Abstract
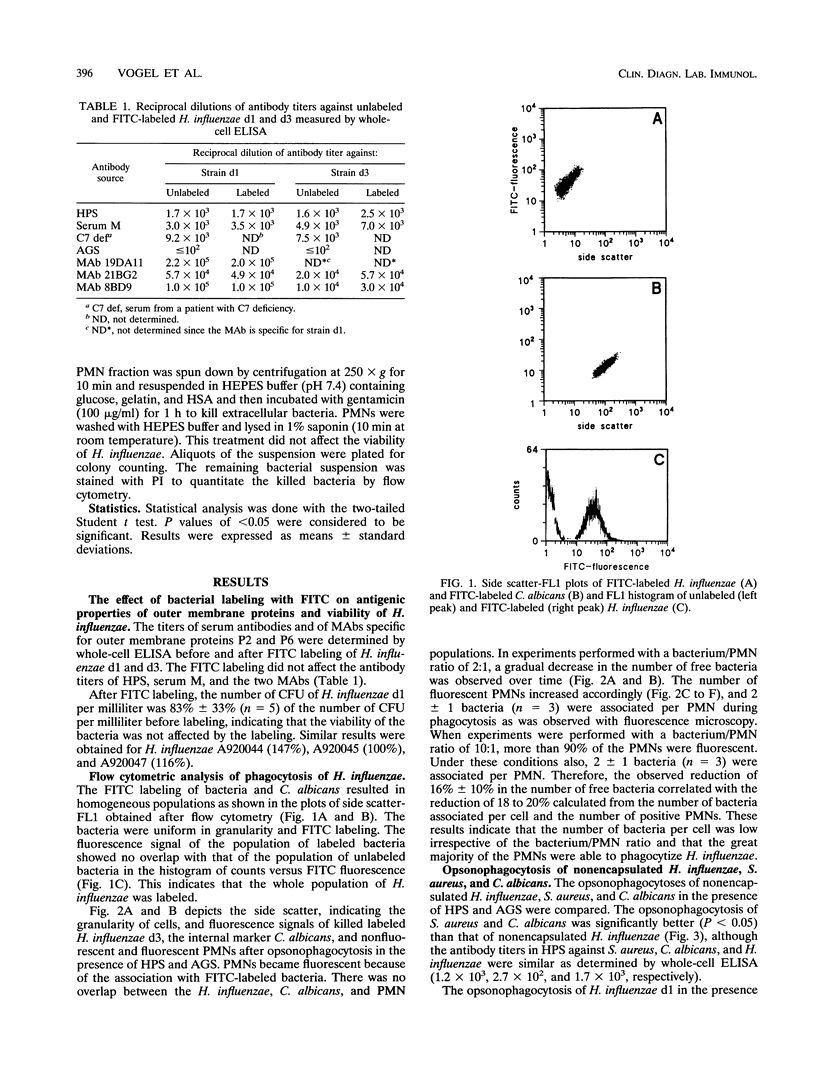
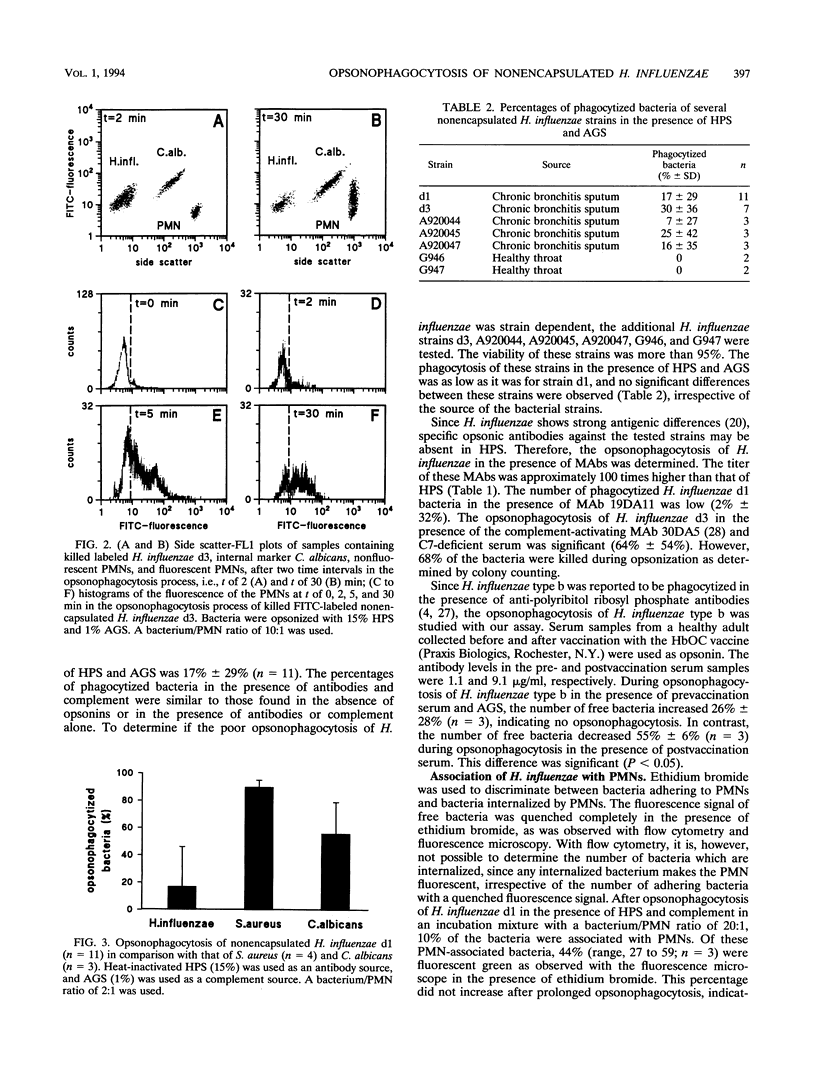
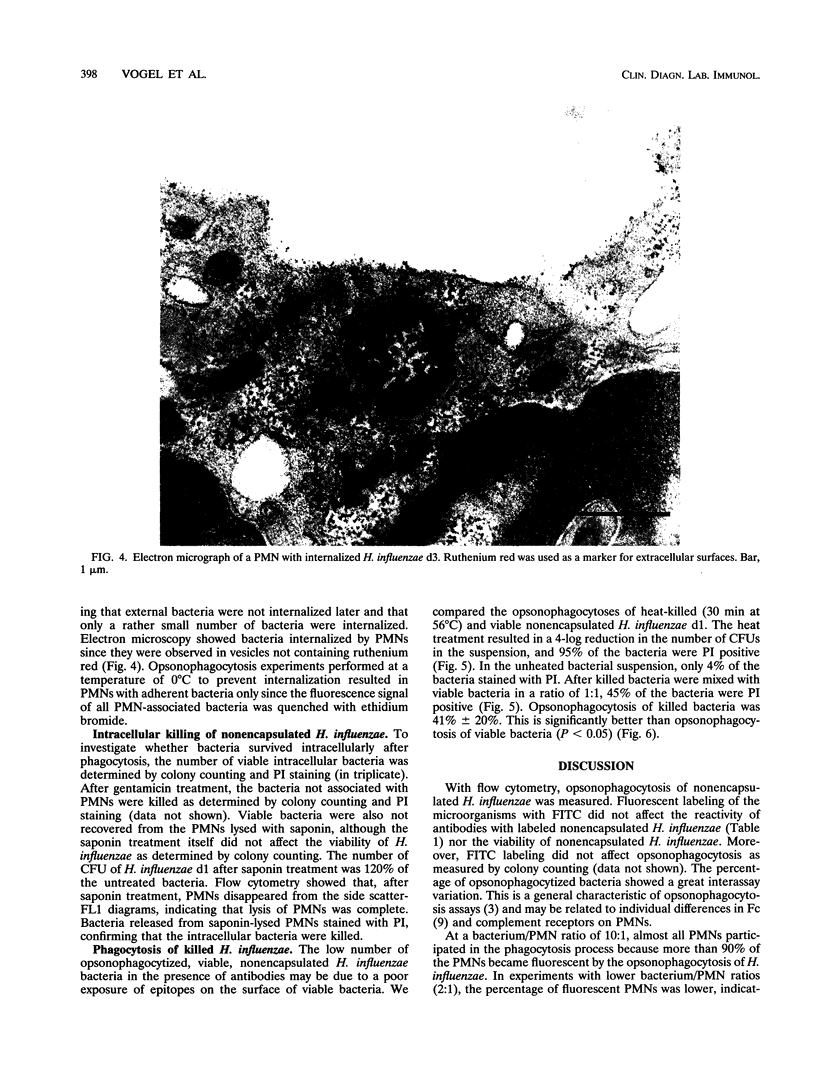
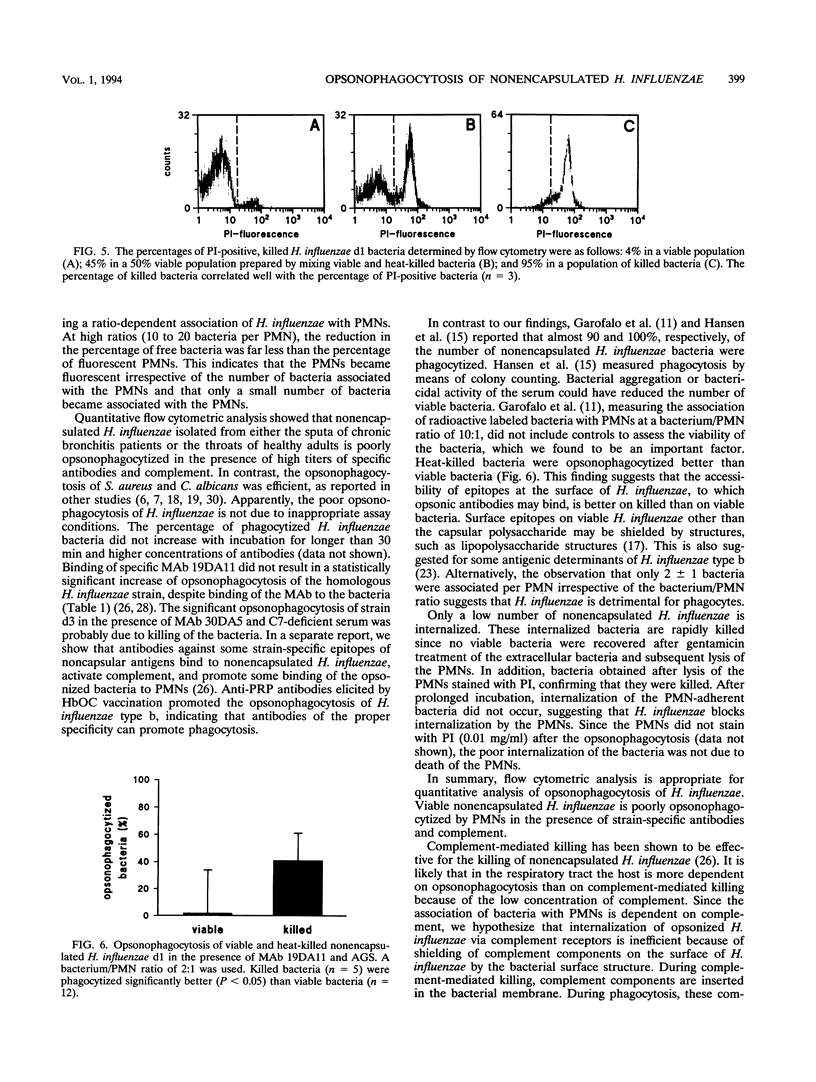
Since nonencapsulated Haemophilus influenzae persists in the lower respiratory tracts of patients with chronic bronchitis despite the presence of specific antibodies, complement, and polymorphonuclear leukocytes (PMNs), opsonophagocytosis of H. influenzae was analyzed. Nonencapsulated H. influenzae isolated from the sputa of chronic bronchitis patients was labeled with fluorescein isothiocyanate and incubated with human PMNs in the presence of complement and antibodies for 30 min at 37 degrees C. Candida albicans was added to each sample as an internal standard, and the reduction of the number of bacteria was determined by flow cytometry. Fluorescence quenching with ethidium bromide was used to discriminate between intracellular and extracellular bacteria. Opsonophagocytosis of viable H. influenzae d1 was 17% +/- 29% in the presence of complement and human pooled sera containing high titers of strain-specific antibodies. Opsonophagocytosis of six other H. influenzae strains was also poor. Under the same conditions, opsonophagocytosis of Staphylococcus aureus was 90% +/- 5%, and opsonophagocytosis of C. albicans was 55% +/- 23%. About half of the number of H. influenzae bacteria associated with PMNs was internalized. Opsonophagocytosis of heat-killed H. influenzae d1 (41% +/- 20%) was higher than that of viable bacteria of the same strain (P < 0.05). This result suggests that the accessibility of epitopes on H. influenzae for opsonizing antibodies is better on killed than on viable bacteria. We conclude that viable nonencapsulated H. influenzae is poorly opsonophagocytized in the presence of strain-specific antibodies and complement.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerström B., Brodin T., Reis K., Björck L. Protein G: a powerful tool for binding and detection of monoclonal and polyclonal antibodies. J Immunol. 1985 Oct;135(4):2589–2592. [PubMed] [Google Scholar]
- Amir J., Scott M. G., Nahm M. H., Granoff D. M. Bactericidal and opsonic activity of IgG1 and IgG2 anticapsular antibodies to Haemophilus influenzae type b. J Infect Dis. 1990 Jul;162(1):163–171. doi: 10.1093/infdis/162.1.163. [DOI] [PubMed] [Google Scholar]
- Anderson P., Johnston R. B., Jr, Smith D. H. Human serum activities against Hemophilus influenzae, type b. J Clin Invest. 1972 Jan;51(1):31–38. doi: 10.1172/JCI106793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassøe C. F., Laerum O. D., Solberg C. O., Haneberg B. Phagocytosis of bacteria by human leukocytes measured by flow cytometry. Proc Soc Exp Biol Med. 1983 Nov;174(2):182–186. doi: 10.3181/00379727-174-41722. [DOI] [PubMed] [Google Scholar]
- Bjerknes R., Bassøe C. F., Sjursen H., Laerum O. D., Solberg C. O. Flow cytometry for the study of phagocyte functions. Rev Infect Dis. 1989 Jan-Feb;11(1):16–33. doi: 10.1093/clinids/11.1.16. [DOI] [PubMed] [Google Scholar]
- Bjerknes R. Flow cytometric assay for combined measurement of phagocytosis and intracellular killing of Candida albicans. J Immunol Methods. 1984 Aug 3;72(1):229–241. doi: 10.1016/0022-1759(84)90451-4. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Humoral immune response patterns of human mucosae: induction and relation to bacterial respiratory tract infections. J Infect Dis. 1992 Jun;165 (Suppl 1):S167–S176. doi: 10.1093/infdis/165-supplement_1-s167. [DOI] [PubMed] [Google Scholar]
- Bredius R. G., de Vries C. E., Troelstra A., van Alphen L., Weening R. S., van de Winkel J. G., Out T. A. Phagocytosis of Staphylococcus aureus and Haemophilus influenzae type B opsonized with polyclonal human IgG1 and IgG2 antibodies. Functional hFc gamma RIIa polymorphism to IgG2. J Immunol. 1993 Aug 1;151(3):1463–1472. [PubMed] [Google Scholar]
- Drevets D. A., Campbell P. A. Macrophage phagocytosis: use of fluorescence microscopy to distinguish between extracellular and intracellular bacteria. J Immunol Methods. 1991 Aug 28;142(1):31–38. doi: 10.1016/0022-1759(91)90289-r. [DOI] [PubMed] [Google Scholar]
- Garofalo R., Faden H., Sharma S., Ogra P. L. Release of leukotriene B4 from human neutrophils after interaction with nontypeable Haemophilus influenzae. Infect Immun. 1991 Nov;59(11):4221–4226. doi: 10.1128/iai.59.11.4221-4226.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneveld K., Eijk P. P., van Alphen L., Jansen H. M., Zanen H. C. Haemophilus influenzae infections in patients with chronic obstructive pulmonary disease despite specific antibodies in serum and sputum. Am Rev Respir Dis. 1990 May;141(5 Pt 1):1316–1321. doi: 10.1164/ajrccm/141.5_Pt_1.1316. [DOI] [PubMed] [Google Scholar]
- Groeneveld K., van Alphen L., Eijk P. P., Jansen H. M., Zanen H. C. Changes in outer membrane proteins of nontypable Haemophilus influenzae in patients with chronic obstructive pulmonary disease. J Infect Dis. 1988 Aug;158(2):360–365. doi: 10.1093/infdis/158.2.360. [DOI] [PubMed] [Google Scholar]
- Groeneveld K., van Alphen L., Eijk P. P., Visschers G., Jansen H. M., Zanen H. C. Endogenous and exogenous reinfections by Haemophilus influenzae in patients with chronic obstructive pulmonary disease: the effect of antibiotic treatment on persistence. J Infect Dis. 1990 Mar;161(3):512–517. doi: 10.1093/infdis/161.3.512. [DOI] [PubMed] [Google Scholar]
- Hansen E. J., Hart D. A., McGehee J. L., Toews G. B. Immune enhancement of pulmonary clearance of nontypable Haemophilus influenzae. Infect Immun. 1988 Jan;56(1):182–190. doi: 10.1128/iai.56.1.182-190.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan E., Soskolne C. L., Zwi S., Hurwitz S., Maier G. M., Ipp T., Rabson A. R. Immunologic studies in patients with recurrent bronchopulmonary infections. Am Rev Respir Dis. 1975 Apr;111(4):441–451. doi: 10.1164/arrd.1975.111.4.441. [DOI] [PubMed] [Google Scholar]
- Kuratana M., Loeb M. R., Hansen E. J., Anderson P. The antigenic specificity of a serum factor-induced phenotypic shift in Hemophilus influenzae type b, strain Eag. J Infect Dis. 1989 Jun;159(6):1135–1138. doi: 10.1093/infdis/159.6.1135. [DOI] [PubMed] [Google Scholar]
- Martin E., Bhakdi S. Flow cytometric assay for quantifying opsonophagocytosis and killing of Staphylococcus aureus by peripheral blood leukocytes. J Clin Microbiol. 1992 Sep;30(9):2246–2255. doi: 10.1128/jcm.30.9.2246-2255.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E., Bhakdi S. Quantitative analysis of opsonophagocytosis and of killing of Candida albicans by human peripheral blood leukocytes by using flow cytometry. J Clin Microbiol. 1991 Sep;29(9):2013–2023. doi: 10.1128/jcm.29.9.2013-2023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Apicella M. A. Antigenic heterogeneity of outer membrane proteins of nontypable Haemophilus influenzae is a basis for a serotyping system. Infect Immun. 1985 Oct;50(1):15–21. doi: 10.1128/iai.50.1.15-21.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Sethi S. Bacterial infection in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1992 Oct;146(4):1067–1083. doi: 10.1164/ajrccm/146.4.1067. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., Parke J. C., Jr, Schneerson R., Whisnant J. K. Quantitative measurement of "natural" and immunization-induced Haemophilus influenzae type b capsular polysaccharide antibodies. Pediatr Res. 1973 Mar;7(3):103–110. doi: 10.1203/00006450-197303000-00001. [DOI] [PubMed] [Google Scholar]
- Robertson S. M., Frisch C. F., Gulig P. A., Kettman J. R., Johnston K. H., Hansen E. J. Monoclonal antibodies directed against a cell surface-exposed outer membrane protein of Haemophilus influenzae type b. Infect Immun. 1982 Apr;36(1):80–88. doi: 10.1128/iai.36.1.80-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos D., de Boer M. Purification and cryopreservation of phagocytes from human blood. Methods Enzymol. 1986;132:225–243. doi: 10.1016/s0076-6879(86)32010-x. [DOI] [PubMed] [Google Scholar]
- Sjursen H., Bjerknes R., Halstensen A., Naess A., Sørnes S., Solberg C. O. Flow cytometric assay for the measurement of serum opsonins to Neisseria meningitidis serogroup B, serotype 15. J Immunol Methods. 1989 Jan 17;116(2):235–243. doi: 10.1016/0022-1759(89)90209-3. [DOI] [PubMed] [Google Scholar]
- Troelstra A., Vogel L., van Alphen L., Eijk P., Jansen H., Dankert J. Opsonic antibodies to outer membrane protein P2 of nonencapsulated Haemophilus influenza are strain specific. Infect Immun. 1994 Mar;62(3):779–784. doi: 10.1128/iai.62.3.779-784.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk D. C. The pathogenicity of Haemophilus influenzae. J Med Microbiol. 1984 Aug;18(1):1–16. doi: 10.1099/00222615-18-1-1. [DOI] [PubMed] [Google Scholar]
- van Alphen L., Eijk P., Geelen-van den Broek L., Dankert J. Immunochemical characterization of variable epitopes of outer membrane protein P2 of nontypeable Haemophilus influenzae. Infect Immun. 1991 Jan;59(1):247–252. doi: 10.1128/iai.59.1.247-252.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alphen L., van den Berghe N., Geelen-van den Broek L. Interaction of Haemophilus influenzae with human erythrocytes and oropharyngeal epithelial cells is mediated by a common fimbrial epitope. Infect Immun. 1988 Jul;56(7):1800–1806. doi: 10.1128/iai.56.7.1800-1806.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]




