene clusters, as it has been investigated in some detail using both genetic and biochemical approaches. ‘Posterior’ Hoxd genes (from Hoxd9 to Hoxd13) are required for the patterning of both proximal (arm and forearm or leg and lower leg) and distal (hands and feet) limb segments [69]. Their expression in growing limb buds follows two independent phases [21,70], controlled by distinct regulatory elements located on either side of the gene cluster [71]. In a first phase, corresponding to the future proximal limb segment, Hoxd genes are activated in a time sequence, which generates a nested pattern of expression along the limb anterior to posterior axis. While regulatory elements controlling this early activation are not yet reported, some genomic rearrangements suggest that they may be located on the telomeric side of the gene cluster, on chromosome 2 ([68,71]; figure 2a). Multiple highly conserved non-coding DNA sequences can be found there and thus represent potential candidates [72].
Figure 2.
Long-range control and regulatory archipelagos. The coordinated transcription of Hox genes in different embryonic territories relies on remote regulatory elements located on either sides of the cluster. (a) Map of the HoxD locus and its flanking centromeric (cen) and telomeric (tel) conserved gene deserts. Multiple regulatory islands (blue ovals) participate in Hoxd13–Hoxd10 regulation in developing digits (blue arrow). Hoxd gene activation in other embryonic structures also relies on long-range controls, yet the corresponding regulatory elements have not yet been identified (dashed arrows). (b) Spatial conformation of the locus. (i) In the silent state, a ground-state structure is formed, involving contacts between a subset of the regulatory elements and the HoxD cluster. (ii) In digits, additional contacts are formed, leading to a fully active conformation paralleled with histone modifications at the regulatory elements, and leading to Hox genes transcriptional activation. (c) Robustness of regulatory archipelagos. Different genetic configurations of the locus are shown in (i), with a scheme of the resulting Hox gene expression in developing limbs in (ii). The wild-type situation is depicted on top, and serial deletions within the archipelago are indicated below. Deleting subsets of the regulatory islands leads to partial downregulation of Hoxd genes in distal limbs, and only a full deletion of the regulatory interval fully abolishes transcription.
Subsequently, the transcription of posterior Hoxd genes (Hoxd13 to Hoxd10) is activated at the distal extremities of the growing limbs, in a territory corresponding to presumptive digits. This ‘late phase’ is controlled by elements located centromeric from Hoxd13 [73]. The mapping of the three-dimensional organization of the cluster revealed that active genes establish numerous long-range interactions with sequences dispersed over an 800 kb interval overlapping a gene desert located centromeric to the cluster [60]; figure 2a). These sites are grouped into ‘islands’ that are labelled with histone marks usually associated with enhancer elements, such as the monomethylation of H3K4 (H3K4me1) and the acetylation of H3K27 (H3K27Ac) [74–76]. The various islands contact each other, as well as the active part of the HoxD cluster, suggesting that they form an active conformation in developing digits.
In contrast, silent genes display the opposite profile of interactions, mostly involving the telomeric gene desert, which is devoid of active histone marks [60]. Therefore, the compartmentalization of Hox clusters in active and silent domains, in growing limb cells, also involves differential association with surrounding sequences. In the developing forebrain, where the entire HoxD cluster is repressed, some of these long-range interactions with distant islands are observed too, yet in this case the islands display a distinct epigenetic signature, involving limited levels of H3K4me1 and no H3K27Ac [60]. This indicates that the transition between a ground-state or poised structure towards a fully active configuration involves only a partial reorganization of the locus micro-architecture, associated with chromatin modifications over the regulatory elements (figure 2b). Interestingly, such megabase-scale regions of chromatin interactions, or ‘topological domains’ are widespread in vertebrate genomes, and appear stable when comparing different cell lines [77], suggesting that ‘poised’ regulatory conformations are not restricted to Hox clusters.
However, when using chromosome conformation capture approaches, it is important to keep in mind a few methodological aspects. First, the data obtained give an average of the contacts established within the cellular population under scrutiny and hence may not reflect the real situation within one particular cell, at a particular time. While this approach does not tell about the dynamics of the interactions, the subsequent use of contact points as baits themselves, may at least give some hints about the complexity of the interactions (for example by documenting interactions amongst various enhancers sequences, rather than between enhancers and the target promoter). In any case, the cellular homogeneity of the sample and its physiological relevance must be carefully assessed beforehand to help interpret the interaction profiles.
Second, the cross-linking step will bring together DNA pieces that can be quite far from one another, provided they are engaged in large DNA–protein complexes. In this respect, the notion of ‘contact’ can be understood both as a direct and close interaction between two pieces of DNA, for example between an enhancer sequence and its target promoter via looping, but also as a more diffuse proximity, where enhancers could act as platforms to recruit factors such as to increase their concentration around particular genomic loci. In this context, investigating these dynamic patterns of contacts at the single cell level and at different stages of limb development should bring more insight into the establishment of this complex conformation. Interestingly, recent observations indicate that the frequencies of some of these interactions, as documented by FISH, display regional differences along the limb anterior–posterior axis [78].
Several of the regulatory islands, as defined by the interaction profile, could elicit a digit-specific transcriptional activation when isolated from the locus and tested in transgenic assays [60,68,79], further validating the 4C approach as a tool to isolate long-range acting enhancers. A genetic dissection of the 800 kb large genomic interval centromeric to Hoxd13 indicated that these various elements contribute in a partially redundant fashion to the transcriptional activation of Hoxd genes ([60]; figure 2c). Such a dispersed enhancer system, somehow reminiscent of shadow enhancers described in Drosophila [80–82], was referred to as a regulatory archipelago, i.e. a collection of regulatory islands, located nearby one another and all devoted to a collective function though with slightly different contributions of the diverse elements. Such a complex regulatory system may both ensure a robust transcriptional response of the target genes, and confer some regulatory flexibility during the development and evolution of distal limbs. Genetic alterations within this interval could indeed impact on the growth and patterning of digits, and thus contribute to the diversity of digit morphologies amongst various tetrapods.
6. Concluding remarks and outlook
The correspondence between the structural organization of Hox clusters and their transcriptional control in space and time has fascinated biologists for decades. While the underlying mechanisms remain to be fully elucidated, recent progress in deciphering the epigenetic status of these loci in developing embryos, as well as their three-dimensional organization in the nuclear space, have provided substantial information.
Future investigations will involve the identification of upstream factors and signals controlling the recruitment of chromatin-modifying activities to Hox loci, as well as the definition of their spatial organization during development. Considerable interest has been focused recently on the involvement of non-coding RNA or structural proteins, such as CTCF, in these processes [56,58,83,84]. For instance, CTCF binding sites are enriched at the boundaries of topological domains within the HoxA cluster, as well as at other genomic loci [77]. Yet, a critical impact of these various factors on Hox clusters regulation has not been confirmed by genetic analysis, so far [85,86]. It will also be critical to isolate the factors involved in the transcriptional activation of Hox genes, to understand the nature of the directionality of the ancestral process, during trunk extension.
Finally, the limb regulatory archipelago may be a paradigm to understand the emergence and evolution of such regulatory systems. Tetrapods indeed present a great variety of distal morphologies, in their limbs, and the analysis of the accompanying regulations will be informative in this context. Also, developing limbs are accessible structures, in the developing embryo, with rather well defined cell types and expression domains, unlike cultured cell systems, which may have little physiological relevance in this context, and where genetic approaches are not available.
Tracing back the emergence of novel regulatory landscapes in vertebrates may also bring some insights to our understanding of genome evolution. For instance, the acquisition of a late and distal phase of Hoxd gene activation in limbs was critical to the evolution of tetrapod digits, and this expression specificity does not seem to have a counterpart in fishes [87]. Likewise, the activation of Hoxd genes in developing external genitalia and digits follows highly similar patterns, which led to the hypothesis of a shared and ancestral regulation in both structures [22,88]. Addressing the regulatory potential of the syntenic regions of different species, as well as comparing the mechanisms controlling Hox clusters expression in different embryonic structures, should help reconstruct the evolutionary history of these regulations.
Similar concepts are likely to apply to other regulatory contexts. For instance, the involvement of gene deserts in long-range transcriptional control is not a specificity of Hox clusters, and regulatory elements were identified within gene deserts flanking other genes displaying intricate expression patterns [89–92]. The prevalence of regulatory archipelagos similar to that described at the HoxD locus nevertheless remains to be established and further efforts in mapping the spatial organization of genomes, combined with large-scale genetic approaches will be necessary.
Acknowledgements
We thank Natalia Soshnikova and colleagues from the Duboule laboratories for discussions. We acknowledge financial support from the Ecole Polytechnique Federale de Lausanne (EPFL), the University of Geneva, the Swiss National Research Council (SNF), the European Research Council (ERC) and the FP7 EU programme IDEAL.
References
- 1.Zhou VW, Goren A, Bernstein BE. 2011. Charting histone modifications and the functional organization of mammalian genomes. Nat. Rev. Genet. 12, 7–18 10.1038/nrg2905 (doi:10.1038/nrg2905) [DOI] [PubMed] [Google Scholar]
- 2.Bernstein BE, Birney E, Dunham I, Green ED, Gunter C, Snyder M. 2012. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 10.1038/nature11247 (doi:10.1038/nature11247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Wit E, de Laat W. 2012. A decade of 3C technologies: insights into nuclear organization. Genes Dev. 26, 11–24 10.1101/gad.179804.111 (doi:10.1101/gad.179804.111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lieberman-Aiden E, et al. 2009. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326, 289–293 10.1126/science.1181369 (doi:10.1126/science.1181369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sexton T, Yaffe E, Kenigsberg E, Bantignies F, Leblanc B, Hoichman M, Parrinello H, Tanay A, Cavalli G. 2012. Three-dimensional folding and functional organization principles of the Drosophila genome. Cell 148, 458–472 10.1016/j.cell.2012.01.010 (doi:10.1016/j.cell.2012.01.010) [DOI] [PubMed] [Google Scholar]
- 6.Edelman LB, Fraser P. 2012. Transcription factories: genetic programming in three dimensions. Curr. Opin. Genet. Dev. 22, 110–114 10.1016/j.gde.2012.01.010 (doi:10.1016/j.gde.2012.01.010) [DOI] [PubMed] [Google Scholar]
- 7.Duboule D. 2007. The rise and fall of Hox gene clusters. Development 134, 2549–2560 10.1242/dev.001065 (doi:10.1242/dev.001065) [DOI] [PubMed] [Google Scholar]
- 8.Lewis EB. 1978. A gene complex controlling segmentation in Drosophila. Nature 276, 565–570 10.1038/276565a0 (doi:10.1038/276565a0) [DOI] [PubMed] [Google Scholar]
- 9.Gaunt SJ, Sharpe PT, Duboule D. 1988. Spatially restricted domains of homeo-gene transcripts in mouse embryos: relation to a segmented body plan. Development 104, 169–170 [Google Scholar]
- 10.Graham A, Papalopulu N, Krumlauf R. 1989. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell 57, 367–378 10.1016/0092-8674(89)90912-4 (doi:10.1016/0092-8674(89)90912-4) [DOI] [PubMed] [Google Scholar]
- 11.Duboule D, Dolle P. 1989. The structural and functional organization of the murine HOX gene family resembles that of Drosophila homeotic genes. EMBO J. 8, 1497–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izpisua Belmonte JC, Falkenstein H, Dollé P, Renucci A, Duboule D. 1991. Murine genes related to the Drosophila AbdB homeotic genes are sequentially expressed during development of the posterior part of the body. EMBO J. 10, 2279–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mallo M, Wellik DM, Deschamps J. 2010. Hox genes and regional patterning of the vertebrate body plan. Dev. Biol. 344, 7–15 10.1016/j.ydbio.2010.04.024 (doi:10.1016/j.ydbio.2010.04.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young T, et al. 2009. Cdx and Hox genes differentially regulate posterior axial growth in mammalian embryos. Dev. Cell 17, 516–526 10.1016/j.devcel.2009.08.010 (doi:10.1016/j.devcel.2009.08.010) [DOI] [PubMed] [Google Scholar]
- 15.Tschopp P, Duboule D. 2011. A genetic approach to the transcriptional regulation of hox gene clusters. Annu. Rev. Genet. 45, 145–166 10.1146/annurev-genet-102209-163429 (doi:10.1146/annurev-genet-102209-163429) [DOI] [PubMed] [Google Scholar]
- 16.Seo H-C, et al. 2004. Hox cluster disintegration with persistent anteroposterior order of expression in Oikopleura dioica. Nature 431, 67–71 10.1038/nature02709 (doi:10.1038/nature02709) [DOI] [PubMed] [Google Scholar]
- 17.Tschopp P, Christen AJ, Duboule D. 2012. Bimodal control of Hoxd gene transcription in the spinal cord defines two regulatory subclusters. Development 139, 929–939 10.1242/dev.076794 (doi:10.1242/dev.076794) [DOI] [PubMed] [Google Scholar]
- 18.Tschopp P, Tarchini B, Spitz F, Zakany J, Duboule D. 2009. Uncoupling time and space in the collinear regulation of Hox genes. PLoS Genet. 5, e1000398. 10.1371/journal.pgen.1000398 (doi:10.1371/journal.pgen.1000398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Hoeven F, Zakany J, Duboule D. 1996. Gene transpositions in the HoxD complex reveal a hierarchy of regulatory controls. Cell 85, 1025–1035 10.1016/S0092-8674(00)81303-3 (doi:10.1016/S0092-8674(00)81303-3) [DOI] [PubMed] [Google Scholar]
- 20.Kmita M, Fraudeau N, Hérault Y, Duboule D. 2002. Serial deletions and duplications suggest a mechanism for the collinearity of Hoxd genes in limbs. Nature 420, 145–150 10.1038/nature01189 (doi:10.1038/nature01189) [DOI] [PubMed] [Google Scholar]
- 21.Tarchini B, Duboule D. 2006. Control of Hoxd genes’ collinearity during early limb development. Dev. Cell 10, 93–103 10.1016/j.devcel.2005.11.014 (doi:10.1016/j.devcel.2005.11.014) [DOI] [PubMed] [Google Scholar]
- 22.Montavon T, Le Garrec J-F, Kerszberg M, Duboule D. 2008. Modeling Hox gene regulation in digits: reverse collinearity and the molecular origin of thumbness. Genes Dev. 22, 346–359 10.1101/gad.1631708 (doi:10.1101/gad.1631708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dollé P, Izpisua Belmonte JC, Falkenstein H, Renucci A, Duboule D. 1989. Coordinate expression of the murine Hox-5 complex homoeobox-containing genes during limb pattern formation. Nature 342, 767–772 10.1038/342767a0 (doi:10.1038/342767a0) [DOI] [PubMed] [Google Scholar]
- 24.Maeda RK, Karch F. 2009. The bithorax complex of Drosophila an exceptional Hox cluster. Curr. Top. Dev. Biol. 88, 1–33 10.1016/S0070-2153(09)88001-0 (doi:10.1016/S0070-2153(09)88001-0) [DOI] [PubMed] [Google Scholar]
- 25.Schwartz YB, Pirrotta V. 2007. Polycomb silencing mechanisms and the management of genomic programmes. Nat. Rev. Genet. 8, 9–22 10.1038/nrg1981 (doi:10.1038/nrg1981) [DOI] [PubMed] [Google Scholar]
- 26.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. 2002. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298, 1039–1043 10.1126/science.1076997 (doi:10.1126/science.1076997) [DOI] [PubMed] [Google Scholar]
- 27.Czermin B, Melfi R, McCabe D, Seitz V, Imhof A, Pirrotta V. 2002. Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell 111, 185–196 10.1016/S0092-8674(02)00975-3 (doi:10.1016/S0092-8674(02)00975-3) [DOI] [PubMed] [Google Scholar]
- 28.Kuzmichev A, Nishioka K, Erdjument-Bromage H, Tempst P, Reinberg D. 2002. Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes Dev. 16, 2893–2905 10.1101/gad.1035902 (doi:10.1101/gad.1035902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller J, et al. 2002. Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell 111, 197–208 10.1016/S0092-8674(02)00976-5 (doi:10.1016/S0092-8674(02)00976-5) [DOI] [PubMed] [Google Scholar]
- 30.Fischle W, Wang Y, Jacobs SA, Kim Y, Allis CD, Khorasanizadeh S. 2003. Molecular basis for the discrimination of repressive methyl-lysine marks in histone H3 by Polycomb and HP1 chromodomains. Genes Dev. 17, 1870–1881 10.1101/gad.1110503 (doi:10.1101/gad.1110503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Min J, Zhang Y, Xu RM. 2003. Structural basis for specific binding of Polycomb chromodomain to histone H3 methylated at Lys 27. Genes Dev. 17, 1823–1828 10.1101/gad.269603 (doi:10.1101/gad.269603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Napoles M, et al. 2004. Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation. Dev. Cell. 7, 663–676 10.1016/j.devcel.2004.10.005 (doi:10.1016/j.devcel.2004.10.005) [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Wang L, Erdjument-Bromage H, Vidal M, Tempst P, Jones RS, Zhang Y. 2004. Role of histone H2A ubiquitination in Polycomb silencing. Nature 431, 873–878 10.1038/nature02985 (doi:10.1038/nature02985) [DOI] [PubMed] [Google Scholar]
- 34.Ruthenburg AJ, Allis CD, Wysocka J. 2007. Methylation of lysine 4 on histone H3: intricacy of writing and reading a single epigenetic mark. Mol. Cell 25, 15–30 10.1016/j.molcel.2006.12.014 (doi:10.1016/j.molcel.2006.12.014) [DOI] [PubMed] [Google Scholar]
- 35.Bracken AP, Dietrich N, Pasini D, Hansen KH, Helin K. 2006. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev. 20, 1123–1136 10.1101/gad.381706 (doi:10.1101/gad.381706) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee TI, et al. 2006. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell 125, 301–313 10.1016/j.cell.2006.02.043 (doi:10.1016/j.cell.2006.02.043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyer LA, et al. 2006. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441, 349–353 10.1038/nature04733 (doi:10.1038/nature04733) [DOI] [PubMed] [Google Scholar]
- 38.Bernstein BE, et al. 2005. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell 120, 169–181 10.1016/j.cell.2005.01.001 (doi:10.1016/j.cell.2005.01.001) [DOI] [PubMed] [Google Scholar]
- 39.Bernstein BE, et al. 2006. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315–326 10.1016/j.cell.2006.02.041 (doi:10.1016/j.cell.2006.02.041) [DOI] [PubMed] [Google Scholar]
- 40.Azuara V, et al. 2006. Chromatin signatures of pluripotent cell lines. Nat. Cell Biol. 8, 532–538 10.1038/ncb1403 (doi:10.1038/ncb1403) [DOI] [PubMed] [Google Scholar]
- 41.Ringrose L, Paro R. 2007. Polycomb/Trithorax response elements and epigenetic memory of cell identity. Development 134, 223–232 10.1242/dev.02723 (doi:10.1242/dev.02723) [DOI] [PubMed] [Google Scholar]
- 42.Lan F, et al. 2007. A histone H3 lysine 27 demethylase regulates animal posterior development. Nature 449, 689–694 10.1038/nature06192 (doi:10.1038/nature06192) [DOI] [PubMed] [Google Scholar]
- 43.De Santa F, Totaro MG, Prosperini E, Notarbartolo S, Testa G, Natoli G. 2007. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of Polycomb-mediated gene silencing. Cell 130, 1083–1094 10.1016/j.cell.2007.08.019 (doi:10.1016/j.cell.2007.08.019) [DOI] [PubMed] [Google Scholar]
- 44.Lee MG, Villa R, Trojer P, Norman J, Yan KP, Reinberg D, Di Croce L, Shiekhattar R. 2007. Demethylation of H3K27 regulates polycomb recruitment and H2A ubiquitination. Science 318, 447–450 10.1126/science.1149042 (doi:10.1126/science.1149042) [DOI] [PubMed] [Google Scholar]
- 45.Mansour AA, et al. 2012. The H3K27 demethylase Utx regulates somatic and germ cell epigenetic reprogramming. Nature 488, 409–413 10.1038/nature11272 (doi:10.1038/nature11272) [DOI] [PubMed] [Google Scholar]
- 46.Soshnikova N. 2011. Dynamics of Polycomb and Trithorax activities during development. Birth Defects Res. A Clin. Mol. Teratol. 91, 781–787 10.1002/bdra.20774 (doi:10.1002/bdra.20774) [DOI] [PubMed] [Google Scholar]
- 47.Surface LE, Thornton SR, Boyer LA. 2010. Polycomb group proteins set the stage for early lineage commitment. Cell Stem Cell 7, 288–298 10.1016/j.stem.2010.08.004 (doi:10.1016/j.stem.2010.08.004) [DOI] [PubMed] [Google Scholar]
- 48.Soshnikova N, Duboule D. 2009. Epigenetic temporal control of mouse Hox genes in vivo. Science 324, 1320–1323 10.1126/science.1171468 (doi:10.1126/science.1171468) [DOI] [PubMed] [Google Scholar]
- 49.Woo CJ, Kharchenko PV, Daheron L, Park PJ, Kingston RE. 2010. A region of the human HOXD cluster that confers Polycomb-group responsiveness. Cell 140, 99–110 10.1016/j.cell.2009.12.022 (doi:10.1016/j.cell.2009.12.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beckers J, Duboule D. 1998. Genetic analysis of a conserved sequence in the HoxD complex: regulatory redundancy or limitations of the transgenic approach? Dev. Dyn. 213, 1–11 (doi:10.1002/(SICI)1097-0177(199809)213:1<1::AID-AJA1>3.0.CO;2-L) [DOI] [PubMed] [Google Scholar]
- 51.Ku M, et al. 2008. Genomewide analysis of PRC1 and PRC2 occupancy identifies two classes of bivalent domains. PLoS Genet. 4, e1000242. 10.1371/journal.pgen.1000242 (doi:10.1371/journal.pgen.1000242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mendenhall EM, Koche RP, Truong T, Zhou VW, Issac B, Chi AS, Ku M, Bernstein BE. 2010. GC-rich sequence elements recruit PRC2 in mammalian ES cells. PLoS Genet. 6, e1001244. 10.1371/journal.pgen.1001244 (doi:10.1371/journal.pgen.1001244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chambeyron S, Bickmore WA. 2004. Chromatin decondensation and nuclear reorganization of the HoxB locus upon induction of transcription. Genes Dev. 18, 1119–1130 10.1101/gad.292104 (doi:10.1101/gad.292104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chambeyron S, Da Silva NR, Lawson KA, Bickmore WA. 2005. Nuclear re-organisation of the Hoxb complex during mouse embryonic development. Development 132, 2215–2223 10.1242/dev.01813 (doi:10.1242/dev.01813) [DOI] [PubMed] [Google Scholar]
- 55.van Steensel B, Dekker J. 2010. Genomics tools for unraveling chromosome architecture. Nat. Biotechnol. 28, 1089–1095 10.1038/nbt.1680 (doi:10.1038/nbt.1680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferraiuolo MA, Rousseau M, Miyamoto C, Shenker S, Wang XQ, Nadler M, Blanchette M, Dostie J. 2010. The three-dimensional architecture of Hox cluster silencing. Nucleic Acids Res. 38, 7472–7484 10.1093/nar/gkq644 (doi:10.1093/nar/gkq644) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fraser J, Rousseau M, Shenker S, Ferraiuolo MA, Hayashizaki Y, Blanchette M, Dostie J. 2009. Chromatin conformation signatures of cellular differentiation. Genome Biol. 10, R37. 10.1186/gb-2009-10-4-r37 (doi:10.1186/gb-2009-10-4-r37) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang KC, et al. 2011. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 472, 120–124 10.1038/nature09819 (doi:10.1038/nature09819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noordermeer D, Leleu M, Splinter E, Rougemont J, De Laat W, Duboule D. 2011. The dynamic architecture of Hox gene clusters. Science 334, 222–225 10.1126/science.1207194 (doi:10.1126/science.1207194) [DOI] [PubMed] [Google Scholar]
- 60.Montavon T, Soshnikova N, Mascrez B, Joye E, Thevenet L, Splinter E, de Laat W, Spitz F, Duboule D. 2011. A regulatory archipelago controls Hox genes transcription in digits. Cell 147, 1132–1145 10.1016/j.cell.2011.10.023 (doi:10.1016/j.cell.2011.10.023) [DOI] [PubMed] [Google Scholar]
- 61.Grau DJ, Chapman BA, Garlick JD, Borowsky M, Francis NJ, Kingston RE. 2011. Compaction of chromatin by diverse Polycomb group proteins requires localized regions of high charge. Genes Dev. 25, 2210–2221 10.1101/gad.17288211 (doi:10.1101/gad.17288211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eskeland R, et al. 2010. Ring1B compacts chromatin structure and represses gene expression independent of histone ubiquitination. Mol. Cell 38, 452–464 10.1016/j.molcel.2010.02.032 (doi:10.1016/j.molcel.2010.02.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lanzuolo C, Roure V, Dekker J, Bantignies F, Orlando V. 2007. Polycomb response elements mediate the formation of chromosome higher-order structures in the bithorax complex. Nat. Cell Biol. 9, 1167–1174 10.1038/ncb1637 (doi:10.1038/ncb1637) [DOI] [PubMed] [Google Scholar]
- 64.Bantignies F, Roure V, Comet I, Leblanc B, Schuettengruber B, Bonnet J, Tixier V, Mas A, Cavalli G. 2011. Polycomb-dependent regulatory contacts between distant Hox loci in Drosophila. Cell 144, 214–226 10.1016/j.cell.2010.12.026 (doi:10.1016/j.cell.2010.12.026) [DOI] [PubMed] [Google Scholar]
- 65.Godwin AR, Capecchi MR. 1998. Hoxc13 mutant mice lack external hair. Genes Dev. 12, 11–20 10.1101/gad.12.1.11 (doi:10.1101/gad.12.1.11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Di-Poi N, Zakany J, Duboule D. 2007. Distinct roles and regulations for HoxD genes in metanephric kidney development. PLoS Genet. 3, e232. 10.1371/journal.pgen.0030232 (doi:10.1371/journal.pgen.0030232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spitz F, Gonzalez F, Peichel C, Vogt TF, Duboule D, Zakany J. 2001. Large scale transgenic and cluster deletion analysis of the HoxD complex separate an ancestral regulatory module from evolutionary innovations. Genes Dev. 15, 2209–2214 10.1101/gad.205701 (doi:10.1101/gad.205701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spitz F, Gonzalez F, Duboule D. 2003. A global control region defines a chromosomal regulatory landscape containing the HoxD cluster. Cell 113, 405–417 10.1016/S0092-8674(03)00310-6 (doi:10.1016/S0092-8674(03)00310-6) [DOI] [PubMed] [Google Scholar]
- 69.Zakany J, Duboule D. 2007. The role of Hox genes during vertebrate limb development. Curr. Opin. Genet. Dev. 17, 359–366 10.1016/j.gde.2007.05.011 (doi:10.1016/j.gde.2007.05.011) [DOI] [PubMed] [Google Scholar]
- 70.Nelson CE, et al. 1996. Analysis of Hox gene expression in the chick limb bud. Development 122, 1449–1466 [DOI] [PubMed] [Google Scholar]
- 71.Spitz F, Herkenne C, Morris MA, Duboule D. 2005. Inversion-induced disruption of the Hoxd cluster leads to the partition of regulatory landscapes. Nat. Genet. 37, 889–893 10.1038/ng1597 (doi:10.1038/ng1597) [DOI] [PubMed] [Google Scholar]
- 72.Lee AP, Koh EG, Tay A, Brenner S, Venkatesh B. 2006. Highly conserved syntenic blocks at the vertebrate Hox loci and conserved regulatory elements within and outside Hox gene clusters. Proc. Natl Acad. Sci. USA 103, 6994–6999 10.1073/pnas.0601492103 (doi:10.1073/pnas.0601492103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tschopp P, Duboule D. 2011. A regulatory ‘landscape effect’ over the HoxD cluster. Dev. Biol. 351, 288–296 10.1016/j.ydbio.2010.12.034 (doi:10.1016/j.ydbio.2010.12.034) [DOI] [PubMed] [Google Scholar]
- 74.Heintzman ND, et al. 2007. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat. Genet. 39, 311–318 10.1038/ng1966 (doi:10.1038/ng1966) [DOI] [PubMed] [Google Scholar]
- 75.Creyghton MP, et al. 2010. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl Acad. Sci. USA 107, 21 931–21 936 10.1073/pnas.1016071107 (doi:10.1073/pnas.1016071107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rada-Iglesias A, Bajpai R, Swigut T, Brugmann SA, Flynn RA, Wysocka J. 2011. A unique chromatin signature uncovers early developmental enhancers in humans. Nature 470, 279–283 10.1038/nature09692 (doi:10.1038/nature09692) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, Hu M, Liu JS, Ren B. 2012. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485, 376–380 10.1038/nature11082 (doi:10.1038/nature11082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Williamson I, Eskeland R, Lettice LA, Hill AE, Boyle S, Grimes GR, Hill RE, Bickmore WA. 2012. Anterior-posterior differences in HoxD chromatin topology in limb development. Development 139, 3157–3167 10.1242/dev.081174 (doi:10.1242/dev.081174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gonzalez F, Duboule D, Spitz F. 2007. Transgenic analysis of Hoxd gene regulation during digit development. Dev. Biol. 306, 847–859 10.1016/j.ydbio.2007.03.020 (doi:10.1016/j.ydbio.2007.03.020) [DOI] [PubMed] [Google Scholar]
- 80.Hong J-W, Hendrix DA, Levine MS. 2008. Shadow enhancers as a source of evolutionary novelty. Science 321, 1314. 10.1126/science.1160631 (doi:10.1126/science.1160631) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frankel N, Davis GK, Vargas D, Wang S, Payre F, Stern DL. 2010. Phenotypic robustness conferred by apparently redundant transcriptional enhancers. Nature 466, 490–493 10.1038/nature09158 (doi:10.1038/nature09158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perry MW, Boettiger AN, Bothma JP, Levine M. 2010. Shadow enhancers foster robustness of Drosophila gastrulation. Curr. Biol. 20, 1562–1567 10.1016/j.cub.2010.07.043 (doi:10.1016/j.cub.2010.07.043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rinn JL, et al. 2007. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129, 1311–1323 10.1016/j.cell.2007.05.022 (doi:10.1016/j.cell.2007.05.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim YJ, Cecchini KR, Kim TH. 2011. Conserved, developmentally regulated mechanism couples chromosomal looping and heterochromatin barrier activity at the homeobox gene A locus. Proc. Natl Acad. Sci. USA 108, 7391–7396 10.1073/pnas.1018279108 (doi:10.1073/pnas.1018279108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Soshnikova N, Montavon T, Leleu M, Galjart N, Duboule D. 2010. Functional analysis of CTCF during mammalian limb development. Dev. Cell 19, 819–830 10.1016/j.devcel.2010.11.009 (doi:10.1016/j.devcel.2010.11.009) [DOI] [PubMed] [Google Scholar]
- 86.Schorderet P, Duboule D. 2011. Structural and functional differences in the long non-coding RNA hotair in mouse and human. PLoS Genet. 7, e1002071. 10.1371/journal.pgen.1002071 (doi:10.1371/journal.pgen.1002071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Woltering JM, Duboule D. 2010. The origin of digits: expression patterns versus regulatory mechanisms. Dev. Cell 18, 526–532 10.1016/j.devcel.2010.04.002 (doi:10.1016/j.devcel.2010.04.002) [DOI] [PubMed] [Google Scholar]
- 88.Dolle P, Izpisua-Belmonte JC, Brown JM, Tickle C, Duboule D. 1991. HOX-4 genes and the morphogenesis of mammalian genitalia. Genes Dev. 5, 1767–1767 10.1101/gad.5.10.1767 (doi:10.1101/gad.5.10.1767) [DOI] [PubMed] [Google Scholar]
- 89.Nobrega MA, Ovcharenko I, Afzal V, Rubin EM. 2003. Scanning human gene deserts for long-range enhancers. Science 302, 413. 10.1126/science.1088328 (doi:10.1126/science.1088328) [DOI] [PubMed] [Google Scholar]
- 90.Jeong Y, El-Jaick K, Roessler E, Muenke M, Epstein DJ. 2006. A functional screen for sonic hedgehog regulatory elements across a 1 Mb interval identifies long-range ventral forebrain enhancers. Development 133, 761–772 10.1242/dev.02239 (doi:10.1242/dev.02239) [DOI] [PubMed] [Google Scholar]
- 91.Sagai T, Amano T, Tamura M, Mizushina Y, Sumiyama K, Shiroishi T. 2009. A cluster of three long-range enhancers directs regional Shh expression in the epithelial linings. Development 136, 1665–1674 10.1242/dev.032714 (doi:10.1242/dev.032714) [DOI] [PubMed] [Google Scholar]
- 92.Tena JJ, Alonso ME, de la Calle-Mustienes E, Splinter E, de Laat W, Manzanares M, Gomez-Skarmeta JL. 2011. An evolutionarily conserved three-dimensional structure in the vertebrate Irx clusters facilitates enhancer sharing and coregulation. Nat. Commun. 2, 310. 10.1038/ncomms1301 (doi:10.1038/ncomms1301) [DOI] [PubMed] [Google Scholar]