Abstract
Interactions between the terrestrial nitrogen (N) and carbon (C) cycles shape the response of ecosystems to global change. However, the global distribution of nitrogen availability and its importance in global biogeochemistry and biogeochemical interactions with the climate system remain uncertain. Based on projections of a terrestrial biosphere model scaling ecological understanding of nitrogen–carbon cycle interactions to global scales, anthropogenic nitrogen additions since 1860 are estimated to have enriched the terrestrial biosphere by 1.3 Pg N, supporting the sequestration of 11.2 Pg C. Over the same time period, CO2 fertilization has increased terrestrial carbon storage by 134.0 Pg C, increasing the terrestrial nitrogen stock by 1.2 Pg N. In 2001–2010, terrestrial ecosystems sequestered an estimated total of 27 Tg N yr−1 (1.9 Pg C yr−1), of which 10 Tg N yr−1 (0.2 Pg C yr−1) are due to anthropogenic nitrogen deposition. Nitrogen availability already limits terrestrial carbon sequestration in the boreal and temperate zone, and will constrain future carbon sequestration in response to CO2 fertilization (regionally by up to 70% compared with an estimate without considering nitrogen–carbon interactions). This reduced terrestrial carbon uptake will probably dominate the role of the terrestrial nitrogen cycle in the climate system, as it accelerates the accumulation of anthropogenic CO2 in the atmosphere. However, increases of N2O emissions owing to anthropogenic nitrogen and climate change (at a rate of approx. 0.5 Tg N yr−1 per 1°C degree climate warming) will add an important long-term climate forcing.
Keywords: terrestrial carbon and nitrogen budget, carbon sequestration, nitrogen deposition, CO2 fertilization, biogeochemistry–climate interactions
1. Introduction
Nitrogen is a fundamental component of living organisms. Ecosystem available forms of nitrogen (ammonium, as well as nitrate among other oxidized nitrogen forms), hereafter reactive N (Nr), are scarce in unperturbed ecosystems owing to low atmospheric inputs, the high energetic costs of assimilating elementary N2 through biological fixation and nitrogen losses to leaching and volatilization, particularly after disturbances [1]. The productivity of plants and soil organisms strongly depends on nitrogen, imposing stoichiometric constraints at the level of an individual organism. These two facts lead to a tight coupling of the terrestrial nitrogen and carbon cycles, as evidenced by the constrained flexibility of ecosystem C : N stoichiometry [2]. N availability thereby plays an important role in controlling the productivity, structure and spatio-temporal dynamics of terrestrial ecosystems: perturbations in the nitrogen cycle will have repercussions in the carbon cycle, and vice versa.
The terrestrial biogeochemical cycles have been disturbed in the past by human actions altering land cover and land-use, by increasing the atmospheric abundance of CO2, and by doubling the inputs of Nr through the burning of fossil fuel and the creation of agricultural fertilizer since 1860 [3,4]. These anthropogenic changes must have had consequences for the terrestrial store and turnover of nitrogen and carbon. However, because of the uncertainty in (i) the global distribution of nitrogen availability and demand in terrestrial ecosystems, (ii) the capacity of the terrestrial biosphere to retain added nitrogen and (iii) the tightness of the coupling between the terrestrial nitrogen and carbon cycles, these consequences are not well understood. The regional distribution of the anthropogenic perturbation is also important to take into account, as the fertilization by anthropogenic CO2—even if regionally constrained by nitrogen availability—is ubiquitous, whereas high levels of anthropogenic Nr only affect a small fraction of the global land surface, and land-use changes mainly act locally.
Quantifying the changes in the terrestrial carbon and nitrogen budgets is relevant not only to understand the fate of the anthropogenic Nr, and the cascading effects of this nitrogen, but also because these changes matter for the climate system [4]. Limited natural N availability reduces the carbon storage potential of the terrestrial biosphere. Anthropogenic Nr deposition generally increases terrestrial C sequestration and thus decreases the rate of anthropogenic CO2 accumulation in the atmosphere, but at the same time enhances nitrogen losses for instance to the greenhouse gas N2O, which might compensate for the C-cycle-related climate benefit [5,6]. This is important because the long atmospheric lifetime of the N2O can transform even subtle but long-term changes in terrestrial emission into a significant climate forcing.
The objective of this paper is to provide an assessment of the present and future nitrogen–carbon cycle interactions with a focus on the role of the natural and perturbed terrestrial nitrogen cycles in shaping the terrestrial net carbon and nitrogen balance and terrestrial carbon–climate feedbacks. A suite of new global ecosystem models that integrates current ecological and biogeochemical understanding with process-based descriptions of the terrestrial energy and water balance at a comparatively high spatial resolution is now available for such a task [7]. However, no systematic and comprehensive analyses have been performed so far with several models that would allow for a systematic model synthesis. I therefore present past, present and future nitrogen and carbon budgets based on one model only, the O–CN model [6,8], and discuss the uncertainties related to the application of this model in the light of other modelling studies and independent estimates.
2. Material and methods
(a). The O–CN model
O–CN [6,8] is a terrestrial biosphere model, which has been developed from the land surface model ORCHIDEE [9], and describes the nitrogen and carbon fluxes and stocks of vegetation and soil organic matter for 10 natural plant functional types, as well as C3 and C4 croplands at a half hourly time scale. The biogeochemical fluxes are tightly coupled to the calculations of the terrestrial energy and water balance. Nitrogen availability directly controls photosynthesis and respiration of vegetation through tissue nitrogen concentrations and effects on plant allocation (e.g. the root : shoot ratio), and thus foliage area and root growth. Nitrogen availability also affects the temperature-sensitive rate of organic matter decomposition and the net mineralization of nitrogen. The stoichiometry of plant tissues, litter and soil organic matter varies prognostically within observed limits, depending on the relative availabilities of nitrogen and carbon. The modelled ecosystem receives Nr inputs from biological nitrogen fixation and atmospheric Nr deposition, and simulates losses of nitrogen to leaching and volatilization based on the process-based simulation of nitrification and denitrification. Fertilizer is applied to the cropland fraction of each model grid cell at distinct dates during the growing season, but the treatment of cropland management and biomass removal is very simple, and manure systems are not taken into account. O–CN does not simulate the industrial sources and atmospheric transport of Nr. The model has been evaluated and applied to study the interactions of nitrogen and carbon cycling over the past few decades, and was found to simulate carbon and nitrogen fluxes that are generally commensurate with current understanding [6,8,10,11].
(b). Modelling protocol
O–CN was applied at a 3.75° × 2.5° spatial resolution. The model was brought into steady-state for 1860 conditions and then run transiently in a factorial design to identify the contribution of the individual driving forces. To isolate the effects of nitrogen dynamics, the model has been run twice, once with explicit accounting for nitrogen dynamics (referred to as O–CN), and once with nitrogen concentrations set to global averages of observed values (referred to as O–C), such that plant productivity and soil organic matter decomposition correspond to an ecosystem with average nitrogen availability not taking account of the spatial–temporal patterns of N availability. Two sets of simulations were performed: a ‘historic’ run driven by observed or reconstructed changes in land use, climate, atmospheric CO2, cropland fertilization and atmospheric deposition (1860–2010), and a ‘future’ run (1860–2100) with a reduced set of forcings (climate, atmospheric CO2 and Nr deposition) for the Intergovernmental Panel on Climate Change (IPCC) Special Report on Emissions Scenarios (SRES) A2 scenario.
(c). Datasets
(i). Historic run
The climate forcing (1901–2010) was taken from the CRU-NCEP dataset (v. 4; [12]) in the spatially degraded form (at 3.75° × 2.5° spatial resolution) provided by C. Huntingford (2012, personal communication). Gridded time series of cropland fertilization rates [6] and annual land-use changes [13] were used for the period 1860–2005, and assumed constant thereafter. Biological nitrogen fixation in natural vegetation was prescribed based on a climatology developed after Cleveland et al. [11,14]. To assess the uncertainty related to the estimation of nitrogen deposition, decadal time slices of monthly nitrogen deposition fields were obtained from two atmospheric chemistry transport models (CTMs), TM5 [15] and NCAR-CTM [16], and linearly interpolated to arrive at annual values. No estimates beyond 2000 were available for TM5. These were constructed by extrapolating the TM5 estimate for 2000 to 2001–2010 using the grid-cell wise monthly trends of the NCAR-CTM.
(ii). Future run
The future projections are described in detail by Zaehle et al. [10]. The simulations were forced with the SRES A2 climate and atmospheric CO2 change scenario of the IPSL-CM4 climate model [17], and a nitrogen deposition scenario, which increases the deposition on land from 10 Tg N yr−1 (1860) to 51 Tg N yr−1 (1993) and 106 Tg N yr−1 (2050), after which it was assumed to be constant [3]. This roughly follows the upper boundary of the representative concentration pathway (RCP) nitrogen deposition scenarios [18].
3. Results
(a). Current global terrestrial nitrogen and carbon budget
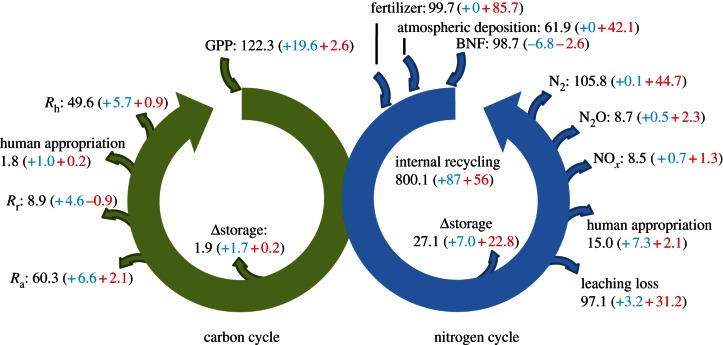
The contemporary nitrogen and carbon budget displayed in figure 1 (see also table 1) for 2001–2010 is based on the ‘unperturbed’ state of the cycles (1860s) and the historic changes in land cover, climate, atmospheric CO2 abundance and anthropogenic Nr inputs from atmospheric deposition and fertilizer application between the years 1860 and 2010.
Figure 1.
The 2001–2010 global carbon and nitrogen cycles of terrestrial ecosystems. The values in parentheses are the changes from the pre-industrial equilibrium fluxes (1860s) owing to land-use, climate and atmospheric CO2 change (blue) and anthropogenic nitrogen additions (red). Carbon fluxes: Pg C yr−1; nitrogen fluxes: Tg N yr−1. NOx, N2O and N2 emissions are from soils only. Ra, Rr and Rh are autotrophic, rhizosphere and heterotrophic respiration, respectively. BNF, biological nitrogen fixation; GPP, gross primary production.
Table 1.
Global and continental carbon and nitrogen budgets for the years 2001–2010 derived from the O–CN simulations driven with NCAR (TM5) nitrogen deposition fields. BNF, biological nitrogen fixation; GPP, gross primary production; NBP, net biome production = net ecosystem production − anthropogenic C losses.
| Africa | Asia | Europe | N. America | S. America | Oceania | global | |
|---|---|---|---|---|---|---|---|
| area (106 km2) | 29.8 | 40.6 | 9.7 | 24.0 | 17.9 | 8.1 | 133.6 |
| nitrogen inputs (Tg N yr−1) | |||||||
| BNF | 26.9 | 20.5 | 4.3 | 11.6 | 29.9 | 5.2 | 98.7 (99.1) |
| deposition | 9.7 | 27.4 | 7.5 | 9.1 | 5.9 | 1.2 | 61.9 (55.6) |
| fertilizer | 7.2 | 53.9 | 13.6 | 17.9 | 4.9 | 1.4 | 99.7 (99.7) |
| nitrogen losses (Tg N yr−1) | |||||||
| leaching loss | 16.1 | 37.2 | 7.2 | 14.2 | 17.4 | 3.6 | 97.1 (92.8) |
| volatilization (NH3 + NOx + N2O + N2) | 26.0 | 47.0 | 12.4 | 16.9 | 17.8 | 4.4 | 124.9 (123.8) |
| soil N2O emission | 2.3 | 2.8 | 0.5 | 1.0 | 1.7 | 0.4 | 8.7 (8.7) |
| soil N2 emission | 20.7 | 41.4 | 10.9 | 15.0 | 14.0 | 3.6 | 105.8 (104.9) |
| N export | 2.5 | 5.4 | 2.8 | 2.3 | 1.8 | 0.2 | 15.0 (15.0) |
| nitrogen balance (Tg N yr−1) | |||||||
| plant uptake | 161.5 | 205.9 | 95.5 | 113.8 | 201.1 | 21.4 | 800.1 (792.2) |
| net ecosystem N storage | 0.6 | 13.3 | 3.3 | 5.6 | 4.0 | 0.1 | 27.1 (26.8) |
| nitrogen pools (Pg N) | |||||||
| vegetation N | 0.7 | 0.9 | 0.3 | 0.4 | 1.1 | 0.1 | 3.5 (3.4) |
| litter and soil N | 13.2 | 35.7 | 16.8 | 21.7 | 13.7 | 2.6 | 103.9 (102.2) |
| soil mineral N (10−3) | 14.8 | 35.5 | 10.8 | 15.8 | 9.8 | 3.1 | 90.8 (85.5) |
| carbon fluxes (Pg C yr−1) | |||||||
| GPP | 25.6 | 29.9 | 11.4 | 16.8 | 34.9 | 3.6 | 122.3 (121.6) |
| NBP | 0.1 | 0.4 | 0.4 | 0.5 | 0.5 | 0.1 | 1.9 (1.9) |
| carbon pools (Pg C) | |||||||
| vegetation C | 114.5 | 145.8 | 42.1 | 75.8 | 187.3 | 12.4 | 581.4 (578.1) |
| litter and soil C | 169.2 | 431.2 | 196.6 | 270.3 | 174.5 | 37.5 | 1287.6 (1268.2) |
(i). Nitrogen budget
During 2001–2010, direct (Nr additions) or indirect (land-use change, climate change and increase in atmospheric CO2) anthropogenic factors are responsible for 0.5 Pg N of the nitrogen stored in vegetation (15% of the global total), and for 1.6 Pg N (2%) stored in soils and litter (excluding wetlands and permafrost soils; figure 2). The most significant cause for vegetation N changes prior to the 1960s has been forest clearance, which has only partly been compensated by increased sequestration owing to CO2 fertilization. Anthropogenic Nr plays an increasing role after 1960, but remains only a modest cause of additional N stored in vegetation compared with the other drivers. Conversely, anthropogenic Nr substantially increases soil organic N—partly by decreasing the soil C : N—contributing thereby the largest share of the significant increase in soil N, whereas the effects of climatic, atmospheric CO2 and land-use changes on soil N storage largely cancel out.
Figure 2.
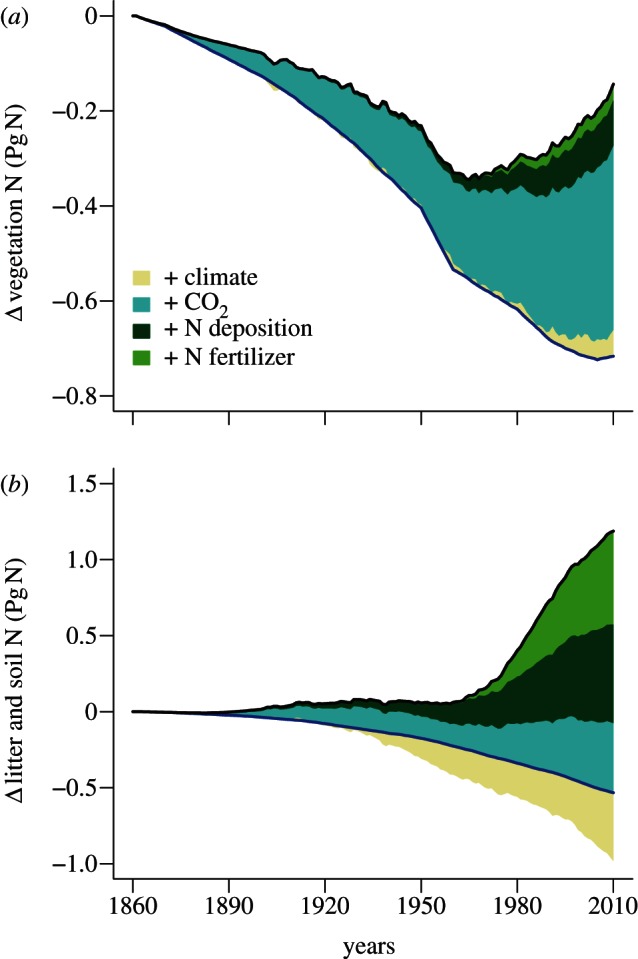
Estimated development of the terrestrial nitrogen stores in (a) vegetation and (b) litter and soil organic matter. The blue line marks the changes from land-use change alone, the shaded areas indicate the changes due to individual driving forces and the black line denotes the total change of the respective nitrogen pool.
The average 2001–2010 rate of terrestrial nitrogen sequestration (27 Tg N yr−1, figure 3) is a very small fraction of annual global terrestrial nitrogen turnover (about 800 Tg N yr−1). This estimate is somewhat smaller than the 60 Tg N yr−1 estimate by Galloway et al. [3] for the 1990s. However, Galloway did not separate sequestration from N exports owing to land use and land cover change (15 Tg N yr−1), which compares well with the export number simulated by the ISAM-CN terrestrial biosphere model (15.6 Tg N yr−1; [19]). The contribution of global Nr deposition to the 2001–2010 N sequestration is 10 Tg N yr−1 (figure 3), which is very close to the estimate of 9 Tg N yr−1 by Schlesinger [20], based on the assumption that 50 per cent of the deposited N over forests would be sequestered. In O–CN, there is a large spatial gradient with close to 100 per cent retention in nutrient poor boreal systems and nearly no retention in nitrogen-saturated tropical and temperate ecosystems. Nitrogen retention is estimated to have declined globally from about 50 per cent in 1860 to 30 per cent at present. The estimated retention rate peaked in the 1980s with about 16 Tg N yr−1, and remained high until the early 1990s when N deposition started to decline regionally (e.g. in Central Europe), and highly polluted ecosystems reached saturation. O–CN predicts gradually increasing terrestrial N losses since the 1950s, stagnating at year 2000 levels as a consequence of the estimate of declining global nitrogen deposition in 2001–2010 simulated by NCAR-CTM. This is a significant difference to cropland ecosystems, which show a modest and stable N sequestration rate since the 1980s, but strongly increasing leaching and volatilization losses with increasing fertilizer consumption.
Figure 3.
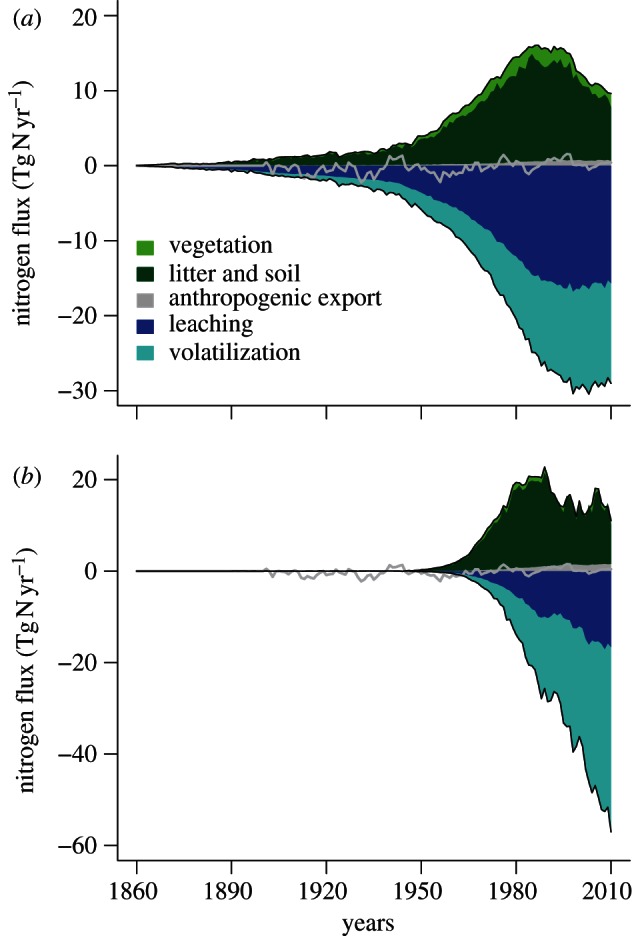
Estimated development of the terrestrial nitrogen balance due to (a) anthropogenic Nr deposition and (b) fertilizer application. The grey line marks the changes in the net balance induced by changes in atmospheric CO2 abundance and climate.
(ii). Nitrogen–carbon couplings
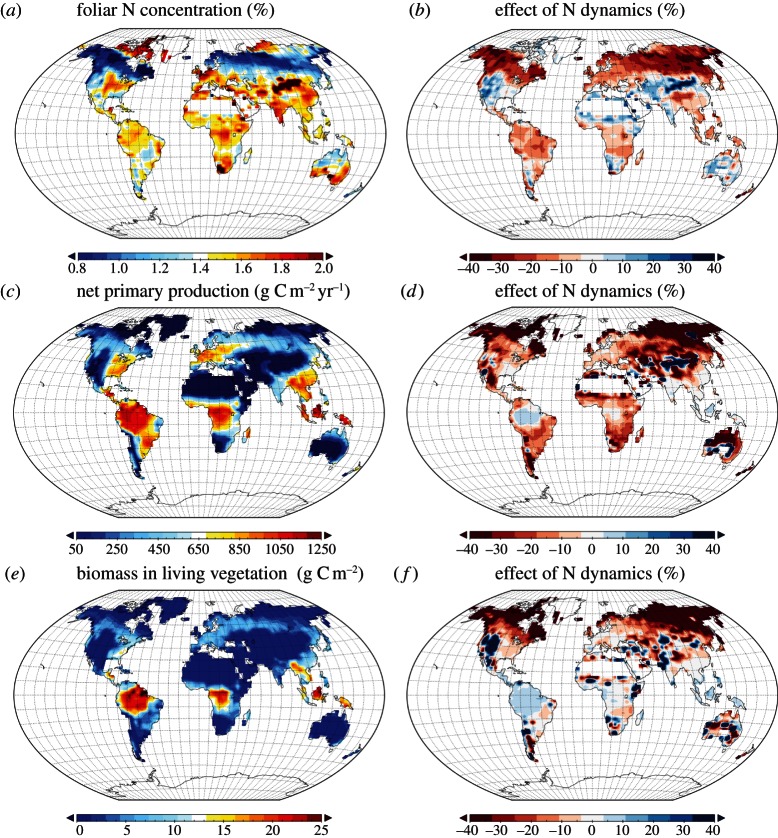
The spatial pattern of N availability shows a strong latitudinal gradient, which is regionally dominated by the signature of the human Nr perturbation due to deposition and fertilizers. Figure 4 displays the resulting patterns of contemporary N limitation of vegetation growth and carbon storage, which follows closely the pattern of N availability: the naturally high N limitation in the boreal and temperate zone due to low natural N fixation is regionally dominated by anthropogenic Nr inputs. This regional pattern is consistent with current understanding [2,11], but difficult to evaluate quantitatively because of the lack of suitable observations. Hyper-spectral remote sensing might be one way forward, as it provides a direct measure of chlorophyll. However, a range of complicating factors in interpreting these data hamper their application at present [21].
Figure 4.
Average estimates and effect of nitrogen dynamics on (a,b) foliar nitrogen concentration, (c,d) net primary production and (e,f) living biomass for the years 2001–2010, as simulated by O–CN. The effect of N dynamics are expressed as per cent deviation between the O–CN and O–C model estimates.
Nitrogen additions to the terrestrial biosphere have increased global productivity by an estimated 2.6 Pg C yr−1, which corresponds to 2 per cent of the global annual total production and 12 per cent of the increase since pre-industrial times (table 2). About 0.2 Pg C yr−1 of this increased production is sequestered in the terrestrial biosphere, corresponding to 10–20 per cent of the global net land carbon uptake (table 2). Earlier studies based on simple biogeochemical models and upscaling of field-based carbon-sequestration estimates have estimated C sequestration based on N deposition estimates as 0.4–0.7 Pg C yr−1 in 1990 [5,22]. The estimate of the process-based O–CN model applied here is somewhat lower, but within the range of model simulations with the current generation of carbon–nitrogen cycle models (0.2–0.6 Tg N yr−1; [7]). Over the period 1860–2010, 1.3 Pg N of the added anthropogenic nitrogen caused an increase of the terrestrial C stocks by 11.3 Pg C (table 2). The tight stoichiometry of this new material results from the large share of N sequestration in soils with a low C : N ratio as well as increases in tissue and soil N concentrations. The anthropogenic Nr additions thus enrich the biosphere with nitrogen relative to carbon. This is a striking difference to the consequences CO2 fertilization, which leads to the sequestration of 135 Pg C, but only 1.2 Pg N, predominantly in vegetation.
Table 2.
Attribution of the changes in the global nitrogen budget from 1860 to 2010 due to changes in land cover and land-use (‘LUCC’), increased atmospheric CO2 abundance (‘CO2’), climatic variability and changes (‘climate’), anthropogenic reactive nitrogen additions (‘deposition’) and industrial fertilizer application (‘fertilizer’). Note that this analysis does not take account of manure additions. Land-use emissions in 2000–2010 are an underestimate, as the dataset for land-use changes stops in 2005 [13]. Values are reported from simulations driven with the NCAR (TM5) nitrogen deposition fields. GPP, Gross primary production; NBP, net biome production = net ecosystem production – anthropogenic C losses.
| 1860s | 2000s | LUCC | CO2 | climate | deposition | fertilizer | |
|---|---|---|---|---|---|---|---|
| fluxes (Tg N yr−1; Pg C yr−1) | |||||||
| natural N input | 112.4 (112.7) | 98.7 (99.0) | −10.0 | 3.1 | 0.1 | −2.5 (−2.3) | −0.1 |
| anthropogenic N input | 28.5 (22.5) | 161.6 (155.2) | 0 | 0 | 0 | 42.1 (44.0) | 85.6 |
| ecosystem N lossesa | 148.2 (144.4) | 237.1 (231.6) | 19.2 | −18.9 | 11.4 | 29.9 (31.2) | 50.0 |
| soil NOx emission | 6.5 (6.3) | 8.5 (8.4) | 0.1 | −0.2 | 0.8 | 0.7 (0.7) | 0.7 |
| soil N2O emission | 6.0 (5.8) | 8.7 (8.6) | 0.3 | −0.6 | 0.8 | 0.8 (0.8) | 1.5 |
| GPP | 99.9 (100.0) | 122.3 (121.6) | −0.4 | 17.1 | 2.9 | 1.66 (1.81) | 0.93 |
| NBP 1990s | 1.3 (1.3) | −0.8 | 2.0 | 0.0 | 0.20 (0.21) | 0.07 | |
| NBP 2000s | 1.9 (1.9) | −0.4 | 2.4 | −0.2 | 0.18 (0.17) | 0.06 | |
| pools (Pg N; Pg C) | |||||||
| vegetation N | 3.4 (3.6) | 3.4 (3.4) | −0.71 | 0.36 | 0.04 | 0.09 (0.10) | 0.03 |
| vegetation C | 615.7 (607.2) | 581.4 (578.1) | −141.3 | 98.6 | 3.8 | 2.7 (2.7) | 0.08 |
| soils N | 103.2 (101.5) | 103.9 (102.2) | −0.50 | 0.83 | −0.39 | 0.61 (0.60) | 0.56 |
| litter and soil C | 1312.3 (1291.3) | 1287.6 (1268.2) | −52.1 | 35.9 | −9.1 | 6.1 (5.8) | 2.4 |
aLeaching + volatilization (NH3 + NOx + N2O + N2).
The additional carbon sequestration due to anthropogenic Nr additions has a perceivable but small cooling effect for the climate system, as it reduces the rate of atmospheric CO2 accumulation due to fossil-fuel burning. The nitrogen–carbon cycle interactions have further climate-relevant consequences, as increased plant N uptake due to CO2 fertilization reduces nitrogen losses globally, including the terrestrial N2O emissions from soils (table 2). This counteracts the strong simulated increase in terrestrial N2O emissions due to recent climate changes (0.8 Tg N yr−1; corresponding to an increase of 13% relative to pre-industrial conditions). Warmer temperatures will enhance nitrogen cycling and probably also N2O production where N is not limiting [23]. However, there is mixed empirical evidence from ecosystem warming experiments, which show varying responses of soil N2O emissions, resulting from the concurrent effects of changes in the moisture regime, plant and microbial N demand and biodiversity [24–27]. In agreement with earlier studies [5,6], the dominant cause for the estimated increase in terrestrial N2O emissions is anthropogenic Nr inputs (table 2), which reduce or even overcompensate the climatic benefits from carbon sequestration in response to anthropogenic Nr inputs. There have also been slight increases in NOx emissions from natural and fertilized soils (table 2), with as yet unquantified effects on the climate system. However, this anthropogenic soil NOx source remains small compared with the anthropogenic NOx from combustion sources, which globally has a strongly negative effect on the climate forcing [28]. While these changes all matter to the climate system, the net effect of anthropogenic Nr on the climate system is still unknown [29].
(b). Future projections of coupled nitrogen–carbon cycle dynamics
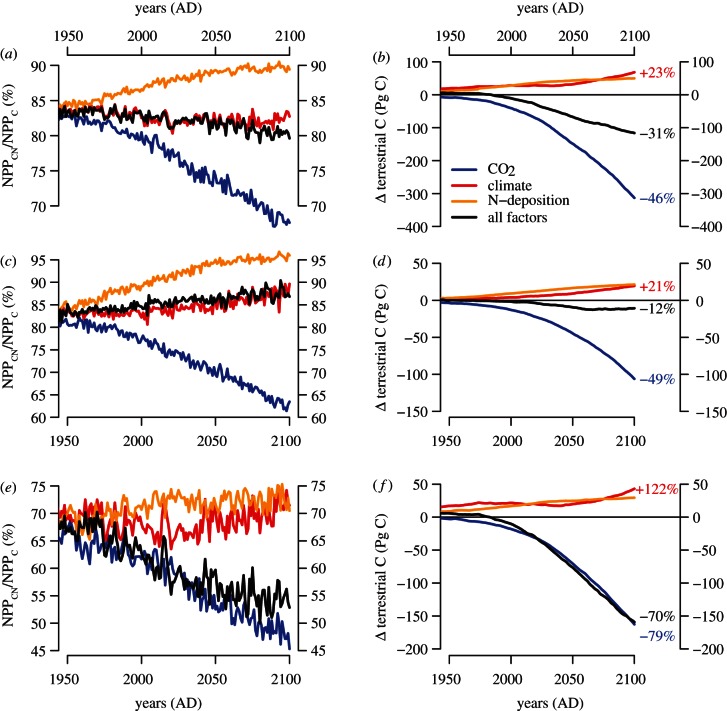
Figure 5 illustrates the development of nitrogen limitation on terrestrial plant production and carbon sequestration between 1950 and 2100, based on projections with the SRES A″ scenario. Increasing atmospheric CO2 enhances plant productivity and, therefore, N demand, which increases nitrogen limitation, as the higher demand cannot be completely met by reduced nitrogen losses, increasing N deposition or biological N fixation. This additional limitation is most pronounced in the boreal zone, where N constraints attenuate the direct CO2 fertilization effect on plant production by more than 50 per cent and on carbon sequestration by nearly 80 per cent (in the year 2100) relative to a projection not taking N limitation explicitly into account (figure 5e,f). These projections are broadly consistent with the importance of a nitrogen constraint in free-air CO2 enrichment experiments [30,31], and the simulated geographical distribution of nitrogen limitation (figure 4).
Figure 5.
Estimated reduction in (a,c,e) terrestrial net primary production and (b,d,f) terrestrial carbon sequestration due to explicitly accounting for N dynamics under the SRES A2 scenario, expressed as difference between the O–CN and O–C simulations. (a,b) The global total, (c,d) the temperate and (e,f) the boreal latitudinal bands. The percentage numbers in (b,d,f) refer to the relative difference between the model simulations with and without N dynamics. Blue lines, CO2; red lines, climate; yellow lines, N-deposition; black lines, all factors.
Consistent with observational evidence from a temperate forest soil warming study [32], warming increases carbon sequestration because of the remineralization of nitrogen from soil, which fertilizes the vegetation and thus increases accumulation of biomass. This climate effect is simulated to have increased productivity during most of the twenty-first century in the boreal and temperate zones, but the global effect is rather small because of opposite trends in tropical regions related to increased respiration costs. The same processes operate in two other global modelling studies [33,34]. However, these two studies suggest a stronger positive effect from climate change, such that in these studies the total carbon balance in the year 2100 is changed from a negative carbon balance to a positive carbon balance owing to the considerations of nitrogen–carbon cycle interactions.
Nitrogen deposition is estimated here to play only a small role in future carbon uptake (figure 5), as also reported by a simulation with the CLM4 terrestrial biosphere model [33]. The C sequestration resulting from anthropogenic Nr deposition (27 Pg C, sequestering also 3.9 Pg N) is slightly less than the sequestration resulting from climate change induced remineralization of soil N and enhanced vegetation growth (44 Pg C, recapturing 1.2 Pg N). A thorough analysis of the effects of future Nr deposition is still lacking. However, given these results, Nr deposition needs to be a component of future global carbon-cycle projections.
N limitation of CO2 fertilization dominates the estimated long-term trend of terrestrial carbon sequestration at all latitudinal bands (figure 5), consistent with two other independent modelling studies [33,34]. The dominance of the reduced CO2 fertilization due to N limitation has important consequences for projections of future climate changes with interactive biogeochemical cycling: neglecting an explicit treatment of N dynamics in coupled carbon-cycle climate modelling studies such as the Coupled Climate–Carbon Cycle Model Intercomparison Project (C4MIP) [35] will lead to an underestimation of the build-up of fossil CO2 in the atmosphere [7]. For the O–CN model and the SRES A2 scenario, nitrogen dynamics reduce global carbon sequestration between 1860 and 2100 by 164 Pg C (358 Pg C for the CO2 fertilization effect only), because of a regional nitrogen deficit of 5.7 (12.0) Pg N. Depending on whether the radiative forcing in Earth system models is prescribed (RCP-type forcing) or calculated based on the greenhouse gas and aerosol burden of the atmosphere, neglecting nitrogen–carbon cycle interactions will lead to an underestimation of the need for emission reductions of carbon-sequestration efforts to meet a certain radiative forcing pathway, or the rate of climate change, respectively. Remineralization of nitrogen due to accelerated soil organic matter turnover, and deposition of reactive nitrogen deposition are not strong enough to counteract this phenomenon, even though they lead to increased carbon sequestration.
The future changes in the terrestrial nitrogen and carbon balance also induce changes in NOx and N2O emissions from soils. O–CN suggests a change of +3.1 (−0.8) Tg N yr−1 from pre-industrial to 2100 soil N2O emissions due to climate change (CO2 fertilization), with similar changes occurring also for the terrestrial soil NOx source. This results implies a positive terrestrial N2O-climate feedback of 0.54 Tg N yr−1 K−1, which would be weakened by a smaller negative carbon-concentration–N2O feedback. However, one should place limited confidence in this estimate from one model and one scenario. A feedback of this magnitude would be important enough to require further consideration in coupled biogeochemistry–climate models, even though the biospheric feedback might, as with anthropogenic CO2, be small compared with future anthropogenic emissions of N2O from managed ecosystems [36].
4. Discussion
This study provides an advance over previous assessments [3,20], as it relies on a process-based ecosystem model that integrates the key carbon–nitrogen cycle interactions and their coupling to biogeophysical processes, while considering the impacts of atmospheric (climate, CO2) and land cover changes. Tables 1 and 2 provide an assessment of the uncertainties related to estimates in nitrogen deposition, and show that the simulated trends and spatial patterns are reasonably robust against these uncertainties. Regionally important ecosystem types (e.g. wetland and peatland ecosystems [37]), land management characteristics (such as nitrogen efficient farming, manure-based agriculture [38]) and effects of Nr-related air pollution (such as tropospheric ozone [39]) have been neglected, because they cannot be simulated by the current version of O–CN, but they might nonetheless be globally significant.
The increased complexity of the analyses introduces new uncertainties. While the simulated trends are considered robust, other carbon–nitrogen cycle models may give notably differing estimates. Key uncertainties in the modelling include: (i) the response of canopy-level photosynthesis to nitrogen additions; (ii) changes in the allocation patterns (root : shoot ratio); (iii) the competition of plants and soil microbes for the added (or reduced) amount of nitrogen, and therefore the temporal dynamics of the fate of the added N; (iv) the change of ecosystem stoichiometry over time; (v) responses of and controls on biological N fixation; and (iv) the fraction of N that is exported from ecosystems. Evaluation against ecosystem manipulation experiments, which were part of the O–CN model evaluation [8,10], help to understand whether the model's sensitivities to perturbations are adequate. However, the interpretation of these experiments is complex, and their regional representativity unclear, such that, whereas O–CN's sensitivities appear reasonable, large uncertainties remain in the modelled responses, requiring further assessments.
Another factor omitted in this assessment is the co-limitation of the terrestrial nitrogen and carbon cycles by phosphorus. Plants have evolved strategies to access soil P using phosphatase exudation, such that P limitation mainly occurs on old, deeply weathered and P-deprived soils [40]. The results presented here are consistent with the hypothesis [41,42] that temperate and boreal ecosystems are limited by N, whereas moist tropics are not. Given that most of the anthropogenic perturbation of the nitrogen cycle so far occurred in predominantly N-limited regions, it is unlikely that the analyses of the fate of anthropogenic N and its consequences for the carbon cycle would be dramatically altered when accounting for the phosphorus cycle. However, future projections of the global carbon cycle will be different in regions where P limitation prevails.
5. Concluding remarks
The estimates presented in this study result from a state-of-the-art terrestrial biosphere model integrating biophysical, biogeochemical and ecological process understanding. There is considerable uncertainty in any such model and a systematic assessment of nitrogen–carbon cycle interactions by an ensemble of such models seems the logical next step to take. Nonetheless, some conclusions appear robust:
— anthropogenic Nr additions currently enhance nitrogen and carbon sequestration in the biosphere (figure 1), but cause at the same time increased emissions of NOx and N2O from soils. Each of the factors is large enough to matter to the climate system, but the net climatic effect is still uncertain;
— nitrogen is limiting terrestrial productivity in many ecosystems, and therefore the capacity of the terrestrial biosphere to sequester carbon in response to increased atmospheric abundance of CO2;
— regional and global strategies for increasing terrestrial carbon storage in either woody biomass or soils need to consider the consequences for nutrient cycling and anticipate the effects of nutrient limitation when discussing the effectiveness of different measures; and
— future projections of the global carbon cycle will underestimate the fraction of anthropogenic fossil-fuel-based CO2 emissions remaining in the atmosphere, unless nitrogen dynamics are taken into account. Because of the tight coupling of the terrestrial nitrogen and carbon cycles and their interactions with climate, nitrogen dynamics need to be accounted for interactively in the next generation of Earth system models designed for long-term studies of biogeochemical–climate interactions.
Acknowledgements
S.Z. was supported by the Marie Curie Reintegration Grant JULIA (PERG02-GA-2007-224775) and the European Community's Seventh Framework Programme under the GREENCYLCES II ITN (grant agreement no. 238366) and the ÉCLAIRE project (grant agreement no. 282910).
References
- 1.Vitousek PM, Howarth RW. 1991. Nitrogen limitation on land and in the sea—how it can occur. Biogeochemistry 13, 87–115 10.1007/BF00002772 (doi:10.1007/BF00002772) [DOI] [Google Scholar]
- 2.Kattge J, et al. 2011. TRY—a global database of plant traits. Glob. Change Biol. 17, 2905–2935 10.1111/j.365-2486.011.02451.x (doi:10.1111/j.365-2486.011.02451.x) [DOI] [Google Scholar]
- 3.Galloway JN, et al. 2004. Nitrogen cycles: past, present and future. Biogeochemistry 70, 153–226 10.1007/s10533-004-0370-0 (doi:10.1007/s10533-004-0370-0) [DOI] [Google Scholar]
- 4.Gruber N, Galloway JN. 2008. An Earth-system perspective of the global nitrogen cycle. Nature 451, 293–296 10.1038/nature06592 (doi:10.1038/nature06592) [DOI] [PubMed] [Google Scholar]
- 5.Liu LL, Greaver TL. 2009. A review of nitrogen enrichment effects on three biogenic GHGs: the CO2 sink may be largely offset by stimulated N2O and CH4 emission. Ecol. Lett. 12, 1103–1117 10.1111/j.1461-0248.2009.01351.x (doi:10.1111/j.1461-0248.2009.01351.x) [DOI] [PubMed] [Google Scholar]
- 6.Zaehle S, Ciais P, Friend AD, Prieur V. 2011. Carbon benefits of anthropogenic reactive nitrogen offset by nitrous oxide emissions. Nat. Geosci. 4, 601–605 10.1038/NGEO1207 (doi:10.1038/NGEO1207) [DOI] [Google Scholar]
- 7.Zaehle S, Dalmonech D. 2011. Carbon–nitrogen interactions on land at global scales: current understanding in modelling climate biosphere feedbacks. Curr. Opin. Environ. Sustain. 3, 311–320 10.1016/j.cosust.2011.08.008 (doi:10.1016/j.cosust.2011.08.008) [DOI] [Google Scholar]
- 8.Zaehle S, Friend AD. 2010. Carbon and nitrogen cycle dynamics in the O–CN land surface model. I. Model description, site-scale evaluation and sensitivity to parameter estimates. Glob. Biogeochem. Cycles 24, GB100. 10.29/2009GB003521 (doi:10.29/2009GB003521) [DOI] [Google Scholar]
- 9.Krinner G, Viovy N, de Noblet-Ducoudre N, Ogee J, Polcher J, Friedlingstein P, Ciais P, Sitch S, Prentice IC. 2005. A dynamic global vegetation model for studies of the coupled atmosphere-biosphere system. Glob. Biogeochem. Cycles 19, GB1015. 10.1029/2003GB002199 (doi:10.1029/2003GB002199) [DOI] [Google Scholar]
- 10.Zaehle S, Friedlingstein P, Friend A. 2010. Terrestrial nitrogen feedbacks may accelerate future climate change. Geophys. Res. Lett. 37, L01401. 10.1029/2009GL041345 (doi:10.1029/2009GL041345) [DOI] [Google Scholar]
- 11.Zaehle S, Friend AD, Dentener F, Friedlingstein P, Peylin P, Schulz M. 2010. Carbon and nitrogen cycle dynamics in the O–CN land surface model. II. The role of the nitrogen cycle in the historical terrestrial carbon balance. Glob. Biogeochem. Cycles 24, GB1006. 10.29/2009GB003522 (doi:10.29/2009GB003522) [DOI] [Google Scholar]
- 12.Viovy N. 2011. CRU-NCEPv4. CRUNCEP dataset. See http://dods.extra.cea.fr/data/p529viov/cruncep/readme.htm.
- 13.Hurtt GC, et al. 2006. The underpinnings of land-use history: three centuries of global gridded land-use transitions, wood-harvest activity, and resulting secondary lands. Glob. Change Biol. 12, 1208–1229 10.1111/j.1365-2486.2006.01150.x (doi:10.1111/j.1365-2486.2006.01150.x) [DOI] [Google Scholar]
- 14.Cleveland CC, et al. 1999. Global patterns of terrestrial biological nitrogen (N-2) fixation in natural ecosystems. Glob. Biogeochem. Cycles 13, 623–645 10.1029/1999GB900014 (doi:10.1029/1999GB900014) [DOI] [Google Scholar]
- 15.Dentener F, et al. 2006. Nitrogen and sulfur deposition on regional and global scales: a multimodel evaluation. Glob. Biogeochem. Cycles 20, GB4003. 10.1029/2005GB002672 (doi:10.1029/2005GB002672) [DOI] [Google Scholar]
- 16.Lamarque J-F, et al. 2010. Historical (1850–2000) gridded anthropogenic and biomass burning emissions of reactive gases and aerosols: methodology and application. Atmos. Chem. Phys. 10, 7017–7039 10.5194/acp-10-7017-2010 (doi:10.5194/acp-10-7017-2010) [DOI] [Google Scholar]
- 17.Marti O, et al. 2005. The new IPSL climate system model: IPSL-CM4. Paris, France: IPSL [Google Scholar]
- 18.van Vuuren DP, Bouwman LF, Smith SJ, Dentener F. 2011. Global projections for anthropogenic reactive nitrogen emissions to the atmosphere: an assessment of scenarios in the scientific literature. Curr. Opin. Environ. Sustain. 3, 359–369 10.1016/j.cosust.2011.08.014 (doi:10.1016/j.cosust.2011.08.014) [DOI] [Google Scholar]
- 19.Jain A, Yang X, Kheshgi H, McGuire AD, Post W, Kicklighter D. 2009. Nitrogen attenuation of terrestrial carbon cycle response to global environmental factors. Glob. Biogeochem. Cycles 23, GB4028. 10.1029/2009GB003519 (doi:10.1029/2009GB003519) [DOI] [Google Scholar]
- 20.Schlesinger WH. 2009. On the fate of anthropogenic nitrogen. Proc. Natl Acad. Sci. USA 106, 203–208 10.1073/pnas.0810193105 (doi:10.1073/pnas.0810193105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ollinger S, Frolking S, Richardson A, Martin M, Hollinger D, Reich P, Plourde L. 2009. Nitrogen-albedo relationship in forests remains robust and thought-provoking reply. Proc. Natl Acad. Sci. USA 106, E172. 10.1073/pnas.0900137106 (doi:10.1073/pnas.0900137106) [DOI] [Google Scholar]
- 22.Townsend A, Braswell BH, Holland E, Penner J. 1996. Spatial and temporal patterns in potential terrestrial carbon storage due to deposition of fossil fuel derived nitrogen. Ecol. Appl. 6, 806–814 10.2307/2269486 (doi:10.2307/2269486) [DOI] [Google Scholar]
- 23.Butterbach-Bahl K, Dannenmann M. 2011. Denitrification and associated soil N2O emissions due to agricultural activities in a changing climate. Curr. Opin. Environ. Sustain. 3, 389–395 10.1016/j.cosust.2011.08.004 (doi:10.1016/j.cosust.2011.08.004) [DOI] [Google Scholar]
- 24.Reich PB, Hobbie SE, Lee T, Ellsworth DS, West JB, Tilman D, Knops JM, Naeem S, Trost J. 2006. Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440, 922–925 10.1038/nature04486 (doi:10.1038/nature04486) [DOI] [PubMed] [Google Scholar]
- 25.van Groenigen KJ, Osenberg C, Hungate B. 2011. Increased soil emissions of potent greenhouse gases under increased atmospheric CO2. Nature 475, 214–216 10.1038/nature10176 (doi:10.1038/nature10176) [DOI] [PubMed] [Google Scholar]
- 26.Kammann C, Müller C, Grünhage L, Jäger H-J. 2008. Elevated CO2 stimulates N2O emissions in permanent grassland. Soil Biol. Biochem. 40, 2194–2205 10.1016/j.soilbio.2008.04.012 (doi:10.1016/j.soilbio.2008.04.012) [DOI] [Google Scholar]
- 27.Barnard R, Leadley PW, Hungate BA. 2005. Global change, nitrification, and denitrification: a review. Glob. Biogeochem. Cycles 19, GB1007. 10.1029/2004GB002282 (doi:10.1029/2004GB002282) [DOI] [Google Scholar]
- 28.Shindell DT, Faluvegi G, Koch DM, Schmidt GA, Unger N, Bauer SE. 2009. Improved attribution of climate forcing to emissions. Science 326, 716–718 10.1126/science.1174760 (doi:10.1126/science.1174760) [DOI] [PubMed] [Google Scholar]
- 29.Butterbach-Bahl K, et al. 2011. Chapter 19. Nitrogen as a threat to the European greenhouse balance. In The European nitrogen assessment (eds Sutton MA, Howard CM, Erisman JW, Billen G, Bleeker A, Grennfelt P.). Cambridge, UK: Cambridge University Press [Google Scholar]
- 30.Norby RJ, Warren JM, Iversen CM, Medlyn BE, McMurtrie RE. 2009. CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proc. Natl Acad. Sci. USA 107, 19 368–19 373 10.1073/pnas.1006463107 (doi:10.1073/pnas.1006463107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finzi AC, et al. 2006. Progressive nitrogen limitation of ecosystem processes under elevated CO2 in a warm-temperate forest. Ecology 87, 15–25 10.1890/04-1748 (doi:10.1890/04-1748) [DOI] [PubMed] [Google Scholar]
- 32.Melillo JM, et al. 2011. Soil warming, carbon–nitrogen interactions, and forest carbon budgets. Proc. Natl Acad. Sci. USA 108, 9508–9512 10.1073/pnas.1018189108 (doi:10.1073/pnas.1018189108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thornton PE, et al. 2009. Carbon–nitrogen interactions regulate climate-carbon cycle feedbacks: results from an atmosphere–ocean general circulation model. Biogeosciences 6, 2099–2120 10.5194/bg-6-2099-2009 (doi:10.5194/bg-6-2099-2009) [DOI] [Google Scholar]
- 34.Sokolov AP, Kicklighter DW, Melillo JM, Felzer BS, Schlosser CA, Cronin TW. 2008. Consequences of considering carbon–nitrogen interactions on the feedbacks between climate and the terrestrial carbon cycle. J. Clim. 21, 3776–3796 10.1175/2008JCLI2038.1 (doi:10.1175/2008JCLI2038.1) [DOI] [Google Scholar]
- 35.Friedlingstein P, et al. 2006. Climate-carbon cycle feedback analysis: Results from the C4MIP model intercomparison. J. Clim. 19, 3337–3353 10.1175/JCLI3800.1 (doi:10.1175/JCLI3800.1) [DOI] [Google Scholar]
- 36.Meinshausen M, et al. 2011. The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Clim. Change 109, 213–241 10.1007/s10584-011-0156-z (doi:10.1007/s10584-011-0156-z) [DOI] [Google Scholar]
- 37.Bragazza L, et al. 2006. Atmospheric nitrogen deposition promotes carbon loss from peat bogs. Proc. Natl Acad. Sci. USA 103, 19 386–19 389 10.1073/pnas.0606629104 (doi:10.1073/pnas.0606629104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davidson EA. 2009. The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nat. Geosci. 2, 659–662 10.1038/ngeo608 (doi:10.1038/ngeo608) [DOI] [Google Scholar]
- 39.Felzer BS, Cronin TW, Melillo JM, Kicklighter DW, Schlosser CA. 2009. Importance of carbon–nitrogen interactions and ozone on ecosystem hydrology during the 21st century. J. Geophys. Res. Biogeosci. 114, G01020. 10.1029/2008JG000826 (doi:10.1029/2008JG000826) [DOI] [Google Scholar]
- 40.Treseder KK, Vitousek PM. 2001. Effects of soil nutrient availability on investment in acquisition of N and P in Hawaiian rain forests. Ecology 82, 946–954 10.1890/0012-9658(2001)082[0946:EOSNAO]2.0.CO;2 (doi:10.1890/0012-9658(2001)082[0946:EOSNAO]2.0.CO;2) [DOI] [Google Scholar]
- 41.Townsend AR, Cleveland CC, Houlton BZ, Alden CB, White JWC. 2011. Multi-element regulation of the tropical forest carbon cycle. Front. Ecol. Environ. 9, 9–17 10.1890/100047 (doi:10.1890/100047) [DOI] [Google Scholar]
- 42.Elser JJ, et al. 2007. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 10, 1135–1142 10.1111/j.1461-0248.2007.01113.x (doi:10.1111/j.1461-0248.2007.01113.x) [DOI] [PubMed] [Google Scholar]