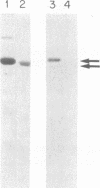
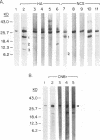
Abstract
The major outer surface protein, OspA, of Borrelia burgdorferi is a lipoprotein which is a particular interest because of its potential as a vaccine candidate. However, serotypic and genetic analysis of OspA from both European and North American strains have demonstrated antigenic and structural heterogeneities. We purified OspA to homogeneity by exploiting its resistance to trypsin digestion. By treating spirochetes with trypsin and then using Triton X-114 extraction and ion-exchange chromatography, we obtained a yield of 2 mg of pure OspA protein per liter of culture. INtrinsic labeling with [14C]palmitic acid confirmed that OspA was lipidated, and partial digestion established lipidation at the amino-terminal end of the molecule. The reactivity of five anti-OspA murine monoclonal antibodies to nine different isolates of B. burgdorferi was ascertained by Western blot (immunoblot) analysis. Purified OspA was fragmented by enzymatic or chemical cleavage, and the monoclonal antibodies were able to define four distinct immunogenic domains. Further resolution of the epitope specificity to determine humoral and cellular immune responses to OspA has implications for vaccine development and for the utility of this protein as a reagent in diagnostic testing for Lyme borreliosis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baranton G., Postic D., Saint Girons I., Boerlin P., Piffaretti J. C., Assous M., Grimont P. A. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992 Jul;42(3):378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Hayes S. F., Heiland R. A., Schrumpf M. E., Tessier S. L. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986 May;52(2):549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Hayes S. F. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984 Jul;45(1):94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Todd W. J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983 Aug;41(2):795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström S., Bundoc V. G., Barbour A. G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989 Apr;3(4):479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Boerlin P., Peter O., Bretz A. G., Postic D., Baranton G., Piffaretti J. C. Population genetic analysis of Borrelia burgdorferi isolates by multilocus enzyme electrophoresis. Infect Immun. 1992 Apr;60(4):1677–1683. doi: 10.1128/iai.60.4.1677-1683.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P. Structure of alpha-1-CB8, a large cyanogen bromide produced fragment from the alpha-1 chain of rat collagen. The nature of a hydroxylamine-sensitive bond and composition of tryptic peptides. Biochemistry. 1970 Jun 9;9(12):2408–2421. doi: 10.1021/bi00814a004. [DOI] [PubMed] [Google Scholar]
- Brandt M. E., Riley B. S., Radolf J. D., Norgard M. V. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect Immun. 1990 Apr;58(4):983–991. doi: 10.1128/iai.58.4.983-991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Coleman J. L., Benach J. L. Isolation of antigenic components from the Lyme disease spirochete: their role in early diagnosis. J Infect Dis. 1987 Apr;155(4):756–765. doi: 10.1093/infdis/155.4.756. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Lade B. N., Barbour A. G. Outer surface protein A (OspA) from the Lyme disease spirochete, Borrelia burgdorferi: high level expression and purification of a soluble recombinant form of OspA. Protein Expr Purif. 1990 Nov;1(2):159–168. doi: 10.1016/1046-5928(90)90011-m. [DOI] [PubMed] [Google Scholar]
- Dykhuizen D. E., Polin D. S., Dunn J. J., Wilske B., Preac-Mursic V., Dattwyler R. J., Luft B. J. Borrelia burgdorferi is clonal: implications for taxonomy and vaccine development. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10163–10167. doi: 10.1073/pnas.90.21.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdile L. F., Brandt M. A., Warakomski D. J., Westrack G. J., Sadziene A., Barbour A. G., Mays J. P. Role of attached lipid in immunogenicity of Borrelia burgdorferi OspA. Infect Immun. 1993 Jan;61(1):81–90. doi: 10.1128/iai.61.1.81-90.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikrig E., Barthold S. W., Kantor F. S., Flavell R. A. Long-term protection of mice from Lyme disease by vaccination with OspA. Infect Immun. 1992 Mar;60(3):773–777. doi: 10.1128/iai.60.3.773-777.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikrig E., Barthold S. W., Kantor F. S., Flavell R. A. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990 Oct 26;250(4980):553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- Fikrig E., Barthold S. W., Persing D. H., Sun X., Kantor F. S., Flavell R. A. Borrelia burgdorferi strain 25015: characterization of outer surface protein A and vaccination against infection. J Immunol. 1992 Apr 1;148(7):2256–2260. [PubMed] [Google Scholar]
- Holloway P. W. A simple procedure for removal of Triton X-100 from protein samples. Anal Biochem. 1973 May;53(1):304–308. doi: 10.1016/0003-2697(73)90436-3. [DOI] [PubMed] [Google Scholar]
- Jonsson M., Noppa L., Barbour A. G., Bergström S. Heterogeneity of outer membrane proteins in Borrelia burgdorferi: comparison of osp operons of three isolates of different geographic origins. Infect Immun. 1992 May;60(5):1845–1853. doi: 10.1128/iai.60.5.1845-1853.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luft B. J., Jiang W., Munoz P., Dattwyler R. J., Gorevic P. D. Biochemical and immunological characterization of the surface proteins of Borrelia burgdorferi. Infect Immun. 1989 Nov;57(11):3637–3645. doi: 10.1128/iai.57.11.3637-3645.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadziene A., Thompson P. A., Barbour A. G. In vitro inhibition of Borrelia burgdorferi growth by antibodies. J Infect Dis. 1993 Jan;167(1):165–172. doi: 10.1093/infdis/167.1.165. [DOI] [PubMed] [Google Scholar]
- Sambri V., Cevenini R. Comparative ability of various detergents to extract proteins of Borrelia burgdorferi and Borrelia hermsii. Microbiologica. 1991 Oct;14(4):307–313. [PubMed] [Google Scholar]
- Schubach W. H., Mudri S., Dattwyler R. J., Luft B. J. Mapping antibody-binding domains of the major outer surface membrane protein (OspA) of Borrelia burgdorferi. Infect Immun. 1991 Jun;59(6):1911–1915. doi: 10.1128/iai.59.6.1911-1915.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears J. E., Fikrig E., Nakagawa T. Y., Deponte K., Marcantonio N., Kantor F. S., Flavell R. A. Molecular mapping of Osp-A mediated immunity against Borrelia burgdorferi, the agent of Lyme disease. J Immunol. 1991 Sep 15;147(6):1995–2000. [PubMed] [Google Scholar]
- Shanafelt M. C., Anzola J., Soderberg C., Yssel H., Turck C. W., Peltz G. Epitopes on the outer surface protein A of Borrelia burgdorferi recognized by antibodies and T cells of patients with Lyme disease. J Immunol. 1992 Jan 1;148(1):218–224. [PubMed] [Google Scholar]
- Shechter Y., Patchornik A., Burstein Y. Selective chemical cleavage of tryptophanyl peptide bonds by oxidative chlorination with N-chlorosuccinimide. Biochemistry. 1976 Nov 16;15(23):5071–5075. doi: 10.1021/bi00668a019. [DOI] [PubMed] [Google Scholar]
- Simon M. M., Schaible U. E., Kramer M. D., Eckerskorn C., Museteanu C., Müller-Hermelink H. K., Wallich R. Recombinant outer surface protein a from Borrelia burgdorferi induces antibodies protective against spirochetal infection in mice. J Infect Dis. 1991 Jul;164(1):123–132. doi: 10.1093/infdis/164.1.123. [DOI] [PubMed] [Google Scholar]
- Wallich R., Helmes C., Schaible U. E., Lobet Y., Moter S. E., Kramer M. D., Simon M. M. Evaluation of genetic divergence among Borrelia burgdorferi isolates by use of OspA, fla, HSP60, and HSP70 gene probes. Infect Immun. 1992 Nov;60(11):4856–4866. doi: 10.1128/iai.60.11.4856-4866.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilske B., Luft B., Schubach W. H., Zumstein G., Jauris S., Preac-Mursic V., Kramer M. D. Molecular analysis of the outer surface protein A (OspA) of Borrelia burgdorferi for conserved and variable antibody binding domains. Med Microbiol Immunol. 1992;181(4):191–207. doi: 10.1007/BF00215765. [DOI] [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Schierz G., Kühbeck R., Barbour A. G., Kramer M. Antigenic variability of Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:126–143. doi: 10.1111/j.1749-6632.1988.tb31846.x. [DOI] [PubMed] [Google Scholar]