Abstract
Nitric oxide (NO) is a reactive gas that plays an important role in atmospheric chemistry by influencing the production and destruction of ozone and thereby the oxidizing capacity of the atmosphere. NO also contributes by its oxidation products to the formation of acid rain. The major sources of NO in the atmosphere are anthropogenic emissions (from combustion of fossil fuels) and biogenic emission from soils. NO is both produced and consumed in soils as a result of biotic and abiotic processes. The main processes involved are microbial nitrification and denitrification, and chemodenitrification. Thus, the net result is complex and dependent on several factors such as nitrogen availability, organic matter content, oxygen status, soil moisture, pH and temperature. This paper reviews recent knowledge on processes forming NO in soils and the factors controlling its emission to the atmosphere. Schemes for simulating these processes are described, and the results are discussed with the purpose of scaling up to global emission.
Keywords: nitric oxide, emission, soil
1. Introduction
Exchange of trace gases between the terrestrial ecosystems and the atmosphere plays an important role in the regulation of atmospheric composition and climate. Therefore, there is a growing need for quantification of trace gas exchange and for mechanistic descriptions that can be used in models for prediction of future climate changes. Obviously, the greenhouse gases such as CO2, N2O and ozone (O3) are of prime interest, but also other gases such as volatile organic carbons and nitrogen oxides (NO and NO2), that are much more reactive in the atmosphere, are important [1].
Nitrogen oxides are an integral part of the Earth system and are constantly exchanged between the atmosphere and ecosystems. The nitrogen cycle has a pronounced effect on ecosystem productivity and affects climate in several ways. Both nitrous oxide (N2O) and nitric oxide (NO) are emitted from the soil, and whereas N2O is a strong greenhouse gas by itself, NO acts indirectly by contributing to the formation of O3, the third largest contributor to positive radiative forcing [2]. The deposition of N compounds to ecosystems has a fertilizing effect and might stimulate primary production and thus CO2 uptake from the atmosphere, whereas O3 is phytotoxic and thus will reduce primary production. Davidson & Kingerlee [3] estimated the amount of NO emitted from soils to the atmosphere globally to be between 13 and 21 Tg N yr–1, depending on the strength of the adsorption onto plant canopy surfaces.
NO reacts rapidly with O3 in the atmosphere and creates NO2; the sum of NO and NO2 is called NOx. The amount of NOx originating from soil emission is comparable in source strength with the anthropogenically emitted NOx from the burning of fossil fuels which amounts to 20–24 Tg N yr–1 [2]. NO emitted from the soil is a significant contributor to the NOx mixing ratio in the atmosphere, and NO may directly be involved in the production of OH, thus influencing the oxidizing capacity of the atmosphere [4,5]. The relative role of soil-emitted NO is highest in areas with the lowest anthropogenic emission, and in forested areas, a significant amount of the soil-emitted NO might be captured as NO2 by the canopy [6].
NO emission from soils has been studied in numerous experiments both in the field and in the laboratory, often together with N2O. The knowledge on rates of emission is thus quite comprehensive, but the knowledge on the mechanisms that control the emission still needs improvement [7–9]. The intention of this paper is to give a short overview of the current knowledge of NO emission from soils with emphasis on new knowledge gained during recent years.
2. Nitric oxide formation in soil
The production and consumption of NO in soil is a result of both microbial activity and chemical reactions. Many processes and micro-organisms are involved; the two most important groups of micro-organisms are nitrifiers and denitrifiers. Generally, both NO and N2O are produced by the same processes; however, the exact ratios of the two products are not yet clearly known. Recently, there has been a much stronger focus on N2O emission from soil rather than on NO, because N2O is a greenhouse gas by itself, whereas NO only indirectly contributes to global warming. However, generally processes producing N2O also produce NO.
The following overview of processes involved in NO production and consumption is mainly based on Conrad [10].
(a). Nitrification
NO and N2O are by-products of nitrification, the oxidation of ammonium to nitrate. The so-called nitrosobacteria that carry out the first part of this process require oxygen. The production mechanism for NO by these bacteria is unknown but might follow the sequential pathway in equation (2.1) [11]:
| 2.1 |
The nitrobacteria oxidize nitrite to nitrate, using oxygen as the terminal electron acceptor:
| 2.2 |
Under anoxic conditions, these bacteria reduce nitrate to nitrite and further to NO.
Nitrosobacteria and nitrobacteria are autotrophic. Heterotrophic nitrifiers also exist (both bacteria and fungi); they produce nitrate by either an inorganic or an organic pathway:
| 2.3 |
and
| 2.4 |
Many of the heterotrophic nitrifiers seem to denitrify under aerobic conditions. They might be especially important as producers of NO and N2O in forest soils.
(b). Denitrification
The process where nitrogen oxides are reduced by micro-organism (mainly bacteria but also fungi) to gaseous products is called denitrification. Both NO and N2O are produced as part of the process that reduces nitrate to the end product N2:
| 2.5 |
Denitrification can take place both under aerobic and anaerobic conditions, but it is believed that anaerobic denitrification is the most important for NO production in soil.
Wrage et al. [12] described a pathway where the oxidation of NH3 to NO−2 is followed by the reduction of NO−2 to N2O and N2. They called this pathway nitrifier denitrification (figure 1). The process is carried out by autotrophic NH3-oxidizers. Only nitrifiers carry out nitrifier denitrification, whereas both nitrifiers and denitrifiers are involved in coupled nitrification–denitrification. The quantitative importance of the nitrifier denitrification pathway is not yet certain, but Wrage et al. [12] estimate that it can be an important source of N2O (and NO) under certain circumstances, i.e. high N content, low organic C content, low O2 pressure and maybe also low pH. Isotope tracing has shown that the process can actually occur in soils, and may in fact be responsible for the greater proportion of total nitrifier-induced N2O production [13]. Recently, Kool et al. [14] studied a poor sandy soil and found that nitrifier denitrification could be a major contributor to N2O emission from the soil, when moisture conditions were suboptimal for denitrification. However, the study did not include analysis of NO emission. Baggs [15] has shown that it is possible to quantify some of the processes involved in NO and N2O production in soil by applying stable isotope enrichment techniques. However, the methods are still only usable in the laboratory, and further technical advances are needed to quantify the processes in natural systems.
Figure 1.
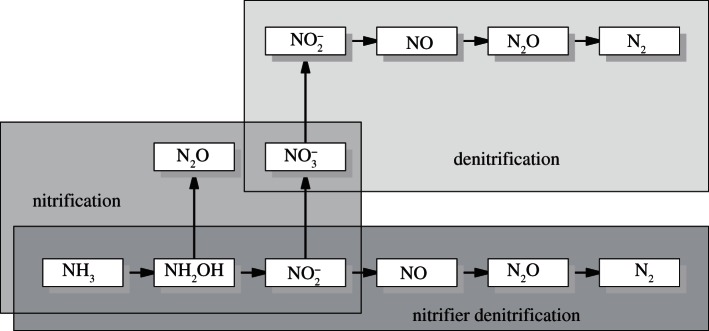
Transformations of mineral nitrogen in soil. The boxes for nitrification and nitrifier denitrification overlap, as nitrifier denitrification is a pathway of nitrification, but separate into the different branches from NO−2 onwards. Adapted from Wrage et al. [12].
New insights into the composition, density and activity of the denitrifying community have been gained from advanced molecular techniques [16]. Of special interest for the NO formation in soil is nitrite reductase, which catalyses the reduction of soluble nitrite into gaseous nitric oxide. Thus, for example, it has become possible to measure the abundance and structural community composition of the functional group involved in nitrite reduction by analysing the nirK gene coding for the enzyme nitrite reductase [17].
Other micro-organisms than the true denitrifiers can reduce nitrate and produce NO. The nitrate respirers produce nitrite as their end product, and other micro-organisms carry out dissimilatory reduction of nitrate to ammonium (DNRA). DNRA is an anaerobic process reducing nitrate via nitrite to ammonium. Thus, DNRA can play an important role in retaining N in the ecosystem. DNRA is of highest significance in wetland ecosystems, but can also be an important process in tropical forest soils with high clay content as well as in temperate grassland soils. The role of DNRA in aerobic soils is not yet clear [9].
(c). Chemodenitrification
The chemical decomposition of nitrite (chemodenitrification) can be important at low pH. Nitrite is normally only present in small amounts in soil and is mainly of microbial origin. Nitrite can be produced from ammonium (by nitrifying microbes) as well as from nitrate (by denitrifying microbes; figure 2). High concentrations of nitrite can be found in water films where H+ is also present due to microbial activity and can lead to NO production:
| 2.6 |
Figure 2.

Coupling of atmospheric HONO with soil nitrite. Red arrows represent the multiphase processes linking gaseous HONO and soil nitrite (acid–base reaction and phase partitioning), green arrows represent biological processes, orange arrows represent heterogeneous chemical reactions converting NO2 and HNO3 into HONO and blue arrows represent other related physico-chemical processes in the N cycle. Adapted from Su et al. [18]. (Reprinted with permission from AAAS).
If ferrous iron or other reduced metals are available, then nitrite can be reduced chemically to NO:
| 2.7 |
A further possible chemical reduction pathway for nitrite is by hydroxylamine. However, this process mainly produces N2O:
| 2.8 |
Recently, a new pathway for soil nitrite has been shown [18]:
| 2.9 |
The release of HONO from soil nitrite can be especially large from fertilized soils with low pH. The process is, however, reversible as also shown by Venterea et al. [19].
(d) Nitric oxide consumption
NO is highly reactive and may therefore easily be decomposed either chemically or biologically. Chemical oxidation plays a minor role in soil owing to the generally low concentrations of NO in the soil atmosphere. NO can be consumed by denitrifiers because they can use it as an electron acceptor in the step that produces N2O (equation (2.5)). This process is stimulated by anoxic conditions. Other micro-organisms in the soil are able to oxidize NO to N2O under oxic conditions.
3. Nitric oxide emission from soil
Firestone & Davidson [11] presented a conceptual model for microbiological and ecological factors controlling soil emissions of NO and N2O. The so-called hole-in-the-pipe (HIP) model [20] linked the two gases through their common processes of microbial production and consumption. Thus, the HIP model relates the sum of NO and N2O production to availability of nitrogen in the soil and to abiotic factors. The complex effects of soil moisture on gaseous N emission could be described by relating the ratio NO : N2O to soil moisture. The HIP model can be explained as nitrogen flowing through two leaky pipes, representing nitrification and denitrification. NO and N2O ‘leak’ out of the pipes, and the rate of leaking is determined by the soil moisture. Soil water both controls oxygen transport in the soil and transport of gaseous N out of the soil. In dry well-aerated soils, nitrification is the dominant process resulting in NO emission from the soil because it can diffuse out of the soil before being consumed. In wet soils, denitrification dominates and much of the NO is consumed before it can leave the soil; thus, N2O is the dominating gas emitted from the soil. In soils that are even more wet and mostly anaerobic N2O is reduced to N2 [20], which is subsequently emitted.
(a). Nitrogen availability
The most important factor for NO emission is the availability of N in the soil, mainly in the form of NO−3 and NH+4 as the sources for denitrification and nitrification. The pool sizes of NO−3 and NH+4 are a net result of N input to the ecosystem and N output and the processes involved. Basically, there are three major forms of nitrogen input: nitrogen fixation, nitrogen deposition and input as fertilizer/manure, and two forms of nitrogen output: gaseous emission and leaching. Leaching is mostly in the form of NO−3 and to a lesser extent dissolved organic nitrogen.
The transformation of atmospheric N2 to NH3 (nitrogen fixation) is an important pathway for nitrogen, because N2 normally cannot be directly used by organisms for their metabolism. Biological fixation can be carried out by certain symbiotic and heterotrophic bacteria in the soil. Fixation by leguminous symbiotic bacteria has an especially large potential and these bacteria can fix up to 200 kg N ha–1 yr–1, whereas fixation by heterotrophic bacteria is generally in the order of 1–5 kg N ha–1 yr–1 [9]. High NO emission has been observed from soils with legumes capable of symbiotic nitrogen fixation [21].
In most agricultural systems, nitrogen input as fertilizer (either natural in the form of livestock manure or as synthetic fertilizer) is the most important source of N in the soil. The mean national input to European agricultural soils varies from 42 in Portugal to 243 kg N ha–1 yr–1 in the Netherlands [22]. A rapid increase in NO emission is normally observed immediately after fertilizer application. The effect of fertilizer input on soil NO emission is very variable and ranges from 0.003 to 11 per cent with an average of approximately 0.5 per cent of the N applied as fertilizer [7].
Nitrogen can be deposited from the atmosphere as both wet deposition (dissolved in rain water) and dry deposition (as gases or particles). Deposition to open land with short vegetation is mainly in the form of wet deposition and amounts to 3–30 kg N ha–1 yr–1 over Europe. Forest canopies act as efficient sinks for gases and particles, and deposition to forest is therefore often twice as high as to open land (5–60 kg N ha–1 yr–1). The lowest deposition is found in northern Europe and the highest in central and western Europe with high industrialization and intensive agriculture [9].
Pilegaard et al. [23] found significant positive correlations between deposition of nitrogen to forests and NO emission. Similarly, elevated fluxes of NO were observed after N addition (+50 and +150 kg N ha–1 yr–1) to coniferous forest soils [24], but only the highest amount had an effect on deciduous forest soil. It was concluded that atmospheric deposition may result in increased NO emission from forest soils by promoting nitrification, and that the response may vary significantly between forest types under similar climatic regimes. However, increased nitrogen input does not always lead to enhanced microbial activity. Burton et al. [25] found that forest soil N availability was enhanced following simulated chronic NO−3 deposition, but after an initial increase, soil respiration was reduced, probably owing to reduced decomposition activity of the microbial community.
Measurements of nitrification potential have shown the highest correlation with NO (and N2O) emission [20], but other indexes such as the litterfall C : N ratio have also shown to be a reasonable proxy for nitrogen availability. However, because many diverse processes lead to NO formation in the soil, it will be difficult to identify a single index for the prediction of soil NO emission.
(b). Soil moisture
Soil water acts as a transport medium for NO−3 and NH+4 and influences the rate of O2 supply and thereby controls whether aerobic processes such as nitrification or anaerobic processes such as denitrification dominate within the soil. In general, nitrification dominates at values of water-filled pore space (WFPS) below 60 per cent and denitrification dominates at values above 60 per cent. Thus, at a WFPS of 60 per cent, the ratio of NO : N2O is often close to 1 (figure 3). Owing to limited substrate diffusion at very low water content and limited gas diffusion at high water content, maximum NO emission generally occurs at intermediate soil moisture. Because NO is highly reactive, it will more readily be consumed at high soil moisture where the residence time is longer.
Figure 3.
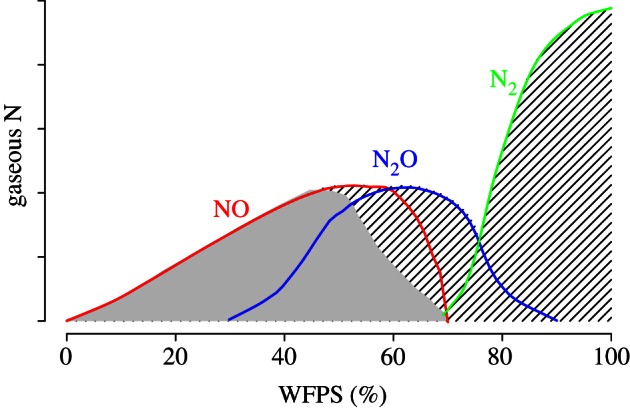
Proposed relative contributions of nitrification (solid grey shading) and denitrification (hatched shading) to gaseous N emissions as a function of water-filled pore space (WFPS). Adapted from Davidson et al. [20].
In a laboratory study with soil cores from European forest soils, Schindlbacher et al. [26] found that soil moisture and temperature explained 74 per cent of the variability in NO emission. NO was emitted from all the soils except from a boreal pine forest soil in Finland, where the laboratory experiment showed net NO consumption. NO emissions from a German spruce forest ranged from 1.3 to over 600 mg NO–N m–2 h–1 and greatly exceeded emissions from other soils. Average N2O emissions from this soil tended also to be the largest (170 ± 40 mg N2O-N m–2 h–1), but did not differ significantly from other soils. The optimal soil moisture for NO emission differed significantly between the soils, and ranged between 15 per cent WFPS in sandy Italian floodplain soil and 65 per cent in loamy Austrian beech forest soils (figure 4). Thus, each soil had its own optimum probably owing to differences in other soil characteristics.
Figure 4.
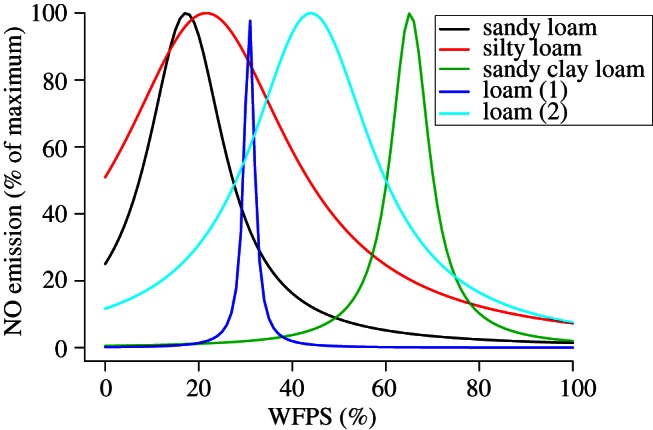
The relationship between NO emission and water-filled pore space (WFPS) from different forest soils in the NOFRETETE project (based on data in Schindlbacher et al. [26]).
Goldberg & Gebauer [27] studied the effect of soil moisture on NO emission by experimental induced drought (throughfall exclusion) in a Norway spruce forest followed by re-wetting. They found a maximum NO emission for this soil at 33 per cent WFPS in the organic layer (figure 5). They observed decreased NO emission during natural rain events, but a large NO emission after re-wetting of the soil following drought. The high emission following drought might be due to accumulation of nutrients during the drought period and re-activation of the micro-organisms immediately after re-wetting.
Figure 5.

Mean daily NO fluxes in throughfall exclusion and control plot dependent on the water-filled pore space in the organic layers (6 cm depth). (Reprinted from Goldberg & Gebauer [27] with permission from Elsevier.)
(c) Soil temperature
As long as other factors such as substrate availability and soil moisture are not limiting, NO emission increases with soil temperature owing to the positive effect of temperature on enzymatic processes and microbial turnover rates. Thus, temperature is not a primary control factor for the magnitude of soil emission, but rather a modulating factor of short-term variations [7]. In general, it has been found that NO emission increases exponentially with soil temperature [26] with Q101 values in the range of 2–3 [28,29]. Some soils, however, have much higher activation energies, resulting in Q10 of up to 10 which could be due to strong chemodenitrification [26].
Based on a 5 year dataset of continuous measurements of soil–atmosphere exchange of N2O, NO and CO2 at the temperate, nitrogen-saturated Norway spruce forest site Höglwald, Wu et al. [28] found that NO emissions were positively correlated with WFPS up to a soil temperature of 15°C, but at soil temperatures above 15°C, highest NO emissions were found at lowest WFPS values. The correlation of trace gas fluxes with soil temperature was stronger than that with soil moisture. However, soil moisture could become the crucial regulator for N2O emission during freeze/thaw periods. The effects of freeze/thaw events on NO emission were negligible, but an increased NO emission was observed following soil re-wetting after long drought periods. The NO : N2O ratio was determined by WFPS rather than by soil temperature. A significant positive correlation between soil temperature and NO : N2O ratio was observed only when WFPS was below 45 per cent. The highest NO : N2O ratio was found under conditions favourable to nitrification (soil temperature around 15°C; WFPS less than 40%). A recent analysis of soil flux measurements over the period 1994–2010 at the Höglwald site [30] confirmed these findings, specifying that NO fluxes were highest when the temperature was high (more than 15°C), but the soil moisture was in the range of 24–30 vol% (figure 6).
Figure 6.

Effects of soil moisture (10 cm depth) and temperature (5 cm depth) on monthly means of soil NO fluxes. Results from NO emission measurements in the Höglwald forest during 1994–2010 (adapted from Luo et al. [30]).
(d). pH
A high pH generally favours nitrification and thus NO emission. Decreasing pH values lead to increased NO production by denitrification, and chemodenitrification (NO production from NO−2) increases at a pH less than 4 [29]. Because different NO producing processes are favoured by different pH values, there is often no direct relationship between the amount of NO emitted and the pH.
(e). Vegetation
Vegetation type seems to have an influence on NO emission. Both the immediate vegetation cover of the soil and the ecosystem as such can influence the emission. Thus, moss cover seems to reduce NO emission [31]. This was confirmed by laboratory measurements of nitric oxide from a spruce forest soil by Bargsten et al. [32], who found the lowest emissions for soil samples taken under moss and grass and highest emissions from soil samples taken under blueberry and spruce.
In the NOFRETETE project [23] of nitrogen oxide emission from soils at 15 forest sites across Europe, it was found that coniferous forest soils had much higher NO emissions than deciduous forest soils. This is probably due to differences in soil and litter properties in the two types of forests. Thus, Finzi et al. [33] found large differences in C and N pools and in net N mineralization in the soil under six different tree species. Similarly, from studies in a species trial, Brüggemann et al. [34] found the highest rates of soil respiration, gross N mineralization and gross nitrification in the organic layer under spruce, followed by beech > larch > oak > pine. Nitrification and thus NO emission is favoured by a coniferous forest floor because of the aerobic conditions in the litter and the lower water content. Another explanation of the large difference in NO emission from spruce and beech forest might be that the filtering capacity of the much denser spruce forests is higher than that of beech forests and thus the throughfall carries a higher amount of deposited N from the atmosphere to the forest floor [35].
Once emitted from the soil, NO might react with O3 to form NO2 that can be taken up by the canopy and thus eventually return to the forest soil. This so-called canopy reduction is important for the estimation of NO emission to the free atmosphere, especially from forest soils. On the other hand, foliar uptake of NO2 might be an important source of N for the forest ecosystems [36].
Figure 7 gives an overview of the main factors controlling NO emission.
Figure 7.
Overview of main factors affecting NO emission from the soil: (a) linear response of NO emission versus N input or N availability, the slope is dependent on vegetation type and type of input (e.g. fertilizer application, wet deposition); (b) temperature sensitivity with a Q10 of approximately 2; (c) maximum NO emission at intermediate soil water content, moderated by soil type; (d) highest NO emission at acid conditions (chemodenitrification) and basic conditions (nitrification).
(f) Range of nitric oxide emission
Ludwig et al. [7] summarized a large number of soil NO emission measurements made in different ecosystems throughout the globe. The mean annual emission ranged from less than 0.01 to 30 kg N ha–1 yr–1. Generally, agricultural fields receiving fertilizer showed the highest emissions followed by grasslands, forests and other natural ecosystems. Some of the lowest values in forests are found in nitrogen-poor forest soils with low N input from the atmosphere such as the Hyytiälä forest in Finland, whereas the highest emission is found in nitrogen-saturated forests in areas with a high N deposition like Höglwald in Germany and Speulderbos in the Netherlands [23].
4. Modelling and upscaling
Owing to the many biotic and abiotic factors controlling soil emission of NO, there is a substantial uncertainty in the scaling up to regional and global estimates. Several attempts have been made to provide modelling schemes for this task. Skiba et al. [37] presented an approach based on a simple relationship of soil NO emissions as a factor of fertilizer-N input. The fraction emitted was set to 0.3 per cent of the added fertilizer N. To account for non-agricultural areas, two additional assumptions were made by Simpson et al. [38]: a background emission of 0.1 ng NO–N m–2 s–1, and the fraction of applied N released as NO was applied to all inputs of N whether as fertilizer, animal manure or as atmospheric deposition. This approach resulted in an estimate of an annual NO emission from European soils of 0.14–1.5 Tg N.
A slightly more complicated and widely used model is the one by Yienger & Levy [39]. This model takes both temperature and precipitation (as a proxy for soil moisture) into account:
 |
4.1 |
where fw/d is some function of temperature (constant, linear, exponential), Aw/d (biome) a coefficient to distinguish between biomes (11 classes), P (precipitation) a scalar function to adjust for pulses (wetting events, four different classes) and CR(LAI, SAI) a scalar reduction factor that accounts for canopy uptake of NOx. The annual estimate of soil emission for Europe was 0.74 and 5.5 Tg N yr–1 for the Globe with a range of 3.3–7.7 Tg N yr–1. The same algorithm, but with different database sources, was used by Ganzeveld et al. [6] who estimated the global soil emission to be 12 Tg N yr–1.
Stehfest & Bouwman [40] compiled information from several hundred measurements of NO emission from soils (189 from agricultural fields and 210 from soils under natural vegetation). Based on statistical models of the factors controlling NO emission (mainly vegetation type and soil C content), they estimated the NO emission from soils globally to be 1.8 Tg N yr–1 (1.4 Tg from fertilized cropland and 0.4 Tg from grassland). A similar statistical approach was used by Yan et al. [41] to estimate global NOx emission from soils based on organic carbon content, soil pH, land-cover type, climate, nitrogen input, soil temperature, soil moisture and vegetation fire. The annual NOx emission from global soils was calculated to be 7.43 Tg N, decreasing to 4.97 Tg N after canopy reduction. The fertilizer emission factor was calculated to be 1.16 per cent above soil and 0.70 per cent above canopy, which is somewhat higher than the factor applied by Skiba et al. [37] and Simpson et al. [38].
To estimate future climate effects on N2O and NO emissions from European forest soils, Kesik et al. [42] used the model PnET-N-DNDC (a combination of the photosynthesis-evapotranspiration model (PnET), the denitrification–decomposition (DNDC) model and a nitrification module). The model was tested against data from the forest soils in the NOFRETETE project [23] and combined with climate scenarios and GIS information. According to the scenario, mean annual surface temperatures in Europe in 2031–2039 will be approximately 1.8°C higher (from 7.6 to 9.4°C) compared with the period 1991–2000. The average precipitation over Europe is predicted to be almost unchanged but with large regional differences such as increases along the western coasts by up to 30 per cent, but decreases in many inland regions and especially in the Mediterranean area by up to 80 per cent. Using this scenario, the forest soil NO emissions were predicted to increase by 9 per cent from 0.068 to 0.074 Tg NO–N yr–1.
The same approach was used to calculate NO emissions from both agricultural and forest soils [43]. Total forest soil NO emission from EU15 countries was estimated at 0.075 Tg NO–N yr–1 and total agricultural soil NO emission at 0.102 Tg NO–N yr–1 which amounts to 4–6% of the total anthropogenic emission from Europe. This model estimate was compared with the approaches described above by Yienger & Levy, Skiba et al., Simpson et al., and Stehfest & Bouwman, and it was found that the estimates for agricultural soils varied by a factor of 4 with the Yienger and Levy approach giving the highest estimate (0.190 Tg NO–N yr–1) and the Skiba/Simpson approach giving the lowest estimate (0.049 Tg NO–N yr–1). In summary, estimates of global soil NO emission ranges from 1.8 Tg NO–N yr–1 [40] to 12 Tg NO–N yr–1 [6] and European soil NO emission from 0.14 to 1.5 Tg NO–N yr–1 [38].
It was recognized by de Bruijn et al. [44] that the data requirements of the process-based models are very high and therefore make their application for regional and global scaling up difficult. They tested a simplified model (DecoNit) for describing biogeochemical processes of C and N in combination with a modular simulation environment where it is combined with soil water balance and forest process submodules. The modelling approach was tested with data from the NOFRETETE forest sites. The model performed better than the PnET-N-DNDC model [45] by providing a more precise simulation of the annual emission rates, and also a somewhat more precise prediction of the variation on shorter time scales.
A new model of medium complexity, MiCNiT, for simulation of soil air NO, N2O and N2 concentrations and net exchange of these gases at the soil–atmosphere interface has recently been published [46]. The MiCNiT model calculates decomposition of soil organic matter, dynamics of microbial biomass, denitrification, autotrophic and heterotrophic nitrification by applying the microbial activity concept, as well as transport of gases and solutes between anaerobic and aerobic soil fractions and through the soil profile. So far, the model has been successful in simulating CO2 and N2O emission from the Höglwald forest. It remains, however, to be demonstrated how well it simulates NO emission.
5. Outlook
Although more focus has been placed on N2O emission, many new field studies have been carried out to quantify emissions of NO from soils, giving us a comprehensive knowledge of NO emission from various ecosystems, soil types and climatic conditions. However, most field studies were short-term, and to elucidate the temporal variation there is a need for continuous long-term (multi-year) measurements.
Recent years have also provided an improved mechanistic understanding of NO formation and consumption in the soil, partly because of the application of new tools from molecular biology. The many laboratory and field studies have provided increased knowledge of the controlling factors for NO emission.
NO emission from soils is the result of many processes with many types of micro-organisms involved. Abiotic factors in the soil influence the processes to different extents and simulation of NO emission is therefore complicated. Models of NO emission generally fall into two categories: very simple models with few factors or very complicated models, including mechanistic processes in the soil. The simple models have the advantage of being more easy to upscale, because of a lower demand on input information, whereas the advanced process models are able to simulate plot-scale measurements quite accurately. Recently, intermediate models have been developed with the hope that more precise estimates of regional and global NO emission can be provided.
The present global or regional estimates are, however, quite far from converging and show a range of values with about a factor of 10 in difference between the lowest and highest estimates. Clearly, there is a need for more field and laboratory experiments to elucidate the mechanisms controlling NO emission from the soil, and especially long-term continuous measurements are needed because of a high temporal variation. Model development should be continued both with respect to models requiring few input parameters (for estimating global and regional emission) and models that can accommodate the detailed knowledge of all the processes and factors controlling NO emission from the soil to be used in areas where sufficient input data are available.
Endnote
Rate of change with a temperature increase of 10.
References
- 1.Arneth A, et al. 2010. From biota to chemistry and climate: towards a comprehensive description of trace gas exchange between the biosphere and atmosphere. Biogeosciences 7, 121–149 10.5194/bg-7-121-2010 (doi:10.5194/bg-7-121-2010) [DOI] [Google Scholar]
- 2.Denman KL, et al. 2007. Couplings between changes in the climate system and biogeochemistry. In Climate change 2007: the physical basis (IPCC Fourth Assessment Report), pp. 499–588 Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.Davidson EA, Kingerlee W. 1997. A global inventory of nitric oxide emissions from soils. Nutr. Cycling Agroecosyst. 48, 37–50 10.1023/A:1009738715891 (doi:10.1023/A:1009738715891) [DOI] [Google Scholar]
- 4.Delon C, Reeves CE, Stewart DJ, Serça D, Dupont R, Chaboureau CMJ-P, Tulet P. 2008. Biogenic nitrogen oxide emissions from soils—impact on NOx and ozone over West Africa during AMMA (African Monsoon Multidisciplinary Experiment): modelling study. Atmos. Chem. Phys. 8, 2351–2363 10.5194/acp-8-2351-2008 (doi:10.5194/acp-8-2351-2008) [DOI] [Google Scholar]
- 5.Steinkamp J, Ganzeveld LN, Wilcke W, Lawrence MG. 2009. Influence of modelled soil biogenic NO emissions on related trace gases and the atmospheric oxidizing efficiency. Atmos. Chem. Phys. 9, 2663–2677 10.5194/acp-9-2663-2009 (doi:10.5194/acp-9-2663-2009) [DOI] [Google Scholar]
- 6.Ganzeveld LN, Lelieveld J, Dentener FJ, Krol MC, Bouwman AJ, Roelofs G-J. 2002. Global soil-biogenic NOx emissions and the role of canopy processes. J. Geophys. Res. 107, ACH9-1–ACH9-17 10.1029/2001JD001289 (doi:10.1029/2001JD001289) [DOI] [Google Scholar]
- 7.Ludwig J, Meixner FX, Vogel B, Förstner J. 2001. Soil–air exchange of nitric oxide: an overview of processes, environmental factors, and modeling studies. Biogeochemistry 52, 225–257 10.1023/A:1006424330555 (doi:10.1023/A:1006424330555) [DOI] [Google Scholar]
- 8.Fowler D, et al. 2009. Atmospheric composition change: ecosystems–atmosphere interactions. Atmos. Environ. 43, 5193–5267 10.1016/j.atmosenv.2009.07.068 (doi:10.1016/j.atmosenv.2009.07.068) [DOI] [Google Scholar]
- 9.Butterbach-Bahl K, et al. 2011. The European nitrogen assessment, ch. 6: nitrogen processes in terrestrial ecosystems, pp. 99–125 Cambridge, UK: Cambridge University Press [Google Scholar]
- 10.Conrad R. 1996. Metabolism of nitric oxide in soil and soil microorganisms and regulation of flux into the atmosphere. In Microbiology of atmospheric trace gases, vol. I 39 (eds Murrell JC, Kelly DP.), pp. 167–203 NATOASI Series. Berlin, Germany: Springer [Google Scholar]
- 11.Firestone MK, Davidson EA. 1989. Microbial basis of NO and N2O production and consumption in soil. In Exchange of trace gases between terrestrial ecosystems and the atmosphere (eds Andreae MO, Schimel DS.), pp. 7–21 Chichester, UK: John Wiley & Sons Ltd [Google Scholar]
- 12.Wrage N, Velthof G, van Beusichem M, Oenema O. 2001. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 33, 1723–1732 10.1016/S0038-0717(01)00096-7 (doi:10.1016/S0038-0717(01)00096-7) [DOI] [Google Scholar]
- 13.Kool DM, Wrage N, Zechmeister-Boltenstern S, Pfefferd M, Brus D, Oenemaa O, Van Groenigen JW. 2010. Nitrifier denitrification can be a source of N2O from soil: a revised approach to the dual-isotope labelling method. Eur. J. Soil Sci. 61, 759–772 10.1111/j.1365-2389.2010.01270.x (doi:10.1111/j.1365-2389.2010.01270.x) [DOI] [Google Scholar]
- 14.Kool DM, Dolfing J, Wrage N, Van Groenigen JW. 2011. Nitrifier denitrification as a distinct and significant source of nitrous oxide from soil. Soil Biol. Biochem. 43, 174–178 10.1016/j.soilbio.2010.09.030 (doi:10.1016/j.soilbio.2010.09.030) [DOI] [Google Scholar]
- 15.Baggs EM. 2008. A review of stable isotope techniques for N2O source partitioning in soils: recent progress, remaining challenges and future considerations. Rapid Commun. Mass Spectrom. 22, 1664–1672 10.1002/rcm.3456 (doi:10.1002/rcm.3456) [DOI] [PubMed] [Google Scholar]
- 16.Philippot L, Hallin S. 2005. Finding the missing link between diversity and activity using denitrifying bacteria as a model functional community. Curr. Opin. Microbiol. 8, 234–239 10.1016/j.mib.2005.04.003 (doi:10.1016/j.mib.2005.04.003) [DOI] [PubMed] [Google Scholar]
- 17.Szukics U, Hackl E, Zechmeister-Bolstenstern S, Sessitsch A. 2009. Contrasting response of two forest soils to nitrogen input: rapidly altered NO and N2O emissions and nirK abundance. Biol. Fertil. Soils 45, 855–863 10.1007/s00374-009-0396-5 (doi:10.1007/s00374-009-0396-5) [DOI] [Google Scholar]
- 18.Su H, et al. 2011. Soil nitrite as a source of atmospheric HONO and OH radicals. Science 333, 1616–1618 10.1126/science.1207687 (doi:10.1126/science.1207687) [DOI] [PubMed] [Google Scholar]
- 19.Venterea RT, Rolston DE, Cardon ZG. 2005. Effects of soil moisture, physical, and chemical characteristics on abiotic nitric oxide production. Nutr. Cycling Agroecosyst. 72, 27–40 10.1007/s10705-004-7351-5 (doi:10.1007/s10705-004-7351-5) [DOI] [Google Scholar]
- 20.Davidson EA, Keller M, Erickson HE, Verchot LV, Veldkamp E. 2000. Testing a conceptual model of soil emissions of nitrous and nitric oxides. Bioscience 50, 667–680 10.1641/0006-3568(2000)050[0667:TACMOS]2.0.CO;2 (doi:10.1641/0006-3568(2000)050[0667:TACMOS]2.0.CO;2) [DOI] [Google Scholar]
- 21.Erickson H, Davidson EA, Keller M. 2002. Former land-use and tree species affect nitrogen oxide emissions from a tropical dry forest. Oecologia 130, 297–308 10.1007/s004420100801 (doi:10.1007/s004420100801) [DOI] [PubMed] [Google Scholar]
- 22.Erisman JW, van Grinsven H, Grizzetti B, Bouraoui F, Powlson D, Sutton MA, Bleeker A, Reis S. 2011. The European nitrogen assessment, ch. 2: the European nitrogen problem in a global perspective, pp. 9–31 Cambridge, UK: Cambridge University Press [Google Scholar]
- 23.Pilegaard K, et al. 2006. Factors controlling regional differences in forest soil emission of nitrogen oxides (NO and N2O). Biogeosciences 3, 651–661 10.5194/bg-3-651-2006 (doi:10.5194/bg-3-651-2006) [DOI] [Google Scholar]
- 24.Venterea RT, Groffman PM, Verchot LV, Magill AH, Aber JD, Steudler PA. 2003. Nitrogen oxide gas emissions from temperate forest soils receiving long-term nitrogen inputs. Glob. Change Biol. 9, 346–357 10.1046/j.1365-2486.2003.00591.x (doi:10.1046/j.1365-2486.2003.00591.x) [DOI] [Google Scholar]
- 25.Burton JL, Pregitzer KS, Crawford JN, Zogg GP, Zak DR. 2004. Simulated chronic NO3– deposition reduces soil respiration in northern hardwood forests. Glob. Change Biol. 10, 1080–1091 10.1111/j.1365-2486.2004.00737.x (doi:10.1111/j.1365-2486.2004.00737.x) [DOI] [Google Scholar]
- 26.Schindlbacher A, Zechmeister-Boltenstern S, Butterbach-Bahl K. 2004. Effects of soil moisture and temperature on NO, NO2, and N2O emissions from European forest soils. J. Geophys. Res. 109, d17302 10.1029/2004JD004590 (doi:10.1029/2004JD004590) [DOI] [Google Scholar]
- 27.Goldberg SD, Gebauer G. 2009. N2O and NO fluxes between a Norway spruce forest soil and atmosphere as affected by prolonged summer drought. Soil Biol. Biochem. 41, 1986–1995 10.1016/j.soilbio.2009.07.001 (doi:10.1016/j.soilbio.2009.07.001) [DOI] [Google Scholar]
- 28.Wu X, Brüggemann N, Gasche R, Shen Z, Wolf B, Butterbach-Bahl K. 2010. Environmental controls over soil-atmosphere exchange of N2O, NO, and CO2 in a temperate Norway spruce forest. Glob. Biogeochem. Cycles 24 10.1029/2009GB003616 (doi:10.1029/2009GB003616) [DOI] [Google Scholar]
- 29.Kesik M, Blagodatsky S, Papen H, Butterbach-Bahl K. 2006. Effect of pH, temperature and substrate on N2O, NO and CO2 production by Alcaligenes faecalis p . J. Appl. Microbiol. 101, 655–667 10.1111/j.1365-2672.2006.02927.x (doi:10.1111/j.1365-2672.2006.02927.x) [DOI] [PubMed] [Google Scholar]
- 30.Luo GJ, Brüggemann N, Wolf B, Gasche R, Grote R, Butterbach-Bahl K. 2012. Decadal variability of soil CO2, NO, N2O, and CH4 fluxes at the Höglwald forest, Germany. Biogeosciences 9, 1741–1763 10.5194/bg-9-1741-2012 (doi:10.5194/bg-9-1741-2012) [DOI] [Google Scholar]
- 31.Pilegaard K, Hummelshøj P, Jensen NO. 1999. Nitric oxide emission from a Norway spruce forest floor. J. Geophys. Res. D 104, 3433–3445 10.1029/1998JD100050 (doi:10.1029/1998JD100050) [DOI] [Google Scholar]
- 32.Bargsten A, Falge E, Pritsch K, Huwe B, Meixner FX. 2010. Laboratory measurements of nitric oxide release from forest soil with a thick organic layer under different understory types. Biogeosciences 7, 1425–1441 10.5194/bg-7-1425-2010 (doi:10.5194/bg-7-1425-2010) [DOI] [Google Scholar]
- 33.Finzi A, van Breemen N, Canham CD. 1998. Canopy tree–soil interactions within temperate forests: species effects on soil carbon and nitrogen. Ecol. Appl. 8, 440–446 [Google Scholar]
- 34.Brüggemann N, Rosenkranz P, Papen H, Pilegaard K, Butterbach-Bahl K. 2005. Pure stands of temperate forest tree species modify soil respiration and N turnover. Biogeosci. Discuss. 2, 303–331 10.5194/bgd-2-303-2005 (doi:10.5194/bgd-2-303-2005) [DOI] [Google Scholar]
- 35.Rothe A, Huber C, Kreutzer K, Weis W. 2002. Deposition and soil leaching in stands of Norway spruce and European beech: results from the Höglwald research in comparison with other European case studies. Plant Soil 240, 33–45 10.1023/A:1015846906956 (doi:10.1023/A:1015846906956) [DOI] [Google Scholar]
- 36.Sparks JP. 2009. Ecological ramifications of the direct foliar uptake of nitrogen. Oecologia 159, 1–13 10.1007/s00442-008-1188-6 (doi:10.1007/s00442-008-1188-6) [DOI] [PubMed] [Google Scholar]
- 37.Skiba U, Fowler D, Smith KA. 1997. Nitric oxide emissions from agricultural soils in temperate and tropical climates: sources, controls and mitigation options. Nutr. Cycling Agroecosyst. 48, 139–153 10.1023/A:1009734514983 (doi:10.1023/A:1009734514983) [DOI] [Google Scholar]
- 38.Simpson D, et al. 1999. Inventorying emissions from nature in Europe. J. Geophys. Res. 104, 8113–8152 10.1029/98JD02747 (doi:10.1029/98JD02747) [DOI] [Google Scholar]
- 39.Yienger JJ, Levy H. 1995. Empirical model of global soil-biogenic NOx emissions. J. Geophys. Res. 100, 11 447–11 464 10.1029/95JD00370 (doi:10.1029/95JD00370) [DOI] [Google Scholar]
- 40.Stehfest E, Bouwman L. 2006. N2O and NO emission from agricultural fields and soils under natural vegetation: summarizing available measurement data and modeling of global annual emissions. Nutr. Cycling Agroecosyst. 74, 207–228 10.1007/s10705-006-9000-7 (doi:10.1007/s10705-006-9000-7) [DOI] [Google Scholar]
- 41.Yan X, Ohara T, Akimoto H. 2005. Statistical modeling of global soil NOx emissions. Glob. Biochem. Cycles 19, 1–16 10.1029/2004GB002276 (doi:10.1029/2004GB002276) [DOI] [Google Scholar]
- 42.Kesik M, Brüggemann N, Forkel R, Kiese R, Knoche R, Li C, Seufert G, Simpson D, Butterbach-Bahl K. 2006. Future scenarios of N2O and NO emissions from European forest soils. J. Geophys. Res. 111 (doi:10.1029/2005JG000115) [Google Scholar]
- 43.Butterbach-Bahl K, Kahl M, Mykhayliv L, Kiese R, Li C. 2009. A Europeanwide inventory of soil NO emissions using the biogeochemical models DNDC/Forest-DNDC. Atmos. Environ. 43, 1392–1402 10.1016/j.atmosenv.2008.02.008 (doi:10.1016/j.atmosenv.2008.02.008) [DOI] [Google Scholar]
- 44.de Bruijn AMG, Grote R, Butterbach-Bahl K. 2011. An alternative modelling approach to predict emissions of N2O and NO from forest soils. Eur. J. Forest Res. 130, 755–773 10.1007/s10342-010-0470-4 (doi:10.1007/s10342-010-0470-4) [DOI] [Google Scholar]
- 45.Kesik M, et al. 2005. Inventories of N2O and NO emissions from European forest soils. Biogeosciences 2, 353–375 10.5194/bg-2-353-2005 (doi:10.5194/bg-2-353-2005) [DOI] [Google Scholar]
- 46.Blagodatsky S, Grote R, Kiese R, Werner C, Butterbach-Bahl K. 2011. Modelling of microbial carbon and nitrogen turnover in soil with special emphasis on N-trace gases emission. Plant Soil 346, 297–330 10.1007/s11104-011-0821-z (doi:10.1007/s11104-011-0821-z) [DOI] [Google Scholar]



