Abstract
Chimeric mice, which have human hepatocytes engrafted in their liver, have been used to study human drug metabolism and pharmacodynamic responses for nearly 20 years. However, there are very few examples where their use has prospectively impacted the development of a candidate medication. Here, three different chimeric mouse models and their utility for pharmacology studies are evaluated. Several recent studies indicate that using these chimeric mouse models could help to overcome traditional (predicting human-specific metabolites and toxicities) and 21st Century problems (strategies for personalized medicine and selection of optimal combination therapies) in drug development. These examples suggest that there are many opportunities in which the use of chimeric mice could significantly improve the quality of preclinical drug assessment.
Keywords: Chimeric Mice, Drug Metabolism, Predicting Drug Toxicity, Liver Humanization
Modeling Human Drug Metabolism and Drug Response
As the time, cost and regulatory hurdles for testing new drug candidates in human subjects have increased, a greater emphasis has been placed on developing preclinical methodologies that more predictive information about human drug metabolism or pharmacodynamic responses. Inter-species differences in drug metabolism create qualitative and quantitative differences between the drug metabolites produced in humans and animal species. As a consequence, the results obtained from in vitro systems and from in vivo animal testing have not always accurately predicted human human-specific drug metabolic pathways 1–3. The inability to pre-clinically identify human-specific drug metabolites is particularly problematic because it is most often a drug metabolite, and not the parent drug itself, that is responsible for an unexpected drug-induced toxicity 4, 5. Thus, interspecies differences in drug metabolism have limited the utility of animal toxicology studies. In addition, two relatively new challenges for 21st century drug development have also appeared. (i) To implement ‘personalized medicine’ strategies, drug development is coupled with the production of diagnostic tests whose results will guide drug or dose selection, which should minimize the toxicity or increase the efficacy of the new medication 6. Identification and characterization of pharmacogenetic factors affecting drug response is the major barrier to implementation of these strategies 7. (ii) In several therapeutic areas (e.g. Hepatitis C Virus (HCV) infection), where there are multiple therapeutic options, identification of optimized combinations of therapies has become a new challenge.
To address these issues, chimeric mice with ‘humanized livers’ have been produced by transplantation of human liver cells into mice that have two significant genetically engineered modifications. First, defects in their immune system enable them to accept transplanted human liver cells. Second, a gene knockout or an expressed transgene causes damage to endogenous murine liver cells, which facilitates engraftment of the transplanted human liver cells. Together, these genetic modifications enable transplanted human liver cells to re-populate the liver of these mice, where they can synthesize human proteins and mediate human drug biotransformation reactions. This review evaluates the advantages and limitations of the different chimeric mouse models based upon the differences in the underlying genetically engineered modifications. Recent results obtained using these chimeric models indicate that they can be used to address both conventional (prediction of human-specific metabolites and toxicity) and emerging 21st century (development of pharmacogenomic and optimized combination strategies) problems in drug development.
Different engineering leads to different performance
When considering their utility for drug development, it is important to recognize that the chimeric mice with different genetically engineered modifications will have intrinsically different properties. Based on the genetic engineering modifications that confer immunodeficiency and cause damage to endogenous murine liver cells, there are three different types of chimeric mouse models (Table 1). Chimeric mice were first generated by Brinster and colleagues in 1991 when a transgene with an albumin promoter that directed the liver-specific expression of a urokinase-type plasminogen activator (uPA) was introduced into an immunodeficient mouse with a DNA-dependent protein kinase (SCID) mutation 8. Expression of the uPA transgene induced progressive liver failure and death within weeks, which is probably caused by uPA-mediated activation of an enzyme (plasminogen) that regulates the activity of matrix metalloproteinases that are critical for liver cell growth. Multiple investigators subsequently demonstrated that immunocompromised uPA/SCID mice could be repopulated with human hepatocytes, and the chimeric mice could produce known human-specific drug metabolites after treatment with test compounds (9–13). Although many pioneering studies were performed using this model, it has significant limitations. The ongoing uPA-induced liver toxicity can alter cell growth and gene expression in the liver, which renders this model less suitable for longer-term toxicology studies or for studies that evaluate stem/progenitor cell development. The liver damage and systemic toxicity has other important consequences that include: a poor breeding efficiency, renal disease, and a very narrow time window for transplantation before the mice succumb to their bleeding diathesis. Another limitation comes from the selective pressure to remove the cells expressing the uPA transgene, which favors the re-growth of endogenous mouse liver cells that have deleted the transgene 14. In addition, the SCID mutation only affects the adaptive immune system, and these mice sometimes require an anti-serum to neutralize NK cells and/or an anti-complement agent to enable the survival of the transplanted human cells 11.
Table 1.
A chart comparing the properties of 3 different chimeric mouse models is shown. The genes and type of immune deficit (adaptive and/or innate), mechanism of induction and the duration of liver injury resulting from the indicated knockout (−/−) or transgene (tg), presence of systemic toxicity, requirement for ongoing drug treatment, and selected references relating to the production of the model or use in drug metabolism are shown.
| Model |
Immune Compromise |
Liver Injury |
Systemic Toxicity |
Ongoing Drug Rx |
Selected References |
|---|---|---|---|---|---|
| uPA/SCID | Adaptive (SCID) | continuous uPA tg | Renal Bleeding | Immune Suppressive | 9–12 |
| FRG | Innate (Il2rg−/−) Adaptive (Rag2−/−) |
continuous Fah−/− | None | NTBC | 15 |
| TK-NOG | Innate Adaptive (Il2rg−/− SCID) |
time limited TK tg | None | None | 18,19,20 |
A second type of chimeric mouse (Rag2−/− Il2rg−/− Fah−/− or FRG) uses a gene knockout in an enzyme that catalyzes the final step in tyrosine degradation (fumarylacetoacetate hydrolase, Fah) to cause damage to endogenous mouse liver cells 15. Accumulation of the substrate (fumarylacetoacetate) leads to oxidative damage and hepatocyte death. Fah−/− also leads to tyrosine accumulation, which is not directly toxic to the liver, but tyrosinemia can cause dermatologic and neurodevelopmental problems in humans. FRG mice contain two other gene knockouts that inactivate both the innate (Il2rg−/−) and adaptive (Rag2−/−) immune systems. FRG mice have two advantages over uPA/SCID mice: additional immunosuppressive therapy is not required and they do not have the systemic morbidities seen in uPA mice. However, the FRG model has three limitations: (i) ongoing Fah−/− induced liver toxicity is inherent in the design of the model; (ii) they develop carcinomas, which is a known consequence of the type I tyrosinemia produced by Fah mutations 16; and (iii) their long-term survival requires treatment with a drug (2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexadione or NTBC) that inhibits 4-hydroxyphenylpyruvate oxidase 15 and prevents fumarylacetoacetate accumulation, which is required to suppress liver toxicity and the development of liver tumors.
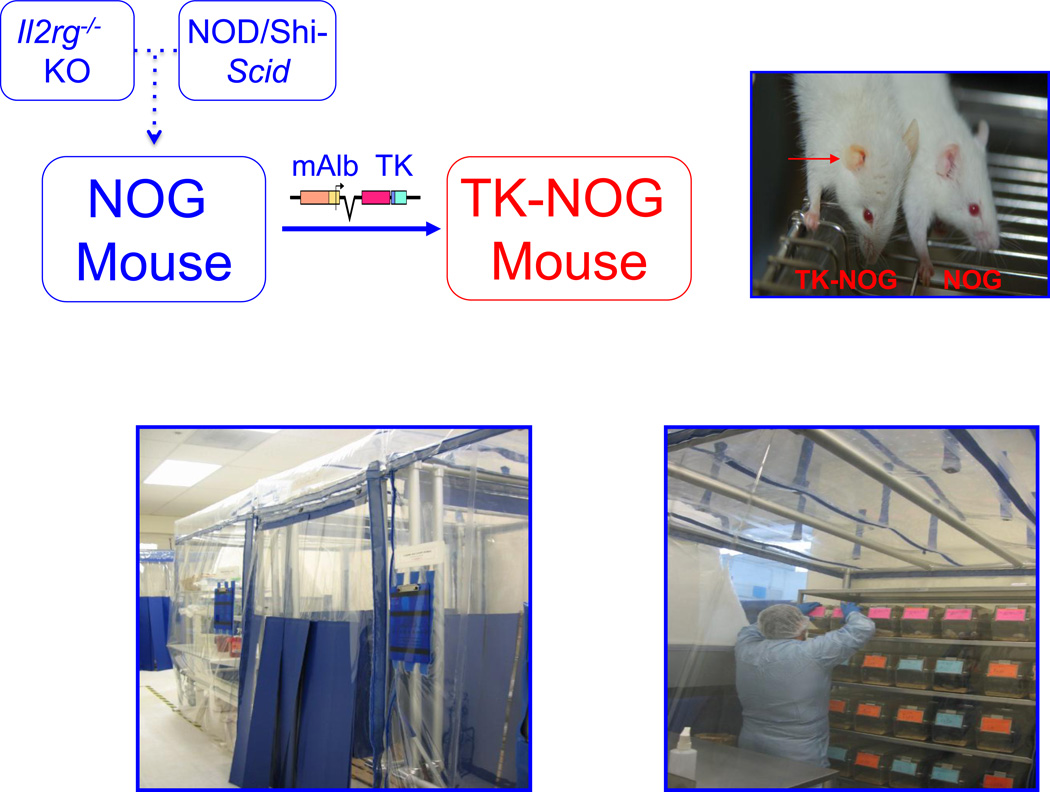
To overcome the limitations inherent in uPA/SCID and FRG mice, a third chimeric mouse model was recently produced. In this model, a herpes simplex virus type 1 thymidine kinase (TK) transgene was expressed within the liver of a highly immunodeficient mouse strain (NOG) 17 to produce the TK-NOG transgenic mouse 18 (Figure 1). NOG mice have mutations that inactivate both the innate (Il2rg−/−) and adaptive (SCID) immune responses 17. A brief exposure to a nontoxic dose of ganciclovir causes the rapid and temporally controlled ablation of mouse liver cells expressing the transgene (Figure 2). Of note, the TK-NOG mouse is only exposed to gancyclovir for a very short period of time prior to cellular transplantation. Because of the temporally limited nature of the period of gancyclovir administration, the TK-transgene expressing liver cells are not subjected to a continuous selection pressure that leads to ablation of transgene expressing cells, as occurs in the uPA/SCID mouse. The most unique feature of TK-NOG mice (relative to the uPA/SCID and FRG models) is that the time-limited toxicity enables the transplanted human liver cells to develop into a mature “human organ” with a 3-dimensional architecture and to maintain a gene expression pattern that is characteristic of mature human liver. The absence of ongoing liver toxicity enabled the humanized liver to be stably maintained for >6 months without exogenous drug treatments. The chimeric TK-NOG liver expresses mRNAs encoding human cytochrome P450 (CYP450) enzymes, transporters and transcription factors affecting drug metabolism at levels that were equivalent to those in the donor human hepatocytes. Moreover, there was extensive human hepatic CYP3A4 protein expression, and chimeric TK-NOG mice could mediate human-specific drug biotransformation reactions 18. TK-NOG mice were used to predict the pattern of human drug metabolism and the occurrence of a human drug-drug interaction (prior to human exposure) for a drug in development 19, and to identify human genetic factors affecting the metabolism of clinically important drugs 20.
Figure 1.
The production, maintenance and assessment of humanized TK-NOG mice. NOG mice were produced by Dr. Mamoru Ito and colleagues at the Central Institute for Experimental Animals (Tokyo, Japan), who crossed NOD-Shi mice with the SCID mutation with an Il2rg−/− knockout mouse 17. A thymidine kinase transgene with an albumin promoter was placed in a NOG mouse to produce the TK-NOG mouse 18 (upper panel). Because TK-NOG mice express the thymidine kinase transgene only in liver, administration of 2 doses of (25 mg/kg) gancyclovir, 7 and 5 days prior to human hepatocyte transplantation leads to injury of the mouse liver. The presence of jaundice in a ganciclovir-treated TK-NOG mouse (shown on left), but not in a treated NOG mouse (shown on right), is indicative of the transgene-dependent and drug-induced liver damage (right upper panel). Because TK-NOG mice have defective innate and adaptive immune responses, they are maintained within a germ-free environment in ‘biobubbles’; and access is limited to personnel wearing sterile gowns, gloves and masks (lower panel).
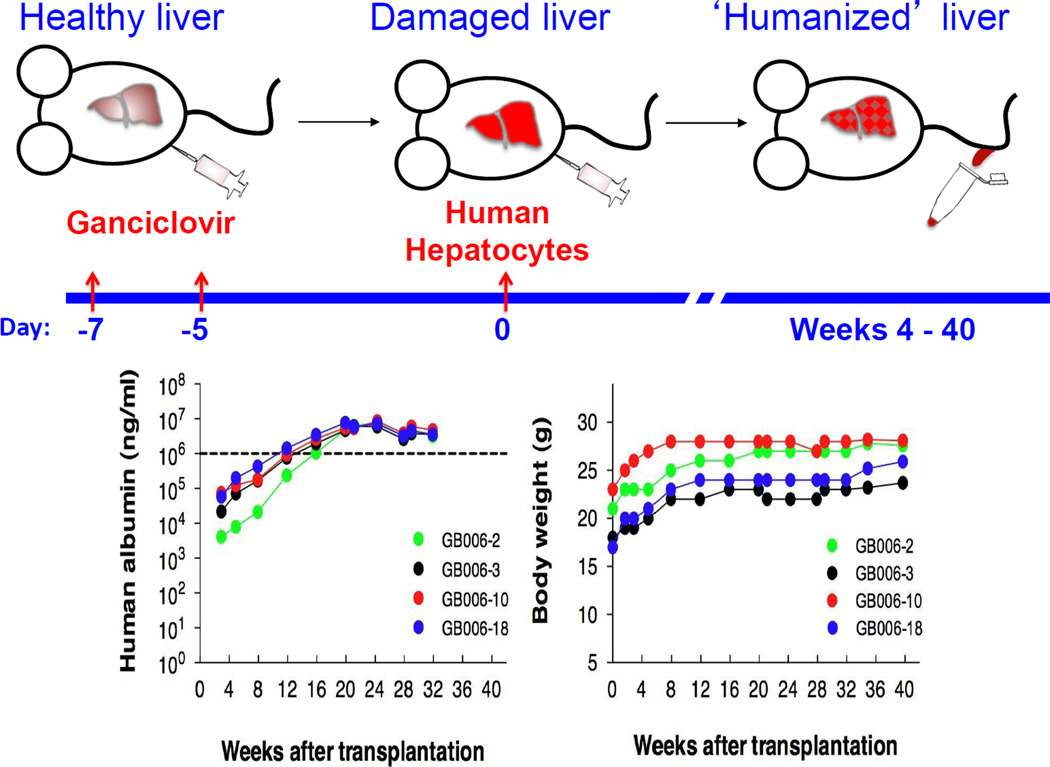
Figure 2.
Ganciclovir conditioning and human hepatocyte transplantation of TK-NOG mice. The basic protocol is diagramed in the upper panel, showing the timeline in which the transplanted human hepatocytes to engraft within the liver of TK-NOG mice. The level of human hepatocyte engraftment is determined by serially measuring the amount of human serum albumin, which initially appears 4 weeks after transplantation and then increases in amount over the next 4–6 weeks. The absence of ongoing toxicity in this model is demonstrated by the ability of four chimeric TK-NOG mice (each represented by a different color) to stably maintain a high level of human biosynthetic function and their body weight for 8 months after transplantation (lower panel). The data in the bottom panel of Figure 2 are reproduced with permission from ref [18].
There are no available studies comparing the properties of the 3 different types of chimeric mice, but their intrinsic properties, which are determined by the genetically engineered modifications, enable their relative utility for future studies to be assessed. The significant underlying differences in the construction of these models will have a greater impact when the current focus on acute human drug metabolite studies recedes, and chimeric mice are used for long-term studies that examine pharmacodynamic responses or toxicologic mechanisms, or when liver progenitor cells are used to produce disease models or for assessment of pharmacogenetic factors. The residual immunity and the ongoing liver and systemic toxicity associated with the uPA/SCID model will limit its utility, whereas the increased level of immune deficiency and the decreased level of systemic toxicity will increase the utility of FRG mice for long-term studies. However, the FRG mouse still has ongoing liver toxicity that is suppressed by administration of an exogenous medication, which is also required to suppress tumor development. In my opinion, there are several important factors that favor the use of TK-NOG mice for the longer-term studies that will become a major component of future academic research and for studies of importance to drug development. Liver damage is induced in a temporally controlled manner over a very limited time period, and is not an ongoing process. Both the innate and adaptive immune responses are compromised; so additional immunosuppression is not required. No exogenous drugs are required to promote its survival.
Looking towards the future, advances in tissue bioengineering may facilitate the production of new types of chimeric mice. Conventionally cultured human hepatocytes rapidly lose key liver functional properties, and they cannot mediate the multi-step drug biotransformation reactions performed by the 3-dimensional organ, which has limited the utility of in vitro systems for preclinical drug development. However, as one example of progress in the tissue bioengineering field, a polymer scaffold was recently developed that enabled cultured human hepatocytes to retain their functional properties. Moreover, detectable amounts of the human-predominant metabolites of debrisoquine (4-hydroxy) and coumarin (7-hydroxy) were found in the urine of mice whose subcutaneous tissue was implanted with these encapsulated human hepatocytes 21. Thus, tissue bioengineering methods may facilitate the production of chimeric mice, or could even produce an ex vivo system that resembles human liver.
Areas of opportunity for 21st Century drug development
At present, there are no regulatory requirements for using chimeric mouse data, nor does any pharmaceutical company routinely use chimeric mice to evaluate candidate medications prior to human clinical studies. Nevertheless, given the high failure rate of new compounds, using chimeric mice to characterize drug candidates could improve many traditional and newly emerging problem areas for drug development. One traditional problem is that existing in vitro systems and in vivo animal testing results have not always accurately predicted human human-specific drug metabolic pathways for candidate medications 1–3 owing to inter-species differences in drug metabolism. Analysis of drug metabolism in chimeric mice can (at least partly) address this issue. High levels of human liver reconstitution can be obtained with any of the chimeric model systems, and all three models have been able to produce human-specific drug metabolites for test drugs 21,18,11,12,22,10,23,24. Moreover, the hepatic clearance and pharmacokinetic properties of selected drugs in chimeric mice have been shown to resemble that of humans 25, 26.
The vast majority of studies performed using chimeric mice have examined whether known human drug metabolites or human patterns of drug metabolism were reproduced in chimeric mice. However, there is much less information about the prospective utility of chimeric mouse data for drug development, nor do we know if they can reliably predict the pattern of human drug metabolism. In one study, the results obtained using chimeric mice could not fully predict the pattern of human drug metabolism for two of the three structurally distinct compounds that were assessed 27. However, a recently published study demonstrated that chimeric TK-NOG mouse data had significant utility for evaluation of a drug (clemizole) in development for treatment of HCV infection 19. Clemizole was recently discovered to be a potent inhibitor (IC50 24 nM) of the interaction between a HCV protein (NS4B) and HCV RNA. Clemizole had a human-specific pattern of metabolism in humanized TK-NOG mice that was substantially different from that in rodents 19, and the chimeric mouse data had significant utility for it development. First, the human-specific metabolite had significant anti-HCV activity, which was an important feature that favored its continued development. A drug-drug interaction between clemizole and a CYP3A4 inhibitor (ritonavir) was also modeled in humanized TK-NOG mice, which enabled development of a ritonavir co-administration strategy to increase its efficacy. Moreover, the inter-species differences in the drug metabolites indicated that there would be potential problems with rodent toxicity studies for this drug. Rodent toxicology testing would provide a false assurance of drug safety if toxicities were caused by the human predominant metabolite, or would raise a false safety concern if a rodent-predominant metabolite caused toxicity. Although this study provides only a single example, it indicates how chimeric mouse data can impact the preclinical assessment of a drug candidate.
Chimeric mice should also be used for pharmacodynamic studies. The ability to control many confounding variables and ready access to tissue are features of chimeric mice that favor their use for pharmacodynamic studies. Hepatitis B virus (HBV) and HCV infection provides an ideal area for pharmacodynamic studies in chimeric mice. Multiple investigators have demonstrated that chimeric mice can be infected with these viruses, and that anti-viral efficacy can be assessed 28. However, because many new types of direct-acting anti-viral agents (DAAA) are emerging, a major challenge is to identify optimal combination therapies for HCV infection. This could be accomplished through expensive clinical trials that require a long time period for completion, and which require multiple cycles for testing different dose combinations. However, it was recently demonstrated that administration of two DAAA and interferon-α for 4 weeks could eradicate HCV in infected uPA/SCID mice, while mono-therapy with any of these agents was much less (or in-) effective 29. These results mimicked those obtained in human clinical studies. This extremely promising result demonstrates how pharmacodynamic studies using different drug combinations in chimeric mice could be used to guide the design of human clinical trials. Alternatively, studies in chimeric mice could be quickly performed to assess aberrant outcomes in human subjects. The use of chimeric mice for these studies could greatly accelerate the development of optimized combination therapies for HCV. Moreover, pharmacodynamic studies could be performed in chimeric mice to test drugs for other human-specific hepatotropic infectious agents (i.e. Dengue), which would accelerate the development of entirely new classes of drugs.
Looking forward, there are two emerging areas in 21st Century drug development where using chimeric mice can help. The first is in ‘personalized medicine.’ Simulation studies have demonstrated that the use of pharmacogenetic information for drug selection 30 or dose adjustment 7 can improve drug efficacy and reduce drug toxicity, and many companies are co-developing drugs with predictive genetic tests. However, large and costly human clinical studies are required for testing pharmacogenetic hypotheses, which has been a major barrier to the implementation of genetically based ‘personalized medicine’ 31. Also, multiple confounding variables (other medications, dietary differences, disease severity) can make it difficult to identify pharmacogenetic variables in human cohorts. However, it was recently demonstrated that chimeric TK-NOG mice could be used to identify human CYP450 alleles affecting the metabolism of two medications 20. Remarkably, analysis of the variance in this model system indicated that allelic affects could be identified using chimeric mice that were reconstituted with hepatocytes obtained from a relatively small number (3–10 per genotype) of human donors. For certain compounds, this innovative model system could enable human pharmacogenetic analyses to be efficiently performed with control of all confounding environmental variables. At present, the drugs analyzed in chimeric mice must have human-predominant metabolites, and the allelic variants affecting its metabolism must be reasonably common in the population. However, advances in cellular reprogramming methodology, which enable induced liver cells to be prepared from individuals with rare genotypes, will expand the breadth of human pharmacogenetic studies that can be performed in chimeric mice.
Secondly, drug-drug interactions (DDIs) have created major problems for patients and for regulatory authorities, since more than 30% of the US population over 57 years of age take five or more prescription drugs at a given time 32. The available in vitro or in vivo animal models have been unable to predict the occurrence of many of clinically important DDIs, which only became apparent after drug development was completed 33. However, a recent study using chimeric TK-NOG mice correctly predicted that a DDI involving a candidate medication would occur in humans 19. Thus, chimeric mice can be used to assess and model DDIs for selected candidate medications that are metabolized by human-specific pathways.
It is also important to point out that there are inherent limitations associated with drug metabolism or toxicity studies performed using any chimeric model. Chimeric mice have both human and mouse liver tissue. Because mouse liver has a much higher rate of drug metabolism, even a small percentage of remnant mouse liver will introduce a significant amount of murine drug metabolism into the system, which could confound toxicologic or other analyses performed using chimeric mice. The variable extent of liver humanization introduces an additional complicating factor, which can affect the results obtained in studies using chimeric mice. The small blood volume of a mouse limits the number of repeat samples that can be obtained from a single mouse within a limited time interval. Human-predominant drug metabolites will have to be identified by a careful comparison of the drug metabolite profiles using a sufficient number of control and chimeric mice. In addition, extra-hepatic human-specific factors affecting drug metabolism or clearance cannot be identified in chimeric mice. Furthermore, since we have not fully characterized the extent of biliary tract humanization in chimeric mice, we do not know how accurately they can predict the clearance of drugs that depend upon human-specific transporter-mediated hepatobiliary clearance. However, the evidence that at least one drug has a humanized profile of liver excretion in chimeric mice 34 suggests that chimeric mice could be useful for this purpose. Finally, because chimeric mice are immunocompromised, they cannot be used to analyze immune-mediated drug toxicities.
Moving forward
Because the liver is affected by many drug-induced toxicities, toxicology studies in chimeric mice could have a large impact on drug development. However, we do not yet have definitive examples of drug-induced toxicities that were detected in chimeric mice, but not in conventional rodent models. Analysis of acetaminophen-induced liver toxicity in chimeric uPA/SCID mice demonstrated that the murine and human liver cells were differentially susceptible to this toxicity 35. Although this provides some suggestive evidence, there is a glaring lack of toxicity data for other drugs in chimeric mice. Many drug candidates induced minimal toxicity in preclinical animal studies, but caused significant un-anticipated hepatic toxicities in humans. The most striking example is provided by a nucleoside analogue (fialuridine or FIAU), which had great potential for treatment of HBV infection. FIAU had potent anti-viral activity in vitro and caused minimal toxicity in preclinical animal studies. However, seven of the 15 subjects in the clinical trial unexpectedly developed severe liver failure after three months of FIAU treatment 36, which led to 5 deaths and two participants required liver transplants. After a decade of research, an inter-species difference in the mitochondrial expression of nucleotide transporter (hENT1) was identified as the probable mechanism for the inter-species difference susceptibility to FIAU-induced toxicity 37. It is of crucial importance to determine if FIAU toxicity (or that of other drugs with un-anticipated human-specific toxicity) could be detected in chimeric mice. Because drug-induced toxicity studies require a longer dosing period (weeks to months) and liver histology is analyzed, these studies will place additional demands on the chimeric models. To minimize confounding effects, the chimeric model should not have ongoing model-related liver toxicity, nor should it require exogenous drugs (for immune suppression or to promote survival). Related to this, several drugs (e.g. bosentan) have caused un-anticipated cholestatic toxicity due to inter-species differences in liver transporters 38. Therefore, a more thorough characterization of biliary transporter expression, canalicular function and measurement of hepatic clearance for a larger set of test drugs in chimeric mice are also needed.
Concluding remarks
Chimeric mice with humanized livers have great potential – so far unrealized – to improve modern drug development by alerting researchers to the possibility of adverse hepatic events early in the development process. Recent publications have demonstrated that studies performed using chimeric mice can predict the pattern of human drug metabolism, and in a few limited cases, human drug responses. However, to impact drug development, studies performed in chimeric mice must shift away from the short-term dosing studies that measure the production of human drug metabolites, which have been the focus of chimeric mouse studies over the past decade. To increase their utility for drug development, it will be important to perform longer-term studies in chimeric mice examining whether human-specific drug-induced toxicities (i.e. FIAU) can be detected, or that characterize the pharmacodynamic effects of drugs (or drug combinations) on infectious agents (HCV). Although these longer term studies are more complex and costly than the studies currently performed in chimeric mice; they will provide very valuable information for drug development, they can be performed under conditions where confounding variables can be controlled, and at a fraction of the cost of similar studies in human subjects.
Acknowledgements
This work is dedicated to the memory of Dr. Tatsuji Nomura, whose vision and leadership enabled the TK-NOG project to proceed. G.P. was partially supported by funding from a transformative RO1 award (1R01DK090992) provided by the NIDDK. I thank Drs. Ying-Ying Guo and Haili Zhang for reviewing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest.
References
- 1.Anderson S, et al. Predicting circulating human metabolites: how good are we? Chem Res Toxicol. 2009;22:243–256. doi: 10.1021/tx8004086. [DOI] [PubMed] [Google Scholar]
- 2.Walker D, et al. A holistic strategy for characterizing the safety of metabolites through drug discovery and development. Chem Res Toxicol. 2009;22:1653–1662. doi: 10.1021/tx900213j. [DOI] [PubMed] [Google Scholar]
- 3.Leclercq L, et al. Which human metabolites have we MIST? Retrospective analysis, practical aspects, and perspectives for metabolite identification and quantification in pharmaceutical development. Chem Res Toxicol. 2009;22:280–293. doi: 10.1021/tx800432c. [DOI] [PubMed] [Google Scholar]
- 4.Smith DA, Obach RS. Metabolites in safety testing (MIST): considerations of mechanisms of toxicity with dose, abundance, and duration of treatment. Chem Res Toxicol. 2009;22:267–279. doi: 10.1021/tx800415j. [DOI] [PubMed] [Google Scholar]
- 5.Guengerich FP, MacDonald JS. Applying mechanisms of chemical toxicity to predict drug safety. Chem Res Toxicol. 2007;20:344–369. doi: 10.1021/tx600260a. [DOI] [PubMed] [Google Scholar]
- 6.Lesko LJ, Schmidt S. Individualization of drug therapy: history, present state, and opportunities for the future. Clin Pharmacol Ther. 2012;92:458–466. doi: 10.1038/clpt.2012.113. [DOI] [PubMed] [Google Scholar]
- 7.Guo Y, et al. Pharmacogenomics and drug development. Pharmacogenomics. 2005;6:857–864. doi: 10.2217/14622416.6.8.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandgren EP, et al. Complete hepatic regeneration after somatic deletion of an albumin-plasminogen activator transgene. Cell. 1991;66:245–256. doi: 10.1016/0092-8674(91)90615-6. [DOI] [PubMed] [Google Scholar]
- 9.Meuleman P, et al. Morphological and biochemical characterization of a human liver in a u PA-SCID mouse chimera. Hepatology. 2005;41:847–856. doi: 10.1002/hep.20657. [DOI] [PubMed] [Google Scholar]
- 10.Lootens L, et al. The uPA(+/+)-SCID mouse with humanized liver as a model for in vivo metabolism of 4-androstene-3,17-dione. Drug Metab Dispos. 2009;37:2367–2374. doi: 10.1124/dmd.109.028183. [DOI] [PubMed] [Google Scholar]
- 11.Tateno C, et al. Near Completely Humanized Liver in Mice Shows Human-Type Metabolic Responses to Drugs. American Journal of Pathology. 2004;165:901–912. doi: 10.1016/S0002-9440(10)63352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katoh M, Yokoi T. Application of Chimeric Mice with Humanized Liver for Predictive ADME. Drug Metabolism Reviews. 2007;39:145–157. doi: 10.1080/03602530601021340. [DOI] [PubMed] [Google Scholar]
- 13.Suemizu H, et al. Establishment of a humanized model of liver using NOD/Shi-scid IL2Rgnull mice. Biochem Biophys Res Commun. 2008;377:248–252. doi: 10.1016/j.bbrc.2008.09.124. [DOI] [PubMed] [Google Scholar]
- 14.Sandgren EP, et al. Complete hepatic regeneration after somatic deletion of an albumin-plasminogen activator transgene. Cell. 1991;66:245–256. doi: 10.1016/0092-8674(91)90615-6. [DOI] [PubMed] [Google Scholar]
- 15.Azuma H, et al. Robust expansion of human hepatocytes in Fah−/−/Rag2−/−/Il2rg−/−mice. Nature Biotechnology. 2007;25:903–910. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cederbaum SD, et al. Amino Acid Metabolism. In: Rimoin DL, et al., editors. Principles and Practice of Medical Genetics. 3 edn. Churchill Livingstone; 1997. pp. 1872–1875. [Google Scholar]
- 17.Ito M, et al. NOD/SCID/gamma c null mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa M, et al. The reconstituted 'humanized Liver' in TK-NOG mice is mature and functional. Biochem Biophys Res Commun. 2011;405:405–410. doi: 10.1016/j.bbrc.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishimura T, et al. Using Chimeric Mice with Humanized Livers to Predict Human Drug Metabolism and a Drug-Drug Interaction. Journal of Pharmacology And Experimental Therapeutics. 2013;344:388–398. doi: 10.1124/jpet.112.198697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu Y, et al. Human Pharmacogenetic Analysis in Chimeric Mice with ‘Humanized Livers’. Pharmacogenetics and Genomics. 2013;344:388–396. doi: 10.1097/FPC.0b013e32835cb2c7. [DOI] [PubMed] [Google Scholar]
- 21.Chen AA, et al. Humanized mice with ectopic artificial liver tissues. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1101791108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katoh M, et al. In vivo drug metabolism model for human cytochrome P450 enzyme using chimeric mice with humanized liver. Journal of Pharmaceutical Sciences. 2007;96:428–437. doi: 10.1002/jps.20783. [DOI] [PubMed] [Google Scholar]
- 23.Pozo OJ, et al. Detection and structural investigation of metabolites of stanozolol in human urine by liquid chromatography tandem mass spectrometry. Steroids. 2009;74:837–852. doi: 10.1016/j.steroids.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Kamimura H, et al. Assessment of chimeric mice with humanized liver as a tool for predicting circulating human metabolites. Drug Metab Pharmacokinet. 2010;25:223–235. doi: 10.2133/dmpk.25.223. [DOI] [PubMed] [Google Scholar]
- 25.Sanoh S, et al. Prediction of in vivo hepatic clearance and half-life of drug candidates in human using chimeric mice with humanized liver. Drug Metab Dispos. 2012;40:322–328. doi: 10.1124/dmd.111.040923. [DOI] [PubMed] [Google Scholar]
- 26.Sanoh S, et al. Predictability of metabolism of ibuprofen and naproxen using chimeric mice with human hepatocytes. Drug Metab Dispos. 2012;40:2267–2272. doi: 10.1124/dmd.112.047555. [DOI] [PubMed] [Google Scholar]
- 27.De Serres M, et al. Evaluation of a chimeric (uPA+/+)/SCID mouse model with a humanized liver for prediction of human metabolism. Xenobiotica. 2011;41:464–475. doi: 10.3109/00498254.2011.560295. [DOI] [PubMed] [Google Scholar]
- 28.Bissig K-D, et al. Human liver chimeric mice provide a model for hepatitis B and C virus infection and treatment. Journal of Clinical Investigation. 2010;120:650–653. doi: 10.1172/JCI40094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohara E, et al. Elimination of hepatitis C virus by short term NS3-4A and NS5B inhibitor combination therapy in human hepatocyte chimeric mice. J Hepatol. 2011;54:872–878. doi: 10.1016/j.jhep.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 30.Trusheim MR, et al. Quantifying factors for the success of stratified medicine. Nat Rev Drug Discov. 2011;10:817–833. doi: 10.1038/nrd3557. [DOI] [PubMed] [Google Scholar]
- 31.Weinshilboum R, Wang L. Pharmacogenomics: Bench to Bedside. Nature Reviews Drug Discovery. 2004;3:739–748. doi: 10.1038/nrd1497. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, et al. Drug interactions evaluation: an integrated part of risk assessment of therapeutics. Toxicol Appl Pharmacol. 2010;243:134–145. doi: 10.1016/j.taap.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 33.Bode C. The nasty surprise of a complex drug-drug interaction. Drug Discov Today. 2010;15:391–395. doi: 10.1016/j.drudis.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Okumura H, et al. Humanization of Excretory Pathway in Chimeric Mice with Humanized Liver. Toxicological Sciences. 2007;97:533–538. doi: 10.1093/toxsci/kfm041. [DOI] [PubMed] [Google Scholar]
- 35.Sato Y, et al. Human hepatocytes can repopulate mouse liver: histopathology of the liver in human hepatocyte-transplanted chimeric mice and toxicologic responses to acetaminophen. Toxicol Pathol. 2008;36:581–591. doi: 10.1177/0192623308318212. [DOI] [PubMed] [Google Scholar]
- 36.McKenzie R, et al. Hepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis B. N Engl J Med. 1995;333:1099–1105. doi: 10.1056/NEJM199510263331702. [DOI] [PubMed] [Google Scholar]
- 37.Lee EW, et al. Identification of the mitochondrial targeting signal of the human equilibrative nucleoside transporter 1 (hENT1): implications for interspecies differences in mitochondrial toxicity of fialuridine. J Biol Chem. 2006;281:16700–16706. doi: 10.1074/jbc.M513825200. [DOI] [PubMed] [Google Scholar]
- 38.Fattinger K, et al. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther. 2001;69:223–231. doi: 10.1067/mcp.2001.114667. [DOI] [PubMed] [Google Scholar]