Abstract
This study investigated the shedding of Escherichia coli O26, O103, O111, O145, and O157 in a cohort of beef calves from birth over a 5-month period and assessed the relationship between shedding in calves and shedding in their dams, the relationship between shedding and scouring in calves, and the effect of housing on shedding in calves. Fecal samples were tested by immunomagnetic separation and by PCR and DNA hybridization assays. E. coli O26 was shed by 94% of calves. Over 90% of E. coli O26 isolates carried the vtx1, eae, and ehl genes, 6.5% carried vtx1 and vtx2, and one isolate carried vtx2 only. Serogroup O26 isolates comprised seven pulsed-field gel electrophoresis (PFGE) patterns but were dominated by one pattern which represented 85.7% of isolates. E. coli O103 was shed by 51% of calves. Forty-eight percent of E. coli O103 isolates carried eae and ehl, 2% carried vtx2, and none carried vtx1. Serogroup O103 isolates comprised 10 PFGE patterns and were dominated by two patterns representing 62.5% of isolates. Shedding of E. coli O145 and O157 was rare. All serogroup O145 isolates carried eae, but none carried vtx1 or vtx2. All but one serogroup O157 isolate carried vtx2, eae, and ehl. E. coli O111 was not detected. In most calves, the temporal pattern of E. coli O26 and O103 shedding was random. E. coli O26 was detected in three times as many samples as E. coli O103, and the rate at which calves began shedding E. coli O26 for the first time was five times greater than that for E. coli O103. For E. coli O26, O103, and O157, there was no association between shedding by calves and shedding by dams within 1 week of birth. For E. coli O26 and O103, there was no association between shedding and scouring, and there was no significant change in shedding following housing.
Verocytotoxigenic Escherichia coli (VTEC) isolates are important animal and human pathogens (18, 20, 31, 40, 49). VTEC O26 and O111 are putative causes of diarrhea in calves, and in pigs VTEC causes edema disease (6, 18, 26, 40, 41). In humans, VTEC is associated with illnesses ranging from uncomplicated watery diarrhea to hemorrhagic colitis (HC) and hemolytic-uremic syndrome (HUS), which may result in death (49). VTEC O26, O103, O111, O145, and O157 are all highly associated with HC and HUS (4, 21, 23, 36, 46).
VTEC infection may result from the ingestion of contaminated food or water or may be associated with animal contact. Reduction of food, water, and environmental contamination by VTEC is an important part of infection control in animals and humans and requires an understanding of the natural history of these organisms. VTEC shedding is common in healthy cattle, and numerous surveys have indicated that cattle are an important source of VTEC O157 (24, 35, 53). VTEC O26, O103, O111, and O145 have also been found in cattle feces (45), but the importance of cattle as a source of these organisms is not known.
The advent of serogroup-specific immunomagnetic separation (IMS) beads and a suitable selective indicator medium greatly improved the detection of E. coli O157 (10, 50). IMS beads specific for E. coli O26, O103, O111, and O145 recently became available and were used in this study in addition to PCR and DNA hybridization assays for VTEC. We decided to use the two methods to maximize the detection of each serogroup and to allow us to detect non-VTEC O26, O103, O111, and O145.
The principal objective of this longitudinal study was to investigate the onset and subsequent pattern of shedding of E. coli O26, O103, O111, O145, and O157 in a cohort of Scottish beef calves over a 5-month period. For each of the five serogroups, the study also aimed to assess the relationship between shedding in calves and shedding in their dams, the relationship between shedding and scouring in calves, and the effect of housing on shedding in calves. All strains of E. coli isolated in this study were screened for genes encoding enterohemolysin (ehl) and the known virulence factors verocytotoxin 1 (vtx1), verocytotoxin 2 (vtx2), and intimin (eae) (12, 20, 25). This study was conducted under and in compliance with U.K. Government Home Office license PPL 60/2615 issued under the Animals (Scientific Procedures) Act of 1986.
MATERIALS AND METHODS
Study group and sampling.
Rectal fecal samples were taken from 49 calves born in the months of August to November 2001 and their 45 dams on a mixed beef and sheep farm in northern Scotland. The farm was selected because it was managed by a compliant owner, supportive of the study. It was not randomly selected. The calves were sampled at birth and then weekly until the end of the sampling period in January 2002. One calf died shortly after birth and was sampled once; a second calf died 4 weeks after birth and was sampled three times. Dams were sampled at the time of calving and at the end of the sampling period, with the exception of one dam which was sampled at calving only, one dam which was sampled at the end of the sampling period only, and a third dam which was sampled neither at calving nor at the end of the sampling period. In addition, as part of a separate study, rectal fecal samples were taken from dams on 3 September and 10 December and screened for the presence of E. coli O157 only. Animals were not sampled for 2 weeks (weeks 18 and 19) during the Christmas and New Year season. The presence or absence of scouring was noted in calves at the time of sampling. A calf with liquid feces was classified as scouring, but it was not graded and, to the best of our knowledge, none of the scouring calves received treatment. From the beginning of the study until they were housed (week 11), the study animals were allowed to run together in the same field on pasture. On 9 November 2002, the group was housed on straw bedding. While housed, the group remained together, although recently calved cows and their offspring were sometimes kept apart from the main group on straw bedding in nursery pens, typically for 1 to 2 weeks. Samples were refrigerated within 2 h of sampling and kept at 5°C.
IMS.
Within 48 h of sampling, 1 g of feces from each sample was suspended in 20 ml of buffered peptone water (BPW) and incubated at 37°C for 6 h. Following incubation, 1 ml of BPW broth was added to 20 μl of serogroup-specific IMS beads (serogroups O26, O103, O111, and O145 [LAB M, Bury, Lancashire, United Kingdom]; serogroup O157 [Dynal Biotech Ltd., Bromborough, Wirral, United Kingdom]) in a screw-cap microcentrifuge tube for each of serogroups O26, O103, O111, O145, and O157. The tube contents were mixed on a blood tube rotator for 30 min, and then the tubes were placed in IMS magnet racks for 5 min. The beads then were washed three times as follows. From each tube, the supernatant was removed and the beads were resuspended in 1 ml of 0.01 M phosphate-buffered saline (PBS) with 0.05% (wt/vol) Tween; each tube was inverted gently four or five times and then placed in a magnet rack for 3 min. Following the final wash, the supernatant was removed and the beads were resuspended in 50 μl of PBS-0.05% Tween. To ensure that the beads were thoroughly suspended, the tubes were held upright and flicked gently several times.
Fifty-microliter suspensions of serogroup O157 beads were plated on sorbitol-MacConkey agar supplemented with cefixime (2.5 mg liter−1) and potassium tellurite (0.05 mg liter−1). Non-sorbitol-fermenting colonies were picked, plated on Chromocult coliform agar (Merck, Poole, Dorset, United Kingdom), and incubated overnight at 37°C; distinctive hazy red-pink colonies were tested with anti-E. coli O157-coated latex reagent (Oxoid, Basingstoke, United Kingdom). Fifty-microliter suspensions of serogroup O26, O103, O111, and O145 beads were plated on Chromocult TBX plates (Merck) and incubated at 37°C overnight. From each Chromocult TBX plate, all morphologically different colonies were tested against serogroup-specific antisera (Statens Serum Institut, Copenhagen, Denmark) by a slide agglutination test. In rare cases, when there were 11 or more morphologically different colonies, the number of colonies tested by slide agglutination was restricted to 10. All strains isolated by IMS were tested for the presence of vtx1, vtx2, eae, and ehl by using the methods described below.
PCR and DNA hybridization assays.
Following the removal of 1 g of feces from samples for IMS testing, fecal samples were forwarded in cool boxes with ice bricks to the Laboratory of Enteric Pathogens, Central Public Health Laboratory, Colindale, United Kingdom, for PCR and DNA hybridization testing. Samples were stored at 5°C upon arrival and tested within 48 h of receipt. From each sample, 1 g of feces was suspended in 4 ml of PBS; 200 μl of the suspension was added to 10 ml of BPW and incubated overnight at 37°C. From each culture, 10 μl of the suspension was plated on MacConkey agar and incubated overnight at 37°C. Nutrient broths were inoculated with a sweep of mixed colonies from the MacConkey agar plates, incubated on an orbital shaker at 37°C for 2 to 4 h, and then tested for the presence of vtx1 and vtx2 by PCR by the method described by Willshaw et al. (46). From MacConkey agar plates with PCR-positive colonies, colonies were transferred by replica plating to nylon membranes placed on nutrient agar plates and incubated at 37°C for 4 to 6 h. These membranes were prepared for hybridization by the method described by Maniatis et al. (27). Individual VTEC colonies were identified by DNA hybridization with a mixture of vtx1 and vtx2 polynucleotide probes (43, 47). vtx probe-positive colonies on the master plate were subcultured on MacConkey agar. All subcultures were checked by PCR as described above to confirm that a verocytotoxin-producing strain had been isolated. eae and ehl were detected by PCR by the methods described by Oswald et al. (34) and Schmidt et al. (38).
PFGE.
Pulsed-field gel electrophoresis (PFGE) analysis was based on previously described techniques (2, 14). A single colony of each strain was incubated overnight in 5 ml of Luria-Bertani broth (Gibco BRL, Life Technologies, Paisley, United Kingdom) at 37°C. Cells suspended in broth cultures were pelleted by centrifugation, washed twice in SE buffer (75 mM NaCl, 25 mM EDTA [pH 8.0]), and resuspended in 2.5 ml of SE buffer. Chromosome-grade agarose (Bio-Rad Laboratories, Hemel Hempstead, United Kingdom) was prepared in 10 mM Tris-0.1 mM EDTA to a final concentration of 1.2%. Plugs were formed by mixing 0.5 ml of cell suspension with 0.5 ml of agarose preparation and pipetting the mixture into plug molds (Bio-Rad). After solidification, the plugs were transferred to lysis buffer (50 mM Tris, 50 mM EDTA [pH 8.0], 1% N-laurylsarcosine, 0.1% [wt/vol] proteinase K). The tubes were incubated overnight at 55°C. After lysis, the lysis buffer was removed and the plugs were washed twice for 15 min each time in TE (10 mM Tris, 1 mM EDTA [pH 8.0]) containing 1.5 mM phenylmethylsulfonyl fluoride and then four times for 15 min each time in TE without phenylmethylsulfonyl fluoride. The plugs were stored at 4°C in TE.
Before restriction, the plugs were preincubated in 0.2 ml of 1× buffer D (Promega, Southampton, United Kingdom). The buffer then was removed and replaced with a fresh mixture containing 50 U of XbaI restriction enzyme (Promega), and the mixture was incubated overnight at 37°C. The plugs were washed briefly in 0.5× Tris-borate-EDTA (TBE) buffer prior to electrophoresis.
PFGE was performed by use of a contour-clamped homogeneous electric field DRII apparatus (Bio-Rad) with pulsed-field-certified agarose (Bio-Rad) in 0.5× TBE buffer. The electrophoresis conditions for XbaI were a switching time linearly ramped from 2.1 to 55 s for 22 h at 14°C and 6.0 V/cm (200 V). After PFGE, gels were stained with ethidium bromide, and gel images were recorded by using a Bio-Rad diversity database software image-capturing system. To normalize bands from one gel to another, a mid-range-molecular-weight lambda marker (New England Biolabs) was included in three lanes of each gel.
Serotyping.
Each isolate analyzed by IMS and by PCR and DNA hybridization assays was biochemically confirmed as E. coli and serotyped by the Laboratory of Enteric Pathogens by the scheme of Kauffmann (22), which depends on the identification of heat-stable lipopolysaccharide somatic (O) antigens (1, 22).
Statistical analysis.
Statistical analysis was performed by using SAS, version 8.2 (SAS Institute Inc., Cary, N.C.). Associations between shedding in dams and shedding in calves, between shedding and scouring in calves, and between housing and shedding were tested by using a two-tailed Fisher exact test (51). Shedding patterns in calves were tested for randomness, clustering, and uniformity by using the runs test (51). The relationship between birth order and time to onset of shedding was tested by using the runs above and below the median test (51). Survival curves for time to onset of first shedding of E. coli O26 and O103 were compared by using the log-rank and Wilcoxon tests. PFGE gels were analyzed by using BioNumerics, version 3.0 (Applied Maths, Ghent, Belgium). Fragments smaller than 48.5 kb in length were not used in the analysis. Dice similarities were subjected to cluster analysis as unweighted matched pair groups.
RESULTS
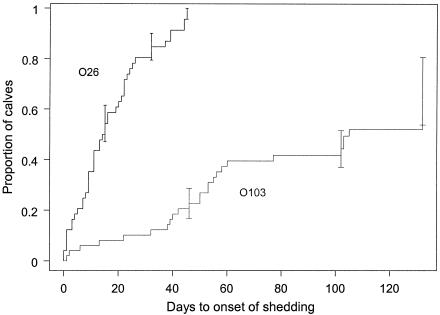
The first calf was born on 28 August, and the 49th calf was born on 29 November. In all, 664 samples from calves and 86 samples from their dams were tested for E. coli O26, O103, O111, O145, and O157; a further 84 samples from the dams were tested for E. coli O157 only. Table 1 shows the number of fecal samples in which E. coli O26, O103, O111, O145, and O157 isolates were detected by either IMS or PCR and DNA hybridization assays and, for each serogroup, the number of samples with isolates carrying vtx1, vtx2, eae, and ehl. Table 2 shows a breakdown of E. coli O26, O103, O111, O145, and O157 isolates by isolation procedure and the carriage of vtx1, vtx2, eae, and ehl. The rates at which calves began shedding E. coli O26 and O103 for the first time are shown in Fig. 1 and differed significantly between the two serogroups (log-rank test: χ2 = 66.53, P < 0.0001) (Wilcoxon test: χ2 = 53.04, P < 0.0001). The rate at which calves began shedding E. coli O26 for the first time was five times faster than that for E. coli O103. Scouring was diagnosed in 12 calves, 2 of which had scouring on two separate occasions.
TABLE 1.
Numbers of fecal samples from calves and dams with E. coli O26, O103, O111, O145, and O157 by carriage of genes encoding verocytotoxin, intimin, and enterohemolysin
| Study group (no. of samples) | Sero- group | No. of fecal samples positive for the following gene(s):
|
|||||
|---|---|---|---|---|---|---|---|
| Total | vtx1 | vtx2 | vtx1 and vtx2 | eae | ehl | ||
| Calves (664) | O26 | 115 | 101 | 1 | 8 | 114 | 105 |
| O103 | 34 | 0 | 4 | 0 | 22 | 22 | |
| O111 | 0 | 0 | 0 | 0 | 0 | 0 | |
| O145 | 4 | 0 | 0 | 0 | 4 | 4 | |
| O157 | 4 | 0 | 3 | 0 | 4 | 4 | |
| Dams (86) | O26 | 8 | 5 | 0 | 0 | 8 | 5 |
| O103 | 13 | 0 | 0 | 0 | 0 | 0 | |
| O111 | 0 | 0 | 0 | 0 | 0 | 0 | |
| O145 | 0 | 0 | 0 | 0 | 0 | 0 | |
| O157a | 11 | 0 | 11 | 0 | 11 | 11 | |
Includes additional samples taken from dams on 3 September 2001 and 10 December 2001 (n = 172).
TABLE 2.
Numbers of E. coli O26, O103, O111, O145, and O157 isolates from calves and dams by isolation procedure and genes encoding verocytotoxin, intimin, and enterohemolysin
| Serogroup | Test | No. of isolates carrying the following gene(s):
|
|||||
|---|---|---|---|---|---|---|---|
| Total | vtx1 | vtx2 | vtx1 and vtx2 | eae | ehl | ||
| O26 | IMS | 114 | 97 | 1 | 6 | 113 | 105 |
| PCR-DNA hybridization | 40 | 36 | 0 | 4 | 40 | 37 | |
| O103 | IMS | 45 | 0 | 1 | 0 | 22 | 21 |
| PCR-DNA hybridization | 3 | 0 | 3 | 0 | 1 | 2 | |
| O145 | IMS | 4 | 0 | 0 | 0 | 4 | 4 |
| PCR-DNA hybridization | 0 | 0 | 0 | 0 | 0 | 0 | |
| O157 | IMSa | 10 (15) | 0 (0) | 9 (14) | 0 (0) | 10 (15) | 10 (14) |
| PCR-DNA hybridization | 0 | 0 | 0 | 0 | 0 | 0 | |
Values in parentheses include additional samples taken from dams on 3 September 2001 and 10 December 2001 (n = 172).
FIG. 1.
Time from birth to onset of first shedding of E. coli O26 and E. coli O103 in calves.
Serogroup O26.
E. coli O26 was found in 115 (17.3%) of 664 calf samples and 8 (9.1%) of 88 samples from dams. Shedding was detected in 46 (93.9%) of 49 calves and 7 (15.6%) of 45 dams. The temporal patterns of E. coli O26 shedding in calves and their dams are shown in Fig. 2. With the exception of two calves that died soon after birth, all calves but one shed E. coli O26 at some point during the study. Twenty-five percent of calves began shedding within 1 week of birth, and 50% did so within 2 weeks of birth; the mean age at which shedding was first detected was 16.5 days (standard deviation, 12.8 days). Age at the onset of the first shedding episode was not related to birth order within the cohort (n1 = 23, n2 = 23, u = 25 [where n1 is the number of calves that began shedding before the median age of onset of shedding among all calves, n2 is the number of calves that began shedding after the median age of onset of shedding among all calves, and u is the number of runs in the sequence of calves, ranked by date of birth]; P > 0.5). Ninety-one percent of shedding episodes lasted 1 to 2 weeks, and the longest lasted 5 weeks; of the 47 calves that lived longer than 3 weeks, 25 (53%) had multiple episodes of shedding. The patterns of E. coli O26 shedding in calves were generally random but clustered in calves 674 (n1 = 15, n2 = 3, u = 3 [where n1 is the number of sampling events in which E. coli O26 was not defected, n2 is the number of sampling events in which E. coli O26 was detected, and u is the number of runs in the sequence of sampling events]; 0.01 < P ≤ 0.025), 677 (n1 = 14, n2 = 3, u = 3; 0.01 < P ≤ 0.025), 683 (n1 = 13, n2 = 4, u = 4; 0.05 < P ≤ 0.025), 688 (n1 = 13, n2 = 3, u = 3; 0.05 < P ≤ 0.025), and 702 (n1 = 9, n2 = 5, u = 3; 0.005 < P ≤ 0.01).
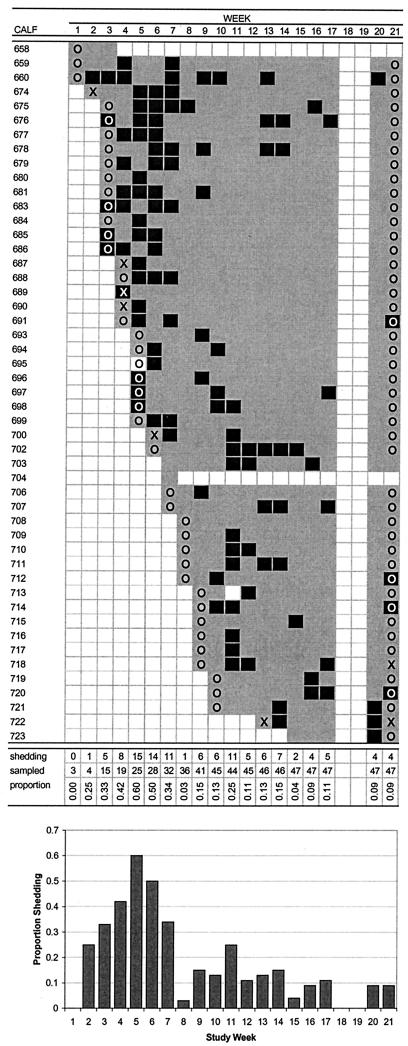
FIG. 2.
Pattern of E. coli O26 shedding in calves, including number of calves shedding, number of calves sampled, and proportion of sampled calves shedding, by week of study. Dams and calves were housed at week 11. Calves shedding, calves not shedding, dams shedding, and dams not shedding are indicated by black squares, gray squares, uppercase “X,” and uppercase “O,” respectively.
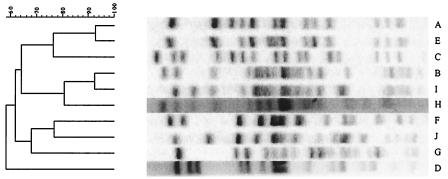
Of the 115 calf fecal samples in which E. coli O26 was detected, 101 (87.8%) harbored E. coli O26 isolates with vtx1, 1 (0.9%) had an isolate with vtx2, and 8 (7.0%) had isolates with both vtx1 and vtx2; 114 samples (99.1%) had isolates with eae; and 105 samples (91.3%) had isolates with ehl. Of the 8 dam fecal samples with E. coli O26, 5 (62.5%) had E. coli O26 isolates with vtx1, but none had isolates with vtx2; all 8 samples had isolates with eae; and 5 samples (62.5%) had isolates with ehl. Serogroup O26 isolates comprised seven PFGE patterns, designated A to G (Fig. 3). The dominant pattern, D, represented 132 (85.7%) of 154 isolates, and of these, 129 were vtx1 positive, vtx2 negative, and eae positive. The next most common pattern, F, represented 11 isolates (7.1%), followed by pattern C, representing 7 isolates (4.5%). Seven of the 11 pattern F isolates possessed vtx1 and vtx2, and all 11 pattern F isolates possessed eae. All pattern C stains were vtx1 and vtx2 negative and eae positive. Six dams shed E. coli O26 at the time of calving: one shed E. coli O26 with PFGE pattern F, two shed pattern C isolates, and three shed pattern D isolates. The calves of all six dams shed E. coli O26, and the first PFGE pattern shed by all of them was pattern D. The calves of the dams shedding E. coli O26 with PFGE pattern C never shed pattern C. The calf of the dam shedding E. coli O26 with PFGE pattern F shed pattern F once, 4 weeks after birth.
FIG. 3.
PFGE patterns (A to G) of E. coli O26 isolates following XbaI digestion.
There was no significant association between shedding of E. coli O26 by dams and shedding by calves either within 1 week of birth (P = 1.00) or at the end of the study (P = 1.00), and there was no significant association between shedding of E. coli O26 and scouring in calves (P = 0.48). No significant change was detected in the rate of shedding 1 week after housing (P = 1.00) or 2 weeks after housing (P = 1.00) compared with the results seen the week before housing.
Serogroup O103.
E. coli O103 was found in 34 (5.1%) of 664 calf samples and 13 (14.8%) of 88 samples from dams. The patterns of E. coli O103 shedding in calves and their dams are shown in Fig. 4. Twenty-five calves (51%) shed serogroup O103 at some point during the study. Twenty-five percent of calves began shedding E. coli O103 within 50 days of birth, and 50% did so within 103 days of birth. Ninety-three percent of shedding episodes lasted 1 to 2 weeks, and the longest lasted 4 weeks. Of the 47 calves that lived longer than 3 weeks, only 2 (4%) had multiple episodes of shedding. Shedding of E. coli O103 was random in all calves except for calf 683, in which shedding was clustered (n1 = 13, n2 = 4, u = 2; P ≤ 0.001). Only one dam was shedding E. coli O103 at calving, but 12 (27.9%) of 43 were shedding on the last day of the study.
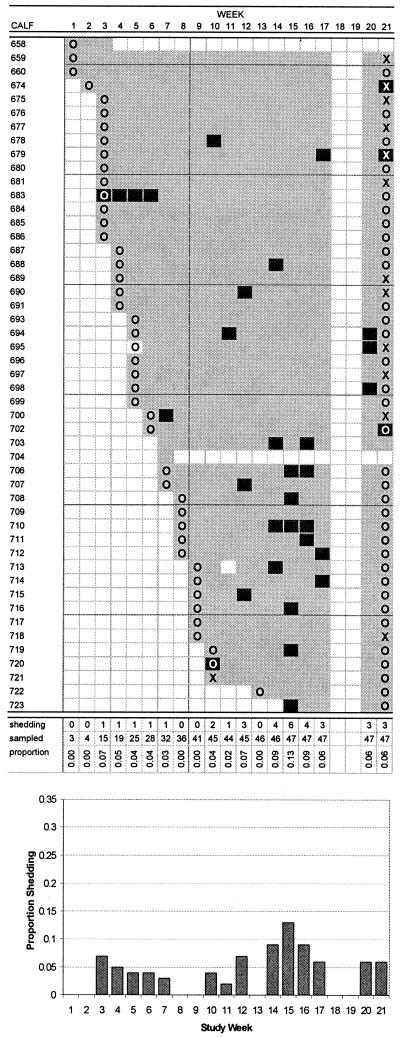
FIG.4.
Pattern of E. coli O103 shedding in calves, including number of calves shedding, number of calves sampled, and proportion of sampled calves shedding, by week of study. Dams and calves were housed at week 11. Calves shedding, calves not shedding, dams shedding, and dams not shedding are indicated by black squares, gray squares, uppercase “X,” and uppercase “O,” respectively.
Of the 34 calf fecal samples in which E. coli O103 was detected, only 4 (11.8%) harbored VTEC O103 isolates, all with vtx2 only; 22 samples (64.7%) had isolates with eae, and the same proportion had isolates with ehl. None of the E. coli O103 isolates from dams possessed vtx1, vtx2, eae, or ehl. Serogroup O103 isolates comprised 10 unrelated PFGE patterns, designated A to J (Fig. 5). There were two dominant patterns: B, representing 17 (35.4%) of 48 isolates, and D, representing 13 (27.1%) of 48 isolates. Fifteen of the 17 pattern B isolates were vtx1 and vtx2 negative, while 2 isolates possessed vtx2 only. All pattern B isolates were eae positive. All pattern D isolates were vtx1, vtx2, and eae negative. All but one of the pattern B isolates were isolated during study weeks 12 to 17 and represented 16 (80.0%) of the 20 isolates obtained during that period; the remaining pattern B isolate was obtained during the final week of the study. All pattern D isolates were obtained during the last week of the study and represented 13 (86.7%) of the 15 isolates obtained during that week.
FIG. 5.
PFGE patterns (A to J) of E. coli O103 isolates following XbaI digestion.
There was no significant association between shedding of E. coli O103 by dams and shedding by calves within 1 week of birth (P = 1.00) or at the end of the study (P = 0.22). There was no significant association between shedding of E. coli O103 and scouring in calves (P = 0.16). There was no change in the rate of shedding 1 week after housing (P = 1.00) compared with the results seen the week before housing. Shedding of E. coli O103 was not detected in the second week after housing.
Serogroups O111 and O145.
Serogroup O111 was not detected in feces from any of the cows or calves sampled. E. coli O145 was detected in 4 (0.6%) of 664 samples. Calf 684 was shedding at 5 and 14 days after birth, and calves 690 and 679 were shedding at 9 and 31 days after birth, respectively. E. coli O145 was not found in any fecal samples from dams. All E. coli O145 isolates were vtx negative but eae and ehl positive. The PFGE patterns of all serogroup O145 isolates obtained during the study were indistinguishable.
Serogroup O157.
E. coli O157 was detected in 4 (0.6%) of 664 calf samples and 11 (6.4%) of 172 samples from dams. Calf 683 was shedding E. coli O157 on the day after birth, and calves 658, 659, and 697 were shedding at 6 days, 8 weeks, and 15 weeks after birth, respectively. No E. coli O157 was detected in feces from the dams of these four calves during the study. Five dams were shedding on 3 September. The other six dams were shedding at the time of calving. E. coli O157 shedding was not detected in any cattle on the farm from 1 October until the end of the study. All but one E. coli O157 isolate carried vtx2, but none carried vtx1; all isolates carried eae; and all but one isolate carried ehl. The PFGE patterns of all serogroup O157 isolates obtained during the study were identical.
Shedding of multiple serogroups.
Two or more of the target E. coli serogroups were detected in 10 (1.3%) of 756 samples. There was a significant association between shedding of E. coli O26 and shedding of E. coli O145 in calves (P = 0.018), but there was no association for shedding of any other pair of serogroups. Only in calf 683 were more than two serogroups found in the same sample: in week 3 of the study, this calf was shedding E. coli O26, O103, and O157.
DISCUSSION
E. coli O26 was the most commonly shed serogroup of those tested in calves and the second most commonly detected serogroup in dams. Shedding of E. coli O26 by calves began within a few weeks of birth and persisted in the cohort throughout the study. There was a marked pulse of shedding among calves during weeks 3 to 7, when the prevalence of shedding was above 30% and peaked at 60%. After this time, the prevalence of shedding dropped abruptly and remained much lower. This fourfold decrease in prevalence resulted from a decrease in the number of calves shedding rather than an increase in the population of calves in the cohort. Shedding was generally random and unrelated to age, although shedding on consecutive weeks was common and clustering of shedding did occur in five calves.
Over 90% of E. coli O26 isolates from calves and dams carried vtx1, eae, and ehl, and vtx1 was carried 13 times more commonly than vtx2. These results agree with the findings of several other studies which found that bovine E. coli O26 strains typically carry vtx1 and eae but not vtx2 (5, 7, 33, 37). However, high rates of carriage of vtx2 among E. coli O26 isolates from cattle have been reported (15). It is notable that among serogroup O26 strains isolated during this study, the carriage of vtx2 was almost invariably associated with the carriage of vtx1. In strains of E. coli O26 associated with diarrhea and HUS in humans, it is common to find vtx1, eae, and ehl (39, 52). Some strains carrying vtx2 have also been associated with diarrhea and HUS (29). The patterns of virulence factors carried by the majority of bovine strains in this study are therefore consistent with those of strains pathogenic for humans.
E. coli O103 was the second most commonly shed serogroup of those tested in calves but the most commonly detected serogroup in dams. The pattern of shedding in calves was sporadic and random; clustering of shedding was apparent in only one calf. It is remarkable that all but one of the E. coli O103 isolates from dams were detected at the end of the study. Half of the E. coli O103 isolates from calves carried eae and ehl, but these genes were absent in isolates from dams. PFGE results showed that only a single strain was being shed by dams at the end of the study. This strain, of PFGE pattern D, was detected only during the last week of the study, suggesting that it was a newly introduced strain. The carriage of eae and ehl may confer a selective advantage for the colonization of the calf gut. Carriage of verocytotoxin-encoding genes by E. coli O103 was rare and occurred only in strains from calves. In this study, the only verocytotoxin-encoding gene detected in E. coli O103 strains was vtx2. This novel result contrasts with the findings of a number of other studies in which VTEC O103 possessed vtx1 only (5, 19, 32, 45, 48).
The patterns of shedding of E. coli O26 and O103 in calves were very different. E. coli O26 was shed much more commonly and earlier in life than E. coli O103. Unlike serogroup O103, serogroup O26 was shed by a higher proportion of dams at calving than at the end of the study; this result suggests higher E. coli O26 activity at calving—and a greater force of infection for calves than at the end of the study. In addition, the vtx1, eae, and ehl genes were much more common in E. coli O26 than in E. coli O103 and may play an important role in facilitating colonization and persistence in the calf gut.
The absence of E. coli O111 shedding was striking, since this serogroup has been found frequently in cattle (32, 37, 45). However, the low prevalence of E. coli O145 shedding is consistent with the findings of other studies (30, 37, 45). Despite shedding of E. coli O157 by six dams at calving and five dams during quarterly sampling in September, shedding in calves was sporadic and infrequent. Other studies have also found the prevalence of shedding of E. coli O157 by young calves to be low (13, 16, 17). All but one isolate of E. coli O157 from calves and dams in this study carried vtx2, eae, and ehl. This finding is significant, since vtx2 and eae are regarded as important virulence factors found in E. coli O157 strains associated with HC and HUS in humans (3, 25, 46).
Concurrent shedding of more than one serogroup was uncommon and, with the exception of E. coli O26 and O145, we found no tendency for two serogroups to be shed simultaneously more than could be expected by chance. Although E. coli O145 was detected in only four samples, in three of these it occurred simultaneously with E. coli O26. In one calf sample, serogroups O26, O103, and O157 were all detected, demonstrating that simultaneous shedding of more than two serogroups may occur.
A study of Australian dairy herds reported that calves are more than twice as likely to shed VTEC when born to a dam shedding VTEC (11). The majority of E. coli O26 and O157 strains isolated in this study were verocytotoxigenic. However, we found no association between shedding of E. coli O26 or O157 by dams and shedding by calves in the first week of life or at the end of the study. Similarly, we found no association between shedding of E. coli O103 by dams and shedding by calves in the first week of life or at the end of the study, but most E. coli O103 isolates were not verocytotoxigenic. These findings suggest that for E. coli O26, O103, and O157, horizontal transmission from the environment or cattle other than the dam was a more important route of infection for calves than vertical transmission from the dam.
Housing of cattle results in a number of major environmental changes. In addition, housing is usually associated with changes in diet. In this study, the diet of dams changed from unsupplemented grass to a mixture of silage, barley, and minerals at the time of housing, and calves were given access to concentrates and commercially prepared calf rations. Changes in diet have been shown to affect the patterns of shedding of E. coli O157 (8), and it has been proposed that stress related to crowding and competition and increased mutual licking and sucking among cattle associated with housing encourage the shedding of E. coli O157 (13). However, our results indicated that housing had no detectable effect on shedding of E. coli O26 and O103 among calves in the short term. There were insufficient data to assess the impact of housing on shedding of E. coli O145 and O157.
Several groups have reported the presence of VTEC in feces from scouring calves (11, 18, 31, 33, 41). One report claimed a significant association between the presence of VTEC and scouring (31), but another found no such association (11). E. coli O26, in particular, has been associated with diarrhea in calves (18, 33, 41). E. coli O26 was commonly found in calf fecal samples in this study, and most isolates carried genes encoding verocytotoxins. However, we did not find any link between shedding of E. coli O26 generally or VTEC O26 more specifically and scouring in calves. Given the level of E. coli O26 shedding activity, we wonder whether dams were transferring protective antibodies in colostrum to their calves, thereby preventing VTEC-related scouring in calves. On reflection, it is regrettable that we did not test colostrum for antibodies against lipopolysaccharide antigens and verocytotoxins. In E. coli O103 isolates, the carriage of genes encoding verocytotoxins was rare. As with E. coli O26, there was no apparent association between shedding of E. coli O103 generally or VTEC O103 more specifically and scouring in calves. We examined calves weekly and may have missed scouring episodes lasting less than 7 days. In human VTEC infections, clearance of VTEC may be rapid following the onset of clinical signs (21, 28, 44). If the clearance of VTEC was rapid following the onset of clinical signs in calf infections, we may have failed to detect VTEC when we diagnosed scouring. More frequent sampling might have strengthened the statistical power of our study of the association between shedding and scouring in calves. The small numbers of E. coli O145 and O157 strains isolated in this study did not permit any meaningful assessment of the association between scouring and shedding of these serogroups in calves.
We know from our study and others (32, 33, 37) that calves may shed VTEC O26, O103, O145, and O157 isolates, which are associated with disease in calves and humans. The concentration of E. coli in calf feces is considerably higher than that in adult cattle feces. In calves that are 1 to 2 weeks old, the density of E. coli in feces is 108 to 109 CFU per g, and at 10 to 20 weeks, it is 106 to 107 CFU per g; in adult cattle, it is merely 104 CFU per g (42). We do not know whether the population of VTEC varies proportionately with the overall E. coli population in cattle feces. We did not obtain counts for E. coli O26, O103, O111, and O145 isolates in fecal samples because, unlike the situation for serogroup O157, no suitable indicator media exist and no practicable method exists for counting isolates of these serogroups in large numbers of samples. If the population of VTEC does vary proportionately with the overall E. coli population in cattle feces, calves would represent an important source of VTEC, capable of shedding greater total numbers of VTEC per head than more mature cattle—and this situation could greatly affect the dynamics of infection within a herd. This notion is pertinent because of the potential for human VTEC infection at petting zoos and open farms (9), the risk of veal carcass contamination at slaughter, and the possibililty of calves acting as a VTEC reservoir, with horizontal transmission to other farm livestock. The management of calves would require extra care to minimize these risks.
We believe that this is the first reported epidemiological study to use IMS for the detection of selected E. coli serogroups other than O157 in cattle. Surveys using PCR and DNA hybridization assays to detect VTEC shedding in cattle provide no information on shedding of non-VTEC O26, O103, O111, and O145. This study demonstrated that IMS enabled detection of nonverocytotoxigenic E. coli O26, O103, O145, and O157 in cattle feces and permitted estimation of the proportion of each serogroup that was verocytotoxigenic. The use of IMS increased the detection of VTEC O26 and O157 substantially. Our results suggest that IMS yielded better recovery of VTEC O26 and O157 than PCR and DNA hybridization assays, but the outperformance by IMS may have arisen because the amount of feces tested in the IMS protocol was larger than that tested in the PCR-DNA hybridization protocol.
Rectal sampling requires animals to be moved and held in restraining facilities. It was not practicable to do this more than once per week on our study farm, although more frequent sampling of study cattle may have increased the sensitivity of detection for our target serogroups. Moving animals for sampling may have altered the transmission of the E. coli serogroups under study and the dynamics of infection, especially since other cattle in the herd were present in the same facilities during the course of the study. The impact of sampling strategies on the results of VTEC surveys has received scant attention, and critical evaluation is urgently required. Finally, our study investigated the epidemiology of shedding in a cohort of autumn-born calves only, and we recommend further research to determine whether our findings can be generalized to calves born in different seasons and under different management practices.
Acknowledgments
We thank Chris Low and Darren Shaw for advice and assistance in the preparation of the manuscript.
This study is part of the International Partnership Research Award in Veterinary Epidemiology (IPRAVE) (Epidemiology and Evolution of Enterobacteriaceae Infections in Humans and Domestic Animals), funded by the Wellcome Trust. The Scottish Agricultural College receives financial support from the Scottish Executive Environment and Rural Affairs Department (SEERAD).
REFERENCES
- 1.Barrow, G. I., and R. K. A. Feltham (ed.). 1993. Cowan and Steel's manual for the identification of medical bacteria. Cambridge University Press, Cambridge, United Kingdom.
- 2.Barrett, T. J., H. Lior, J. H. Green, R. Khakhria, J. G. Wells, B. P. Bell, K. D. Greene, J. Lewis, and P. M. Griffin. 1994. Laboratory investigation of a multistate food-borne outbreak of Escherichia coli O157:H7 by using pulsed-field gel electrophoresis and phage typing. J. Clin. Microbiol. 32:3013-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beutin, L., S. Aleksic, S. Zimmermann, and K. Gleier. 1994. Virulence factors and phenotypical traits of verotoxigenic strains of Escherichia coli isolated from human patients in Germany. Med. Microbiol. Immunol. 183:13-21. [DOI] [PubMed] [Google Scholar]
- 4.Beutin, L., S. Zimmermann, and K. Gleier. 2001. Association between serotypes, virulence markers and disease in a group of 679 verocytotoxin-producing Escherichia coli (VTEC) strains isolated from human patients in Germany (1997-1999), p. 5-11. In G. Duffy, P. Garvey, J. E. Coia, Y. Wasteson, and D. A. McDowell (ed.), Epidemiology of verocytotoxigenic E. coli. Proceedings of the 5th Meeting of Concerted Action CT98-3935: verocytotoxigenic E. coli in Europe. Teagasc, Dublin, Ireland.
- 5.Blanco, M., J. E. Blanco, J. Blanco, A. Mora, C. Prado, M. P. Alonso, M. Mouriño, C. Madrid, C. Balsalobre, and A. Juarez. 1997. Distribution and characterization of faecal verotoxin-producing Escherichia coli (VTEC) isolated from healthy cattle. Vet. Microbiol. 54:309-319. [DOI] [PubMed] [Google Scholar]
- 6.Bosworth, B. T., J. E. Samuel, H. W. Moon, A. D. O'Brien, V. M. Gordon, and S. C. Whipp. 1996. Vaccination with genetically modified Shiga-like toxin IIe prevents edema disease in swine. Infect. Immun. 64:55-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnens, A. P., A. Frey, H. Lior, and J. Nicolet. 1995. Prevalence and clinical significance of verocytotoxin-producing Escherichia coli (VTEC) isolated from cattle in herds with and without calf diarrhoea. J. Vet. Med. Ser. B 42:311-318. [DOI] [PubMed] [Google Scholar]
- 8.Callaway, T. R., R. O. Elder, J. E. Keen, R. C. Anderson, and D. J. Nisbet. 2003. Forage feeding to reduce preharvest Escherichia coli populations in cattle, a review. J. Dairy Sci. 86:852-860. [DOI] [PubMed] [Google Scholar]
- 9.Chapman, P. A., J. Cornell, and C. Green. 2000. Infection with verocytotoxin-producing Escherichia coli O157 during a visit to an inner city open farm. Epidemiol. Infect. 125:531-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman, P. A., D. J. Wright, and C. A. Siddons. 1994. A comparison of immunomagnetic separation and direct culture for the isolation of verocytotoxin-producing Escherichia coli O157 from bovine faeces. J. Med. Microbiol. 40:424-427. [DOI] [PubMed] [Google Scholar]
- 11.Cobbold, R., and P. M. Desmarchelier. 2000. A longitudinal study of Shiga-toxigenic Escherichia coli (STEC) prevalence in three Australian dairy herds. Vet. Microbiol. 71:125-137. [DOI] [PubMed] [Google Scholar]
- 12.Frankel, G., A. D. Phillips, I. Rosenshine, G. Dougan, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30:911-921. [DOI] [PubMed] [Google Scholar]
- 13.Garber, L. P., S. J. Wells, D. D. Hancock, M. P. Doyle, J. Tuttle, J. A. Shere, and T. Zhao. 1995. Risk factors for fecal shedding of Escherichia coli O157:H7 in dairy calves. J. Am. Vet. Med. Assoc. 207:46-49. [PubMed] [Google Scholar]
- 14.Gautom, R. K. 1997. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J. Clin. Microbiol. 35:2977-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geue, L., M. Segura-Alvarez, F. J. Conraths, T. Kuczius, J. Bockemühl, H. Karch, and P. Gallien. 2002. A long-term study on the prevalence of shiga toxin-producing Escherichia coli (STEC) on four German cattle farms. Epidemiol. Infect. 129:173-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hancock, D. D., T. E. Besser, M. L. Kinsel, P. I. Tarr, D. H. Rice, and M. G. Paros. 1994. The prevalence of Escherichia coli O157:H7 in dairy and beef cattle in Washington state. Epidemiol. Infect. 113:199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hancock, D. D., T. E. Besser, D. H. Rice, D. E. Herriott, and P. I. Tarr. 1997. A longitudinal study of Escherichia coli O157 in fourteen cattle herds. Epidemiol. Infect. 118:193-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janke, B. H., D. H. Francis, J. E. Collins, M. C. Libal, D. H. Zeman, D. D. Johnson, and R. D. Neiger. 1990. Attaching and effacing Escherichia coli infection as a cause of diarrhea in young calves. J. Am. Vet. Med. Assoc. 196:897-901. [PubMed] [Google Scholar]
- 19.Jenkins, C., H. Chart, T. Cheasty, G. A. Willshaw, M. C. Pearce, G. Foster, G. J. Gunn, H. R. Smith, G. Dougan, B. A. Synge, and G. Frankel. 2002. Verocytotoxin-producing Escherichia coli (VTEC) other than serogroup O157 from Scottish cattle. Vet. Rec. 151:58-60. [DOI] [PubMed] [Google Scholar]
- 20.Karmali, M. A. 1989. Infection by verocytotoxin-producing Escherichia coli. Clin. Microbiol. Rev. 2:15-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karmali, M. A., M. Petric, C. Lim, P. C. Fleming, G. S. Arbus, and H. Lior. 1985. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J. Infect. Dis. 151:775-782. [DOI] [PubMed] [Google Scholar]
- 22.Kauffmann, A. F. 1944. Zur Serologie der Coli-Gruppe. Acta Pathol. Microbiol. Scand. 21:20-45. [PubMed] [Google Scholar]
- 23.Kleanthous, H., H. R. Smith, S. M. Scotland, R. J. Gross, B. Rowe, C. M. Taylor, and D. V. Milford. 1990. Haemolytic uraemic syndromes in the British Isles, 1985-8: association with Verocytotoxin producing Escherichia coli. Part 2. Microbiological aspects. Arch. Dis. Child. 65:722-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laegreid, W. W., R. O. Elder, and J. E. Keen. 1999. Prevalence of Escherichia coli O157:H7 in range beef calves at weaning. Epidemiol. Infect. 123:291-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Law, D. 2000. Virulence factors of Escherichia coli O157 and other Shiga toxin-producing E. coli. J. Appl. Microbiol. 88:729-745. [DOI] [PubMed] [Google Scholar]
- 26.MacLeod, D. L., C. L. Gyles, and B. P. Willcock. 1991. Reproduction of edema disease of swine with purified Shiga-like toxin II variant. Vet. Pathol. 28:66-73. [DOI] [PubMed] [Google Scholar]
- 27.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 28.Milford, D. V., C. M. Taylor, B. Guttridge, S. M. Hall, B. Rowe, and H. Kleanthous. 1990. Haemolytic uraemic syndromes in the British Isles, 1985-8: association with verocytotoxin producing Escherichia coli. Part 1. Clinical and epidemiological aspects. Arch. Dis. Child. 65:716-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Misselwitz, J., H. Karch, M. Bielazewska, U. John, F. Ringelmann, G. Rönnefarth, and L. Patzer. 2003. Cluster of hemolytic-uremic syndrome caused by Shiga toxin-producing Escherichia coli O26:H11. Pediatr. Infect. Dis. J. 22:349-354. [DOI] [PubMed] [Google Scholar]
- 30.Miyao, Y., T. Kataoka, T. Nomoto, A. Kai, T. Itoh, and K. Itoh. 1998. Prevalence of verotoxin-producing Escherichia coli harbored in the intestine of cattle in Japan. Vet. Microbiol. 61:137-143. [DOI] [PubMed] [Google Scholar]
- 31.Mohammad, A., J. S. M. Peiris, E. A. Wijewanta, S. Mahalingam, and G. Gunasekara. 1985. Role of verocytotoxigenic Escherichia coli in cattle and buffalo calf diarrhoea. FEMS Microbiol. Lett. 26:281-283. [Google Scholar]
- 32.Orden, J. A., D. Cid, J. A. Ruiz-Santa-Quiteria, S. García, S. Martinez, and R. de la Fuente. 2002. Verotoxin-producing Escherichia coli (VTEC), enteropathogenic E. coli (EPEC) and necrotoxigenic E. coli (NTEC) isolated from healthy cattle in Spain. J. Appl. Microbiol. 93:29-35. [DOI] [PubMed] [Google Scholar]
- 33.Orden, J. A., J. A. Ruiz-Santa-Quiteria, D. Cid, S. García, R. Sanz, and R. de la Fuente. 1998. Verotoxin-producing Escherichia coli (VTEC) and eae-positive non-VTEC in 1-30-days-old diarrhoeic dairy calves. Vet. Microbiol. 63:239-248. [DOI] [PubMed] [Google Scholar]
- 34.Oswald, E., H. Schmidt, S. Morabito, H. Karch, O. Marchès, and A. Caprioli. 2000. Typing of intimin genes in human and animal enterohemorrhagic and enteropathogenic Escherichia coli: characterization of a new intimin variant. Infect. Immun. 68:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paiba, G. A., J. C. Gibbens, S. J. S. Pascoe, J. W. Wilesmith, S. A. Kidd, C. Byrne, J. B. M. Ryan, R. P. Smith, I. M. McLaren, R. J. Futter, A. C. S. Kay, Y. E. Jones, S. A. Chappell, G. A. Willshaw, and T. Cheasty. 2002. Faecal carriage of verocytotoxin-producing Escherichia coli O157 in cattle and sheep at slaughter in Great Britain. Vet. Rec. 150:593-598. [DOI] [PubMed] [Google Scholar]
- 36.Pierard, D., D. Stevens, L. Moriau, H. Lior, and S. Lauwers. 1994. Three years PCR screening for VTEC in human stools in Brussels, p. 33-36. In M. A. Karmali and A. G. Goglio (ed.), Recent advances in verocytotoxin producing Escherichia coli infections. Proceedings of the 2nd International Symposium and Workshop on Verocytotoxin (Shiga-Like Toxin)-Producing Escherichia coli Infections. Elsevier, Amsterdam, The Netherlands.
- 37.Sandhu, K. S., R. C. Clarke, K. McFadden, A. Brouwer, M. Louie, J. Wilson, H. Lior, and C. L. Gyles. 1996. Prevalence of the eaeA gene in verotoxigenic Escherichia coli strains from dairy cattle in southwest Ontario. Epidemiol. Infect. 116:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt, H., L. Beutin, and H. Karch. 1995. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL-933. Infect. Immun. 63:1055-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt, H., C. Geitz, P. I. Tarr, M. Frosch, and H. Karch. 1999. Non-O157:H7 pathogenic Shiga toxin-producing Escherichia coli: phenotypic and genetic profiling of virulence traits and evidence for clonality. J. Infect. Dis. 179:115-123. [DOI] [PubMed] [Google Scholar]
- 40.Schoonderwoerd, M., R. C. Clarke, A. A. van Dreumel, and S. A. Rawluk. 1988. Colitis in calves: natural and experimental infection with a verotoxin-producing strain of Escherichia coli O111:NM. Can. J. Vet. Res. 52:484-487. [PMC free article] [PubMed] [Google Scholar]
- 41.Sherwood, D., D. R. Snodgrass, and A. D. O'Brien. 1985. Shiga-like toxin production from Escherichia coli associated with calf diarrhoea. Vet. Rec. 116:217-218. [DOI] [PubMed] [Google Scholar]
- 42.Smith, H. W., and W. E. Crabb. 1961. The faecal bacterial flora of animals and man: its development in the young. J. Pathol. Bacteriol. 82:53-66. [Google Scholar]
- 43.Thomas, A., H. R. Smith, G. A. Willshaw, and B. Rowe. 1991. Non-radioactively labelled polynucleotide and oligonucleotide DNA probes for selectively detecting Escherichia coli strains producing verocytotoxins VT1, VT2 and VT2 variant. Mol. Cell. Probes 5:129-135. [DOI] [PubMed] [Google Scholar]
- 44.Wells, J. G., B. R. Davis, I. K. Wachsmuth, L. W. Riley, R. S. Remis, R. Sokolov, and G. K. Morris. 1983. Laboratory investigation of hemorrhagic colitis outbreaks associated with a rare Escherichia coli serotype. J. Clin. Microbiol. 18:512-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wells, J. G., L. D. Shipman, K. D. Greene, E. G. Sowers, J. H. Green, D. N. Cameron, F. P. Downes, M. L. Martin, P. M. Griffin, S. M. Ostroff, M. E. Potter, R. V. Tauxe, and I. K. Wachsmuth. 1991. Isolation of Escherichia coli serotype O157:H7 and other Shiga-like-toxin-producing E. coli from dairy cattle. J. Clin. Microbiol. 29:985-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willshaw, G. A., T. Cheasty, H. R. Smith, S. J. O'Brien, and G. K. Adak. 2001. Verocytotoxin-producing Escherichia coli (VTEC) O157 and other VTEC from human infections in England and Wales: 1995-1998. J. Med. Microbiol. 50:135-142. [DOI] [PubMed] [Google Scholar]
- 47.Willshaw, G. A., H. R. Smith, S. M. Scotland, A. M. Field, and B. Rowe. 1987. Heterogeneity of Escherichia coli phages encoding Vero cytotoxins: comparison of cloned sequences determining VT1 and VT2 and development of specific gene probes. J. Gen. Microbiol. 133:1309-1317. [DOI] [PubMed] [Google Scholar]
- 48.Wilson, J. B., R. C. Clarke, S. A. Renwick, K. Rahn, R. P. Johnson, M. A. Karmali, H. Lior, D. Alves, C. L. Gyles, K. S. Sandhu, S. A. McEwen, and J. S. Spika. 1996. Vero cytotoxigenic Escherichia coli infection in dairy farm families. J. Infect. Dis. 174:1021-1027. [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization. 1998. Zoonotic non-O157 Shiga toxin-producing Escherichia coli (STEC). Report of a W.H.O. scientific working group meeting. W.H.O./CSR/APH/98.8. World Health Organization, Geneva, Switzerland.
- 50.Zadik, P. M., P. A. Chapman, and C. A. Siddons. 1993. Use of tellurite for the selection of verocytoxigenic Escherichia coli O157. J. Med. Microbiol. 39:155-158. [DOI] [PubMed] [Google Scholar]
- 51.Zar, J. H. 1999. Biostatistical analysis. Prentice Hall, Inc., Upper Saddle River, N.J.
- 52.Zhang, W.-L., M. Bielaszewska, A. Liesegang, H. Tschäpe, H. Schmidt, M. Bitzan, and H. Karch. 2000. Molecular characteristics and epidemiological significance of Shiga toxin-producing Escherichia coli O26 strains. J. Clin. Microbiol. 38:2134-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao, T., M. P. Doyle, J. Shere, and L. Garber. 1995. Prevalence of enterohemorrhagic Escherichia coli O157:H7 in a survey of dairy herds. Appl. Environ. Microbiol. 61:1290-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]