Abstract
Objective
Considering cardiovascular (CV) risk could make clinical care more efficient and individualized, but most practice guidelines focus on single risk factors. We sought to see if hypertension treatment intensification (TI) is more likely in patients with elevated CV risk.
Study design
Prospective cohort study of 856 US Veterans with diabetes and elevated blood pressure (BP).
Methods
We used multilevel logistic regression to compare TI across three CV risk groups – those with history of heart disease, a high-risk primary prevention group (10-year event risk > 20% but no history of heart disease), and those with low/medium CV risk (10-year event risk < 20%).
Results
There were no significant differences in TI rates across risk groups, with adjusted odds ratios (ORs) of 1.19 (95% confidence interval 0.77–1.84) and 1.18 (0.76–1.83) for high-risk patients and those with a history of CVD, respectively, compared with those of low/medium-risk. Several individual risk factors were associated with higher rates of TI: systolic BP, mean BP in the prior year, and higher hemoglobin A1C. Self-reported home BP < 140/90 was associated with lower rates of TI. Incorporating CV risk into TI decision algorithms could prevent an estimated 38% more cardiac events without increasing the number of treated patients.
Conclusions
While an individual’s blood pressure alters clinical decisions about TI, overall CV risk does not appear to play a role in clinical decision-making. Adoption of TI decision algorithms that incorporate CV risk could substantially enhance the efficiency and clinical utility of CV preventive care.
Keywords: Prevention, hypertension, decision making, veterans
Background
Preventing cardiovascular disease efficiently and effectively should be a primary goal of healthcare organizations, but clinical focus on non-patient-centered endpoints can limit efficiency. Clinical decision-making and organizational guidance for prevention of cardiovascular (CV) disease has often focused on reduction of individual risk factors, such as hyperlipidemia and hypertension. Care could be more efficient and effective if decision-making focused more on processes that reduce overall CV risk, which can be measured by the UK Prospective Diabetes Study Risk Engine (UKPDS)1 or Framingham Heart Score,2. Overall risk is a better indicator of treatment benefit because those with a higher likelihood of having an event have a higher absolute benefit from treatment.3,4 Even among patients with diabetes there exist large variations of potential benefit.3–5 For example, a patient with diabetes and hypertension in the lowest decile of risk has one-eighth the benefit from a treatment to reduce CV events than a patient in the highest decile of risk.6 However, given the current focus of guidelines on discrete risk factors, clinicians may be less likely to take CV risk into account when making decisions about modifying individual CV risk factors.
One way to assess how clinicians prioritize overall CV risk in patients with a known CV risk factor is by assessing hypertension treatment intensification (TI) decisions in those with elevated blood pressure. While failure of TI has been considered an indicator of poor clinical quality,7 more recent research5, 8 has shown that it often occurs due to clinical circumstances that make the potential benefits of TI less clear, such as clinical uncertainty about the validity of a blood pressure measurement or the presence of comorbidities. Given the variation in benefit as a function of CV risk, if clinicians think about CV risk in decision-making, patients at higher CV risk should have more consistent, reliable TI than those of lower CV risk and treatment benefit. This strategy of individually tailored care would maximize benefits and minimize risks for patients.
In this study we examine if clinicians account for overall CV risk when making TI decisions in response to an elevated blood pressure. Using data from a study of Veterans with diabetes and an elevated measured blood pressure, we assess whether patients with higher CV risk are more likely to have TI than those with lower CV risk. We also examine if individual clinical risk factors predict clinical action. We then developed a decision analysis to estimate the potential benefit of making treatment more risk-focused.
Methods
Setting and participants
The ABATe (Addressing Barriers to Treatment for Hypertension) study was a prospective cohort study of patients with diabetes from 9 Veterans Health Administration (VHA) facilities in 3 Midwestern states. The study conducted a detailed examination of the factors that influence blood pressure management. As has been described elsewhere,5, 9 the study enrolled 1,169 US Veterans with diabetes who were found to have elevated triage blood pressure (≥ 140 systolic mmHg or ≥ 90 mmHg diastolic) before an index primary care visit. Participants were patients of 96 attending-level primary care providers with at least 2 half-days per week of clinic at the involved sites. 87% of all approached and eligible patients and 83% of approached and eligible clinicians participated. Data were obtained from baseline provider and patient surveys, brief post-visit provider and patient surveys, an electronic medical record review, and data from automated VHA data sources. Because of the very small number of women in the population, they were excluded from analysis. We have removed from our analyses patients whose measurements were < 140/90 when repeated by the clinician that day, because of the clinical uncertainty about the appropriate clinical action in these circumstances. The original study examined patients with diabetes because of their greater cardiac risk and benefit from hypertension treatment. The VA EHR does not have an automated Framingham or UKPDS risk calculator, although they are available on many web-sites.
Institutional review boards of all participating facilities approved the study protocol and all patients and providers gave written informed consent before participating.
Outcome variable: Intensification of hypertension treatment
Our dependent variable was whether a provider intensified a patient’s blood pressure medication within 3 months after the index visit in response to the elevated measured blood pressure. We considered a treatment intensified if the dosage was increased on any anti-hypertensive medication or if any anti-hypertensive medications were started or switched. We included any actions taken within 3 months after the initial visit to allow time for laboratory work and blood pressure reassessments.
Cardiovascular Risk (CV) variable
We defined three mutually exclusive categories of CV risk: 1) the highest-risk group, which consisted of those in need of secondary prevention (individuals with a history of MI or congestive heart failure (HF)); 2) the high-risk primary prevention group, which consisted of those with a UK Prospective Diabetes Study1 (UKPDS) 10-year event risk of > 20% but without a history of MI or CHF; and 3) the low/medium risk primary prevention group, which included those with a UKPDS 10-year event risk of < 20% and no history of MI or CHF. We could not estimate an overall risk for secondary prevention patients or create an overall continuous risk score because we know of no validated risk predictor that integrates primary and secondary prevention. We also examined a continuous measure of CV risk in a secondary analysis. For this analysis, we excluded patients with a history of MI or CHF. The UKPDS risk score has better discrimination than the Framingham scores for patients with diabetes.18,19
Covariates for the primary model
Following a previously-developed conceptual model,5 we built sequential models based on 4 categories of potential confounders. The first category, ‘baseline BP’,’ includes SBP at study entry and the mean SBP in the year prior to entry. As in clinical practice, we used the clinic measurement of BP for this value. The second category, ‘clinical factors,’ includes comorbidity count10 and number of hypertension medication classes. Comorbidity count was used by a method developed by the Veterans Affairs Health Economics Resource Center using visit codes from the International Classification of Diseases, Ninth Revision.10 The third category, ‘clinical uncertainty,’ includes information that might make a provider question the patient’s elevated blood pressure, specifically patient-reported lower home blood pressure. The final category, ‘uncertain benefit,’ includes clinical characteristics that are associated with decreased benefit from TI, specifically being on four or more classes of antihypertensive at baseline.
Data analysis
We were principally interested in the association of patient CV risk with rates of blood pressure treatment intensification. We created four categories of variables apart from cardiovascular risk that might influence treatment intensification decisions and sequentially added these categories as covariates. These were blood pressure (visit systolic blood pressure and mean systolic BP in the year before the study), clinical factors (comorbidity scale10 and number of medications), clinical uncertainty (self-report of good home blood pressure) and uncertain benefit (use of four blood pressure medications). We included blood pressure as a covariate even though it is a component of cardiovascular risk as we wanted to look for evidence of any effect of CV risk status on intensification beyond that represented directly by the blood pressure.
To account for treatment differences between physicians and patient clustering within physicians, we used a multilevel logistic regression model with physician as a random effect. We started by estimating the relationship between CV risk level and intensification, and then sequentially added the covariates to the CV risk level: first ‘baseline blood pressure’ variables, then ‘clinical factors,’ then ‘clinical uncertainty’ variables, and finally variables indicating ‘uncertain benefit.’
In a secondary analysis we assessed if clinicians use any specific facets of CV risk in decision-making. To do this, we split the aggregate CV risk variable into its component predictors (patient age, race, hemoglobin A1C, duration of diabetes, the presence of atrial fibrillation, ratio of total cholesterol to HDL, smoking status, and number of hypertension medication classes) and used each of them in a model containing the same covariates described above.
Estimation of Clinical Impact
We also estimated the clinical implications of failing to guide treatment by overall CV risk. To do this we created an estimate of the clinical benefits of observed practice compared to the possible benefits of risk-based decision-making. To estimate intensification’s benefit we used data from a large meta-analysis with meta-regression.11, 12 The meta-regression calculated the decrease in coronary heart disease risk associated with adding a new, normal-dose blood pressure medication. The study found larger relative risk reductions from treating patients with higher SBP and older age. For example, a 55-year-old man with an SBP of 160 would have a 29% relative risk reduction (RRR) of having a CV event. If his BP were 150 the RRR would be 26%. For a 65-year-old with an SBP of 160 the reduction would also be 26%.
We then used these estimates to examine the clinical implications of failing to make treatment decisions based on risk. We used the estimate just described to compare the likely benefit from treatment intensification among the 55% of observed patients who actually received TI in the ABATe study to the likely benefit if those 55% of persons in the ABATe study with the highest CV risk had instead been treated. This compares the number of CV events that were likely prevented by treatment in the observed population to the number that would have been prevented if the highest-risk patients were treated. This comparison demonstrates the potential benefit of basing intensification decisions on overall risk as opposed to usual practice. We used only primary prevention patients because the UKPDS Risk Engine was developed exclusively in primary prevention patients.
Results
There were 856 eligible participants: 159 (19%) were low/medium CV risk primary prevention patients; 324 (38%) were high-risk primary prevention, and 373 (44%) had a history of MI or HF. Average index visit SBP was 155 (+/− 15 SD) mmHg and DBP 79 (+/− 12). The 10th percentile had a 10% 10-year estimated cardiac event risk by UKPDS, the 90th percentile had a 65% risk. More clinical information is provided in Table 1.
Table 1.
Description of the dataset.
| Full Population | Low/ medium risk | High riska, b | History of MI/CHFa | |
|---|---|---|---|---|
| Participants, N, (%) | 856 | 159 (19%) | 324 (38%) | 373 (44%) |
| Age, N (SD) | 65.5 (10.7) | 57 (8.7) | 67 (10)*** | 68 (10)*** |
| Percent African American, N (%) | 169 (20%) | 88 (55%) | 24 (7%)*** | 57 (15%)*** |
| High school education or less, N (%) | 407 (52%) | 67 (46%) | 163 (54%) | 177 (54%) |
| Income < $30,000/year | 175 (24%) | 37 (26%) | 73 (26%) | 64 (21%) |
| Visit systolic blood pressure, mean (SD) | 155 (14) | 152 (12) | 155 (14)** | 156 (14)** |
| Visit diastolic blood pressure, mean (SD) | 79 (12) | 83 (12) | 79 (11)** | 76 (12)*** |
| Systolic blood pressure in prior year, mean (SD) | 145 (15) | 144 (14) | 148 (16)** | 145 (15) |
| A1C , in %, mean (SD) | 7.4 (1.5) | 7.0 (1.3) | 7.6 (1.6)*** | 7.5 (1.5)*** |
| Atrial fibrillation, N (%) | 54 (6%) | 1 (0.6%) | 13 (4%)* | 40 (11%)*** |
| HDL, in mmHg, mean (SD) | 41 (12) | 48 (16) | 40 (10)*** | 40 (11)*** |
| Total Cholesterol, in mmHg, mean (SD) | 175 (50) | 169 (34) | 188 (56)*** | 167 (48) |
| Cigarette use (%) | 222 (26%) | 41 (26%) | 98 (30%) | 83 (22%) |
| Reports lower blood pressure at home (%) | 73 (9%) | 7 (4%) | 37 (11%)* | 29 (8%) |
| On 4+ hypertensive classes | 134 (16%) | 18 (11%) | 41 (13%) | 75 (20%)* |
| UKPDS 10-year risk | N/A | 11% (0.5) | 43 % (10)*** | N/A |
Abbreviations: MI = myocardial infarction; CHF = congestive heart failure; SD = standard deviation; UKPDS = United Kingdom Prospective Diabetes Study
All significance testing is compared to low/medium risk
High risk’ is 10-year UKPDS risk score > 20% in patients without a history of MI or CHF
p < 0.05, > 0.01
p < 0.01, > 0.001
p < 0.001
There were no significant associations between CV risk and the probability of TI, either before or after adjusting for potential confounders (Table 2). In the final model the OR of TI was 1.19 (95% CI 0.77–1.84, p = 0.43) for high-risk primary prevention vs. low/medium risk and 1.18 (0.76–1.83, p = 0.46) for history of MI/HF vs. low/medium risk. This analysis did not have substantial collinearity in any variable.
Table 2.
Effect of overall cardiac risk on probability of treatment intensification for elevated blood pressure in diabetic patients.
| High Riska, b | History of MI/CHF | |||
|---|---|---|---|---|
| Odds Ratio (CI) | P value | Odds Ratio (CI) | P value | |
| Model 1: Unadjusted | 1.20 (0.80–1.81) | 0.37 | 1.24 (0.83–1.85) | 0.29 |
| Model 2: Blood pressure adjustmentc | 1.08 (0.71–1.65) | 0.73 | 1.15 (0.76–1.75) | 0.50 |
| Model 3: + clinical factors | 1.07 (0.70–1.63) | 0.77 | 1.10 (0.72–1.69) | 0.66 |
| Model 4: + clinical uncertainty | 1.18 (0.76–1.82) | 0.46 | 1.16 (0.75–1.79) | 0.51 |
| Model 5: + uncertainty of benefit | 1.19 (0.77–1.84) | 0.43 | 1.18 (0.76–1.83) | 0.46 |
Abbreviations: CI = confidence interval, MI = myocardial infarction, CHF = congestive heart failure
All significance testing is compared to Low/Medium Risk Group (individuals with a 10-year UKPDS risk score < 20% and no history of MI or CHF)
High risk’ includes participants with a 10-year UKPDS risk score > 20%, but no history of patients without a history of MI or CHF
Model 2, BP adjustment, adjusts for measured systolic blood pressure (BP) and mean systolic BP in the year before the study
Model 3, clinical factors, adjusts for the variables in Model 1 plus clinical factors (comorbidity scale and number of medications)
Model 4, clinical uncertainty, adjusts for the variables in Model 2 plus clinical uncertainty (self-report of good home blood pressure)
Model 5, uncertainty of benefit, adjusts for the variables in Model 3 plus uncertainty of benefit (use of four blood pressure medications)
Of the 430 primary prevention patients with complete data, the average 10-year predicted event rate for the 235 intensified patients (55%) was 34.1% vs. 30.6% for the 195 (45%) not intensified. In contrast, if the same number of primary prevention patients had received TI but care had been prioritized by overall CV risk, then the intensified group would have had an estimated pre-intensification 10-year risk of 47.5% vs. only 14.3% in the non-intensified group. Among the 55% of primary prevention patients who would be intensified, this would eliminate an estimated 10.8 events per 100 treatment years, as opposed to 8.0 with the observed practice, resulting in 35% more CV events averted overall with no increase in overall treatment use or costs (Figure).
Figure.
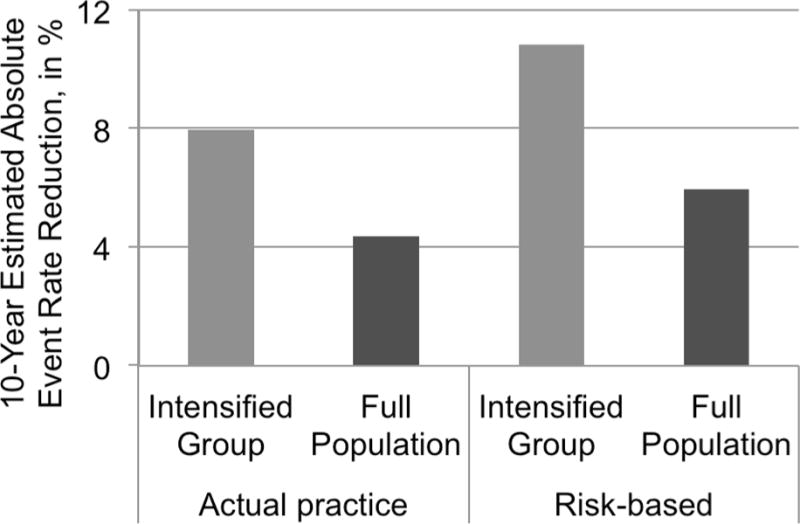
Comparison of 10-year estimated event rate reduction by currently observed treatment intensification vs. possible benefit if treatment were based on risk, among primary prevention patients. ‘Intensified’ is the rate reduction among those 55% who received treatment intensification in actual practice vs. those who would in a risk-based treatment. ‘Full population’ is the risk reduction among the entire population likely from the observed intensification vs. the same benefit if only the highest-risk patients were treated.
While clinicians did not seem to account for overall CV risk, the degree and consistency of systolic hypertension, which is the individual risk factor that is the explicit target of the treatment and the focus of most guidelines, was strongly associated with TI decisions (Table 3). Even though all patients in the study had a measured SBP > 140 mmHg and diabetes, higher systolic BP at the initial visit (OR 1.17, 95% CI 1.02–1.3, p = 0.01 per 10 mmHg), mean SBP from the prior year (OR 1.18, 95% CI 1.05–1.3, p = 0.007 for 10 mmHg), and patient-reported home blood pressures being at goal (OR 0.25, 95% CI 0.13–0.43, p < 0.001) were all independently associated with likelihood of TI. The patient’s A1c was also associated with intensification (OR 1.18, 95% CI 1.04–1.3, p = 0.01 for 10 mmHg). The reliability of this analysis may be limited by collinearity, with a Variance Inflation Factor of 7.0. Adding use of angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB) to this model did not change the results of the model.
Table 3.
Do clinical factors associated with CV risk influence the likelihood of TI?a
| Odds ratio (95% CI) | P value | |
|---|---|---|
| Age, per 10 years | 1.04 (0.9–1.2) | 0.69 |
| Nonwhite | 0.98 (0.6–1.5) | 0.94 |
| A1C | 1.18 (1.04–1.3) | 0.01 |
| Systolic BP (per 10 mmHg) | 1.17 (1.02–1.3) | 0.02 |
| Mean prior year systolic BP (per 10 mmHg) | 1.18 (1.05–1.3) | 0.007 |
| Reports home BP < 140/90 | 0.24 (0.13–0.44) | < 0.001 |
| Comorbidity scale | 1.02 (0.9–1.2) | 0.78 |
| Duration of diabetes, per 10 years | 0.86 (0.7–1.04) | 0.11 |
| Atrial fibrillation | 1.43 (0.7–2.9) | 0.32 |
| Active smoker | 0.85 (0.6–1.3) | 0.43 |
| Cholesterol (per 10 units) | 1.0 (0.96–1.04) | 0.99 |
| HDL (per 10 units) | 0.97 (0.83–1.13) | 0.66 |
| On 4+ BP Classes | 1.3 (0.7–2.4) | 0.39 |
| Total medication classes (count) | 1.0 (0.8–1.2) | 0.88 |
Abbreviations: CI = confidence interval, BP = blood pressure, HDL = high density lipoprotein,
All models are adjusted for systolic blood pressure, mean prior SBP in the prior year, and comorbidity index.
However, patient comorbidities, number of overall medications, and the patient already being on 4 or more antihypertension medications were not significantly associated with TI.
Discussion
Although it may seem intuitive that patient blood pressure level should guide anti-hypertensive treatment, overall cardiovascular (CV) risk is likely a better guide. In fact, CV risk predicts benefit from CV preventive treatments much more strongly than any individual cardiac risk factor.3, 6, 13 Our study is the first to examine the relationship of provider medical decision-making to patients’ CV risk. In keeping with our hypothesis that physician treatment decisions are driven by single risk factors and their corresponding treatments, we found that treatment intensification (TI) was not significantly more likely to occur in those at higher CV risk, but did find evidence that physicians are more likely to advance therapy in people with higher and more consistently-elevated blood pressures. We also demonstrated how care could be dramatically more effective if clinicians did use CV risk to guide their treatment decisions.
Treatment strategies that focus on single risk factors ignore that even people with identical blood pressures or lipid values and diabetes can have tremendously varied cardiac risk and equally-large variation in treatment benefit.3, 6, 13 Indeed, the potential benefit of hypertension treatment among people with diabetes and identical SBP often varies by orders of magnitude.6,4 The primary determinant of these differences in estimated benefit is overall CV risk. Even in our high-risk population of patients with diabetes and high blood pressure, there was a large range in CV risk.
Although we did not find evidence that clinicians use CV risk in treatment intensification decisions, our study showed that real-world clinical care is more nuanced than the dichotomous recommendations of many guidelines. If the guidelines were followed automatically, every patient in our cohort would have had TI. In reality, treatment is much more likely to be intensified in patients with higher systolic blood pressure and less likely in patients with lower BP measurements at home. This is further evidence that failure to intensify treatment is often a clinical decision, not necessarily “clinical inertia”5 or a mental lapse. If failure to intensify were purely a mental lapse, there would be no relationship between TI and SBP or home BP readings.
Progress in using overall risk assessment for treatment decisions has important implications for clinical management, efficiency, and quality. Indeed, as we have shown, accounting for CV risk would result in more efficient care, saving resources and time, and improve population outcomes. Moreover, it would result in more patient-centered care, because only those patients likely to benefit would be subjected to increasing doses and numbers of medications. Most important, clinical information systems that allow capture of relevant variables and automated display of calculated risk information, with recommendations for treatment, could make assessing and acting upon overall CV risk information dramatically simpler for clinicians. These systems would be fairly easy for clinical organizations to create. The ability to harness the power of information systems and routinely use such data and decision tools in personalized clinical care is not only within our grasp technically, it is within our responsibility as healthcare providers and managers. The focus in the Affordable Care Act on clinical efficiency and value-based health purchasing in Accountable Care Organizations will make identifying patients by event risk and potential benefit from treatment even more important in coming years.16, 17
We also found that those with a higher A1C were more likely to be intensified. The most likely reason for this is the observed phenomenon that patients with multiple comorbidities, especially ‘concordant comorbidities’ receive more aggressive care.15 When related problems have concordant solutions (such as diabetes and high blood pressure), patient care seems to be better for all of the conditions.9, 15
The primary strength of our study is the clinical detail of the data. The ABATe study has information from surveys of clinicians and patients, chart review, and information from the electronic health record about many facets of the clinical encounter.5 This enabled us to tests various factors that might influence TI, including the importance of clinical uncertainty of hypertension and uncertainty of the benefit of treatment.
Our study does have limitations. Since we know of no risk assessment score applicable to both primary and secondary prevention, we were not able to use a single, continuous variable of CV risk, which would have been considerably more statistically efficient. This resulted in reduced statistical power, and therefore we are not able to rule out that clinicians consider CV risk to a low to moderate extent. However, the study did have sufficient power to demonstrate that clinicians put greater importance on individual risk factors that have much less impact on patient benefits than risk. The highest risk patients were found to have a trend-level effect towards greater treatment based on risk (p = 0.46). Our study had just fewer than 1000 patients. Perhaps with substantially larger sample size we may have found an effect.
Also, this study was conducted within the VHA in which most patients are male and at high CV risk, resulting in a quite high cardiovascular risk profile. However, the impact of using CV risk is likely to be greater when there is more heterogeneity in CV risk as would be expected in other clinical populations. Although it is always possible that insensitivity to patient CV risk is unique to VA clinicians, we cannot think of any reason why this would be true.
This study suggests that clinicians frequently target single risk factors rather than overall CV risk when making hypertension TI decisions. Organizational policies and guidelines that focus clinical decisions on CV risk could guide towards more efficient and effective prevention of cardiac disease. Currently, this opportunity is missed.
Take-away points.
– Cardiac risk is easy to calculate and the major predictor of benefit for blood pressure medications.
– Even among patients with high blood pressure and diabetes, those with higher blood pressure are more likely to have treatment changes.
– Those with higher cardiac risk are not more likely to have treatment changes.
– This is a major loss in potential efficiency and effectiveness of blood pressure treatment.
Acknowledgments
Funding/Support: This work was supported by the Robert Wood Johnson Clinical Scholars Program and an associated VA Advanced Fellowship, as well as by research grants from the US Department of Veterans Affairs Health Services Research and Development Service (IIR02-225), the Veterans Affairs Quality Enhancement Research Initiative – Diabetes Mellitus (QUERI DM, DIB 98-001) and the Michigan Diabetes Research and Training Center Grant (P60DK-20572).
Role of the Sponsor: The funding sources had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Footnotes
A Previous version of this work was presented at the Society of General Internal Medicine National Meeting on May 6, 2011.
Conflict of Interest: None
References
- 1.Stevens RJ, Kothari V, Adler AI, Stratton IM. The UKPDS risk engine: a model for the risk of coronary heart disease in Type II diabetes (UKPDS 56) Clin Sci (Lond) 2001 Dec;101(6):671–679. [PubMed] [Google Scholar]
- 2.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991 Jan;121(1 Pt 2):293–298. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 3.Hayward RA, Krumholz HM, Zulman DM, Timbie JW, Vijan S. Optimizing statin treatment for primary prevention of coronary artery disease. Ann Intern Med. Jan 19;152(2):69–77. doi: 10.7326/0003-4819-152-2-201001190-00004. [DOI] [PubMed] [Google Scholar]
- 4.Timbie JW, Hayward RA, Vijan S. Diminishing efficacy of combination therapy, response-heterogeneity, and treatment intolerance limit the attainability of tight risk factor control in patients with diabetes. Health Serv Res. Apr;45(2):437–456. doi: 10.1111/j.1475-6773.2009.01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerr EA, Zikmund-Fisher BJ, Klamerus ML, Subramanian U, Hogan MM, Hofer TP. The role of clinical uncertainty in treatment decisions for diabetic patients with uncontrolled blood pressure. Ann Intern Med. 2008 May 20;148(10):717–727. doi: 10.7326/0003-4819-148-10-200805200-00004. [DOI] [PubMed] [Google Scholar]
- 6.Timbie JW, Hayward RA, Vijan S. Variation in the net benefit of aggressive cardiovascular risk factor control across the US population of patients with diabetes mellitus. Arch Intern Med. Jun 28;170(12):1037–1044. doi: 10.1001/archinternmed.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001 Nov 6;135(9):825–834. doi: 10.7326/0003-4819-135-9-200111060-00012. [DOI] [PubMed] [Google Scholar]
- 8.Kmetik KS, O’Toole MF, Bossley H, et al. Exceptions to outpatient quality measures for coronary artery disease in electronic health records. Ann Intern Med. Feb 15;154(4):227–234. doi: 10.7326/0003-4819-154-4-201102150-00003. [DOI] [PubMed] [Google Scholar]
- 9.Zulman DM, Kerr EA, Hofer TP, Heisler M, Zikmund-Fisher BJ. Patient-Provider Concordance in the Prioritization of Health Conditions Among Hypertensive Diabetes Patients. J Gen Intern Med. Feb 2; doi: 10.1007/s11606-009-1232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003 Sep;60(3 Suppl):146S–167S. doi: 10.1177/1077558703257000. [DOI] [PubMed] [Google Scholar]
- 11.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. Bmj. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009 Mar;122(3):290–300. doi: 10.1016/j.amjmed.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 13.Zulman DM, Vijan S, Omenn GS, Hayward RA. The relative merits of population-based and targeted prevention strategies. Milbank Q. 2008 Dec;86(4):557–580. doi: 10.1111/j.1468-0009.2008.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. Bmj. 1998 Sep 12;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen LA, Woodard LD, Henderson LM, Urech TH, Pietz K. Will hypertension performance measures used for pay-for-performance programs penalize those who care for medically complex patients? Circulation. 2009 Jun 16;119(23):2978–85. doi: 10.1161/CIRCULATIONAHA.108.836544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fendrick AM, Smith DG, Chernew ME. Applying value-based insurance design to low-value health services. Health Aff (Millwood) Nov;29(11):2017–2021. doi: 10.1377/hlthaff.2010.0878. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman SM, Bertko JM. Building regulatory and operational flexibility into accountable care organizations and ‘shared savings’. Health Aff (Millwood) Jan;30(1):23–31. doi: 10.1377/hlthaff.2010.0928. [DOI] [PubMed] [Google Scholar]
- 18.Davis WA, Colagiuri S, Davis TM. Comparison of the Framingham and United Kingdom Prospective Diabetes Study cardiovascular risk equations in Australian patients with type 2 diabetes from the Fremantle Diabetes Study. Med J Aust. 2009 Feb 16;190(4):180–184. doi: 10.5694/j.1326-5377.2009.tb02684.x. [DOI] [PubMed] [Google Scholar]
- 19.van der Heijden AA, Ortegon MM, Niessen LW, Nijpels G, Dekker JM. Prediction of coronary heart disease risk in a general, pre-diabetic, and diabetic population during 10 years of follow-up: accuracy of the Framingham, SCORE, and UKPDS risk functions: The Hoorn Study. Diabetes Care. 2009 Nov;32(11):2094–2098. doi: 10.2337/dc09-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]


