Abstract
More than 750 million people are at risk of infection with food-borne liver flukes. Opisthorchis viverrini is considered among the most important of these parasites, due to its strong association with cholangiocarcinoma (CCA). O. viverrini infection results in a chronic inflammatory challenge to the host, which can lead to advanced, pathogen-specific disease sequelae including obstructive jaundice, hepatomegaly, cholecystitis, as well as CCA. However, before disease sequelae are apparent, important inflammatory changes to the liver can be detected early during O. viverrini infection. In a case-control study involving 328 men and women with O. viverrini infection, we determined the presence of advanced periductal fibrosis in asymptomatic, O. viverrini-infected individuals and then measured cytokine responses to O. viverrini excretory/secretory products (ES). In the 200 participants with advanced periductal fibrosis (cases), levels of Interleukin (IL)-6 to O. viverrini ES were 8 times higher than levels of the 128 O. viverrini-infected individuals without advanced periductal fibrosis (controls). Moreover, elevated IL-6 to parasite ES was associated with increased risk of advanced periductal fibrosis by 63% in a model adjusted for sex and age. The risk of advanced periductal fibrosis was also found to increase with higher levels of IL-6: individuals in the third quartile of IL-6-ES production had a 127% higher risk of developing advanced periductal fibrosis than individuals in the first quartile of IL-6 production. O. viverrini-infected individuals with advanced periductal fibrosis showed other hepatobiliary abnormalities, including reduced gallbladder contractility and the presence of gallbladder sludge.
Conclusion
These data strongly implicate a role for parasite specific IL-6 in the pathogenesis of advanced periductal fibrosis in opisthorchiasis, with possible links to other hepatobiliary abnormalities, including cholangiocarcinoma.
Keywords: chronic inflammation, opisthorchiasis, cytokine, periductal fibrosis, case-control study
Introduction
Food-borne trematodiases represent an important group of communicable diseases, and one of the most clinically significant infectious pathogens behind malaria, tuberculosis, and HIV (1, 2). At least 750 million people (10% of the world’s population) are at risk of food-borne trematodiases, with more than 40 million people currently infected (1, 3). Opisthorchis viverrini is considered among the most important of the food-borne trematodes due to its strong association with liver disease (4) and cholangiocarcinoma (CCA) (5, 6). Humans become infected with O. viverrini by consuming raw or undercooked fish that contain the infective stage. The parasites migrate up the biliary tract, establish a chronic infection (on average 4–5 years) in the intrahepatic bile ducts, and produce eggs that are excreted in the feces. While the infection is effectively eliminated by the anthelminthic praziquantel, environmental and cultural factors of East Asia strongly favor the process of re-infection (7, 8).
O. viverrini infection represents a chronic inflammatory challenge to the host. The sustained production of growth factors and fibrogenic cytokines in response to local mechanical, toxic, and immune irritation results in a persistent inflammatory condition in the liver that progressively remodels and destroys the normal tissue architecture of the biliary epithelium (3, 9, 10). The cumulative damage caused by this chronic inflammation can lead to advanced, pathogen-specific disease sequelae including obstructive jaundice, hepatomegaly, cholecystitis, and CCA (4, 11). Whereas these are the acknowledged signs of morbidity in opisthorchiasis, important inflammatory changes to the liver can occur early in the course of the disease (12, 13), most of which will remain clinically silent unless actively detected by ultrasound or other imaging modalities (12, 14). Previous community-based ultrasound studies in O. viverrini endemic areas of Northeastern Thailand suggest that hepatobiliary abnormalities such as enlargement of the left hepatic lobe and the gallbladder, loss of gallbladder contractility, presence of sludge, and increased periportal fibrosis are common (14, 15). Due to the high prevalence of O. viverrini infection in East Asia, which can be as high 60–70% in populations resident in endemic areas (11), asymptomatic hepatobiliary abnormalities may represent the greatest part of the disease burden associated with opisthorchiasis. Multiple risk factors are likely to determine whether the host develops advanced periductal fibrosis from O. viverrini infection, including the duration of infection (15), the intensity of the infection (14, 15), and diet (nitrosamines) (16). However, the risk factors that induce periductal fibrosis are likely to pass through the pro-inflammatory cytokine network, which includes Transforming Growth Factor-β, Interleukin (IL)-1-α, and IL-6 (17).
IL-6 is a pleiotropic cytokine with a broad range of humoral and cellular immune effects relating to inflammation, host defense, and tissue injury (18). It is of particular importance in diseases where the pathology arises from chronic inflammation (see references 17 and 19 for review). Although crucial for the quick induction of an innate immune response (19), the persistent production of IL-6 may be central to the state of chronic inflammation induced during O. viverrini infection. Elevated levels of IL-6 have been reported in almost every chronic inflammatory disease of the liver (17), including alcoholic hepatitis (20) and hepatitis B (HBV) and hepatitis C (HCV) viruses (21–26). More importantly, IL-6 appears to be a pivotal cytokine for cholangiocarcinogenesis. In chronically inflamed biliary epithelium, (e.g., during O. viverrini infection), epithelial cells are constantly stimulated to participate in the inflammation by continuously secreting chemokines and cytokines (3, 4), creating a cellular microenvironment beneficial to cancer growth. In this regard, IL-6 appears to be a pivotal cytokine for both inflammation and cholangiocarcinogenesis (17, 19).
On the basis of this background information, we sought to determine whether levels of pro-inflammatory cytokines are elevated among O. viverrini-infected individuals with advanced periductal fibrosis. We further sought to determine whether the association between markers of chronic inflammation and their association with advanced periductal fibrosis were modified by other factors including age, sex, and the intensity of O. viverrini infection.
Materials and Methods
Study Design and Setting
The current study represents an analysis of baseline data collected from a community–based, case-control study of the risk factors associated with the development of advanced periductal fibrosis in opisthorchiasis. Individuals from seven villages in the vicinity of the regional capital, Khon Kaen, Khon Kaen Province, Thailand were surveyed (27) (Supplemental Figure S1). For the current study, 1,032 individuals were screened for O. viverrini infection, with 554 positive (53.7 %) for O. viverrini infection. From this group of infected individuals, 200 males and females between the ages of 20 and 60 years old with advanced periductal fibrosis were enrolled as “cases” and asked to provide 30 ml of whole blood for baseline immunology and hematological parameters. Individuals who were positive for O. viverrini but negative for advanced fibrosis were defined as “controls” and matched with cases based on geographic location of residence (nearest-neighbor). As such, 128 individuals were identified as controls and were asked to provide 30 ml of blood for baseline immunology and hematological parameters. All individuals (cases and controls) positive for O. viverrini were referred to the local public health outpost for treatment with praziquantel, (standard of care). This study was approved by the Ethics Committee of Khon Kaen University School of Medicine, Khon Kaen, Thailand (reference number HE480528) and the Institutional Review Board of the George Washington University School of Medicine, Washington, D.C (GWUMC IRB# 020864).
Ultrasonography
A mobile, high resolution ultrasound (US) machine (GE model LOGIQ Book XP) was used. Hepatobiliary Abnormalities (HBA) including portal vein radical echoes, echoes in liver parenchyma, indistinct gallbladder wall, gallbladder size, sludge and suspected CCA were graded and recorded as previously described (28, 29). For the study of gallbladder function (contractility), the gallbladder was measured before and then 30 minutes after consumption of a fatty meal (28). Individuals were classified as periductal fibrosis grade 0 when no echoes were observed in any segment of the liver (Figure 1; Panel A); 1+ when echoes were observed in 1 segment of the liver (Figure 1; Panel B); 2+ when echoes were observed in 2 or 3 segments of the liver (Figure 1; Panel C); and, 3+ when echoes were observed in greater than 3 segments of the liver (Figure 1; Panel D). Individuals were then dichotomized into cases and possible controls as follows: “Non-Advanced Fibrosis” or “control” if the US grade was 0 or 1, and “Advanced Fibrosis” or “case” if the US grade was 2 or 3. Two radiologists performed US readings over the course of the study. A weighted kappa score was used to quantify level of agreement between the two readers (30). If a kappa fell below 0.6, a third ultrasonographer was consulted to read the image and determine the final outcome.
Figure 1.
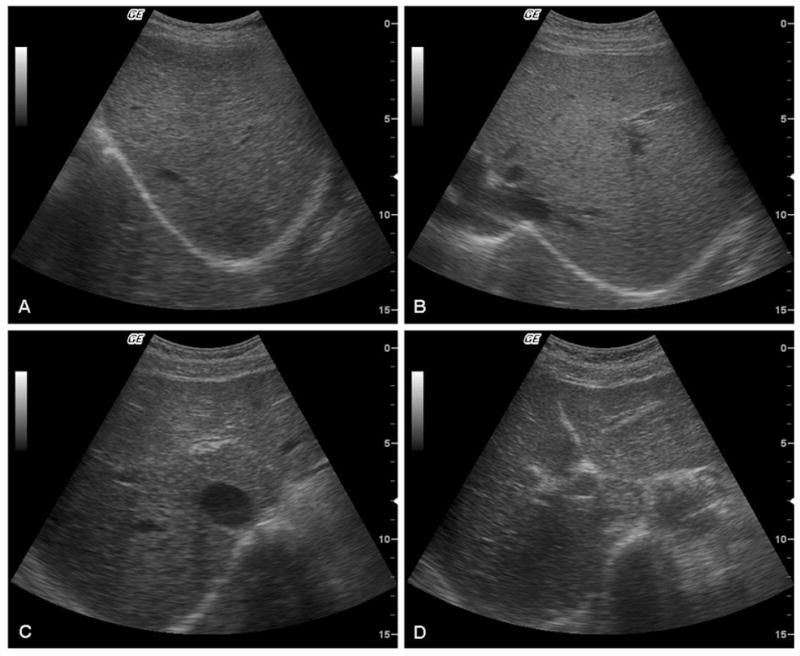
Representative ultrasonographs, revealing increasing periportal echoes and markedly enlarged gallbladder, in individuals categorized as grade 0 (panel A), 1+ (B), 2+ (C) and 3+ (D) periportal fibrosis. ). Individuals were classified as periductal fibrosis grade 0 when no echoes were observed (A); 1+ when echoes were observed in 1 segment of the liver (B); 2+ when echoes were observed in 2 or 3 segments of the liver (C); and, 3+ when echoes were observed in greater than 3 segments of the liver (D). Individuals were then dichotomized into Non-advanced fibrosis” or “control” if the ultrasonography (US) grade was 0 or 1 and “Advanced Fibrosis” or “case” if the US grade was 2 or 3.
Fecal exams
Two grams of feces were weighed and preserved in 10 ml of 10% formalin in a 15 ml screw-cap centrifuge tube, labeled, mixed and kept at room temperature until processing for quantitative formalin/ethyl acetate concentration technique (15). The presence and intensity of O. viverrini infection was determined by quantitative formalin/ethyl acetate technique and expressed as eggs per gram of feces (epg). Eggs/parasite identification and egg counts were under light microscopy at 10× and 40× magnifications, with the results reported as the number of eggs per gram of stool.
Urine pregnancy testing
β-hCG testing was performed at the study site using urine pregnancy test kits approved by Thai and/or US regulatory agencies.
Parasite antigen preparation
Excretory/secretory antigen (ES) of cultured O. viverrini adult worms obtained from experimentally infected hamsters was prepared as described (31). In order to eliminate any possible stimulatory effects in tissue culture from bacterial lipopolysaccharide (LPS) in crude O. viverrini antigen preparations, LPS was removed from the crude antigen extract by phase separation using Triton X-114 (32). These studies were approved by the Animal Ethics Committee of Khon Kaen University (#0514.1.12.2/23) and the Institutional Animal Care and Use Committee, George Washington School of Medicine.
Blood collection by venipuncture
Approximately 2 ml of blood was collected in EDTA-coated tubes for hematology, and approximately 20 ml of whole peripheral blood was obtained in heparinized tubes for cellular immunology and cytokines assays.
Liver Functions Tests
Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), and Alkaline phosphatase (ALP) assays in serum samples from cases and controls were performed on an automated Synchron CX-4 system (Beckman Coulter, Fullerton, CA) according to the manufacturer’s instructions.
ELISA
O. viverrini-specific IgG, IgG1, IgG2, IgG3 and IgG4 were determined by indirect ELISA and parasite-specific IgE was examined by indirect avidin-biotin ELISA as described in Sripa and Kaewkes (31).
Cellular immune response and cytokine measurements
Cytokine production by PBMC in the presence of O. viverrini ES were examined at 0, 48 and 72 hours for levels of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, INFγ, TNFα, and TNFβ production. A whole blood culture technique was used for cytokine stimulation. Appropriately diluted (1:16) heparinized whole blood was cultured in RPMI 1640 medium in 24-well tissue culture plates in the presence of 35 μg/ml of O viverrini ES crude antigen extract or 2.5 μg/ml for phytohaemagglutinin (PHA)-L (Difco Laboratories, Detroit, MI, USA). Cells were cultured at 37°C in a humidified 5% CO2 incubator. Supernatants were removed at predetermined times (when maximum secretion of each cytokine was obtained between 48–120 hours of culture) and stored in aliquots at −80°C. Twenty five to 50 μl of supernatant was used for all cytokine assays. The quantification of secreted cytokines was analyzed using commercial FlowCytomix bead-based multiplexing assays kits (Beckman-Coulter). Values were quantified from standard curves using human recombinant cytokines.
Statistical analyses
Cases and controls were compared for dichotomous and categorical variables by percent and then differences tested with Fisher’s Exact test. In the case of continuous variables, two methods were used to assess for statistical significance. When continuous variables were normally distributed, Student’s T-test was used to determine differences between cases and controls. In the special case of gallbladder contractility, where the difference in gallbladder dimensions between pre and post fatty meal were the variable of interest, an Analysis of Covariance (ANCOVA) was used to test for differences between cases and controls. When the data were (a) highly skewed, (b) had a number of outlier values, or (c) were refractory to transformation (e.g. log-transformation), a quantile regression model was used to determine the median along with 95% Confidence Intervals (95% CI) to estimate the differences between cases and controls: e.g., cytokine level, liver function tests, and antibody levels. Where the data suggested a significant difference between cases and controls, such as in the levels of IL-6 to O. viverrini ES, the risk of this parameter on advanced periductal fibrosis was assessed using a logistic regression model. Odds Ratios (OR) were estimated on the risk of advanced periductal fibrosis in crude models and models adjusted for age and sex. In the case of IL-6 production to O. viverrini ES, the variable was treated in its original scale, a categorical scale (using the median value as a cutoff), and an ordinal scale determined by quartiles of IL-6 production to ES. Stata version 10 (StataCorp, College Park, TX) statistical software was used for all analyses. Level of significance in statistical tests was 0.05. All tests were two-tailed tests.
RESULTS
Study Design
The baseline characteristics of the enrolled participants are shown in Table 1, with 200 cases and 128 controls enrolled in the study. Overall, the sample included slightly more females (52%) than males (48%) (P = 0.083). The study sample had a mean age of 45.8 and an age range of 20 to 60 years, with 74% of the study sample over 40 years of age. The mean ages for cases (46.7 years of age) and controls (45.2 years of age) did not differ significantly (P = 0.115). As part of the inclusion criteria of the study, both cases and controls were positive for O. viverrini as determined by a single ovum in feces. No statistically significant difference (P > 0.811) was observed in the intensity of O. viverrini infection between cases (median = 36 epg) and controls (median = 38 epg). As shown in Table 1, in both case and control groups, there was a similar distribution of in the proportion of individuals when intensity of O. viverrini infection was stratified by tertiles of eggs per gram of feces, with the majority of cases (77%) and controls (77%) having less than 499 eggs per gram of feces.
Table 1.
The relationship between advanced periductal fibrosis status as determined by ultrasonography in Opisthorchis viverrini-infected individuals by age, sex and level of infection.
| Advanced Periductal Fibrosis |
||
|---|---|---|
| Negative (0 and 1) | Positive (2 and 3) | |
| Total | 128 | 200 |
| Sex | ||
| Male | 54 (42.2%) | 104 (52.0%) |
| Female | 74 (57.8%) | 96 (48.0%) |
| Age (years) | ||
| 20–29 | 4 (3.1%) | 17 (8.5%) |
| 30–39 | 21 (16.4%) | 42 (21.0%) |
| 40–49 | 56 (43.8%) | 75 (37.5%) |
| 50+ | 47 (36.7%) | 66 (33.0%) |
| Intensity of infection | ||
| 1–499 epg | 98 (76.6%) | 154 (77.0%) |
| 500–999 epg | 16 (12.5%) | 18 (9.0%) |
| >1000 epg | 14 (11.0%) | 28 (14.0%) |
epg refers to eggs per gram of feces.
Individuals with advanced periductal fibrosis show poor gallbladder function
Table 2 shows that the gallbladder did not contract as much after a fatty meal in individuals with advanced periductal fibrosis compared to individuals without advanced periductal fibrosis. In addition, individuals with advanced periductal fibrosis had a greater presentation of sludge than individuals without advanced periductal fibrosis for whom sludge was entirely absent. These data indicate that individuals with advanced periductal fibrosis from O. viverrini have reduced gallbladder function.
Table 2.
The relationship between gall bladder dimensions “pre” and “post” fatty mean and sludge with the presence of advanced periductal fibrosis by case and control status
| Advanced Periductal Fibrosis |
|||
|---|---|---|---|
| Gall bladder abnormalities | Negative Mean (SD) | Positive Mean (SD) | P |
| Post Fatty Meal Reduction† | |||
| Length | 1.88 (1.35) | 1.59 (1.20) | 0.007 |
| Width | 0.71 (0.55) | 0.68 (0.56) | 0.154 |
| Cross-sectional | 0.61 (0.47) | 0.55 (0.57) | 0.261 |
| n (%) | n (%) | ||
| Presence of sludge | 0 | 13 (6.5) | 0.002 |
Refers to the difference in gall bladder dimensions (measured in centimeters) “pre” and “post” fatty meal.
Advanced periductal fibrosis in opisthorchiasis does not appear to affect liver function
There was no difference in the levels of the liver function tests ALT, AST and ALP between cases and controls (Supplementary Table S1), indicating that pathology may be limited to advanced periductal fibrosis and did not affect liver function.
Intensity of infection was not a risk factor for advanced periductal fibrosis in O. viverrini infection
Table 1 shows the individuals by the intensity of O. viverrini infection: “≤ 499 epg” (77%), “500–999 epg” (10%), and “≥ 1,000 epg” (13%). No significant difference was found (P = 0.696) between the median epg for individuals with advanced periductal fibrosis (median, 141 epg) compared to controls (median, 121 epg). Table 2 shows that intensity of infection (expressed as epg) was not a risk factor for advanced periductal fibrosis. When epg was measured at an interval of 1 egg per gram of feces, the odds ratio was 0.99 (95% CI 0.99 to 1.00, P = 0.811) and when measured in the larger interval of 500 eggs per gram of feces the odds ratio remained unchanged 0.99, (95% CI 0.93 to 1.00, P = 0.811). The relationship between baseline levels of epg and advanced periductal fibrosis was not altered by analyses that adjusted for age, sex, or age and sex simultaneously.
Levels of IL-6 to O. viverrini ES were significantly higher in individuals with advanced periductal fibrosis
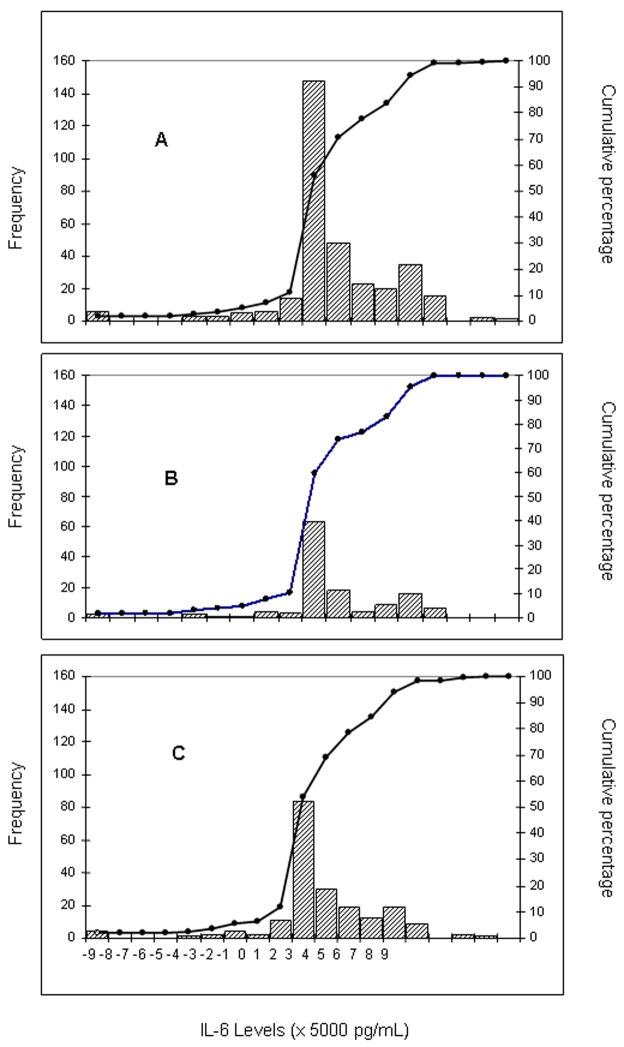
Of the 11 cytokines tested (Supplementary Table S2), only levels of the inflammatory cytokine IL-6 were significantly elevated in individuals with advanced periductal fibrosis (Table 4). The frequency distributions of baseline IL-6 levels to O. viverrini ES are presented for cases and controls combined in Figure 2 Panel A, for cases only in Figure 2 Panel B, and for controls only in Figure 2 Panel C. IL-6 production from PBMC to O. viverrini ES among both cases and controls ranged between −2.7791 and 2.3082 pg/ml, with a range much greater in controls (−27,791 to 23,082 pg/ml) than in cases (−116.4 pg pg/ml to 14,221 pg/ml), indicating far less variation in the IL-6 response to O. viverrini ES among cases than controls. Median baseline levels of IL-6 in the presence of O. viverrini ES were 8 times higher in PBMC from cases than controls (3981 versus 507 pg/ml; P = 0.001). Individuals with “<499 epg” showed 12 times higher levels of IL-6 than controls (4305.9 versus 348.9 pg per ml, (95% CI 1, 500.9 to 5568.6, P = 0.001).
Table 4.
Baseline concentrations (median) of IL-6 in supernatants from peripheral blood mononuclear cells (PBMC) stimulated with Opisthorchis viverrini excretory/secretory products after 48 hours according to Case (Advanced Periportal Fibrosis Positive) and Control (Advanced Periportal Fibrosis Negative) Status and in relationship to sex, age, and intensity of infection
| Unstimulated PBMC |
Stimulated PBMC |
IL-6 Production by PBMC |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Control 128 | Case 200 | P | Control 128 | Case 200 | P | Control 128 | Case 200 | P |
| Total | 999.2 | 1408.9 | 0.478 | 4478.6 | 8415.3 | 0.145 | 507.1 | 3981.2 | 0.001 |
| Sex | |||||||||
| Male | 1842.3 | 1288.0 | 0.501 | 908.3 | 7436.4 | 0.016 | 4.1 | 2772.6 | 0.039 |
| Female | 739.0 | 1506.4 | 0.138 | 8032.1 | 10440.6 | 0.680 | 1404.7 | 4966.2 | 0.082 |
| Age (years) | |||||||||
| 20–29 | 2452.4 | 421.6 | 0.036 | 19.7 | 21.5 | 0.999 | −154.7 | 2.5 | 0.963 |
| 30–39 | 404.4 | 2540.5 | 0.018 | 8262.3 | 3729.6 | 0.530 | 495.9 | 1298.6 | 0.344 |
| 40–49 | 1872.4 | 104.6 | 0.143 | 636.0 | 8790.8 | 0.005 | 3.2 | 3648.2 | 0.014 |
| 50–59 | 820.9 | 1920.4 | 0.030 | 8604.9 | 9217.9 | 0.873 | 1687.8 | 6030.0 | 0.116 |
| Intensity of infection (epg) | |||||||||
| <499 | 959.5 | 1532.0 | 0.318 | 4286.7 | 8603.0 | 0.113 | 348.9 | 4305.9 | <0.001 |
| 500–999 | 3645.7 | 280.5 | <0.001 | 3817.5 | 3918.1 | 0.939 | 2135.9 | 3473.4 | 0.333 |
| 1000–2000 | 2877.2 | 2572.2 | 0.984 | 12694.4 | 9077.3 | 0.991 | 9017.3 | 2870.9 | 0.524 |
Production refers to the median of the differences between Stimulated PBMC and Unstimulated PBMC (control wells) as expressed by the equation: Production = Median (Stimulated PBMC – Unstimulated PBMC).
Figure 2.
The frequency and cumulative percentage of production of Interleukin (IL)-6 to Opisthorchis viverrini excretory/secretory (ES) products among cases and controls in a community-based case control study. The bars refer to frequency of production of IL-6 and the line refers to cumulative frequency of IL-6. Panel A refers to all individuals in the study (n = 328); Panel B refers only to cases (n = 200), and Panel C refers only to controls (n = 128)
Elevated levels of IL-6 to O. viverrini ES significantly increase the risk of developing advanced periductal fibrosis
Table 5 shows that elevated levels of IL-6 to O. viverrini ES at baseline significantly increased the risk (OR = 1.63) of advanced periductal fibrosis in a model adjusted for age and sex (95% CI 1.01 to 2.54, P = 0.048). Moreover, as shown in Table 6, the risk of advanced periductal fibrosis increased with increasing quartiles of IL-6 production to O. viverrini ES: OR = 1.00 for Quartile 1 (reference quartile); OR = 1.39 for Quartile 2; OR = 2.27 for Quartile 3; and OR = 1.64 for Quartile 4. However, only for individuals in Quartile 3 of IL-6 production to O. viverrini ES was the risk (OR = 2.27) statistically significant for advanced periductal fibrosis (95%CI = 1.18 to 4.368, P = 0.014).
Table 5.
Crude and Adjusted Odds Ratios for levels of IL-6 by Peripheral Blood Mononuclear Cells (PBMC) stimulated by Opisthorchis viverrini excretory/secretory products (ES) for advanced periductal fibrosis†.
| Odds Ratio | 95% CI | P-value | |
|---|---|---|---|
| Crude | 1.51 | 0.94, 2.41 | 0.089 |
| Adjusted | |||
| Age | 1.56 | 0.97, 2.51 | 0.066 |
| Sex | 1.55 | 0.96, 2.50 | 0.070 |
| Age and Sex | 1.63 | 1.01, 2.54 | 0.048 |
Refers to” in vitro cultures of PBMC with O. viverrini ES after 48 hours. CI refers to Confidence Interval.
Table 6.
Crude and Adjusted Odds Ratios by quartiles of the difference between IL-6 produced by PBMC that were unstimulated or stimulated by Opisthorchis viverrini excretory/secretory products (ES)for advanced periductal fibrosis among O. viverrini infected individuals.
| Quartile IL-6 in production to O. viverrini ES (range, pg/ml) |
|||||
|---|---|---|---|---|---|
| 1 (−47073.0 to −2.44) | 2 (−2.45 to 2695.8) | 3 (2695.9 to 12519.1) | 4 (12519.2 to 44055.3) | P for Trend | |
| Crude | |||||
| OR | 1.00 | 1.35 | 2.07 | 1.49 | |
| 95% CI | — | 0.73 – 2.50 | 1.09 – 3.92 | 0.80 – 2.78 | P = 0.106 |
| P | — | 0.346 | 0.026 | 0.207 | |
| Adjusted | |||||
| OR | 1.00 | 1.38 | 2.27 | 1.64 | |
| 95% CI | — | 0.74 – 2.59 | 1.18 – 4.38 | 0.87 – 3.08 | NA |
| P | 0.312 | 0.014 | 0.127 | ||
OR indicates Odds Ratio. CI refers to Confidence Interval. Adjusted models include age and sex simultaneously. NA refers to not applicable to adjusted models.
Antibody to O. viverrini ES did not associate with advanced periductal fibrosis
There was no significant increase between cases and controls in levels of IgG and its subclasses (IgG1, IgG2, IgG3, and IgG4) or IgE to O. viverrini ES (Supplementary Table S3). While important for O. viverrini infection, these data indicate that humoral immunity may not be a risk factor for advanced fibrosis.
Discussion
Liver fluke infection with O. viverrini is associated with a number of inflammation-induced hepatobiliary pathologies, the most common of which is periductal fibrosis (28). To our knowledge, this is the first report of an association between an inflammatory cytokine and periductal fibrosis in liver fluke infection. IL-6 levels to O. viverrini ES were on average 8 times higher in O. viverrini-infected individuals with advanced periductal fibrosis compared to O. viverrini-infected individuals without periductal fibrosis. Moreover, baseline PBMC production of IL-6 to O. viverrini ES significantly elevated the risk (63%) of advanced periductal fibrosis in a model adjusted for age and sex, with the risk of advanced periductal fibrosis increasing with increasing IL-6 levels to parasite antigen. The current data strongly support our hypothesis that opisthorchiasis represents a chronic inflammatory disease mediated by pro-inflammatory cytokines, specifically IL-6, which is significantly associated with the development of advanced periductal fibrosis.
Previous community-based ultrasound studies in O. viverrini endemic areas of Northeastern Thailand suggest that hepatobiliary abnormalities such as enlargement of the left hepatic lobe and the gallbladder, loss of gallbladder contractility, presence of sludge, and increased periductal fibrosis are common (15, 28, 34). In the current study, we also document deficits in gallbladder function, specifically reduced gallbladder contractility and the presence of sludge in O. viverrini-infected individuals with advanced periductal fibrosis. Recent studies in animals (34) and humans (4) have shown that opisthorchiasis is associated with chronic cholecystitis, which is characterized by inflammatory cell infiltration and prominent fibrosis of the gallbladder wall. The chronic inflammation and fibrosis of the gallbladder wall (14) can impair gallbladder contractility as observed in the current study among the individuals with advanced periductal fibrosis (14). The poor contractility of the gallbladder as well as the periductal fibrosis of the intrahepatic bile ducts may also cause functional bile stasis (or sludge). This can lead to precipitation of bile contents, which may even include O. viverrini eggs (35). Periportal and periductal fibrosis are among the most prominent histological features in chronic O. viverrini infection in humans (36) and in long term O. viverrini infection in hamsters (37). In a series of human autopsies conducted during the 1970s (38), no detectable changes were observed in the biliary epithelium and periductal areas of the liver in individuals with recent O. viverrini infection, while individuals with chronic O. viverrini infection had a proliferation of epithelial cells and the formation of acini and periductal fibrosis. Our observations support the hypothesis that, as with other chronic helminth infections (8), the asymptomatic abnormalities represent an important component of the disease burden in opisthorchiasis.
Chronic inflammatory disorders such as those seen in opisthorchiasis are induced by persistent irritants that sustain the production of growth factors and fibrogenic cytokines, which in turn stimulate the deposition of connective tissue elements that progressively remodel and destroy normal tissue architecture, resulting in fibrotic elements (10). In opisthorchiasis, the persistent irritation can come from one or both of the following sources: (1) the feeding and migration of flukes (mechanical irritation), when oral and ventral suckers hook onto the biliary epithelium and damage tissue or (2) metabolic products that are released from the tegument and excretory openings of the parasite and enter into the bile duct epithelium of the human host (4). From the current data, we hypothesize that the persistent irritant derives from some component(s) of the crude ES antigen preparation that we employed to stimulate PBMC in vitro, which resulted in elevated IL-6 production. ES antigens have proven to be important immune modulators in a number of helminth infections (39), usually skewing the host cytokine response to create a microenvironment favorable to parasite survival. In the current study, we offer a novel role for helminth ES products: the idea that ES products from O. viverrini may be toxic or may interact with the biliary epithelium as a mitogen (36) which results in chronic inflammation and subsequent periductal fibrosis and possibly even CCA. This hypothesis is consistent with much of the experimental literature on O. viverrini ES products in laboratory models. For example, when O. viverrini adults worms were co-cultured with human biliary cell lines in a non-contact transwell system, they induced a proliferative response (4), indicating that soluble products excreted or secreted from the parasite are indeed capable of stimulating cells to proliferate. A similar observation was also found when O. viverrini adult worms were co-cultured with mouse NIH-3H3 fibroblasts (40). There is also evidence that these immunological responses are, at least in part, specifically evoked by O. viverrini products: in this same non-contact transwell system, T-cell deprived hamsters did not show a proliferative response when co-cultured with adult worms (41). Our study extends these findings by indicating that ES products in O. viverrini-infected humans may cause a persistent irritation, resulting in a chronic inflammatory state, mediated by IL-6, which is strongly associated with advanced periductal fibrosis.
IL-6 is known to play a role in a number of chronic inflammatory conditions of the liver, e.g., alcoholic and viral hepatitis (42, 43) and other well known fibrotic lesions such as keloid pathogenesis (44). One route by which IL-6 may be responsible for periductal fibrosis in opisthorchiasis is the up-regulation of pro-fibrotic cytokines such as TGF–β1 (10), which has been observed in experimental O. viverrini infection in hamsters (45, 46). In addition, IL-6 is a pleiotropic cytokine involved in a variety of inflammation associated cancers (17), including CCA (47). In particular, in chronically inflamed biliary epithelium, epithelial cells are constantly stimulated to participate in the inflammation by continuously secreting chemokines and cytokines, which establishes a cellular microenvironment that is conducive to neoplasia (48). Opisthorchis may be the first helminth infection linking the development of fibrosis with parasite specific IL-6 production.
This study emphasizes the significant relationship between the inflammatory cytokine IL-6 and advanced periductal fibrosis in opisthorchiasis. Although less visible in clinical presentation, asymptomatic hepatobiliary abnormalities in opisthorchiasis may actually represent the greatest part of the chronic disease burden associated with this very prevalent but also neglected tropical disease (7, 8). Further studies are needed to enhance our knowledge of the relationship between pro-inflammatory cytokines, hepatobiliary pathology, and cholangiocarcinogenesis. Such studies will then allow us to identify additional risk factors and biomarkers for disease progression, highlighting those individuals with the highest risk of developing pathologic sequelae and CCA.
Supplementary Material
Supplementary Figure 1 Panel A, outline map of Thailand to indicate the provinces; panel B, map of Khon Kaen province, North-East Thailand, with box showing the location of the study site villages; panel C, remote sensing image of the region of Khon Kaen where the seven villages addressed in the present study are located. The specific location of the seven study villages in relation to the Chi River, which flows from south west to north east are indicated with the yellow colored stars, as follows: 1, Nongnangkwan; 2, Nongmuang; 3, Loopka; 4, Nongkham; 5, Nongno; 6, Lawa; 7, Chikokor. Panel C modified from Digital Thailand image at http://digitalthailand.gistda.or.th/
Table 3.
Crude and Adjusted Odds Ratios for intensity of infection, expressed as egg per gram of feces, for Opisthorchis viverrini infection and advanced periductal fibrosis
| Odds Ratio | 95% CI | P value | |
|---|---|---|---|
| Intervals of 1 epg | |||
| Crude | 0.99 | 0.99, 1.00 | 0.811 |
| Adjusted | |||
| Age | 0.99 | 0.99, 1.00 | 0.987 |
| Sex | 0.99 | 0.99, 1.00 | 0.646 |
| Age and sex | 0.99 | 0.99, 1.00 | 0.818 |
| Interval of 500 epg | |||
| Crude | 0.99 | 0.93, 1.00 | 0.811 |
| Adjusted | |||
| Age | 0.99 | 0.94, 1.06 | 0.987 |
| Sex | 0.99 | 0.93, 1.05 | 0.646 |
| Age and sex | 0.99 | 0.93, 1.06 | 0.818 |
epg refers to eggs per gram of feces. CI refers to Confidence Interval.
Acknowledgments
Financial Support
This research was supported by award number UO1AI065871 from the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the NIH.
We sincerely thank the volunteers who participated in this study. We also thank our regulatory affairs and data management colleagues including Preeyaporn Plaimee, Sangduan Wannachart, Sombat Thinkhamrop, Wilaiporn Thinkhamrop and Jittiawadee Pukdeepromma, and our laboratory and field technicians, including Arpa Surapitoon for assistance with the flow cytometric work. We thank Dr. Pyatat Tatsanavivat and Tarika Samor of the Clinical Research Collaboration Network, Thailand for guidance with compliance issues. This research was supported by award number UO1AI065871 from the National Institute of Allergy and Infectious Diseases (the content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the NIH).
Abbreviations
- CCA
Cholangiocarcinoma
- PBMC
Peripheral Blood Mononuclear Cells
- ES
Excretory/Secretory
- IL
Interleukin
- CI
Confidence Interval
- INF
Interferon
- TNF
Timor Necrosis Factor
- ml
Milliliter
- US
Ultrasonography
- HBA
Hepatobiliary Abnormalities
- LPS
Lipopolysaccharide
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- ALP
Alkaline Phosphatase
- Ig
Immunoglobulin
- ELISA
Enzyme Linked Immunosorbent Assay
- M
Molar
- PBS
Phosphate Buffered Saline
- HRP
Horseradish Peroxidase
- Hr
Hour
- OD
Optical Density
- OPD
Ortho-Phenylenediamine
- ANCOVA
Analysis of Covariance
- OR
Odds Ratio
- Epg
Eggs per Gram of Feces
References
- 1.Keiser J, Utzinger J. Emerging foodborne trematodiasis. Emerg Infect Dis. 2005;11:1507–14. doi: 10.3201/eid1110.050614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311–21. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sripa B, Kwaekes S, Sithithaworn P, Mairiang E, Laha T, Smout M, et al. Liver fluke induces cholangiocarcinoma. PLoS Medicine. 2007:e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sripa B. Pathobiology of opisthorchiasis: an update. Acta Trop. 2003;88:209–20. doi: 10.1016/j.actatropica.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 5.IARC. Schistosomes, liver flukes and Helicobacter pylori. IARC working group on the evaluation of carcinogenic risks to humans. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 6.Parkin DM. The global health burden of infection-associated cancers in the year. Int J Cancer. 2002;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 7.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–9. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- 8.King CH, Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illn. 2008;4:65–79. doi: 10.1177/1742395307084407. [DOI] [PubMed] [Google Scholar]
- 9.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–9. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sithithaworn P, Haswell-Elkins M. Epidemiology of Opisthorchis viverrini. Acta Trop. 2003;88:187–94. doi: 10.1016/j.actatropica.2003.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Mairiang E, Chaiyakum J, Chamadol N, Laopaiboon V, Srinakarin J, Kunpitaya J, et al. Ultrasound screening for Opisthorchis viverrini-associated cholangiocarcinomas: experience in an endemic area. Asian Pac J Cancer Prev. 2006;7:431–3. [PubMed] [Google Scholar]
- 13.Lim JH, Mairiang E, Ahn GH. Biliary parasitic diseases including clonorchiasis, opisthorchiasis and fascioliasis. Abdominal Imaging. 2008;33:157–65. doi: 10.1007/s00261-007-9326-x. [DOI] [PubMed] [Google Scholar]
- 14.Mairiang E, Mairiang P. Clinical manifestation of opisthorchiasis and treatment. Acta Tropica. 2003;88:221–7. doi: 10.1016/j.actatropica.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Elkins DB, Mairiang E, Sithithaworn P, Mairiang P, Chaiyakum J, Chamadol N, et al. Cross-sectional patterns of hepatobiliary abnormalities and possible precursor conditions of cholangiocarcinoma associated with Opisthorchis viverrini infection in humans. Am J Trop Med Hyg. 1996;55:295–301. doi: 10.4269/ajtmh.1996.55.295. [DOI] [PubMed] [Google Scholar]
- 16.Satarug S, Haswell-Elkins MR, Sithithaworn P, Bartsch H, Ohshima H, Tsuda M, et al. Relationships between the synthesis of N-nitrosodimethylamine and immune responses to chronic infection with the carcinogenic parasite, Opisthorchis viverrini, in men. Carcinogenesis. 1998;19:485–91. doi: 10.1093/carcin/19.3.485. [DOI] [PubMed] [Google Scholar]
- 17.Naugler WE, Karin M. The wolf in sheep’s clothing: the role of Interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14:109–19. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Nishimoto N, Kishimoto T. Interleukin 6: from bench to bedside. Nature Clinical Practice Rheumatology. 2006;2:619–626. doi: 10.1038/ncprheum0338. [DOI] [PubMed] [Google Scholar]
- 19.Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–35. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Khoruts A, Stahnke L, McClain CJ, Logan G, Allen JI. Circulating tumor necrosis factor, interleukin-1 and interleukin-6 concentrations in chronic alcoholic patients. Hepatology. 1991;13:267–76. [PubMed] [Google Scholar]
- 21.Kakumu S, Shinagawa T, Ishikawa T, Yoshioka K, Wakita T, Ito Y, Takayanagi M, Ida N. Serum interleukin 6 levels in patients with chronic hepatitis B. Am J Gastroenterol. 1991;86:1804–8. [PubMed] [Google Scholar]
- 22.Mauad TH, van Nieuwkerk CM, Dingemans KP, Smit JJ, Schinkel AH, Notenboom RG, et al. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol. 1994;145:1237–45. [PMC free article] [PubMed] [Google Scholar]
- 23.Malaguarnera M, Trovato BA, Laurino A, Di Fazio I, Romeo MA, Motta M. Interleukin-6 in hepatitis C cirrhosis. Panminerva Med. 1996;38:207–10. [PubMed] [Google Scholar]
- 24.Malaguarnera M, Di Fazio I, Laurino A, Ferlito L, Romano M, Trovato BA. Serum Interleukin 6 concentrations in chronic hepatitis C patients before and after interferon-alpha treatment. Int J Clin Pharmacol Ther. 1997b;35:385–8. [PubMed] [Google Scholar]
- 25.Malaguarnera M, Di Fazio I, Romeo MA, Restuccia S, Laurino A. Trovato BA Elevation of interleukin 6 levels in patients with chronic hepatitis due to hepatitis C virus. J Gastroenterol. 1997b;32:211–5. doi: 10.1007/BF02936370. [DOI] [PubMed] [Google Scholar]
- 26.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–6. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 27.Sriamporn S, Pisani P, Pipitgool V, Suwanrungruang K, Kamsa-ard S, Parkin DM. Prevalence of Opisthorchis viverrini infection and incidence of cholangiocarcinoma in Khon Kaen, Northeast Thailand. Trop Med Int Health. 2004;9:588–94. doi: 10.1111/j.1365-3156.2004.01234.x. [DOI] [PubMed] [Google Scholar]
- 28.Mairiang E, Elkins DB, Mairiang P, Chaiyakum J, Chamadol N, Loapaiboon V, et al. Relationship between intensity of Opisthorchis viverrini infection and hepatobiliary disease detected by ultrasonography. J Gastroenterol Hepatol. 1992;7:17–21. doi: 10.1111/j.1440-1746.1992.tb00928.x. [DOI] [PubMed] [Google Scholar]
- 29.Mairiang E, Haswell-Elkins MR, Mairiang P, Sithithaworn P, Elkins DB. Reversal of biliary tract abnormalities associated with Opisthorchis viverrini infection following praziquantel treatment. Trans R Soc Trop Med Hyg. 1993;87:194–7. doi: 10.1016/0035-9203(93)90489-d. [DOI] [PubMed] [Google Scholar]
- 30.Landis J, Koch G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 31.Sripa B, Kaewkes S. Relationship between parasite-specific antibody responses and intensity of Opisthorchis viverrini infection in hamsters. Parasite Immunol. 2000;22:139–45. doi: 10.1046/j.1365-3024.2000.00286.x. [DOI] [PubMed] [Google Scholar]
- 32.Aida Y, Pabst MJ. Neutrophil responses to lipopolysaccharide. Effect of adherence on triggering and priming of the respiratory burst. J Immunol. 1991;146(4):1271–6. [PubMed] [Google Scholar]
- 33.Dhiensiri T, Eua-Ananta Y, Bunnag D, Harinasuta T, Schelp PF. Roentgenographically controlled healing of gallbladder lesions in opisthorchiasis after praziquantel treatment. Arzneimittelforschung. 1984;34:1175–7. [PubMed] [Google Scholar]
- 34.Sripa B, Kaewkes S. Gall bladder and extrahepatic bile duct changes in Opisthorchis viverrini-infected hamsters. Acta Trop. 2002;83:29–3635. doi: 10.1016/s0001-706x(02)00052-9. [DOI] [PubMed] [Google Scholar]
- 36.Harinasuta T, Riganti M, Bunnag D. Opisthorchis viverrini infection: pathogenesis and clinical features. Arzneimittelforschung. 1984;34:1167–9. [PubMed] [Google Scholar]
- 37.Bhamarapravati N, Thammavit W, Vajrasthira S. Liver changes in hamsters infected with a liver fluke of man, Opisthorchis viverrini. Am J Trop Med Hyg. 1978;27:787–94. doi: 10.4269/ajtmh.1978.27.787. [DOI] [PubMed] [Google Scholar]
- 38.Tansurat P. Opisthorchiasis. In: Marcial-Rojas RA, editor. Pathology of Protozoal and Helminthic Diseases. Baltimore: Williams and Wilkins; 1971. pp. 536–545. [Google Scholar]
- 39.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites--masters of regulation. Immunol Rev. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 40.Thuwajit C, Thuwajit P, Kaewkes S, Sripa B, Uchida K, Miwa M, et al. Increased cell proliferation of mouse fibroblast NIH-3T3 in vitro induced by excretory/secretory product(s) from Opisthorchis viverrini. Parasitology. 2004;129:455–64. doi: 10.1017/s0031182004005815. [DOI] [PubMed] [Google Scholar]
- 41.Flavell DJ, Flavell SU. Opisthorchis viverrini: pathogenesis of infection in immunodeprived hamsters. Parasite Immunol. 1986;8:455–66. doi: 10.1111/j.1365-3024.1986.tb00861.x. [DOI] [PubMed] [Google Scholar]
- 42.McClain CJ, Song Z, Barve SS, Hill DB, Deaciuc I. Recent advances in alcoholic liver disease. IV. Dysregulated cytokine metabolism in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;287:G497–502. doi: 10.1152/ajpgi.00171.2004. [DOI] [PubMed] [Google Scholar]
- 43.Sheikh MY, Choi J, Qadri I, Friedman JE, Sanyal AJ. Hepatitis C virus infection: molecular pathways to metabolic syndrome. Hepatology. 2008;47:2127–33. doi: 10.1002/hep.22269. [DOI] [PubMed] [Google Scholar]
- 44.Ghazizadeh M, Tosa M, Shimizu H, Hyakusoku H, Kawanami O. Functional implications of the IL-6 signaling pathway in keloid pathogenesis. J Invest Dermatol. 2007;127:98–105. doi: 10.1038/sj.jid.5700564. [DOI] [PubMed] [Google Scholar]
- 45.Jittimanee J, Sermswan RW, Puapairoj A, Maleewong W, Wongratanacheewin S. Cytokine expression in hamsters experimentally infected with Opisthorchis viverrini. Parasite Immunol. 2007;29:159–67. doi: 10.1111/j.1365-3024.2006.00929.x. [DOI] [PubMed] [Google Scholar]
- 46.Prakobwong S, Pinlaor S, Yongvanit P, Sithithaworn P, Pairojkul C, Hiraku Y. Time profiles of the expression of metalloproteinases, tissue inhibitors of metalloproteases, cytokines and collagens in hamsters infected with Opisthorchis viverrini with special reference to peribiliary fibrosis and liver injury. Int J Parasitol. 2009;39:825–35. doi: 10.1016/j.ijpara.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Malhi H, Gores GJ. Cholangiocarcinoma: modern advances in understanding a deadly old disease. J Hepatol. 2006;45:856–67. doi: 10.1016/j.jhep.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;12:131–50. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Panel A, outline map of Thailand to indicate the provinces; panel B, map of Khon Kaen province, North-East Thailand, with box showing the location of the study site villages; panel C, remote sensing image of the region of Khon Kaen where the seven villages addressed in the present study are located. The specific location of the seven study villages in relation to the Chi River, which flows from south west to north east are indicated with the yellow colored stars, as follows: 1, Nongnangkwan; 2, Nongmuang; 3, Loopka; 4, Nongkham; 5, Nongno; 6, Lawa; 7, Chikokor. Panel C modified from Digital Thailand image at http://digitalthailand.gistda.or.th/