Abstract
Objective
Persons developing schizophrenia (SCZ) manifest various premorbid neuropsychological deficits, studied most often by measures of IQ. Far less is known about premorbid neuropsychological functioning in individuals who later develop bipolar psychoses (BP). We evaluated the specificity and impact of family history (FH) of psychosis on premorbid neuropsychological functioning.
Methods
We conducted a nested case-control study investigating the associations of neuropsychological data systematically collected at age 7 years for 99 adults with psychotic diagnoses (including 45 SCZ and 35 BP) and 101 controls, drawn from the New England cohort of the Collaborative Perinatal Project. A mixed model approach evaluated Full Scale IQ, four neuropsychological factors derived from principal components analysis, and the profile of 10 intelligence and achievement tests, controlling for maternal education, race, and intrafamilial correlation. We used a deviant responder approach (< 10%tile) to calculate rates of impairment.
Results
There was a significant linear trend, with the SCZ group performing worst. The profile of childhood deficits for persons with SCZ did not differ significantly from BP. 42.2% of SCZ, 22.9% of BP, and 7% of controls were neuropsychologically impaired. Presence of psychosis in first-degree relatives (FH+) significantly increased the severity of childhood impairment for SCZ but not for BP.
Conclusions
Premorbid neuropsychological deficits are found in a substantial proportion of children who later develop SCZ, especially in the SCZ FH+ subgroup, but less so in BP, suggesting especially impaired neurodevelopment underlying cognition in pre-SCZ children. Future work should assess genetic and environmental factors that explain this FH effect.
Introduction
Kraepelin’s (Kraepelin 1919) differentiation of the psychoses into schizophrenia (SCZ) and “Manic-Depressive Insanity” (Bipolar Disorder, BD) as two distinct neuropsychiatric disorders has been a fundamental nosological distinction for over a century. However, there is evidence identifying common as well as distinctive neurobiological features between the two disorders (Lewandowski et al., 2011). For example, there is now some evidence of shared genetic liability between SCZ and BD (Craddock et al., 2006). Regarding pathophysiological similarities and differences, Kraepelin emphasized early cognitive dysfunction in SCZ (i.e, “dementia praecox”), also noted by Bleuler (Bleuler, 1950), whereas these claims were not made about BD. This distinction was highlighted by Murray et al (Murray et al., 2004), who hypothesized that “on a background of shared genetic predisposition to psychosis, schizophrenia, but not bipolar disorder, is subject to additional genes or early insults, which impair neurodevelopment..” (p. 405). Studies directly comparing SCZ and BD with psychotic features (“BP”) provide a strong test of Kraepelin’s model, because the disorders share psychotic symptoms. The fact that neuropsychological deficits are more severe in individuals with BP compared to BD without psychosis (Glahn et al., 2007; Bora et al., 2010a) supports the idea that contrasting BP with SCZ provides an informative test of specificity and severity.
There is overwhelming evidence of neuropsychological impairment in SCZ, from the first episode of psychosis onward (Heinrichs and Zakzanis, 1998; Mesholam-Gately et al., 2009). The evidence for neuropsychological impairment in BD, especially BP, is growing (Bearden et al., 2001; Bora et al. 2010b), but this literature is substantially less comprehensive (Lewandowski et al., 2011). There is, additionally, evidence that persons with SCZ have more severe neuropsychological impairment than individuals with BP (Seidman et al., 2002; Bora et al. 2010c; Lewandowski et al., 2011). Characterizing neuropsychological impairments prior to the onset of psychosis could shed light on which specific cognitive functions are shared or distinct apart from the confounds introduced after the disorders manifest, particularly the effects of medications.
Retrospective and prospective studies of individuals with SCZ indicate that premorbid neurocognitive deficits can be demonstrated in childhood. This was definitively shown by a meta-analysis of 18 English language studies of premorbid IQ demonstrating a consistent IQ decrement of approximately .50 standard deviations (SD) in children and adolescents who later develop SCZ (Woodberry et al., 2008). IQ (Cannon et al., 2000; Seidman et al., 2006; Woodberry et al., 2008), reasoning (Niendam et al., 2003; Reichenberg et al., 2010), attention (Cornblatt et al., 1999; Niendam et al. 2003), and language (Cannon et al., 2002; Niendam et al., 2003) deficits have been reported as early as age 4 in children who later develop SCZ.
There are fewer studies comparing youth who later go on to develop BD and even fewer examining premorbid neurocognition in BP. A prospective investigation using the Wisconsin Card Sort in adolescence found significantly more participants who later developed BD had impairments than those who developed unipolar depression or no mood disorder (Meyer et al., 2004). Four conscript studies directly compared premorbid intellectual functioning in affective psychosis or BD versus SCZ (Reichenberg et al., 2002; Zammit et al., 2004; Tiihonen et al., 2005; Urfer-Parnas et al., 2010), but none specifically distinguished a BP group from BD or from a mixed group of affective psychoses. Moreover, conscript studies that recruit adolescents at ages 16–25 likely include some participants with attenuated psychotic (“prodromal”) symptoms that manifest qualitatively different and more severe neuropsychological impairments than observed premorbidly in early childhood (Seidman et al., 2010).
In general, participants who developed major affective disorders or affective psychoses did not differ on neurocognition from unaffected comparisons. An exception was in a Finnish cohort study in which male conscripts had premorbid visuospatial deficits that were associated with later development of both BD and SCZ (Tiihonen et al., 2005). Because the four conscript studies either excluded individuals with BP, or did not distinguish between BP and BD, the association between childhood cognitive impairments and adult BP remains largely unexplored.
It is of interest that none of these studies examined the effect of family history (FH) on neurocognition. This is a key gap because FH is the strongest known susceptibility factor for SCZ and affective psychoses. Moreover, there is a large literature indicating a significant association between FH of SCZ, and neurocognitive impairments in IQ, attention and memory (Johnstone et al., 2005; Keshavan et al., 2010; Agnew-Blais and Seidman, 2012), brain structure (Boos et al., 2007) and brain function (MacDonald et al., 2009) in children, adolescents and adults. IQ deficits (Goldstein et al., 2000) were somewhat greater in children at high risk (HR) for SCZ than for affective psychoses, and attention impairments were worse in HR for SCZ than affective disorders (Ott et al., 1998), but neither directly studied BP. Moreover, the presence of FH augmented neuropsychological impairment in those who converted to psychosis amongst putatively prodromal adolescents (Seidman et al., 2010). However, to our knowledge, no one has reported whether a FH of psychosis amplifies neuropsychological impairment in non-prodromal younger children assessed decades before they develop SCZ or BP. Finally, it is of potential clinical significance to identify children who are neuropsychologically impaired. If neuropsychological impairments contribute to childhood disability in school or to prediction of later psychosis, then the amelioration of such deficits may be an important early intervention strategy. Thus, use of deviant responder approaches may identify clinically meaningful subgroups of impaired individuals.
In this study, we assessed neuropsychological functioning of preteen children who later went on to develop psychosis, using a more extensive battery of neuropsychological tests than used in most prior studies. We also stratified the samples by presence or absence of FH of psychosis. We tested three hypotheses: 1). Children who later develop psychoses have neuropsychological impairment compared to controls; 2). Children who develop SCZ will show the most impaired neuropsychological functioning compared to children who develop BP and control children, both at the group level and in frequency of individuals identified as impaired; 3). Those with a positive FH of psychosis will be most impaired.
Methods
The New England Family Study Sample
Participants were selected from the Boston and Providence cohorts of the Collaborative Perinatal Project (CPP), currently known as the New England Family Study (NEFS). The CPP was initiated over 50 years ago to investigate prospectively the prenatal and familial antecedents of pediatric, neurological, and psychological disorders of childhood (Niswander and Gordon, 1972). Details of the CPP methodology and NEFS studies are reported in previous publications (Myrianthopoulos and French, 1968; Buka et al., 1999; Goldstein et al., 2000; Seidman et al., 2000; Seidman et al., 2006; Goldstein et al., 2010). Approximately 55,000 pregnancies were followed, including about 17,000 in New England. Many types of assessments, including psychological examinations, were conducted through 7 years of age when the study officially ended in 1973.
Ascertainment and Assessment of the Adult Psychotic Cases in NEFS
Cohort members with psychosis were identified through a systemic follow-up of the entire NEFS cohort of the CPP. NEFS parents and offspring with history of psychiatric hospitalization and/or possible psychotic and bipolar illness were identified from the following sources: 1) record linkages with public hospitals, mental health clinics, and the Massachusetts and Rhode Island Departments of Mental Health; 2) several follow-up and case-control studies nested within the larger NEFS cohort, involving direct interviews with approximately 20% of the cohort; and 3) reports from participants in these interview studies of a family member with a history of psychotic or bipolar symptoms or diagnosis. Adult offspring with major psychoses within NEFS cohorts were identified through a two-stage diagnostic assessment procedure from 1996 to 2007, approximately 30 years after and blind to prior assessments. In the first stage, 249 individuals with possible psychotic illness were identified through record linkages and from personal interviews. This included 109 subjects who reported psychotic symptoms identified through interviews (Robins et al., 1989), and 140 subjects with a history of treatment for psychotic illnesses identified through record linkage.
In the second stage, those who consented to participate in follow-up efforts were interviewed using the Structured Clinical Interview for DSM-IV (SCID, First et al., 1996). Based on interview data and medical record review, trained Ph.D.- and M.D.-level diagnosticians then completed best-estimate consensus diagnoses according to DSM-IV criteria (American Psychiatric Association, 1994) for lifetime prevalence of psychotic and other psychiatric disorders.
Diagnostic interviews were completed for 173 subjects; medical charts alone were available for the remaining 76 subjects. 114 subjects were determined to have a non-organic psychotic disorder, and 99 of them had been tested at age 7. Nine of 99 cases were determined to have psychosis from medical chart alone. Those tested are:
Schizophrenia disorders (SCZ), n = 45 (schizophrenia [n = 41], schizoaffective, depressed type (SAD) [n = 4]).
Other non-affective psychoses (NAP), n = 10 (delusional disorder [n = 2], brief psychosis [n = 1], nonaffective psychoses – type not specified [n = 7]).
Affective psychoses (AP), n = 44 (schizoaffective bipolar type (SAB) [n = 11], bipolar disorder with psychotic features (BP) [n= 24], major depressive disorder with psychosis (MDD-P) [n = 9].
Ascertainment and Assessment of the Controls
Controls were selected from families participating in the control arm of a NEFS family HR study in which the control population was a random stratified sample of parents selected from the entire NEFS cohort, with no known history of psychosis or other major Axis I disorders. Further, parents and grandparents, as well as the parents’ siblings, had to be free of any known lifetime history of psychosis, bipolar, schizotypal, recurrent major depressive disorder, suicide attempts, or psychiatric hospitalizations (described in Goldstein et al., 2010). Siblings of the controls had to also be free of any lifetime history of psychosis or bipolar disorder.
Among the 132 control parents included in the NEFS HR study, there were 186 offspring potential controls for this study. Of these offspring, 145 (78%) were interviewed. Of the 145 prospective controls 37 were excluded (25.5%): 15 (9.5%) had a diagnosis of recurrent MDD, 8 (4.8%) had personality disorders, 11 (6.8%) had psychosis or bipolar disorder, 1 (0.7%) was a sibling of a case, and 2 (1.4%) had a history of psychosis in a relative. Of the 108 in the final control sample, 101 had testing at age 7.
Measurement of Family History of Psychosis
Family history of psychiatric disorders was determined from direct interviews of parents and siblings using the Diagnostic Interview Schedule (Robins et al., 1989) and the Family Interview for Genetic Studies (FIGS, Maxwell, 1996), and interviews of all available adult offspring using the SCID and the FIGS. FH was dichotomized as FH positive (FH+: presence of psychosis in 1st degree relatives) and FH negative (FH−: no psychosis in 1st degree relatives). Based on these criteria, there were 16 FH+ and 13 FH− individuals in the BP/SAB group and 8 FH+ and 28 FH− individuals in the SCZ group. The remaining subjects did not have FIGS data.
Exclusion criteria
Exclusion criteria for all adult participants were a history of neurological disease, traumatic brain injury, medical illness or alcohol-related disease with documented cognitive sequelae, major sensory impairments (e.g., deafness), IQ less than 65 in adulthood or inability to understand the procedures, fewer than eight years of formal education, and severe substance abuse within the past six months. All psychotic cases were living in the community when assessed. Human subjects approval was granted by Institutional Review Boards at Harvard University, Brown University, and local psychiatric facilities. Written consent was obtained from all interviewed participants, and subjects were compensated for their participation.
Relationship to Prior Reports
In a preliminary report (Seidman et al., 2006), 46% of individuals analyzed for this report (31 SCZ, 61 controls) were studied, focusing only on IQ measures.
Neuropsychological Measures at Age 7
Neurocognitive data was collected at 7 years of age (1966–1973). The assessment used a battery of 13 psychological tasks including seven subtests from the Wechsler Intelligence Scale for Children (WISC, Wechsler,1949), three Wide Range Achievement Test (WRAT) measures of reading, spelling and arithmetic, and three tests of auditory-vocal associations, visual-motor integration, and tactile form recognition (Seidman et al., 2000). The WISC subtests were the scaled scores for Vocabulary, Information, Comprehension, Digit Span, Digit Symbol Coding, Block Design, and Picture Arrangement. Details of the tests and the original principal components analysis (PCA) from data acquired on 11,889 children (Seidman et al., 2000) are reported in the Supplement as is our approach to producing four factors for these analyses: “Academic Achievement Skills”, “Verbal-Conceptual Ability”, “Perceptual- Motor Abilities” and “Attention-Working Memory”.
Demographics
An index of socioeconomic status (SES), adapted from the Bureau of the Census, derived from the education and occupation of the head of household along with household income, was assigned to each pregnancy (Myrianthopoulos and French, 1968), along with other demographic variables including child’s age at interview, mother’s age, mother’s education, and ethnicity.
Data Analysis
Neuropsychology
The measures of cognitive functioning comprised a Full Scale intelligence quotient (IQ) based on 7 WISC subtests, four normalized factor scores derived from all 13 tests, and ten individual scores from the tests of intelligence (n=7) and achievement (n=3) used in profile analysis.
Group Contrasts
To test hypothesis 1, we compared Psychotic cases with controls. To test hypothesis 2 regarding specificity, we compared controls, SCZ and BP/SAB. BP and SAB groups were combined to increase power. To test hypothesis 3, FH+ versus FH− within group, and with controls were compared.
Statistical Approach
Data analyses employed mixed linear models. Least-squares means and standard errors were adjusted for intrafamilial correlation and potential confounders were examined: age at childhood testing, maternal age, SES, maternal education, ethnicity, and gender. Covariates that were significantly associated with the outcome in univariate analyses were retained. Given the potential collinearity between SES and maternal education, maternal education was selected for the final model, as it explained a greater amount of variance. The final models controlled for maternal education and race. Subject gender was added to those models in which it was significant. For models that included FH of psychosis, models were adjusted for maternal age as it was found to be a significant predictor.
For profile analyses using the 10 WISC and WRAT subtests, parameters derived from regression equations based on the control sample were used to predict the neuropsychological test scores for all subjects in the study, conditional on maternal education and race. The residuals (predicted minus the observed scores) for the whole study sample were then standardized based on the control distribution for each test such that each had a mean of 0 and standard deviation (SD) of 1 in the control group. The average of each cognitive test score in cases, represented as the mean standardized residual, was measured as the number of SDs above or below the control mean. When comparing more than two groups (hypothesis 2), analyses of linear trend preceded pairwise comparisons. For profiles, using generalized linear models, we assessed magnitude of each group’s mean (level) across cognitive domains (group effect). If this was significant, univariate tests followed as well as flatness of the groups across domains (domain effect), and profile shape or parallelism of the groups between domains (domain by group interaction). We used p < .05 as our significance level for a priori overall tests (i.e., tests for linear trends, overall profile, schizophrenia < controls). For subsequent pair-wise comparisons after a significant overall test (i.e, BP/SAB vs SCZ or controls), we used .05/3 = < .0167 for significance.
For the deviant responder analyses for the 10 WISC and WRAT variables, we chose two cut-offs commonly used in neuropsychology: < one SD, or < the 10%tile below the control mean. For identifying individuals as impaired, we chose the threshold of at least 3 or more of 10 test scores (30%) as abnormal at the above thresholds. Data are presented on analyses below the 10th percentile because the < one SD approach was too liberal, identifying more than 20% of controls as neuropsychologically impaired. Comparisons between case and control groups were made using χ2 tests. Fisher’s exact test was used for those comparisons based on one or more cells with 5 or less subjects.
Results
Demographics
Cases with psychoses and controls were similar on age at testing, maternal age at birth of subject, SES, and offspring sex (see Table 1). Mothers of cases had fewer years of education and were more often of African- American ethnicity than mothers of controls. There were a higher percentage of males among those with SCZ than in BP/SAB or controls. As adults, SCZ and BP/SAB groups were comparable on duration of illness, number of hospitalizations, total positive symptoms, and differed marginally on total negative symptoms (table 1, P’s > .05).
Table 1.
Demographic Data for Cases of Psychosis and Controls: Childhood and Adult Characteristics
| Childhood Characteristics | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Controls | 2. All psychoses† | 3. BP/SAB‡ | 4. Schizophrenia disorders (SD) | Statistical Comparisons | |||||
| (N=101) | (N=99) | (N=35) | (N=45) | ||||||
| Continuous variables† | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age at interview | 7.02 | 0.1 | 7.05 | 0.2 | 7.05 | 0.1 | 7.07 | 0.2 | NS |
| Maternal age | 26.4 | 6.0 | 25.4 | 6.3 | 25.2 | 5.9 | 25.8 | 6.9 | NS |
| Maternal education, yrs | 11.4 | 2.4 | 10.5 | 2.1 | 10.8 | 2.0 | 10.4 | 2.2 | 1>2* |
| Categorical variables‡ | N | % | N | % | N | % | N | % | |
| Sex: Male | 49 | 48.5 | 59 | 59.6 | 17 | 48.6 | 35 | 77.8 | 1<4**; 3<4** |
| Ethnicity: Non-Caucasian | 6 | 5.9 | 21 | 21.2 | 7 | 20.0 | 10 | 22.2 | 1<2*, 1<3*, 1<4** |
| Parental SES | NS | ||||||||
| 1 – lowest quartile | 21 | 21.7 | 27 | 27.8 | 7 | 20.0 | 14 | 32.6 | |
| 2 | 28 | 28.9 | 24 | 24.7 | 8 | 22.9 | 10 | 23.3 | |
| 3 | 20 | 20.6 | 27 | 27.8 | 11 | 31.4 | 12 | 27.9 | |
| 4 – highest quartile | 28 | 28.9 | 19 | 19.6 | 9 | 25.7 | 7 | 16.3 | |
| Adult characteristics | |||||||||
| Continuous variables† | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Duration of psychosis, yrs | -- | -- | -- | -- | 17.3 | 5.1 | 15.4 | 4.9 | NS |
| Number of psychiatric hospitalizations | -- | -- | -- | -- | 3.6 | 2.4 | 3.5 | 1.6 | NS |
| SANS total | -- | -- | -- | -- | 6.0 | 3.6 | 8.1 | 4.9 | NS |
| SAPS total | -- | -- | -- | 3.8 | 3.4 | 4.7 | 4.3 | NS | |
p<0.05,
p<0.01,
p<0.001. P values from mixed models adjusted for intrafamilial correlation. Variables were log transformed prior to analysis if there were significant skew in the distribution |>0.8|, these variables were Age at Interview, Maternal Age, Number of psychiatric hospitalizations, and SAPS Total.
All psychoses includes major depressive disorder with psychotic features, (N=9), other psychoses (N=10) and groups 3 and 4 for total N=99
BP/SAB is Bipolar Psychosis/Schizoaffective Disorder, Bipolar Type
Maternal age, SES (social-economic status) and Maternal Education calculated at study entry
Sample sizes for adult clinical data on BP/SAB and SD subjects based on available data: Duration of Psychosis (BP/SAB, n = 17, SD, n = 26); Number of Hospitalizations (BP/SAB, n = 32, SD, n = 34); SANS Total,(BP/SAB, n = 24, SD, n = 25); SAPS Total,(BP/SAB, n = 24, SD = 26)
Neuropsychological Data
The age 7 IQ scores were significantly lower for children who developed psychoses compared to controls (9.8 points) as well as on academic achievement, verbal ability and attention/working memory factors (all p <.01, Table 2 and Figure 1). Though cases scored lower than controls on the perceptual-motor factor, the difference was not significant.
Table 2.
Prospective Age 7 Childhood Data from Adult Cases and Controls: Full Scale IQ and Four Neuropsychological Factor Scores (Means and Standard Deviations)
| Controls | All psychoses∂ | Affective psychoses | Schizophrenia disorders | Linear test for trend¶ | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | BP/SAB‡ (Group 2 & 3) | 2. BP w/psychosis | 3. Schizoaffective bipolar disorder (SAB) | Schizophrenia disordersξ (Group 4 & 5) | 4. Schizophrenia | 5. Schizoaffective depressed-type | F value | p value | ||||||||||
| N=101 | (N=99) | (N=35) | (N=24) | (N=11) | (N=45) | (N=41) | (N=4) | |||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Full scale IQ | 106.8 | 12.6 | 97.0*** | 14.9 | 100.7 | 12.8 | 100.7 | 12.4 | 100.5 | 14.1 | 95.8** | 15.3 | 96.2 | 15.8 | 91.3 | 10.0 | 20.3 | <0.001 |
| Factor 1 (academic achievement) | 0.23 | 0.8 | −0.31** | 1.2 | 0.08 | 1.0 | 0.01 | 1.0 | 0.25 | 1.0 | −0.52** | 1.2 | −0.55 | 1.2 | −0.21 | 1.4 | 11.5 | <0.001 |
| Factor 2 (verbal ability) | 0.34 | 0.9 | −0.36** | 1.1 | −0.13 | 0.9 | −0.13 | 0.9 | −0.14 | 0.9 | 0.43**ξ | 1.1 | −0.41 | 1.2 | −0.60 | 0.5 | 10.3ξ | 0.002 |
| Factor 3 (perceptual motor) | 0.15 | 1.0 | −0.31 | 1.1 | −0.23 | 0.9 | −0.07 | 0.7 | −0.56 | 1.3 | −0.30 | 1.1 | −0.31 | 1.1 | −0.22 | 1.2 | 1.4 | 0.237 |
| Factor 4 (attention and working memory) | 0.34 | 0.8 | −0.31*** | 1.1 | 0.06 | 1.1 | 0.07 | 1.1 | 0.05δ | 1.1 | −0.51*** | 1.1 | −0.48 | 1.1 | −0.82 | 0.6 | 14.8 | <0.001 |
p<0.05,
p<0.01,
p<0.001. Mixed model adjusted for maternal education, maternal age, child’s age and race, and intrafamilial correlation. Pair-wise comparisons were performed comparing each diagnostic subgroup (all psychoses, BP/SAB, schizophrenia disorders) to column 1 (controls). Subgroups presented for descriptive purposes.
BP/SAB is Bipolar Psychosis/Schizoaffective Disorder, Bipolar Type
Tests of significance additionally adjusted for child’s sex
There were no significant pair-wise differences between BP/SAB and schizophrenia disorders groups
Linear Tests for Trend compare Controls (N=101), BP/SAB (N=35) and Schizophrenia Disorders (N=45)
Figure 1.
Full Scale IQ and Standardized Measures of Neuropsychological functioning at Age 7 in Adults who Developed Psychosis Including Persons with Schizophrenia Disorders (n=45), Bipolar Psychosis and Schizoaffective, Bipolar type combined (n=35), and Controls (n=101) *p < .05, **p<.01, ***p<.001 compared to controls (Least Square Means)
Analyses of a priori predictions of linear trend showed that the SCZ group was lowest and the BP/SAB group was intermediate. The SCZ group was significantly lower than controls on IQ, academic achievement, verbal ability and attention/working memory (p < .01). None of the comparisons between psychosis subgroups were significant (Table 2 and figure 1).
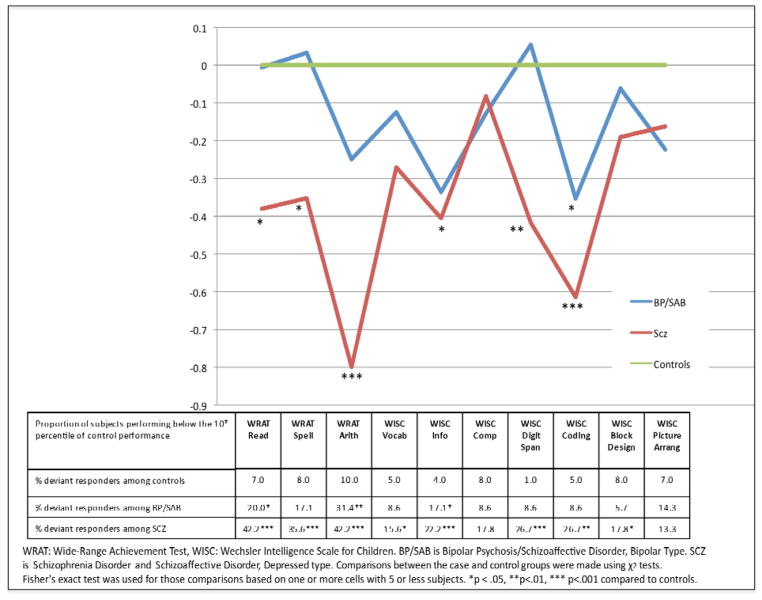
The overall profile of the IQ and achievement test scores (Figure 2) for the SCZ group was significantly lower than for those in the control group (overall protected F = 5.38, P = 0.005). Furthermore, the profiles of the BP/SAB and SCZ groups were not “flat”, suggesting significant variation in performance across cognitive domains (F = 2.33, P = 0.017). However, the results did not indicate an interaction between cognitive subtest and diagnostic group (F = 1.22, P = 0.242).
Figure 2.
Profile analysis of mean performance and rates of abnormal performance on individual tests (<10%tile) of WRAT and WISC Performance at Age 7 for Adults who Subsequently Developed Psychosis, Including Schizophrenia and Schizoaffective Disorder, Depressed type (n=45), Bipolar Psychosis and Schizoaffective, Bipolar type combined (n=35), and Controls (n=101)
Visual inspection (see Supplemental Table 1) and exploratory analyses on individual IQ subtests shows that the SCZ group had non-significantly lower scores than the BP/SAB group on all 10 individual measures. Compared with controls, SCZ were significantly lower on WRAT Arithmetic and WISC Coding (p < .001) and WISC Digit Span (p < .01). Raw scores and effect sizes are presented in supplemental table 1. There were no significant differences between BP/SABs and controls.
Deviant Responder Analyses
At the < 10th %tile threshold at p ≤ .05, 8/10 tests were abnormal in SCZ compared to controls, all except Comprehension and Picture Arrangement. At that threshold, 3/10 tests were abnormal in BP/SABs compared to controls: Information, Reading and Arithmetic. The children who later developed SCZ were significantly more likely (p< .05) to be impaired than BP/SAB on Digit Span (26.7% >8.6%), Digit Symbol Coding (26.7% > 8.6%) and Reading (42.2% > 20%). Forty two percent of SCZ, 22.9% of BP, and 7% of controls were neuropsychologically impaired using this threshold.
Family History
SCZ FH+ subjects had significant IQ impairment compared to controls (17.2 points lower), whereas the SCZ FH-was lower by about 1/2 SD (7.9 points) but not significantly. The difference in IQ between the SCZ FH+ and FH− subjects was significant (9.1 points, P = 0.003) (Table 3, figure 3). Within SCZ, there was a significant linear trend (Controls > FH−/SCZ > FH+/SCZ) for verbal ability and attention/working memory factors, yielding a significant pair-wise difference (FH− > FH+) for verbal ability. There were no significant effects of FH on BP/SAB. The majority of the BP/SAB cases with FH+ (87.5%) had a parent with a diagnosis of affective psychosis. Among the eight SCZ cases FH was more mixed: 25% were SCZ, four had psychosis NOS, and two had affective psychosis.
Table 3.
Prospective Age 7 Childhood Data from Adult Cases and Controls: Full Scale IQ and Four Neuropsychological Factor Scores, by Presence of Family History of Psychosis (Means and Standard Deviations)
| Controls | All psychoses∂ (N=80) | BP/SAB‡ (N=29) | Schizophrenia disorders (N=36) | Linear Tests for trend | fam hx+ vs fam hx− within diagnostic subgroups | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | NC > FH−BP/SAB > FH+BP/SAB | NC> FH−/SCZ > FH+/SCZ | |||||||||||
| Fam hx + | Fam hx− | Fam hx + | Fam hx− | Fam hx + | Fam hx− | F value | p value | F value | p value | ||||||||||
| N=101 | N=32 | N=48 | N=16 | N=13 | N=8 | N=28 | |||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||||
| Full scale IQ | 106.8 | 12.6 | 96.1** | 15.1 | 98.6* | 13.2 | 99.7 | 12.0 | 101.7 | 14.7 | 89.6** | 20.6 | 98.7 | 11.9 | 2.4 | 0.123 | 8.7 | 0.004 | 6<7** |
| Factor 1 (academic achievement) | 0.23 | 0.8 | −0.23 | 1.2 | −0.27* | 1.1 | 0.06 | 1.0 | 0.29 | 1.1 | −0.61* | 1.7 | −0.48* | 1.1 | 0.2 | 0.691 | 3.8 | 0.054 | NS |
| Factor 2 (verbal ability) | 0.34 | 0.9 | −0.46*** | 1.1 | −0.25* | 0.9 | −0.25 | 0.9 | −0.10 | 0.9 | −0.86* | 1.7 | −0.24 | 0.9 | 3.3δ | 0.070 | 9.3δ | 0.003 | 6<7** |
| Factor 3 (perceptual motor) | 0.15 | 1.0 | −0.41 | 1,2 | −0.24 | 1.1 | −0.32 | 1.1 | 0.11 | 0.8 | −0.67 | 1.7 | −0.27 | 1.0 | 1.5 | 0.221 | 1.6 | 0.213 | NS |
| Factor 4 (attention and working memory) | 0.34 | 0.8 | −0.24** | 1.0 | −0.18** | 1.1 | 0.00 | 1.0 | 0.19 | 1.3 | −0.82** | 1.2 | −0.24* | 1.0 | 1.6 | 0.210 | 8.5 | 0.004 | NS |
p<0.05,
p<0.01,
p<0.001. Mixed model adjusted for maternal education, maternal age, race, and intrafamilial correlation. Pair-wise comparisons were performed comparing each group in columns 2–7 (all psychoses fam hx +, all psychoses fam hx −, BP/SAB fam hx+, BP/SAB fam hx−, schizophrenia disorders fam hx + and schizophrenia disorders fam hx−) to column 1 (controls).
BP/SAB is Bipolar Psychosis/Schizoaffective Disorder, Bipolar Type
Tests of significance additionally adjusted for child’s sex
Fam hx + is a positive family history of psychotic illness in first-degree relatives; Fam hx− is absence of family history of psychotic illness in first-degree relatives.
Figure 3.

Full Scale IQ at 7 Years of Age for Adults That Subsequently Developed Psychosis With and Without a Family History of Psychosis in First-Degree Relatives, *p < .05, **p<.01, compared to controls. Controls (n=101), BP/SAB with no family history (N=13), BP/SAB with family history (N=16), Schizophrenia with no family history (N=28) and Schizophrenia with a positive family history of psychosis (N=8) (Least Square Means)
Discussion
This study demonstrated that schizophrenia was associated with significantly lower IQ, academic achievement, verbal ability and attention/working memory in childhood. There was a clear linear trend in performance with controls > BP/SAB > SCZ. Children who developed BP/SAB were statistically indistinguishable from controls. Premorbid neuropsychological deficits in children who later develop SCZ averaged a moderate effect (Cohen’s d = ~ 0.57), whereas the effect in BP/SAB was about 1/2 of that (d = ~0.30) across 13 tests (Supplemental table 1). FH of psychosis had a significant influence on cognitive performance for SCZ but not for BP/SAB. Neurocognitive deficit was particularly pronounced in the SCZ FH+ subgroup (mean d of the 4 factors = 1.08).
Analyses of individual IQ subsets were exploratory and should be interpreted with caution given the possibility of Type I error. The greatest impairments on these subtests were on WRAT Arithmetic (d = 0.91), WISC Digit Span (d = 0.70), and WISC Coding (d = 0.64), tests that involve numbers, rapid processing of mental computation, working memory and temporal information. The WRAT Arithmetic is a timed, written task of computations requiring organization and executive functioning skills including working memory. While we cannot definitively identify the specific cognitive processes impaired in these complex neuropsychological tasks, it is of interest that Digit Symbol Coding is one of the most severely impaired tasks during the prodromal, first episode and chronic phases of illness (Heinrichs and Zakzanis, 1998; Mesholam-Gately et al., 2009; Seidman et al., 2010). Moreover, our findings are consistent with a study from the Philadelphia CPP (Niendam et al., 2003) showing premorbid deficits on Digit Symbol Coding. This suggests that some core neuropsychological deficits found in individuals with schizophrenia are observed in nascent form many years prior to psychosis, and well before the prodromal phase.
These data indicate that family history of schizophrenia plays a major role in childhood cognitive vulnerabilities, consistent with the robust demonstration of cognitive impairments in family HR studies of schizophrenia (Keshavan et al., 2010; Agnew-Blais and Seidman, 2012). Effect sizes of neuropsychological deficits in offspring or young siblings of persons with schizophrenia typically range between 0.3–0.8 on cognitive measures of IQ, verbal ability, attention and working memory (Agnew-Blais and Seidman, 2012). In our study, compared with family HR studies, the effects of FH on cognition are much larger, perhaps because all of the subjects “go on” to develop psychosis compared to about 10% in family HR studies of SCZ.
In general, BP/SAB disorder manifests minor premorbid neuropsychological impairment. While the schizophrenia group performance was not significantly lower than the BP/SAB group, there was a clear linear trend, and the mean effect size differences across the 10 WISC and WRAT measures for those with BP/SAB was d = 0.28, ranging from clinically meaningful differences of d = 0.51 on Information to trivial differences of d = 0.09 on Digit Span. These small differences are consistent with family HR studies showing modest associations with IQ (Goldstein et al., 2000) and neuropsychological functioning in BD (Reichenberg et al., 2002; Meyer et al., 2004; Zammit et al., 2004; Tiihonen et al., 2005; Urfer-Parnas et al., 2010).
Limitations and Strengths of the study
The main limitations of this study are relatively modest power to detect differences in individual diagnostic groups and the constraint of using tests that were selected in the 1960s that preclude precise clarification of cognitive mechanisms. While the clinical tests used are multifactorial and preclude identifying specific neuropsychological processes, the intelligence and achievement tests are commonly used in clinical settings and have utility for clinicians. Moreover, the positive results with the SCZ group are clear and consistent with prior studies, while the marginal results in the BP group are also consistent with a smaller but growing body of work indicating mild premorbid deficits. While the sample size of the BP group is modest, when combined with the SAB group, its size was similar to that for the SCZ group. The direct comparison of these groups in premorbid neuropsychological functioning is novel and consistent with Murray’s hypothesis regarding more clear-cut neurodevelopmental impairments in SCZ than BP (Murray et al., 2004).
The constraint of a limited sample size is particularly relevant in the comparison of the FH+ and FH− groups, where a relatively small group of FH+ individuals show significantly larger deficits compared to FH− SCZ individuals. So while the striking impact of FH+ on premorbid neuropsychological functioning was specific to schizophrenia, future research on larger samples are needed to confirm these findings. Given that some of the cases were identified through the companion HR study (Goldstein et al., 2010) from which we ascertained our controls, our rate of FH of psychosis was higher than typical in part due to our identifying a number of the cases through personal interview of the offspring of parents with psychosis.
Our study also raises some issues regarding ascertainment. We anticipate that our linkage procedures with tertiary public hospitals would tend to identify persons with greater severity and of lower SES, and would under-represent high functioning cases with no hospitalizations and those receiving treatment exclusively from private hospital settings. However in contrast, inclusion of persons and family members identified through direct follow-up and interview studies tends to identify participants with greater residential stability, levels of independent functioning and SES. Due to the various methods of case ascertainment used, we anticipate that both poles of the psychosis severity spectrum are likely to be slightly overrepresented in the current study, with no extreme bias towards patients of higher or lower severity. Moreover, all of the patients were stable outpatients, and their IQ estimate was typical of premorbid IQ derived from meta-analysis (Woodberry et al., 2008). Thus, from the point of view of the best-studied premorbid neurocognitive measure, they are representative of schizophrenia. Finally, we considered the possibility that ascertainment of a presumably episodic disorder (BP/SAB) versus a more chronic one (SCZ) might account for the results. We consider this unlikely because the two psychosis groups did not differ on duration of illness, number of hospitalizations or total positive symptoms, although there was a trend for more negative symptoms in the SCZ group (table 1).
Clinical Implications and Future Directions
The neuropsychological deficits in pre-SCZ were observed on tests commonly used in schools and clinical settings (e.g., WISC, WRAT). This may be clinically meaningful because deficits were roughly 1.0 SD in the context of a FH+ for schizophrenia. Moreover, a sizeable proportion of future cases (42.2% SCZ and 22.9% BP/SAB) were considered abnormal. School psychologists need to be aware of the constellation of cognitive impairments and a positive family history of psychosis in a school-age child and not misdiagnose this syndrome as attention-deficit/hyperactivity disorder or other disorder given some cognitive similarities.
The greater severity of cognitive findings for SCZ versus BP suggests that FH for SCZ may implicate genes associated with greater cognitive impairment compared with genes associated with BP. That is, FH for BP did not augment cognitive deficits but it did augment risk for BP diagnosis (Goldstein et al., 2010), whereas FH for SCZ augmented cognitive impairment as well as its role in risk for SCZ. Of course, while the FH+ group suggests “genetic risk” and is probably associated with the neural substrates of the illness, it is imperative to directly study the FH+ factors before concluding that they are attributable largely to inherited variations, as other environmental factors (e.g., perinatal complications, environmental stress) may prove to be equally important.
Supplementary Material
Acknowledgments
Grant Support and Other Acknowledgements: This research was supported by grants from the Stanley Medical Research Institute (LJS & SLB); the National Association for Research on Schizophrenia and Depression (LJS), the National Institute of Mental Health, MH-63951 (LJS), MH-56956 (JMG), MH-50647 (MTT/JMG), and the Commonwealth Research Center (SCDMH82101008006), Massachusetts Department of Mental Health (LJS). We thank the following people for help in carrying out this study: Lindsay Barker, Jennifer Burbridge, Michael Cirillo, Ph.D., Lisa Denny, M.D., Jo-Anne Donatelli, Ph.D., Christine Fetterer, Jennifer Koch, MPH, William S. Kremen, Ph.D., Elizabeth Olson, Ph.D, Christiana Provencale, Anne Peters Remington, William S. Stone, Ph.D.
Footnotes
Presentations: Dr. Seidman presented some of this data at the 6th annual Mt. Sinai Conference on Cognition in Schizophrenia, Colorado Springs, Col. April 2003, at the Society of Biological Psychiatry annual meeting, Toronto, Canada, May, 2006, at the 1st Schizophrenia International Research Society meeting, in Venice, Italy, June, 2008, and at the International Early Psychosis Association meeting in Amsterdam, Holland, November, 2010.
Conflict of Interest Statement: The following authors do not have any conflicts of interest to declare: Larry J. Seidman, Sara Cherkerzian, Jill M. Goldstein, Jessica Agnew-Blais, Ming T. Tsuang, Stephen L. Buka.
References
- Agnew-Blais J, Seidman LJ. Neurocognition in youth and young adults under age 30 at familial risk for schizophrenia: A quantitative and qualitative review. 2012. Submitted Manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Hoffman KM, Cannon TD. The neuropsychology and neuroanatomy of bipolar affective disorder: A critical review. Bipolar Disorders. 2001;3:106– 150. doi: 10.1034/j.1399-5618.2001.030302.x. [DOI] [PubMed] [Google Scholar]
- Bleuler E. Dementia Praecox or the Group of Schizophrenias. International Universities Press; New York, New York: 1950. [Google Scholar]
- Boos HB, Aleman A, Cahn W, Hulshoff Pol H, Kahn RS. Brain volumes in relatives of patients with schizophrenia: a meta-analysis. Archives of General Psychiatry. 2007;64:297–304. doi: 10.1001/archpsyc.64.3.297. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, D.C: 1994. [Google Scholar]
- Bora E, Yücel M, Pantelis C. Neurocognitive markers of psychosis in bipolar disorder: A meta-analytic study. Journal of Affective Disorders. 2010a;127:1–9. doi: 10.1016/j.jad.2010.02.117. [DOI] [PubMed] [Google Scholar]
- Bora E, Yücel M, Pantelis C. Cognitive impairment in affective psychoses: A meta-analysis. Schizophrenia Bulletin. 2010b;36:112–125. doi: 10.1093/schbul/sbp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Yücel M, Pantelis C. Cognitive impairment in schizophrenia and affective psychoses: Implications for DSM-V and beyond. Schizophrenia Bulletin. 2010c;36:36–42. doi: 10.1093/schbul/sbp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buka SL, Goldstein JM, Seidman LJ, Zornberg GL, Donatelli JA, Denny LR, Tsuang MT. Impacts of perinatal hypoxia and genetic vulnerability on schizophrenia: The New England longitudinal studies of schizophrenia. Psychiatric Annals. 1999;29:151–156. [Google Scholar]
- Cannon M, Caspi A, Moffitt TE, Harrington H, Taylor A, Murray RM, Poulton R. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Archives of General Psychiatry. 2002;59:449–456. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Bearden C, Hollister JM, Rosso IM, Sanchez LE, Hadley T. Childhood cognitive functioning in schizophrenia patients and their unaffected siblings. Schizophrenia Bulletin. 2000;26:379–393. doi: 10.1093/oxfordjournals.schbul.a033460. [DOI] [PubMed] [Google Scholar]
- Cornblatt B, Obuchowski M, Roberts S, Pollack S, Erlenmeyer-Kimling L. Cognitive and behavioral precursors of schizophrenia. Developmental Psychopathology. 1999;11:487–508. doi: 10.1017/s0954579499002175. [DOI] [PubMed] [Google Scholar]
- Craddock N, O’Donovan MC, Owen MJ. Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophrenia Bulletin. 2006;32:9–16. doi: 10.1093/schbul/sbj033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JWB. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition, Version 2. American Psychiatric Press; Washington, D.C: 1996. [Google Scholar]
- Heinrichs R, Zakzanis K. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, Soares JC, Velligan DI. The neurocognitive signature of psychotic bipolar disorder. Biological Psychiatry. 2007;62:910–916. doi: 10.1016/j.biopsych.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Buka SL, Horton N, Donatelli J, Rieder RO, Tsuang MT. Impact of genetic vulnerability and hypoxia on overall intelligence by age 7 in offspring at high risk for schizophrenia compared with affective psychosis. Schizophrenia Bulletin. 2000;26:323–334. doi: 10.1093/oxfordjournals.schbul.a033456. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Buka SL, Seidman LJ, Tsuang MT. Specificity of familial transmission of schizophrenia psychosis spectrum and affective psychoses in the New England family study’s high-risk design. Archives of General Psychiatry. 2010;67:458–67. doi: 10.1001/archgenpsychiatry.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone EC, Ebmeier KP, Miller, Owens DG, Lawrie SM. Predicting schizophrenia: findings from the Edinburgh High-Risk Study. British Journal of Psychiatry. 2005;186:18–25. doi: 10.1192/bjp.186.1.18. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Kulkarni SR, Bhojraj T, Francis A, Diwadkar V, Montrose DM, Seidman L, Sweeney J. Premorbid cognitive deficits in young relatives of schizophrenia patients. Frontiers in Human Neuroscience. 2010;3:1–14. doi: 10.3389/neuro.09.062.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E. Dementia Praecox and Paraphrenia. E. & S. Livingston; Edinburgh: 1919. [Google Scholar]
- Lewandowski KE, Cohen BM, Öngur D. Evolution of neuropsychological dysfunction during the course of schizophrenia and bipolar disorder. Psychological Medicine. 2011;41:225–241. doi: 10.1017/S0033291710001042. [DOI] [PubMed] [Google Scholar]
- MacDonald A, Thermenos HW, Barch D, Seidman LJ. Imaging genetic liability to schizophrenia: Systematic review of fMRI studies of patients’ nonpsychotic relatives. Schizophrenia Bulletin. 2009;35:1142–1162. doi: 10.1093/schbul/sbn053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell ME. FIGS. Clinical Neurogenetics Branch, Intramural Research Program, NIMH; Bethesda, MD: 1996. [Google Scholar]
- Mesholam-Gately R, Giuliano AJ, Faraone SV, Goff KP, Seidman LJ. Neurocognition in first-episode schizophrenia: A meta-analytic review. Neuropsychology. 2009;23:315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Meyer SE, Carlson GA, Wiggs EA, Martinez PE, Ronsaville DS, Klimes-Dougan B, Gold PW, Radke-Yarrow M. A prospective study of the association among impaired executive functioning, childhood attentional problems, and the development of bipolar disorder. Developmental Psychopathology. 2004;16:461–76. doi: 10.1017/s095457940404461x. [DOI] [PubMed] [Google Scholar]
- Murray RM, Sham P, Van Os J, Zanelli J, Cannon M, McDonald C. A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophrenia Research. 2004;71:405–416. doi: 10.1016/j.schres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Myrianthopoulos NC, French KS. An application of the US Bureau of the Census socioeconomic index to a large, diversified patient population. Social Science and Medicine. 1968;2:283–299. doi: 10.1016/0037-7856(68)90004-8. [DOI] [PubMed] [Google Scholar]
- Niendam T, Bearden C, Rosso I, Sanchez LE, Hadley T, Nuechterlein KH, Cannon TD. A prospective study of childhood neurocognitive functioning in schizophrenic patients and their siblings. American Journal of Psychiatry. 2003;160:2060–2062. doi: 10.1176/appi.ajp.160.11.2060. [DOI] [PubMed] [Google Scholar]
- Niswander KR, Gordon M. The Women and Their Pregnancies: The Collaborative Perinatal Study of the National Institute of Neurological Diseases and Stroke. U.S. Department of Health, Education, and Welfare; Government Printing Office; Washington, DC: 1972. [Google Scholar]
- Ott SL, Spinelli S, Rock D, Roberts S, Amminger GP, Erlenmeyer-Kimling The New York high-risk project: Social and general intelligence in children at risk for schizophrenia. Schizophrenia Research. 1998;31:1–11. doi: 10.1016/s0920-9964(98)00010-3. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Weiser M, Rabinowitz J, Caspi A, Schmeidler J, Mark M, Kaplan Z, Davidson M. A population-based cohort study of premorbid intellectual, language, and behavioral functioning in patients with schizophrenia, schizoaffective disorder, and nonpsychotic bipolar disorder. American Journal of Psychiatry. 2002;159:2027–35. doi: 10.1176/appi.ajp.159.12.2027. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Caspi A, Harrington H, Houts R, Keefe RSE, Murray RM, Poulton R, Moffitt TE. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: A 30-year study. American Journal of Psychiatry. 2010;167:160–169. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LD, Helzer TE, Cottler L. NIMH Diagnostic Interview Schedule, Version III-R. Washington University Medical School; St. Louis, MO: 1989. [Google Scholar]
- SAS Software. SAS Institute Inc; Cary, NC, USA: 2008. Version 9.2. [Google Scholar]
- Seidman LJ, Buka SL, Goldstein JM, Horton N, Rieder RO, Donatelli J, Tsuang MT. The relationship of prenatal and perinatal complications to cognitive functioning at age 7 in the New England Cohorts of the National Collaborative Perinatal Project. Schizophrenia Bulletin. 2000;26:309–321. doi: 10.1093/oxfordjournals.schbul.a033455. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Kremen WS, Koren D, Faraone SV, Goldstein JM, Tsuang MT. A comparative profile analysis of neuropsychological functioning in patients with schizophrenia and bipolar psychoses. Schizophrenia Research. 2002;53:31–44. doi: 10.1016/s0920-9964(01)00162-1. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Buka SL, Goldstein JM, Tsuang MT. Intellectual decline in schizophrenia: Evidence from a prospective birth cohort 28 year follow-up study. Journal of Clinical and Experimental Neuropsychology. 2006;28:225–242. doi: 10.1080/13803390500360471. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, McGlashan TH, Perkins DO, Tsuang MT, Walker EF, Woods SW, Bearden CE, Christensen BK, Hawkins K, Heaton R, Keefe RSE, Heinssen R, Cornblatt B. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: Relationship to family history and conversion to psychosis. Archives of General Psychiatry. 2010;67:578–588. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J, Haukka J, Henriksson M, Cannon M, Kieseppa T, Laaksonen I, Sinivuo J, Lonnqvist J. Premorbid intellectual functioning in bipolar disorder and schizophrenia: results from a cohort study of male conscripts. American Journal of Psychiatry. 2005;162:1904–1910. doi: 10.1176/appi.ajp.162.10.1904. [DOI] [PubMed] [Google Scholar]
- Urfer-Parnas A, Mortensen EL, Saebye D, Parnas J. Pre-morbid IQ in mental disorders: a Danish draft-board study of 7486 psychiatric patients. Psychological Medicine. 2010;40:547–556. doi: 10.1017/S0033291709990754. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler Intelligence Scale for Children. The Psychological Corporation; New York, New York: 1949. [Google Scholar]
- Woodberry K, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: A meta-analytic review. American Journal of Psychiatry. 2008;165:579–587. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- Zammit S, Allebeck P, David AS, Dalman C, Hemmingsson T, Lundberg I, Lewis G. A longitudinal study of premorbid IQ score and risk of developing schizophrenia, bipolar disorder, severe depression and other nonaffective psychoses. Archives of General Psychiatry. 2004;61:354–360. doi: 10.1001/archpsyc.61.4.354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.