Abstract
Background
Cytomegalovirus (CMV) is the most common opportunistic infection after solid-organ transplant. Valganciclovir prophylaxis significantly reduces disease, but limited data are available on its use in children. Recently, an increase in delayed-onset CMV disease has been noted with some arguing that longer prophylaxis may decrease late-onset disease.
Methods
Single-center, retrospective analysis of pediatric renal transplant patients receiving 24 weeks valganciclovir prophylaxis (15 mg/kg/day, maximum 900 mg/day) from January 2004 to December 2008, aiming to measure the incidence of CMV disease and toxicity of valganciclovir.
Results
We enrolled 111 patients, 60% males, 46% African Americans, and median age at transplant 14.5 years (range 1.4–20.4 years). Sixty-nine percent of donors and 44% of recipients were seropositive pretransplant. Median duration of valganciclovir use was 5.9 months (range 0.5–24 months). CMV viremia and disease occurred in 27% and 4.5%, respectively. All patients with disease presented after prophylaxis ended and all were D+/R−. Thymoglobulin use (P=0.04) and positive donor CMV status (P=0.02) were associated with a higher risk of CMV viremia. Twenty-four percent had hematologic toxicity directly associated with valganciclovir.
Conclusions
Valganciclovir use in children was effective as prophylaxis against CMV disease; no children at our institution developed disease while on therapy. Our regimen of 24 weeks of prophylaxis was associated with a lower rate of late-onset disease than previous reports with 12-week regimens. Further controlled studies should be considered to compare longer versus shorter periods of prophylaxis and dose reductions and their impact on prevention of late-onset disease, resistance, cost, and toxicity.
Keywords: CMV, Valganciclovir, Children
Cytomegalovirus (CMV) is the most common opportunistic infection after solid-organ transplant and is responsible for both direct and indirect complications. Prevention and treatment of CMV primary infection or reactivation has become increasingly important in the management of this patient population. Multiple medications have been used, and all of them have resulted in significant decreases in direct and indirect complications when compared with placebo (1–4). Ganciclovir and more recently, valganciclovir have become the standard drugs used for prophylaxis against CMV infection (2). Valganciclovir is a prodrug of ganciclovir with improved oral bioavailability (2). Currently, there are two main strategies used for prevention of CMV disease—“prophylaxis” and “preemptive therapy.” In prophylaxis, all high-risk patients are started on antiviral medication soon after the transplant and continue it for the first 90 to 100 days posttransplantation. Preemptive therapy, by comparison, uses weekly laboratory surveillance for CMV viremia with initiation of medication only if there is active replication. Arguments in favor and against each of these strategies exist but both have been shown to prevent CMV disease (5, 30).
Since the introduction of valganciclovir as the medication of choice for CMV prevention in adult transplant patients, an increase in the incidence of late-onset CMV disease has been noted. Some have postulated that the increased level of viral suppression seen with valganciclovir may inhibit the host’s ability to mount an appropriate immune response to CMV, limiting the patient’s response once prophylaxis is discontinued (2, 3, 6, 7). Delayed-onset CMV disease has been associated with increased mortality and allograft failure in the first year posttransplantation (6, 8). Prolonging prophylaxis time to 6 months has been proposed to decrease the burden of late-onset disease, but there is still lack of consensus on this approach. Doyle et al. compared 3 versus 6 months of oral ganciclovir for prophylaxis of CMV disease in adult kidney transplant recipients. A lower risk of CMV infection was seen in the group that received 6 months of oral therapy, without significant increase in adverse effects in the first year post-transplant (9). Major concerns related to a longer duration of prophylaxis are increased development of antiviral resistance, drug toxicity, decreased compliance, increased cost, and further delay in the incubation of CMV disease.
Although the pediatric population is at higher risk of acquiring disease because of the increased frequency of seronegative pediatric recipients receiving CMV-seropositive adult organs, there is a lack of data regarding CMV infection and the use of valganciclovir as a prophylactic agent in this population. Studies have shown significant pharmacokinetic variability depending on the child’s age, weight, and creatinine clearance, suggesting that pediatric dosing should take into account those parameters and may need pharmacokinetic monitoring (10, 11). Vaudry et al. (12) recently reported good tolerance of oral valganciclovir in a pediatric solid-organ transplant cohort (n=63) at doses based on body surface area and creatinine clearance: 11% of patients were reported as having serious adverse events, most commonly hematologic laboratory abnormalities, and no patients were found to have CMV disease. No data regarding the administration of 6 months of prophylactic therapy with valganciclovir for prevention of CMV disease have been reported in the pediatric population. At our institution, we have been using valganciclovir prophylaxis for 6 months in the pediatric renal transplant population for the past 4 years. We report retrospective data on the safety and efficacy of valganciclovir in children and the role of 6 months of therapy in the prevention of late-onset CMV disease and graft loss from this cohort of patients.
RESULTS
Patient Sample and Demographics
One hundred thirty patients received a renal transplant from January 2004 to December 2008. Of those, a total of 19 patients were excluded: 13 because they received prophylaxis with an agent other than valganciclovir, 4 moved to another state after transplantation, and 2 lost their graft within the first 24 hr after the procedure, leaving a total of 111 eligible patients for analysis. Most patients (83 [75%]) were followed up for 2 years with a mean duration of follow-up of 21.8 months (range 1.5–24 months; Fig. 1). Of the 111 patients, 101 (90.9%) received prophylaxis with valganciclovir with a mean duration of 5.9 months (range 0.5–24 months). Ten patients did not receive prophylaxis. Of the patients who received prophylaxis, 88 (87%) were considered to be at risk for CMV infection (39 of these were D+/R−), and 13 (13%) were not considered to be at risk (D−/R−) and received prophylaxis for unclear reasons. Sixty-seven (60%) were males, and 46% were African American. The median age at transplant was 14.5 years (range 1.4–20.4 years). Sixty-nine percent of donors and 44% of recipients were seropositive before transplant. Sixty-five percent of the transplants were from cadaveric origin (Table 1). Some degree of rejection, subclinical (stable allograft function with a biopsy consistent with rejection) or clinical, was seen in 50 patients (45%) in the first 2 years posttransplantation, with 13 (12%) requiring thymoglobulin therapy. Graft loss was seen in three patients (3%), mainly due to poor compliance; none of the patients who lost their graft had a history of a positive CMV polymerase chain reaction (PCR). Posttransplant lymphoproliferative disease was present only in one patient (<1%).
FIGURE 1.
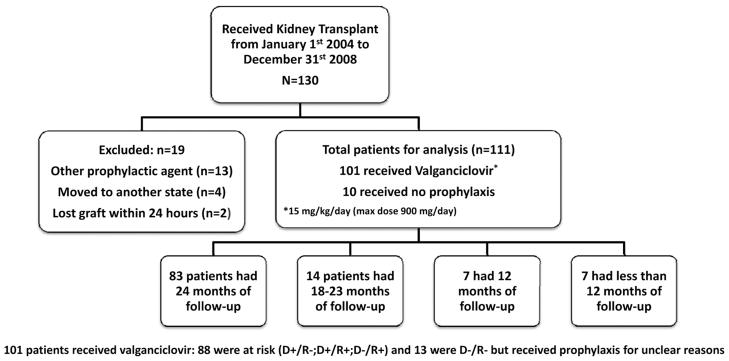
Patient flow-chart. D, donor; R, recipient.
TABLE 1.
Patient demographics and baseline characteristics
| Characteristic | No. patients treated with valganciclovir (n=101) | Total No. patients (N=111) |
|---|---|---|
| Sex, n (%) | ||
| Male | 62 (61) | 67 (60) |
| Female | 39 (39) | 44 (40) |
| Race, n (%) | ||
| African American | 47 (46) | 51 (46) |
| White | 39 (39) | 45 (40) |
| Hispanic | 10 (10) | 10 (9) |
| Asian | 5 (5) | 5 (5) |
| Median age of transplant (range) | 14.4 (1.4–20.4) | 14.5 (1.4–20.4) |
| Donor/recipient status, n (%) | ||
| +/− | 39 (39) | 40 (36) |
| +/+ | 36 (36) | 37 (33.3) |
| −/+ | 10 (10) | 12 (10.8) |
| −/− | 13 (13) | 19 (17.1) |
| Equivocal | 1 (1) | 1 (0.9) |
| Unknown | 2 (2) | 2 (1.8) |
| Origin of transplant, n (%) | ||
| Cadaveric | 67 (66) | 72 (65) |
| Living related/unrelated | 34 (34) | 39 (35) |
| Rejection | 43 (43) | 50 (45) |
| Thymoglobulin use | 11 (11) | 13 (12) |
| Treatment (mo) | ||
| Mean duration of valganciclovir prophylaxis (range) | 5.9 (0.5–24) | |
| No. patients receiving prophylaxis | ||
| <3 mo (mean of 58.5 d [SD 29.6]) | 10 | |
| 3–6 mo (mean of 158.3 d [SD 23.1]) | 48 | |
| >6 mo (mean of 265.4 d [SD 127.3]) | 43 |
CMV Infection
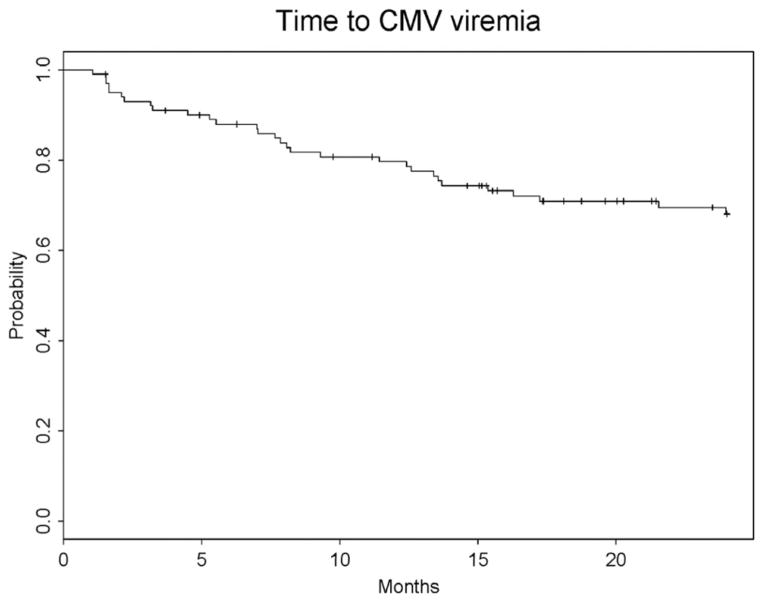
Thirty (27%) patients had CMV infection, and five (4.5%) had CMV disease. Disease occurred only in D+/R− recipients. Of the 30 patients with viremia, 2 patients had detectable CMV viremia before prophylaxis began, 9 patients had detectable virus while on prophylaxis (all except 1 had a positive donor), 1 patient had detectable virus the day prophylaxis ended, and the remaining 18 patients were positive for CMV after prophylaxis ended. In these patients, the median interval between the end of prophylaxis and the development of viremia was 105 days (range 27–475 days; Fig. 2). Of those with disease, only two (40%) received 6 months of prophylaxis, and the remaining patients discontinued therapy at 15, 16, and 87 days because of valganciclovir-induced leukopenia. In patients who received the full 24 weeks of prophylaxis, disease developed at 36 and 49 weeks after completion of therapy, whereas the patients who received shorter duration relapsed at 7, 10, and 68 weeks after stopping valganciclovir. CMV disease manifestations included fever and neutropenia (two), pneumonitis (one), gastrointestinal manifestations (one), and liver disease (one).
FIGURE 2.
Time to development of cytomegalovirus (CMV) viremia from transplantation.
Relationship Between CMV Disease and Associated Risk Factors
For the analysis of potential risk factors involved in the development of CMV viremia, we considered only the 101 patients who received valganciclovir therapy. The distribution of these subjects is shown in Table 1. Univariate analysis showed that being a seropositive donor (D+) was a risk factor for CMV disease and CMV viremia (P=0.02). The highest risk group (D+/R−) showed a similar trend (P=0.08); all patients with disease were D+/R−. In addition, thymoglobulin administration was also a risk factor for CMV viremia (P=0.04). None of the patients with CMV disease received thymoglobulin therapy. Patients with shorter periods of prophylaxis had a higher cumulative CMV viremia rate at 6 months (Table 2). We attempted to construct a multivariable model for development of CMV viremia. However, only donor CMV status was found to be a significant independent prognostic variable. There was no observed relationship between time to acute rejection, origin of transplant, sex, or race with an increased risk of CMV viremia (Table 2).
TABLE 2.
Risk factors for CMV viremia: Univariate analysis
| Covariate | No. patients (n) | 6-mo cumulative CMV viremia rate (95% CI) | P |
|---|---|---|---|
| Sex | |||
| Male | 62 | 13.05 (10.91–15.39) | 0.4233 |
| Female | 39 | 15.79 (12.58–19.33) | |
| Race | |||
| African American | 47 | 15.23 (12.39–18.34) | 0.16 |
| White | 39 | 12.98 (10.33–15.95) | |
| Hispanic | 10 | 0 | |
| Asian | 5 | 60 (45.62–71.7) | |
| Donor serostatus before transplant | |||
| D+ | 76 | 16.15 (13.77–18.70) | 0.02 |
| D− | 23 | 8.70 (6.46–11.35) | |
| Recipient serostatus before transplant | |||
| R+ | 48 | 12.98 (10.54–15.68) | 0.63 |
| R− | 52 | 13.46 (11.08–16.08) | |
| D+R− | |||
| Yes | 39 | 17.95 (14.4,21.8) | 0.08 |
| No | 62 | 11.6 (9.67,13.72) | |
| Rejection | |||
| Yes | 43 | 21.46 (17.51–25.69) | 0.28 |
| No | 58 | 8.75 (7.27–10.39) | |
| Origin of transplant | |||
| Cadaveric | 67 | 16.73 (14.13–19.52) | 0.57 |
| Living related/unrelated | 34 | 9.02 (7.05–11.28) | |
| Duration of valganciclovir | |||
| <3 mo | 10 | 55.6 (44.35–65.39) | 0.1 |
| 3–6 mo | 48 | 12.7 (10.30–15.25) | |
| >6 mo | 43 | 9.4 (7.54–11.41) | |
| Thymoglobulin use | |||
| Yes | 11 | 36.36 (26.07–46.70) | 0.04 |
| No | 90 | 11.3 (9.74–12.99) |
CMV, cytomegalovirus; CI, confidence interval; D, donor; R, recipient; SD, standard deviation.
Valganciclovir-Associated Comorbidities
Valganciclovir-associated comorbidities were seen in 24% of patients (24/101). Of these, all had leukopenia; 13 (54%) of these patients presented with severe neutropenia (absolute neutrophil count <500 cells/μL). In addition, 14 of the 24 had anemia (58%). Fifteen (15%) patients stopped the medication because of these associated comorbidities.
DISCUSSION
Late-onset CMV disease is an emerging problem in transplanted patients receiving CMV prophylaxis. Official guidelines recommend 3 months of oral prophylaxis in renal transplant patients who are seronegative and are receiving an organ from a seropostive donor, and prophylactic or preemptive therapy in seropositive recipients (13). Although a significant reduction in CMV morbidity and mortality occurred as a result of this approach, approximately 18% of patients still develop late-onset CMV disease (2). Although the reasons for this are not clear, prophylactic therapy with a potent agent such as valganciclovir may blunt the ability of the host to mount specific CMV responses by inhibiting viral replication during this period of time. Based on the above finding, opponents of longer courses of CMV prophylaxis argue that increasing the duration of prophylaxis would just continue to postpone the onset of CMV disease, while increasing the chances of medication toxicity and resistance. Results from our study suggest that pediatric kidney transplant patients receiving prophylaxis therapy for 6 months had a low incidence of late-onset CMV disease (4.5% after 2 years of follow-up). Few studies have been conducted looking at the incidence of CMV infection in the pediatric kidney transplant population, and none of them have looked at late-onset CMV disease. In our institution, we had previously reported an incidence of CMV disease of 12.3% in our pediatric kidney transplant population in an era without CMV prophylaxis (14). A Canadian study reported an incidence of CMV viremia and CMV disease of 35% and 9.6%, respectively (15); however, no real comparisons can be made between these two studies because they had different prophylactic approaches (the latter used a preemptive approach with valganciclovir and hyperimmunoglobulin administration). No data have been reported in the pediatric population looking at 3 versus 6 months of valganciclovir prophylaxis therapy and onset of CMV disease.
Among the adult literature, the incidence of late-onset disease has been reported to be approximately 8% to 12% in previous reports of patients receiving only 3 months of therapy (2, 16). Studies comparing 3 and 6 months have suggested better outcomes with the 6-month regimen without a significant increase in toxicity and resistance. Zamora et al. (17) showed that freedom from CMV infection and disease after valganciclovir prophylaxis was greater in patients receiving more than 180 days of therapy in the lung transplant population. A study of 70 adult patients comparing 3 versus 6 months of oral ganciclovir prophylaxis documented a lower risk of symptomatic CMV disease in the 6-month group (9). Recently, results from The Improved Protection Against Cytomegalovirus in Transplantation (IMPACT), a randomized, prospective, double-blinded study in high-risk kidney transplant recipients, showed a significant reduction of CMV disease out to 2 years posttransplantation in patients who received 200 days of valganciclovir prophylaxis compared with those who received 100 days of therapy (21.3 vs. 38.7, P<0.008) (18).
It is known that seronegative recipients are at higher risk of developing CMV disease when receiving organs from a seropositive donor. This is extremely important in children because a greater proportion of patients are seronegative at the time of transplant when compared with adults. Donor seropositivity before transplant is an important risk factor that has been corroborated in our study. In a similar way, thymoglobulin use has been associated with an increased risk of CMV viremia. This was also confirmed in our patient population (P=0.04). There was no relationship between CMV viremia with female gender, transplant origin, or organ rejection as other studies have suggested (6).
Other concerns related to the use of prolonged courses of prophylactic therapy compared with a preemptive approach are the development of toxicity, resistance, and increased cost. Leukopenia with or without neutropenia is the most common side effect reported with valganciclovir therapy. The combination of valganciclovir with other bone marrow suppressive medications such as mycophenolate mofetil (MMF), tacrolimus, or trimethoprim-sulfamethoxazole makes it difficult to implicate a specific drug as the sole cause of toxicity. In our patient population, we found that a 24% incidence of toxicity believed to be secondary to valganciclovir, leukopenia with or without neutropenia, and anemia were reported most frequently. Thirteen percent had severe neutropenia (absolute neutrophil count less than 500 cells/μL), leading to a decrease in dose or termination of prophylaxis. No opportunistic infections were reported as a consequence of the neutropenia. The rates of toxicity seen in our population are in the range of what has been reported in patients receiving 3 months of therapy (19, 20). This highlights the need for close follow-up for hematologic toxicity and development of opportunistic infections in patients on valganciclovir.
There are concerns regarding development of resistance with prolonged exposure to valganciclovir therapy. CMV resistance testing is not routinely performed in our institution, but none of our patients who developed CMV disease were unresponsive to therapy with intravenous ganciclovir despite previous prophylaxis. Studies have shown a low risk of development of resistance in patients receiving 3 months of therapy (21), but further research is needed to better define the risk in patients with longer exposures.
Cost-analysis studies have shown that routine prophylaxis is less expensive or comparable with preemptive therapy (16, 22). Prolonging the course of prophylaxis may have an immediate impact in cost, but in the long-term, it may be less expensive if late-onset CMV disease or graft failures are prevented.
Although our study was limited because of its retrospective nature and the fact that we did not have a control group, it is the largest study in the pediatric population showing the safety and efficacy of valganciclovir prophylaxis in renal transplant patients. Our dosing strategy in addition to a longer prophylactic period resulted in a decreased incidence of both early- and late-onset CMV disease. However, a relatively high prevalence of leukopenia was still seen. Adult studies have demonstrated promising results obtaining similar levels of prevention but with less toxicity by using lower doses of valganciclovir (450 mg/day) (20, 23–25). It is yet uncertain the degree of resistance generated with this approach, considering a longer exposure with lower levels of the medication. Further studies in pediatrics are needed to address these issues of toxicity and resistance.
Although our study was not intended or designed to compare differences among patients receiving different durations of prophylaxis, there seems to be a lower cumulative CMV viremia rate at 6 months in those patients who received longer periods of prophylaxis. Nonetheless, these results need to be interpreted with caution, and a prospective, randomized, controlled study is necessary to better address this question in the pediatric population.
In conclusion, valganciclovir prophylaxis in the pediatric renal transplant population was safe and effective in preventing CMV infection. Prolonging valganciclovir prophylaxis to 6 months may decrease the incidence of late-onset CMV disease without a significant increase in toxicity and resistance.
MATERIALS AND METHODS
Pediatric patients between the ages of birth to 18 years who underwent renal transplants at Emory University/Children’s Healthcare of Atlanta between January 1, 2004 and December 31, 2008 were included in the study. The study was approved by the Institutional Review Board of Children’s Healthcare of Atlanta.
Immunosuppressive Strategy
The immunosuppressive strategy used in our institution has been described previously (26, 27). In brief, all patients received induction therapy with basiliximab and maintenance immunosuppression with prednisone, MMF, and tacrolimus. All graft recipients underwent surveillance biopsy at 3 months posttransplant. If subclinical acute rejection was present, the patient was treated with high-dose methylprednisolone. If no rejection was present, tacrolimus was discontinued, and sirolimus was started. All children continued treatment with MMF and prednisone after conversion to sirolimus.
Antiviral Prophylaxis
Patients who were seropositive or received a transplant from a positive donor were given valganciclovir for 6 months after transplantation. If a patient required thymoglobulin at any point, a minimum of 3 months of valganciclovir administration postthymoglobulin was given. Therefore, some patients received more than 6 months of therapy. Doses used for prophylaxis were 15 mg/kg per day (maximum 900 mg/day) once daily. Surveillance for CMV infection was done with PCR once monthly for the first 6 months followed by every 3 months thereafter.
Definition of CMV Infection and CMV Disease
CMV infection is defined as detection of CMV viral nucleic acid by PCR in body fluids or tissue specimens. Any level of detection was considered positive. CMV quantitative PCR assay was performed at Emory Medical Laboratories by previously described methodology (28). This assay will report quantitative results if there are more than or equal to 300 copies/mL of plasma and a “low-positive” result if there are between 100 and 300 copies/mL.
CMV disease is defined as isolation of CMV or detection of viral proteins or nucleic acid in any body fluid or tissue specimen by PCR or other means, in association with symptoms of CMV syndrome (fever, malaise, myalgias, and arthalgias) or end-organ disease in the lungs, gastrointestinal tract, liver, kidney, myocardium, or pancreas. Primary disease was diagnosed in patients who were previously found to be seronegative and recurrent disease in those who were known to be seropositive and had clinical symptoms and a detection of CMV by PCR or other means (29, 30).
Acute rejection: patients who presented with an increase in creatinine levels who responded to rejection treatment or those who had biopsyproven rejection (29).
Statistical Analysis
Information on patient demographics and clinical events was identified by reviewing medical records, clinical transplant databases, clinical laboratory data, and pathology reports and was entered into a standard Excel (Microsoft, Seattle, WA) based data collection form. Descriptive variables were analyzed and reported as means or medians. Time to CMV viremia was calculated as the time between transplant and laboratory confirmation of CMV viremia or date of last follow-up if no CMV viremia was present. Patients were followed up for a maximum of 24 months from the time of transplantation. The log-rank test was used to compare the estimates of the time to CMV viremia distribution by subgroup. Because most subgroups never achieved a median time to CMV viremia, we reported cumulative 6-month CMV viremia rate using the method of Kaplan-Meier. We used a Cox proportional hazards model to examine the relationship between covariates.
Acknowledgments
This work was supported in part by PHS Grant UL1 RR025008 and KL2 rR025009 from the Clinical and Translational Science Award program, National Institutes of Health, and National Center for Research Resources (J.G.); research support from Bristol-Myers Squibb (A.F.C.-G.); and research support from Pfizer pharmaceutical (J.A.H.).
Footnotes
The authors declare no conflict of interest.
A.F.C.-G. participated in research design, performance of the research, data analysis, and writing of the manuscript; J.G. and T.L. participated in data analysis and writing of the manuscript; L.C.H. participated in research design and writing of the manuscript; and J.A.H. participated in research design, data analysis, writing of the manuscript.
References
- 1.Hodson EM, Craig JC, Strippoli GF, et al. Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev. 2008:CD003774. doi: 10.1002/14651858.CD003774.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Paya C, Humar A, Dominguez E, et al. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2004;4:611. doi: 10.1111/j.1600-6143.2004.00382.x. [DOI] [PubMed] [Google Scholar]
- 3.Singh N. Antiviral drugs for cytomegalovirus in transplant recipients: Advantages of preemptive therapy. Rev Med Virol. 2006;16:281. doi: 10.1002/rmv.513. [DOI] [PubMed] [Google Scholar]
- 4.Snydman DR. The case for cytomegalovirus prophylaxis in solid organ transplantation. Rev Med Virol. 2006;16:289. doi: 10.1002/rmv.514. [DOI] [PubMed] [Google Scholar]
- 5.Mwintshi K, Brennan DC. Prevention and management of cytomegalovirus infection in solid-organ transplantation. Expert Rev Anti Infect Ther. 2007;5:295. doi: 10.1586/14787210.5.2.295. [DOI] [PubMed] [Google Scholar]
- 6.Arthurs SK, Eid AJ, Pedersen RA, et al. Delayed-onset primary cytomegalovirus disease and the risk of allograft failure and mortality after kidney transplantation. Clin Infect Dis. 2008;46:840. doi: 10.1086/528718. [DOI] [PubMed] [Google Scholar]
- 7.Cervera C, Pineda M, Linares L, et al. Impact of valganciclovir prophylaxis on the development of severe late-cytomegalovirus disease in high-risk solid organ transplant recipients. Transplant Proc. 2007;39:2228. doi: 10.1016/j.transproceed.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 8.Limaye AP, Bakthavatsalam R, Kim HW, et al. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation. 2006;81:1645. doi: 10.1097/01.tp.0000226071.12562.1a. [DOI] [PubMed] [Google Scholar]
- 9.Doyle AM, Warburton KM, Goral S, et al. 24-week oral ganciclovir prophylaxis in kidney recipients is associated with reduced symptomatic cytomegalovirus disease compared to a 12-week course. Transplantation. 2006;81:1106. doi: 10.1097/01.tp.0000204048.90367.97. [DOI] [PubMed] [Google Scholar]
- 10.Zhao W, Baudouin V, Zhang D, et al. Population pharmacokinetics of ganciclovir following administration of valganciclovir in paediatric renal transplant patients. Clin Pharmacokinet. 2009;48:321. doi: 10.2165/00003088-200948050-00004. [DOI] [PubMed] [Google Scholar]
- 11.Vethamuthu J, Feber J, Chretien A, et al. Unexpectedly high inter- and intrapatient variability of ganciclovir levels in children. Pediatr Transplant. 2007;11:301. doi: 10.1111/j.1399-3046.2006.00669.x. [DOI] [PubMed] [Google Scholar]
- 12.Vaudry W, Ettenger R, Jara P, et al. Valganciclovir dosing according to body surface area and renal function in pediatric solid organ transplant recipients. Am J Transplant. 2009;9:636. doi: 10.1111/j.1600-6143.2008.02528.x. [DOI] [PubMed] [Google Scholar]
- 13.Cytomegalovirus. Am J Transplant. 2004;4(suppl 10):51. doi: 10.1111/j.1600-6135.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 14.Robinson LG, Hilinski J, Graham F, et al. Predictors of cytomegalovirus disease among pediatric transplant recipients within one year of renal transplantation. Pediatr Transplant. 2002;6:111. doi: 10.1034/j.1399-3046.2002.01049.x. [DOI] [PubMed] [Google Scholar]
- 15.Renoult E, Clermont MJ, Phan V, et al. Prevention of CMV disease in pediatric kidney transplant recipients: Evaluation of pp67 NASBA-based pre-emptive ganciclovir therapy combined with CMV hyperimmune globulin prophylaxis in high-risk patients. Pediatr Transplant. 2008;12:420. doi: 10.1111/j.1399-3046.2007.00799.x. [DOI] [PubMed] [Google Scholar]
- 16.Khoury JA, Storch GA, Bohl DL, et al. Prophylactic versus preemptive oral valganciclovir for the management of cytomegalovirus infection in adult renal transplant recipients. Am J Transplant. 2006;6:2134. doi: 10.1111/j.1600-6143.2006.01413.x. [DOI] [PubMed] [Google Scholar]
- 17.Zamora MR, Nicolls MR, Hodges TN, et al. Following universal prophylaxis with intravenous ganciclovir and cytomegalovirus immune globulin, valganciclovir is safe and effective for prevention of CMV infection following lung transplantation. Am J Transplant. 2004;4:1635. doi: 10.1111/j.1600-6143.2004.00571.x. [DOI] [PubMed] [Google Scholar]
- 18.Humar A, Lebranchu Y, Vincenti F, et al. Long term results of the IMPACT study: 200 vs 100 days of valganciclovir prophylaxis in kidney recipients [abstract 350]. American Transplant Congress; May 1–5, 2010; San Diego, CA. [Google Scholar]
- 19.Parreira L, Bruges M, Gaspar A, et al. Prevention of cytomegalovirus disease in renal transplantation: Single-center experience. Transplant Proc. 2009;41:877. doi: 10.1016/j.transproceed.2009.01.067. [DOI] [PubMed] [Google Scholar]
- 20.Brum S, Nolasco F, Sousa J, et al. Leukopenia in kidney transplant patients with the association of valganciclovir and mycophenolate mofetil. Transplant Proc. 2008;40:752. doi: 10.1016/j.transproceed.2008.02.048. [DOI] [PubMed] [Google Scholar]
- 21.Boivin G, Goyette N, Gilbert C, et al. Absence of cytomegalovirus-resistance mutations after valganciclovir prophylaxis, in a prospective multicenter study of solid-organ transplant recipients. J Infect Dis. 2004;189:1615. doi: 10.1086/382753. [DOI] [PubMed] [Google Scholar]
- 22.Annemans L, Moeremans K, Mutimer D, et al. Modeling costs and cost-effectiveness of different CMV management strategies in liver transplant recipients as a support for current and future decision making. Value Health. 2002;5:347. doi: 10.1046/j.1524-4733.2002.54117.x. [DOI] [PubMed] [Google Scholar]
- 23.Gabardi S, Magee CC, Baroletti SA, et al. Efficacy and safety of low-dose valganciclovir for prevention of cytomegalovirus disease in renal transplant recipients: A single-center, retrospective analysis. Pharmacotherapy. 2004;24:1323. doi: 10.1592/phco.24.14.1323.43152. [DOI] [PubMed] [Google Scholar]
- 24.Park JM, Lake KD, Arenas JD, et al. Efficacy and safety of low-dose valganciclovir in the prevention of cytomegalovirus disease in adult liver transplant recipients. Liver Transpl. 2006;12:112. doi: 10.1002/lt.20562. [DOI] [PubMed] [Google Scholar]
- 25.Weng FL, Patel AM, Wanchoo R, et al. Oral ganciclovir versus low-dose valganciclovir for prevention of cytomegalovirus disease in recipients of kidney and pancreas transplants. Transplantation. 2007;83:290. doi: 10.1097/01.tp.0000251371.34968.ca. [DOI] [PubMed] [Google Scholar]
- 26.Hymes LC, Greenbaum L, Amaral SG, et al. Surveillance renal transplant biopsies and subclinical rejection at three months post-transplant in pediatric recipients. Pediatr Transplant. 2007;11:536. doi: 10.1111/j.1399-3046.2007.00705.x. [DOI] [PubMed] [Google Scholar]
- 27.Hymes LC, Warshaw BL, Amaral SG, et al. Tacrolimus withdrawal and conversion to sirolimus at three months post-pediatric renal transplantation. Pediatr Transplant. 2008;12:773. doi: 10.1111/j.1399-3046.2008.00906.x. [DOI] [PubMed] [Google Scholar]
- 28.Caliendo AM, St George K, Kao SY, et al. Comparison of quantitative cytomegalovirus (CMV) PCR in plasma and CMV antigenemia assay: Clinical utility of the prototype AMPLICOR CMV MONITOR test in transplant recipients. J Clin Microbiol. 2000;38:2122. doi: 10.1128/jcm.38.6.2122-2127.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34:1094. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 30.Legendre C, Pascual M. Improving outcomes for solid-organ transplant recipients at risk from cytomegalovirus infection: Late-onset disease and indirect consequences. Clin Infect Dis. 2008;46:732. doi: 10.1086/527397. [DOI] [PubMed] [Google Scholar]
- 31.Kotton CN, Kumar D, Caliendo AM, et al. International Consensus Guidelines on the Management of Cytomegalovirus in Solid Organ Transplantation. The Transplantation Society International CMV Consensus Group. Transplantation. 2010;89:779. doi: 10.1097/TP.0b013e3181cee42f. [DOI] [PubMed] [Google Scholar]