Abstract
Liver fluke infection caused by Opisthorchis viverrini is a major public health problem in Thailand and adjacent countries. In addition to infection-associated morbidity, infection with O. viverrini and the related Clonorchis sinensis are unarguable risk factors for cholangiocarcinoma, bile duct cancer. Here we review the pathogenesis of opisthorchiasis and the association of O. viverrini infection and bile duct cancer, focusing on the molecular parallels between wound healing, chronic inflammation and cancer development. We review a schema for human disease progression from fluke infection, chronic opisthorchiasis, advanced periductal fibrosis, and cholangiocarcinogenesis, and present a rationale for biomarker discovery to facilitate early intervention. We conclude by addressing post-genomic advances with a view to developing new control strategies to combat this infectious cancer.
Keywords: Opisthorchis, liver fluke, cholangiocarcinoma, liver cancer, granulin, periductal fibrosis, excretory/secretory, wound healing
A liver fluke that causes cancer
Liver fluke infection with Opisthorchis viverrini, Opisthorchis felineus and Clonorchis sinensis remains a major public health problem in many parts of Asia and Eastern Europe. For the purposes of this review, we will restrict our focus to O. viverrini – for comprehensive reviews on C. sinensis see [1–3] and O. felineus see [4]. O. viverrini is a food-borne trematode that encysts as a metacercaria in the fins, skin and musculature of cyprinoid fish. Infection occurs when individuals ingest raw or uncooked fish infected with the metacercariae. Adult flukes reside within the human host for over 10 years, feeding on the epithelial cells that line the intrahepatic bile ducts. O. viverrini is endemic in Southeast Asia, including Thailand, Lao PDR, Vietnam and Cambodia [3, 5]. Opisthorchiasis has been most comprehensively studied in Thailand, where approximately 6 million people are infected with the parasite (calculated from an overall countrywide prevalence of infection of 8.7% in the population in 2009) [6]. The infection is associated with a number of hepatobiliary abnormalities, including cholangitis, obstructive jaundice, hepatomegaly, periductal fibrosis, cholecystitis and cholelithiasis (reviewed in [7]).
Both experimental and epidemiological evidence strongly implicate O. viverrini infection in the etiology of cholangiocarcinoma (CCA) or bile duct cancer [5, 8–10], which has one of the highest mortality rates of any cancer. It has been observed that, of the five major regions of Thailand, the incidence of CCA is highest in the region(s) with the highest prevalence of O. viverrini infection [11]. Moreover, in these regions CCA is the leading cause of cancer-associated mortality, especially cancers among males. Hepatocellular carcinoma (HCC), which is the most common type of liver cancer worldwide, is second to CCA in these regions and shows no association with the endemicity of O. viverrini; i.e., the incidence of HCC remains evenly distributed throughout Thailand [11]. Khon Kaen province in Northeast Thailand has both the highest prevalence of O. viverrini infection and the highest incidence of CCA in the world (Figure 1) [12]. Much of the work we describe here was performed in this endemic region. In Lao PDR, an estimated 2.5 million individuals (~37% of the population) are infected with O. viverrini [13], with prevalence figures approaching 64% in some regions of the Mekong River Basin [14]. A recent study utilized Bayesian geostatistical models to identify risk factors and to investigate the spatial pattern of O. viverrini infection in Lao PDR [15]. Infection with O. viverrini was strongly associated with exposure to infected fish, human behavior and culture, whereas high transmission was sustained by lack of sanitation.
Figure 1. Incidence of liver cancer and prevalence of liver fluke infections.

Liver cancer rates are divided into the major subtypes of hepatocellular carcinoma (HCC), cholangiocarcinoma (CCA) and other less common subtypes (a). Regions within Thailand are highlighted with red stars. The prevalence of O. viverrini and C. sinensis in the Mekong Basin sub-region (b). Endemicity level is defined based on prevalence of infections: low – 1–5%; medium - 5.1–15%; high - >15%. Taken from [6].
The association between CCA and liver flukes has been observed for approximately 60 years (Figure 1) [16]. To our knowledge, chronic O. viverrini infection and the development of CCA is the strongest association between a parasitic infection and cancer - stronger than the better-known link between Schistosoma haematobium infection and squamous cell carcinoma [17]. More than 1000 new cases of CCA are diagnosed each year at Srinagarind University Hospital at Khon Kaen University (B. Sripa, unpublished), an incidence which has not declined since Viranuvatti s observations in 1955 [16], despite government-initiated Mass Drug Administration (MDA) programs with praziquantel [18]. In 1994, the World Health Organization s International Agency for Research on Cancer (IARC) listed O. viverrini as a group 1 carcinogen, i.e., they considered it a direct risk factor for cholangiocarcinoma. In northern Thailand an estimated 5000 cases of cholangiocarcinoma are diagnosed annually [5, 19], which translates into 5000 deaths superimposed on a chronic burden of liver and bile duct disease. A disproportionate number of deaths occur in male heads of households who ordinarily represent a family s major wage earner. Therefore, opisthorchiasis and cholangiocarcinoma also exerts a heavy economic toll that in Thailand results in US$120 million annually in both medical care and lost wages [20, 21].
The challenge: an infection-associated cancer in less developed countries
Approximately 750 million people are at risk of infection with fish borne liver flukes [3]. An estimated 40 million people are currently infected with liver flukes in the Mekong Basin subregion alone (Figure 1) [6]. Whereas the infection can be resolved by chemotherapy (praziquantel), environmental and socioeconomic factors in the Mekong Basin sub-region strongly favor rapid reinfection [6, 21]. Hence, individuals resident in fluke endemic areas in the Mekong Basin subregion often remain infected for decades, and in some cases, for a lifetime, with CCA as a common outcome [6, 21].
As with other infection-related cancers in low and middle-income countries (LMIC) [22], O. viverrini-induced liver cancer ranks first in mortality among cancers for men and second among cancers in women in the Mekong Basin sub-region [6, 21]. Moreover, O. viverrini-induced CCA is expected to increase sharply in the near future as a result of the demographic and economic factors occurring in Thailand, Lao PDR, Cambodia and Vietnam, including (i) increases in the populations [22]; (ii) rapid aging as they continue national focus on combating diseases that result in premature deaths [22]; (iii) health disparities related to behavior, education, socioeconomics, access to care, and other post-diagnostic factors that strongly influence clinical outcomes beyond disease etiology [23, 24]. Another important factor will be the spread of liver fluke infection in the region due to increased migration among the ASEAN Economic Community (AEC) countries (Thailand, Laos, Cambodia, Vietnam and Myanmar) as a result of an open borders policy starting in 2015. Despite the high infection-related cancer burden in the LMICs such as those of the Mekong Basin subregion and the poor prognosis for the future, international awareness of the problem is minimal. This reality is reflected in the omission of infection-related cancers from the Millennium Development Goals of the United Nations [22].
Opisthorchis-induced CCA – a ‘perfect storm’ of carcinogenic stimuli
Three main mechanisms are proposed to contribute to CCA through chronic infection with O. viverrini: (i) mechanical damage to the biliary epithelia caused by the feeding activities of the parasites; (ii) immunopathology due to infection-related inflammation; and (iii) toxic effects of parasite excretory/secretory (ES) molecules. The interplay of these mechanisms aligns with current knowledge of malignancies, suggesting that formation and progression relies on many interrelated factors creating a microenvironment that is conducive for malignant transformation [25]. In Figure 2 we summarize the various insults that culminate in a continuous state of chronic inflammation and wound healing in opisthorchiasis and eventually lead to CCA.
Figure 2. Hypothesized pathways of pathogenesis of liver fluke-induced cholangiocarcinoma (CCA).
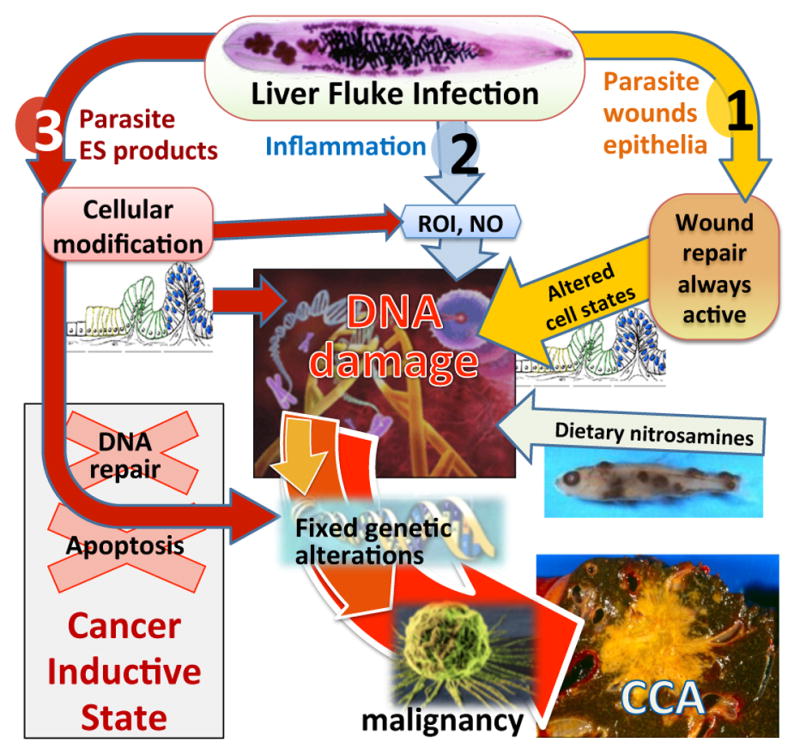
O. viverrini damages bile duct epithelia via three pathways: (1) mechanical damage by feeding parasites; (2) immunopathology, particularly due to reactive oxygen intermediates (ROI) and nitric oxide (NO); (3) direct effects of fluke secreted proteins including induction of cell proliferation and inhibiting cancer prevention pathways (DNA repair/apoptosis). These pathways converge, resulting in genetic lesions that become fixed after successive replications, eventually leading to malignant transformation of cholangiocytes into CCA. Adapted from [121].
Mechanical injury
Physical damage induced by the feeding activities of liver flukes contributes to biliary damage. The suckers of the fluke hook into the biliary epithelia, damaging the bile ducts, even in early infection. As the flukes mature, the lesions enlarge and ulcerate. For the rest of this review, we will focus primarily on the latter two carcinogenic mechanisms described above, immunopathology and direct toxic effects of parasite ES molecules.
Immunopathology
It has long been thought that host immune responses and immunopathologic processes mediate hepatobiliary damage in opisthorchiasis [7]. We recently implicated both parasite-specific [26] and non-specific (serum – [27]) IL-6 in the pathogenesis of advanced periductal fibrosis (APF) in opisthorchiasis, with possible links to other hepatobiliary abnormalities, including CCA. Inflammation around infected hamster bile ducts is a consequence of host cellular responses to Opisthorchis antigens [28], and infiltration of inflammatory cells in periductal sites of infected hamster liver is associated with the presence of fluke antigens in the bile duct epithelium.
Toll-like receptors (TLRs) recognize and respond to diverse molecules, the so-called Pathogen Associated Molecular Patterns (PAMPs). These receptors mediate initial responses in innate immunity including to mucosal pathogens and are required for the development of the acquired immune response [29]. Engagement of the TLR by a cognate PAMP activates cellular signaling pathways to induce immune response genes including pro-inflammatory cytokines. To address the early stages of immunopathology in the biliary tract of O. viverrini infected people, a normal immortalized human cholangiocyte cell line (H69) was stimulated with ES products from O. viverrini and TLR signaling was assessed [30]. ES products induced increased expression of TLR4 mRNA, induced IκB-degradation in a MyD88-dependent manner, and activated NF-κB nuclear translocation. In support of human immunoepidemiology studies [26, 27], O. viverrini ES products induced expression and secretion of IL-6 and IL-8 from cholangiocytes [30].
Liver fluke secretes mitogenic factors for host cells
To survive long periods in hostile environs, parasitic helminths excrete and secrete a range of soluble proteins and other mediators that perform many roles at the host-parasite interface, including digestion of nutrients, tissue invasion and regulating the host immune system. This interaction has long been thought to modify host cellular homeostasis and contribute to malignant transformations [31, 32]. A routine observation in fluke infections is altered cell states, one of the gateways to genomic instability that can be induced by a variety of growth factors [33]. Lesions of epithelial dysplasia and metaplasia including goblet cell metaplasia and adenomatous hyperplasia are common [34]. As flukes feed on the biliary epithelium and induce damage via mechanical injury and the inflammatory response to this process, they actively release ES proteins from the tegument and excretory pore into the bile, or culture medium in vitro, some of which are highly immunogenic [28, 35]. These metabolic products, aside from inducing immune responses, may be toxic to or interact with the biliary epithelium. Murine fibroblasts (NIH-3T3) co-cultured with O. viverrini (but physically separated from the worms in Transwell plates) proliferate compared to cells in media alone [36], confirming pioneering reports of hyperplasia of opisthorchiasis-associated biliary epithelial cells [28, 37]. With recent advances in the characterization of transcriptome [38, 39] and ES proteome [35] of O. viverrini, the molecular identities of some liver fluke proteins has been revealed [40, 41]. Below we focus on one specific parasite-derived growth factor, granulin, highlighting its potential roles in wound healing and carcinogenesis.
Granulin: a parasite growth factor that causes proliferation of host cells
To identify parasite proteins central to liver fluke survival, the host-parasite relationship and the aetiology of CCA, proteomic approaches were employed to characterise 300 O. viverrini secreted and surface membrane proteins [35]. The ES products included a complex mixture of parasite proteins, some of which had homologues in the human host that were associated with cancers, including proteases, protease inhibitors, orthologues of mammalian growth factors and anti-apoptotic proteins. Of note was the identification of Ov-GRN-1, a protein with sequence similarity to the mammalian growth factor, granulin. Ov-GRN-1 is the only helminth-derived growth factor reported to date that causes proliferation of mammalian cells [41, 42]. Ov-GRN-1 was secreted by adult flukes where it bound to biliary epithelial cells of O. viverrini-infected hamsters (Figure 3).
Figure 3. Immunolocalization of Ov-GRN-1 in adult O. viverrini and bile ducts of experimentally infected hamsters.
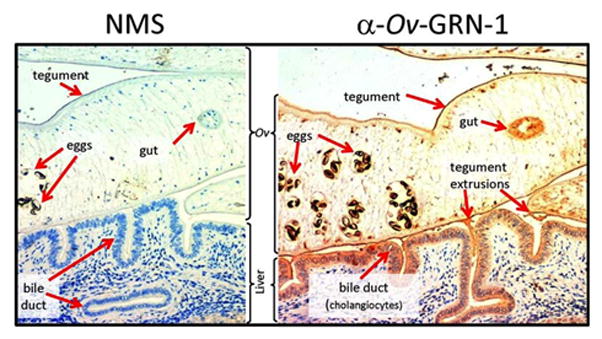
The left panel was probed with IgG purified from normal mouse serum (NMS); the right panel was probed with anti-Ov-GRN-1 IgG. Sections were stained with immunoperoxidase revealed as a brown/rust colored deposit and Mayer s Haematoxylin counterstained the nuclei in blue. Red arrows highlight the regions within the O. viverrini parasite and bile duct tissue that stained positive for Ov-GRN-1. From [41].
GRN protein family members occur in diverse taxa including bacteria, plants and animals. Human pro-GRN (PGRN) is associated with many aggressive cancers. It is overexpressed in many human tumours [43] and stimulates angiogenesis, suppresses apoptosis, and promotes tumour invasion and anchorage independence, all of which support tumour expansion in an unfavourable interstitial environment [44–46]. Indeed, PGRN expression driven by IL-6 results in increased growth of CCA [47]. Preventing the activity of GRN in a range of tumour types, either through gene silencing or antibody neutralization, reduces or entirely inhibits tumour progression [48]. It is pertinent that like human GRN, liver fluke ES products promote tumour cell growth [41], suppress apoptosis [49] and induce potent IL-6 production in vitro [30] and in vivo [26, 27]. Moreover, antibodies raised to recombinant Ov-GRN-1 from O. viverrini blocked ES-driven proliferation of fibroblasts and CCA cell lines [41], attributing all of the growth-factor activity in O. viverrini ES products to Ov-GRN-1 and highlighting the functional conservation between host and parasite granulins.
Similarities between healing wounds and feeding liver flukes
Why O. viverrini secretes a potent growth factor that acts on host cells is unclear, but one potential role is apparent – wound healing. Mammalian inflammatory cells secrete peptides derived from PGRN [50], and PGRN mRNA is highly induced in dermal fibroblasts and epithelial cells following transcutaneous puncture wounds [51]. Furthermore, human recombinant PGRN increased the accumulation of inflammatory cells, blood vessels and fibroblasts at puncture sites, implying a direct role as a wound-healing growth factor [51]. O. viverrini adult worms grasp the bile duct wall with their suckers and feed on the biliary cells, often severely damaging the epithelium. Additional inflammation occurs as a result of the local immune response to resident worms (reviewed in [7]). It therefore seems reasonable to hypothesize that Ov-GRN-1 plays a role in wound repair at and around the feeding site to minimize the pathology that the parasite causes to the host. However, a potential consequence of this parasite-assisted wound repair is the elevated risk of DNA damage and genomic instability in proliferating cells, promoting development of a tumorigenic environment. In preliminary studies, we detected proliferation of hamster biliary epithelial cells and expression of DNA damage markers in the tissues adjacent to O. viverrini in situ, further supporting the notion that biliary cells surrounding liver flukes undergo DNA damage as a result of excessive proliferation (Figure 4).
Figure 4. Epithelial hyperplasia and DNA damage in the bile ducts of O. viverrini infected hamsters.
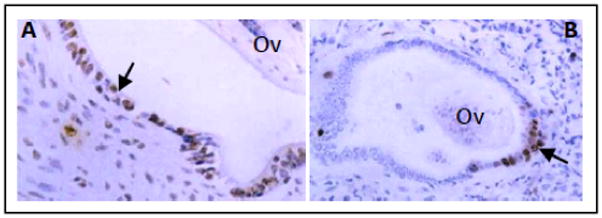
(a) Proliferating epithelial cells were detected in the bile ducts surrounding adult flukes (Ov) using bromodeoxyuridine (arrow); ×200 magnification. (b) DNA damage in epithelial cells (arrow) revealed by 8-oxodG staining in the liver of hamsters that were chronically infected with O. viverrini (Ov). Normal liver did not stain positive for either stain (not shown). Photo provided by B. Sripa (unpublished).
Liver flukes cause mechanical injury to the bile ducts, readily visualized as tissue damage from the oral and ventral suckers hooking into the bile duct epithelium to secure the parasite in place [52]. If the parasite is removed the damage resolves, and the regenerative growth of the wound response halts. But in a fluke infection, persistent damage of the epithelium resulting from constant feeding and migratory activities hinders complete wound repair and recovery from the injury. This is evident from individuals who develop a persistent form of APF that remains, and can even proliferate, after O. viverrini infection is cured by anthelmintic drugs. The relentless cell division associated with this tissue injury (both mechanical damage and immunopathogenesis), in the presence of exogenous co-factors, such as dietary nitrosamines, is thought to result in DNA damage and subsequent oncogenesis. These carcinogenic properties of fluke-induced wounding have been illustrated in hamsters, where the bile ducts were surgically ligated (simulating damage seen in fluke infections), followed by co-administration of sub-carcinogenic oral doses of nitrosamines. The hamsters showed significant biliary lesion development whereas the controls, which received biliary ligation or nitrosamines alone, did not [53]. Therefore, invoking the wound repair response likely triggers cell proliferation, which in the presence of co-factors, significantly contributes to cancer development [54].
Wound repair has long been implicated in tumorigenesis, with striking similarities between tumour stroma and wounded tissues [55, 56]. Wound healing responses disrupt the cellular microenvironment by inducing production of enzymes, including matrix metalloproteases, that degrade the extracellular matrix, interfere with epithelial cell-cell adhesion, and induce cell division to create a cellular covering of the wound [25, 57]. In a normal situation, after the wound has been repaired, a series of secreted factors act to halt the process. Malignant transformation is a particularly severe untoward complication of non-healing ulcers, and the development of cancer in fibrotic tissue is a frequent event [58]. Clinical observations show that cancer is commonly a result of chronic inflammatory disease in diverse organs and tissues. Examples are the elevated risk of developing cancer in patients with chronic viral hepatitis and H. pylori-induced gastric inflammation. Moreover, there is a striking similarity between genes that are upregulated in wound healing and cancer. Of particular note, growth factors and associated signaling molecules are overexpressed in healing wounds and cancer [58]. Perhaps not surprisingly, microarray analysis of murine fibroblasts co-cultured with O. viverrini ES products showed upregulation of the same gene families, with a predominance of genes encoding growth factors and associated signaling molecules [59]. Box 1 highlights some features shared among chronic opisthorchiasis, wound repair and cancer.
Box 1. Parallels between chronic opisthorchiasis, wound repair, and cancer.
Malignant tumours often develop at sites of chronic injury, re-epithelialization, and inflammation. Gene expression profiles of cancers, healing wounds, and cells exposed to ES are very similar – genes that have opposing expression profiles are linked to the irreversible development of malignancy.
Over-representation of upregulated genes involved in cell proliferation, most notably growth factors (notably EGF) and signaling molecules.
Cellular composition of the tumour stroma strongly resembles the granulation tissue of healing skin wounds and biliary tract of animals/humans with chronic opisthorchiasis.
Extensive fibrosis – transient and self-limiting in wounds, but chronic in cancer and opisthorchiasis; excessive collagen deposition.
Inflammatory cell infiltrates characterized by mononuclear cells; M2 (alternatively activated) macrophages and reactive oxygen intermediates are a feature.
Neovascularization (angiogenesis).
IL-6 upregulated in opisthorchiasis (and biliary cell lines exposed to parasite antigens), and healing wounds; IL-6 drives human progranulin expression in CCA.
Disease progression from chronic O. viverrini infection to CCA: data from Thailand
The pathological consequences of chronic O. viverrini infection occur mainly in the liver, the intra- and extra-hepatic bile ducts, and the gall bladder, and have been described in both humans from autopsy studies. The severity of the pathology is associated with both the intensity and the duration of infection (reviewed in [60]). During the decades of O. viverrini infection, a continuum of clearly defined, subclinical and clinical events, starting with bile duct inflammation, proceeding through advanced periductal fibrosis, and, in some cases, concluding with a diagnosis of CCA can be mapped out for chronic infection (Figure 5). In this regard, O. viverrini infection does not differ greatly from other chronic inflammatory disorders, where (as described above) the persistent mechanical, toxic and immunologic irritation from the parasite sustained over decades leads to the continuous production of growth factors and fibrogenic cytokines, which stimulates the deposition of connective tissue that progressively remodels the normal tissue architecture of the biliary epithelium, resulting in the accumulation of fibrotic elements along the intrahepatic biliary tract. When the accumulation of fibrosis advances along the entire intrahepatic bile duct, it is referred to as advanced periductal fibrosis , which can engulf the outflow system of exocrine bile from the liver, resulting in the loss of bile duct contractility and the accumulation of bile sludge, a process that is readily detected by ultrasonography (Box 2 and Figure 5) [61–67]. This process has also been observed in the hamster model of O. viverrini-induced CCA. After 12 weeks of infection with O. viverrini, the biliary epithelium of the hamster is markedly inflamed and displays fibrosis advancing along its length; and, more importantly, there is fibrotic deposition in the biliary epithelium that leads to CCA [37, 68–71]. However, not all individuals who are chronically infected with O. viverrini develop advanced hepatobiliary abnormalities. From community based ultrasound studies in endemic areas in Thailand (Box 2), we have found that only a subset of individuals infected with this food borne trematode respond with a prolonged and intense inflammatory response and have termed these individuals as having a pro-inflammatory phenotype [26, 27]. We hypothesize that this phenotype predisposes them to advanced periductal fibrosis (APF) and elevates their risk of developing CCA. We suspect that individuals with pro-inflammatory phenotype have a dysregulation of inflammatory cytokine production in response to chronic fluke infection [26, 27]. In some cases, the dysregulation of inflammatory cytokine production continues even after treatment by chemotherapy removes the established infection, with a subset of individuals continuing to produce the smoldering and polarized inflammatory immune response and presenting with an unresolved or persistent form of APF, months or even years after treatment [72–75].
Figure 5. Conceptual framework for O. viverrini-induced cholangiocarcinoma (CCA).
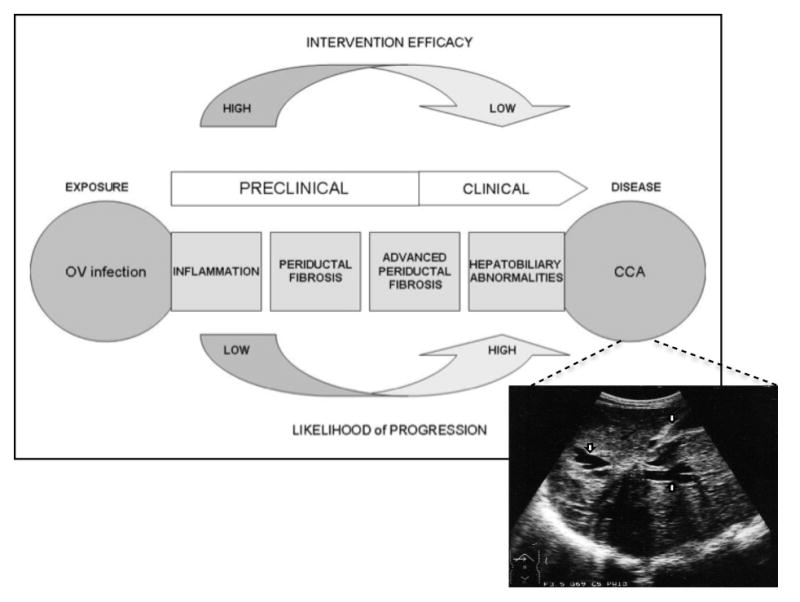
The primary exposure (OV infection) is identified and the progression of disease events to CCA are tracked in well-defined stages. Linked to the CCA window of the schematic is an ultrasonograph depicting a CCA. Note liver mass surrounded by dilated intrahepatic ducts (arrows). Image provided by EM. Figure adapted and modified for O. viverrini-induced CCA from [27].
Box 2. Chronic opisthorchiasis: a steady and stealthy disease burden.
The chronic inflammatory challenge from O. viverrini infection can lead to obvious clinical sequelae including obstructive jaundice, hepatomegaly, cholecystitis, as well as bile duct cancer.
However, important changes occur to the liver early in O. viverrini infection that are clinically silent unless actively detected by ultrasound or other imaging modalities [67].
Community-based ultrasound studies estimate that 45% of adults in O. viverrini endemic areas have advanced hepatobiliary abnormalities, including enlargement of the left hepatic lobe, deficits in gallbladder function (including reduced gallbladder contractility), gallbladder sludge, and advanced periductal fibrosis, a precursor stage to bile duct cancer [26, 27].
Multiple risk factors are likely to determine who evolves advanced hepatobiliary abnormalities from chronic O. viverrini infection, including the duration of the infection, the intensity of the infection, and diet (nitrosamines).
Studies have also identified a pro-inflammatory phenotype characterized by a persistent form of advanced periductal fibrosis and elevated levels of circulating pro-inflammatory cytokines in plasma, such as transforming growth factor-β, interleukin (IL)-1-α, and IL-6 [26, 27].
Asymptomatic hepatobiliary abnormalities from chronic opisthorchiasis may represent the greatest part of the disease burden associated with this neglected tropical disease.
Biomarkers of opisthorchiasis induced APF and CCA
Due to its role in systemic inflammation, IL-6 is readily detected in plasma [76]. In community-based studies along the Chi River Basin in Khon Kaen Thailand, we sought to determine if the concentration of IL-6 in the plasma of O. viverrini infected individuals with APF and CCA was higher than individuals infected with O. viverrini but without these advanced pathologies. Given the poor prognosis for CCA, especially in resource-poor settings such as Thailand, an early marker for the risk of the hepatobiliary pathologies related to O. viverrini infection is urgently needed. The data showed that elevated plasma concentrations of IL-6 were associated with a marked and significant increase in the risk of O. viverrini-associated APF and CCA (Figure 6) in a dose-dependent manner: i.e., individuals with the highest quartiles of plasma IL-6 concentration had a 19 times greater risk of developing APF and a 150 times greater risk of developing CCA than individuals with undetectable levels of plasma IL-6 [27]. As shown in a number of other studies, elevated plasma concentrations of IL-6 may play a key role in the pathogenic processes by creating an inflammatory milieu that favors fibrotic deposition and carcinogenesis in the bile duct (for reviews see [56, 58, 77–79]). Notably, the data showed that O. viverrini infection alone did not result in elevated IL-6 levels circulating in the plasma: e.g., O. viverrini infected individuals without APF or CCA had negligible levels of IL-6 in their plasma. It is only in the presence of an advanced pathology from chronic opisthorchiasis that plasma concentrations of IL-6 are significantly elevated.
Figure 6. Plasma IL-6 concentrations for detection of O. viverrini-induced advanced periductal fibrosis (left panel) or cholangiocarcinoma (right panel).
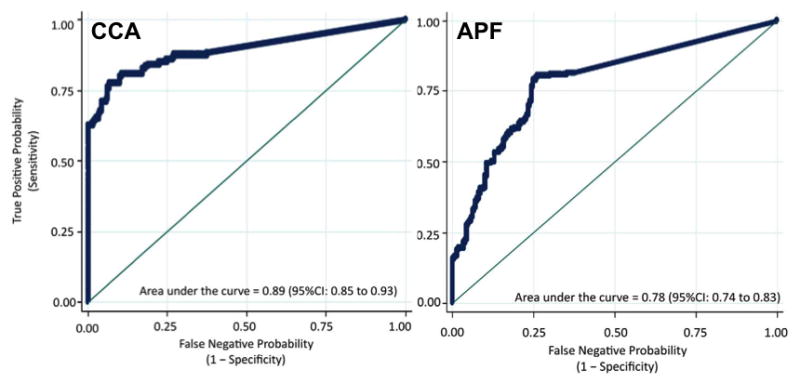
A receiver-operating-characteristic (ROC) curve plots the True Positive Probability (sensitivity) against the False Negative Probability (1 – specificity) for the full range of IL-6 cut-off points for the detection of O. viverrini-induced APF or CCA. The area under the ROC curve is interpreted as the probability of correctly identifying (accuracy) a randomly selected participant as either a case (APF positive or CCA) or a non-case (APF negative or CCA negative). The 45-degree line in the graph subsumes an area equal to 0.50 (50%), which is equivalent to using a coin toss procedure to classify participants as either cases or controls. Figure adapted from [27].
The ability of a single IL-6 measurement to detect risk for O. viverrini associated CCA and O. viverrini associated fibrosis in the bile duct (APF) is especially important in regions where O. viverrini is endemic. In Northeast Thailand, for example, the prevalence of O. viverrini infection can reach as high as 85%; hence, an easily accessible immune marker, which can distinguish between infection with O. viverrini and the advanced pathology induced by O. viverrini infection would be particularly useful [27]. Currently, Thai individuals in O. viverrini endemic areas are diagnosed only in the most advanced stages of CCA, where prognosis is dire and treatment essentially palliative.
Proteomic biomarker discovery in O. viverrini-induced CCA
Outside of Southeast Asia the risk factors for CCA are ill defined and the rarity of the disease means that few samples are available for use in biomarker discovery. In Thailand, the existence of a clear risk factor and ample biobanked samples available for analysis makes O. viverrini-induced CCA more amenable to the use of proteomic methods for protein-based biomarker discovery. Current tumour biomarkers for both O. viverrini- and non-O. viverrini-CCA are of little value [80], and despite a great deal of research, no plasma biomarker has proven to be of sufficient sensitivity or specificity for accurate and early diagnosis of CCA [81, 82]. The carbohydrate antigen, CA-19–9, is widely used for diagnosis of CCA, but it lacks specificity as it is also a marker for pancreatic cancer, gastric cancer, primary biliary cirrhosis and in smokers [80]. Similarly, carcinoembryonic antigen (CEA), a marker for colorectal cancer that is sometimes cited as a CCA marker, is only elevated in approximately 30% of CCA patients [83, 84]. In studies using non-O. viverrini induced models of CCA a number of other proteins have been shown to have some potential as biomarkers, including trypsinogen-2 [85], mucin-5AC [86] and soluble fragment of cytokeratin 19 although none of these are currently in clinical use [87, 88].
Given the seriousness of the problem and the need for early detection to improve outcomes for surgical intervention, much effort has been dedicated to determining diagnostic biomarkers for O. viverrini-induced CCA in Thailand. In some cases markers similar to those identified in non-O. viverrini-induced CCA have been identified in O. viverrini-induced CCA, for instance a glycan epitope of mucin-5AC. However, a number of potential biomarkers of infection, inflammation, and cancer have also been identified in human and hamster models of O. viverrini-induced CCA. Proteins, or other molecules, found to be over-expressed in O. viverrini-induced CCA include the platelet-derived growth factor alpha, found to be overexpressed in the hamster model of O. viverrini-induced CCA and in eight of ten human subjects tested [89], the myristoylated alanine rich C kinase substrate [90], oxysterol binding protein isoforms [91], the master coregulator MTA1 [92], peroxiredoxin 6 [93], and urinary 8-oxodG [94]. In addition to these a number of markers have been found to have prognostic significance, including annexin A2 [95], α-enolase [96], peroxiredoxin 1, and ezrin-radixin-moesin-binding phosphoprotein 50 [96]. In all cases little has been done in the validation of these findings, but they do represent a pool of potential biomarkers that may eventually be of clinical significance.
Biomarker discovery for CCA, and for other malignancies, has traditionally relied on relatively low-throughout proteomic or immunohistochemistry techniques. However, recent advances including techniques for the relative quantification of protein levels in different samples, such as an isobaric tag for relative and absolute quantitation (iTRAQ), stable isotope labeling by amino acids in cell culture (SILAC), and similar techniques [97], as well as improvements in mass spectrometer sensitivity and speed (increased coverage of proteomes) and the development of techniques such as multiple reaction monitoring (MRM) [97] for the targeted detection of multiple protein biomarkers in a single experiment, are accelerating biomarker discovery and validation. Of particular interest is the ability to detect patterns of protein expression. Using MRM, theoretically up to 60 proteins can be assayed in a single experiment, offering the advantages of increased specificity and specificity over single marker approaches [98]. In O. viverrini-induced CCA these emerging techniques offer the opportunity for rapid biomarker detection and validation in the Khon Kaen Cancer Cohort. The ability to assay biomarkers in the cohort, and biobanked samples, can be expected to provide an unparalleled testing ground for the ability of biomarkers to detect cancer in the earliest stages of carcinogenesis. Moreover, the ability to determine biomarker expression in those with advanced symptoms of O. viverrini infection who do not progress to CCA and compare with those who did progress will provide insight into the complex interplay of genetics, infection, inflammation and environment that culminates in cancer.
The Opisthorchis viverrini genome - functional genomics
As noted, the transcriptomes of O. viverrini and C. sinensis have been examined in depth [38, 39]. Deep sequencing in tandem with integrated genomic-bioinformatic approaches identified more than 50 000 unique sequences being in each species. The genome of C. sinensis was described recently; it is predicted to have a haploid genome size of 516 Mb and to include ~16 000 protein-encoding genes [99]. It can be anticipated that the genome sizes of O. viverrini and C. sinensis would be similar based on their similar karyotypes [100] and transcriptomes [39]. However, a recent report indicated that the haploid genome size of O. viverrini was 76 Mb [101]. Functional genomics have not been reported for O. viverrini or C. sinensis; nonetheless, RNA interference (RNAi) and other methods for genetic manipulation will facilitate characterization of the role and importance of genes for these flukes [102, 103]. The first report of RNAi in O. viverrini targeted the cathepsin B cysteine protease [104] as a model gene for development of RNAi in food-borne trematodes. The cathepsin B gene was susceptible to RNAi knockdown, as evidenced by both reductions in transcript levels and indeed in cathepsin B enzyme activity [104]. These findings thereby confirmed the presence of an intact RNAi pathway in these flukes, and provide a method for functional investigation of O. viverrini genes with hypothesized roles in liver disease and cholangiocarcinogenesis.
Opisthorchiasis vaccine - anti-parasite and anti-cancer?
Reliance on a sole drug, praziquantel, for treatment of human opisthorchiasis is concerning, particularly given recent reports of diminished cure rates [105]. Moreover, repeated drug cure followed by repeated reinfections is associated with increased pathology in opisthorchiasis, potentially increasing the risk of CCA (see next section). Subsequently there is a real need for alternative control methods for opisthorchiasis. The goal for development of a vaccine against O. viverrini infection is two-fold. Just as all other vaccines for infectious diseases, a successful vaccine will limit the pathologic sequelae of acute and chronic liver fluke infections; however, a vaccine targeting carcinogenic pathogens such as human papilloma virus has added value in its protection against cancer [106]. A vaccine for opisthorchiasis or clonorchiasis would therefore be of enormous benefit for people throughout endemic areas in SE Asia.
In earlier studies to establish an animal model for vaccination against opisthorchiasis, infectivity of metacercariae (MC) for hamsters was established by feeding them O. viverrini infected fish via oro-gastric tube [28]. As noted earlier, infection in hamsters closely models the proposed carcinogenic processes in humans. Phase 1 is characterized by edema and desquamation of the bile duct epithelium, followed by epithelial hyperplasia, pseudo-stratification of biliary epithelium, and mucin-secreting cell metaplasia. During phase 2, metaplastic squamous cells appear in conjunction with glandular proliferation, periductal infiltrates are composed of plasma cells, lymphocytes, and other mononuclear cell types with extensive infiltration of inflammatory cells producing high levels of pro-inflammatory cytokines. In phase 3, the final phase (>12 wk), the now chronically inflamed biliary tree shows advancing fibrosis along its length. Periductal fibrosis is considered the precursor event to CCA in this hamster model of liver fluke induced CCA [28], and like human opisthorchiasis, progression of infection to CCA is accelerated by the inclusion of dietary nitrosamines [10]. Hamsters are a permissive experimental host for the human hookworm, Necator americanus, and have been used in challenge models for the development of vaccines for human hookworm infection [107]. Hamsters can therefore serve as an excellent model to assess vaccine efficacy in a pre-clinical setting under conditions that mimic human exposure to the parasite.
Pertinent information on the use of the hamster model for testing vaccines is already available for C. sinensis. Irradiated MC afford significant protection in rats against C. sinensis infection [108], and a number of recombinant proteins have been assessed using distinct vaccine delivery systems. Recombinant C. sinensis tegumental protein 22.3 kDa (CsTP22.3) was produced on Bacillus subtilis spores and administered to rats orally to induce mucosal immune responses [109]. Recombinant spores induced production of secretory IgA and induced a significant level of protection in rats challenged with MC. In another study rats vaccinated intradermally with DNA encoding C. sinensis cysteine proteinase (CsCP) developed a CsCP-specific Th-1 response and displayed a significant level of protection upon challenge with MC [110]. Based on this information, we are currently optimizing the irradiated MC model for the establishment of a pre-clinical opisthorchiasis vaccine development and testing platform in hamsters (T. Laha et al., unpublished).
Fluke antigens such as secreted proteases or granulin have potential as intervention target(s) including as vaccine candidates, given recent successes with chemotherapy targeting related enzymes in schistosomes [111] and with vaccines in other liver flukes [112]. Indeed, in view of the recent implementation of an acclaimed vaccination of adolescents against papilloma-virus infection to provide protection from cervical cancer [113], there is the appealing prospect that vaccination to prevent infection with O. viverrini and/or C. sinensis could provide protection against another infection-related cancer, liver fluke-induced CCA. As with other human helminth vaccine development programs, a liver fluke vaccine is unlikely to induce sterilizing immunity [114], so we envisage that any such vaccine would be linked with chemotherapy to achieve maximum efficacy.
Repeated infection and repeated treatment with praziquantel – a cautionary tale?
Opisthorchiasis associated CCA follows several reasonably well-characterized pathological changes in the bile ducts, as described above. The changes include chronic inflammation and fibrosis combined with nitrative stress from either endogenous and/or exogenous nitrosamines, leading to DNA damage and fixation of mutations [7]. A strong correlation between elevated immune responses to fluke antigens and hepatobiliary abnormalities has been described. IgG levels were most markedly elevated in disease cases compared with healthy individuals and were closely associated with gall bladder size and dysfunction. This is consistent with the hypothesis that an immunopathologic mechanism is involved in opisthorchiasis [115]. Repeated infection of hamsters induced greater inflammation and more severe pathology in association with parasite-specific antibody during chronic inflammation, induced the expression of iNOS in inflammatory cells and in the epithelium of bile ducts, and subsequently caused nitrosative and oxidative damage to nucleic acids (Figure 7) [116, 117]. Repeated infection of hamsters followed by repeated chemotherapy with praziquantel resulted in increased inflammation but did not increase the induction potential of CCA [118].
Figure 7. Effect of praziquantel on expression of inducible nitric oxide synthase (iNOS) and nuclear factor- κB (NF-κB) in infected hamsters.

Expression of iNOS and NF-κB in hamster livers was assessed by double immunofluorescence. O. viverrini infection induced iNOS expression (red) in the cytoplasm and NF-κB accumulation (green) in the nucleus of bile duct epithelial cells. Treatment with praziquantel gradually increased expression of these proteins 6 hours post-treatment. Expression reached its highest level 12 hours post-treatment and then decreased 24 hours post-treatment. Normal hamsters treated with praziquantel and analyzed 12 hours after treatment (N + PZ, 12 hr) showed weak immunoreactivity after prolonged exposure (microscopy) to highlight minimal background fluorescence. Magnification × 400; magnification × 200 for N + PZ. OV - O. viverrini; Bd - bile duct. Taken from [74].
In naturally acquired human infections with O. viverrini, individuals who have been repeatedly infected then repeatedly treated with praziquantel are exposed to continued antigenic assaults and presumably develop elevated parasite-specific antibody titers. When these people are exposed to nitrative stresses via endogenous and/or exogenous nitrosamines, they may be at increased risk of cholangiocarcinogenesis. Supporting data for an elevated risk of CCA with repeated rounds of chemotherapy and reinfection in humans are, for the most part, lacking. However, Chernrungroj described a case-control study on the risk factors for O. viverrini-induced CCA and use of praziquantel, adjusted for other covariates (Gun Chernrungroj, Ph.D. thesis, Yale University, 2000). In this study, the odds ratio (OR) of CCA increased significantly with repeated rounds of chemotherapy: 0, 1 and 2–4 rounds of praziquantel treatment had ORs for developing CCA of 1.0 (reference), 3.04 and 4.60 respectively. If indeed repeated rounds of chemotherapy followed by reinfection (and associated acute inflammation) increases the risk of CCA, this would have a profound influence on the widely perceived benefit of MDA programs, and clearly warrants urgent further exploration. This is particularly pertinent in areas such as Lao PDR where MDA is widespread, and the incidence of CCA is higher than that observed in Thailand where chemotherapy is more intermittent. Only through a robust randomized controlled trial can such an important issue be resolved.
Concluding remarks
Despite the MDA programs throughout Thailand, oriental liver fluke infection is still a major public health concern, and its prevalence in some areas is increasing [21]. The association between the parasite and bile duct cancer is undisputed; however, the mechanisms by which carcinogenesis occurs from chronic infection is less well understood. Moreover, despite its classification as a group I carcinogen, and the poor prognosis faced by the millions of people who either have or are at risk of developing CCA, very little research is conducted outside of Thailand on this insidious pathogen. Recent advances have capitalized on the genomics and proteomics revolutions, setting the scene for post-genomic applications of the enormous wealth of sequence information generated. This will hopefully accelerate the development of programs focusing on desperately needed drugs and vaccines [3, 119, 120]. The identification of biomarkers of disease progression is also urgently required. If robust biomarkers that signal progression of chronic infection along the path of periductal fibrosis towards CCA can be identified, targeted intervention strategies can be developed and rapidly deployed into endemic areas. Indeed, based on our recent findings describing the utility of IL-6 as a marker for APF and early CCA, the Thai Ministry of Public Health has introduced plasma IL-6 screening in conjunction with other tests to detect early liver cancer in O. viverrini endemic areas of Northeast Thailand. Finally, there are lessons for cancer biologists in this story. Unlike most other types of cancer, liver fluke-induced CCA in humans is characterized by a relatively established etiology. There is an excellent animal model (hamsters) that closely parallels human opisthorchiasis and the subsequent biliary abnormalities that culminate in CCA: however, in the animal model, the time frame required to progress through these disease stages is months rather than the decades observed in humans. We hope that the work reviewed herein (mostly pioneered by and conducted in Southeast Asian laboratories) will stimulate more researchers to explore this intriguing marriage of an infectious disease and cancer biology alike, and address some fundamental key questions, not the least of which is “why does this parasite cause cancer while most others do not?”
Acknowledgments
Research described here was partially supported by awards UO1AI065871 from the National Institute of Allergy and Infectious Disease and R01CA155297 from the National Cancer Institute as well as project grant and fellowship support (A.L., J.M.) from the National Health and Medical Research Council of Australia. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID, NCI, NIH or NHMRC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lun ZR, et al. Clonorchiasis: a key foodborne zoonosis in China. Lancet Infect Dis. 2005;5:31–41. doi: 10.1016/S1473-3099(04)01252-6. [DOI] [PubMed] [Google Scholar]
- 2.Hong ST, Fang Y. Clonorchis sinensis and clonorchiasis, an update. Parasitol Int. 2012;61:17–24. doi: 10.1016/j.parint.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Keiser J, Utzinger J. Food-borne trematodiases. Clin Microbiol Rev. 2009;22:466–483. doi: 10.1128/CMR.00012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mordvinov VA, et al. Opisthorchis felineus and Metorchis bilis are the main agents of liver fluke infection of humans in Russia. Parasitol Int. 2012;61:25–31. doi: 10.1016/j.parint.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 5.IARC. Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 6.Sithithaworn P, et al. The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitol Int. 2012;61:10–16. doi: 10.1016/j.parint.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sripa B, et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4:e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thamavit W, et al. Strong promoting effect of Opisthorchis viverrini infection on dimethylnitrosamine-initiated hamster liver. Cancer Letts. 1994;78:121–125. doi: 10.1016/0304-3835(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 9.Vatanasapt VSB, Sithithaworn P, Mairiang P. Liver flukes and liver cancer. Cancer Surveys. 1999;33:313–343. [Google Scholar]
- 10.Thamavit W, et al. Effects of dimethylnitrosamine on induction of cholangiocarcinoma in Opisthorchis viverrini-infected Syrian golden hamsters. Cancer Res. 1978;38:4634–4639. [PubMed] [Google Scholar]
- 11.Srivatanakul P, et al. Opisthorchis viverrini infestation and endogenous nitrosamines as risk factors for cholangiocarcinoma in Thailand. Int J Cancer. 1991;48:821–825. doi: 10.1002/ijc.2910480606. [DOI] [PubMed] [Google Scholar]
- 12.Vatanasapt V, et al. Cholangiocarcinoma in north-east Thailand. Lancet. 1990;335:116–117. doi: 10.1016/0140-6736(90)90591-r. [DOI] [PubMed] [Google Scholar]
- 13.Furst T, et al. Global burden of human food-borne trematodiasis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:210–221. doi: 10.1016/S1473-3099(11)70294-8. [DOI] [PubMed] [Google Scholar]
- 14.Sayasone S, et al. Helminth and intestinal protozoa infections, multiparasitism and risk factors in Champasack province, Lao People's Democratic Republic. PLoS Neglect Trop Dis. 2011;5:e1037. doi: 10.1371/journal.pntd.0001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forrer A, et al. Spatial distribution of, and risk factors for, Opisthorchis viverrini infection in southern Lao PDR. PLoS Neglect Trop Dis. 2012;6:e1481. doi: 10.1371/journal.pntd.0001481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viranuvatti V, et al. Retention cyst of liver caused by opisthorchiasis associated with carcinoma; case report. Am J Gastroenterol. 1955;23:442–446. [PubMed] [Google Scholar]
- 17.Newton RW, WW, Anwar WA. Schistosomes and human cancer. Cancer Surveys. 1999;33:291–311. [Google Scholar]
- 18.Jongsuksuntigul P, Imsomboon T. Opisthorchiasis control in Thailand. Acta Trop. 2003;88:229–232. doi: 10.1016/j.actatropica.2003.01.002. [DOI] [PubMed] [Google Scholar]
- 19.IARC. Infection with liver flukes (Opisthorchis viverrini, Opisthorchis felineus and Clonorchis sinensis) IARC Monogr Eval Carcinog Risks Hum. 1994;61:121–175. [PMC free article] [PubMed] [Google Scholar]
- 20.Loaharanu P, Sornmani S. Preliminary estimates of economic impact of liver fluke infection in Thailand and the feasibility of irradiation as a control measure. SE Asian J Trop Med Pub Health. 1991;22(Suppl):384–390. [PubMed] [Google Scholar]
- 21.Andrews RH, et al. Opisthorchis viverrini: an underestimated parasite in world health. Trends Parasitol. 2008;24:497–501. doi: 10.1016/j.pt.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bray F, et al. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol. 2012 Jun 1; doi: 10.1016/S1470-2045(12)70211-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Rebbeck TR. Conquering cancer disparities: new opportunities for cancer epidemiology, biomarker, and prevention research. Cancer Epidemiol Biomarkers Prev. 2006;15:1569–1571. doi: 10.1158/1055-9965.EPI-06-0613. [DOI] [PubMed] [Google Scholar]
- 24.Rebbeck TR, et al. Genetics, epidemiology, and cancer disparities: is it black and white? J Clin Oncol. 2006;24:2164–2169. doi: 10.1200/JCO.2005.05.1656. [DOI] [PubMed] [Google Scholar]
- 25.Bissell MJ, Radisky D. Putting tumours in context. Nature Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sripa B, et al. Advanced periductal fibrosis from infection with the carcinogenic human liver fluke Opisthorchis viverrini correlates with elevated levels of interleukin-6. Hepatol. 2009;50:1273–1281. doi: 10.1002/hep.23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sripa B, et al. Elevated plasma IL-6 associates with increased risk of advanced fibrosis and cholangiocarcinoma in individuals infected by Opisthorchis viverrini. PLoS Neglect Trop Dis. 2012;6:e1654. doi: 10.1371/journal.pntd.0001654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sripa B, Kaewkes S. Localisation of parasite antigens and inflammatory responses in experimental opisthorchiasis. Int J Parasitol. 2000;30:735–740. doi: 10.1016/s0020-7519(00)00054-0. [DOI] [PubMed] [Google Scholar]
- 29.Venugopal PG, et al. Activation and regulation of toll-like receptors (TLRs) by helminth parasites. Immunol Res. 2009;43:252–263. doi: 10.1007/s12026-008-8079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ninlawan K, et al. Opisthorchis viverrini excretory/secretory products induce toll-like receptor 4 upregulation and production of interleukin 6 and 8 in cholangiocyte. Parasitol Int. 2010;59:616–621. doi: 10.1016/j.parint.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pairojkul C, et al. Multistage carcinogenesis of liver-fluke-associated cholangiocarcinoma in Thailand. Princess Takamatsu Symp. 1991;22:77–86. [PubMed] [Google Scholar]
- 32.Vatanasapt V, et al. Cancer survival in Khon Kaen, Thailand. IARC Sci Publ. 1998:123–134. [PubMed] [Google Scholar]
- 33.Schwartz L, et al. Cancer: the role of extracellular disease. Med Hypotheses. 2002;58:340–346. doi: 10.1054/mehy.2001.1539. [DOI] [PubMed] [Google Scholar]
- 34.Moore MA, et al. Early lesions induced by DHPN in Syrian golden hamsters: influence of concomitant Opisthorchis infestation, dehydroepiandrosterone or butylated hydroxyanisole administration. Carcinogenesis. 1988;9:1185–1189. doi: 10.1093/carcin/9.7.1185. [DOI] [PubMed] [Google Scholar]
- 35.Mulvenna J, et al. The secreted and surface proteomes of the adult stage of the carcinogenic human liver fluke Opisthorchis viverrini. Proteomics. 2010;10:1063–1078. doi: 10.1002/pmic.200900393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thuwajit C, et al. Increased cell proliferation of mouse fibroblast NIH-3T3 in vitro induced by excretory/secretory product(s) from Opisthorchis viverrini. Parasitol. 2004;129:455–464. doi: 10.1017/s0031182004005815. [DOI] [PubMed] [Google Scholar]
- 37.Bhamarapravati N, et al. Liver changes in hamsters infected with a liver fluke of man, Opisthorchis viverrini. Am J Trop Med Hyg. 1978;27:787–794. doi: 10.4269/ajtmh.1978.27.787. [DOI] [PubMed] [Google Scholar]
- 38.Laha T, et al. Gene discovery for the carcinogenic human liver fluke, Opisthorchis viverrini. BMC Genomics. 2007;8:189. doi: 10.1186/1471-2164-8-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young ND, et al. Unlocking the transcriptomes of two carcinogenic parasites, Clonorchis sinensis and Opisthorchis viverrini. PLoS Neglect Trop Dis. 2010;4:e719. doi: 10.1371/journal.pntd.0000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daorueang D, et al. Secreted Opisthorchis viverrini glutathione S-transferase regulates cell proliferation through AKT and ERK pathways in cholangiocarcinoma. Parasitol Int. 2012;61:155–161. doi: 10.1016/j.parint.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 41.Smout MJ, et al. A granulin-like growth factor secreted by the carcinogenic liver fluke, Opisthorchis viverrini, promotes proliferation of host cells. PLoS Pathog. 2009;5:e1000611. doi: 10.1371/journal.ppat.1000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smout MJ, et al. Expression, refolding and purification of Ov-GRN-1, a granulin-like growth factor from the carcinogenic liver fluke, that causes proliferation of mammalian host cells. Protein Expr Purif. 2011;79:263–270. doi: 10.1016/j.pep.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 43.Bateman A, Bennett HP. Granulins: the structure and function of an emerging family of growth factors. J Endocrinol. 1998;158:145–151. doi: 10.1677/joe.0.1580145. [DOI] [PubMed] [Google Scholar]
- 44.Ong CH, Bateman A. Progranulin (granulin-epithelin precursor, PC-cell derived growth factor, acrogranin) in proliferation and tumorigenesis. Histol Histopathol. 2003;18:1275–1288. doi: 10.14670/HH-18.1275. [DOI] [PubMed] [Google Scholar]
- 45.Monami G, et al. Proepithelin promotes migration and invasion of 5637 bladder cancer cells through the activation of ERK1/2 and the formation of a paxillin/FAK/ERK complex. Cancer Res. 2006;66:7103–7110. doi: 10.1158/0008-5472.CAN-06-0633. [DOI] [PubMed] [Google Scholar]
- 46.Zanocco-Marani T, et al. Biological activities and signaling pathways of the granulin/epithelin precursor. Cancer Res. 1999;59:5331–5340. [PubMed] [Google Scholar]
- 47.Frampton G, et al. Interleukin-6-driven progranulin expression increases cholangiocarcinoma growth by an Akt-dependent mechanism. Gut. 2012;61:268–277. doi: 10.1136/gutjnl-2011-300643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones MB, et al. The granulin-epithelin precursor/PC-cell-derived growth factor is a growth factor for epithelial ovarian cancer. Clin Cancer Res. 2003;9:44–51. [PubMed] [Google Scholar]
- 49.Kim YJ, et al. Resistance of cholangiocarcinoma cells to parthenolide-induced apoptosis by the excretory-secretory products of Clonorchis sinensis. Parasitol Res. 2009;104:1011–1016. doi: 10.1007/s00436-008-1283-y. [DOI] [PubMed] [Google Scholar]
- 50.Bateman A, et al. Granulins, a novel class of peptide from leukocytes. Biochem Biophys Res Comm. 1990;173:1161–1168. doi: 10.1016/s0006-291x(05)80908-8. [DOI] [PubMed] [Google Scholar]
- 51.He Z, et al. Progranulin is a mediator of the wound response. Nature Med. 2003;9:225–229. doi: 10.1038/nm816. [DOI] [PubMed] [Google Scholar]
- 52.Kullavanijaya P, et al. Current status of infection-related gastrointestinal and hepatobiliary diseases in Thailand. SE Asian J Trop Med Pub Health. 1999;30:96–105. [PubMed] [Google Scholar]
- 53.Thamavit W, et al. Promotion of cholangiocarcinogenesis in the hamster liver by bile duct ligation after dimethylnitrosamine initiation. Carcinogenesis. 1993;14:2415–2417. doi: 10.1093/carcin/14.11.2415. [DOI] [PubMed] [Google Scholar]
- 54.Kenny PA, Bissell MJ. Tumor reversion: correction of malignant behavior by microenvironmental cues. Int J Cancer. 2003;107:688–695. doi: 10.1002/ijc.11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haddow A. Molecular repair, wound healing, and carcinogenesis: tumor production a possible overhealing? Adv Cancer Res. 1972;16:181–234. doi: 10.1016/s0065-230x(08)60341-3. [DOI] [PubMed] [Google Scholar]
- 56.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 57.Sripa J, et al. Secreted cysteine proteases of the carcinogenic liver fluke, Opisthorchis viverrini: regulation of cathepsin F activation by autocatalysis and trans-processing by cathepsin B. Cell Microbiol. 2010;12:781–795. doi: 10.1111/j.1462-5822.2010.01433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schafer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nature Rev Mol Cell Biol. 2008;9:628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 59.Thuwajit C, et al. Gene expression profiling defined pathways correlated with fibroblast cell proliferation induced by Opisthorchis viverrini excretory/secretory product. World J Gastroenterol. 2006;12:3585–3592. doi: 10.3748/wjg.v12.i22.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sripa B, et al. Opisthorchis viverrini and opisthorchiasis: the 21st century review. Acta Trop. 2003;88:169–170. doi: 10.1016/j.actatropica.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Elkins DB, et al. A high frequency of hepatobiliary disease and suspected cholangiocarcinoma associated with heavy Opisthorchis viverrini infection in a small community in north-east Thailand. Trans R Soc Trop Med Hyg. 1990;84:715–719. doi: 10.1016/0035-9203(90)90159-c. [DOI] [PubMed] [Google Scholar]
- 62.Elkins DB, et al. Cross-sectional patterns of hepatobiliary abnormalities and possible precursor conditions of cholangiocarcinoma associated with Opisthorchis viverrini infection in humans. Am J Trop Med Hyg. 1996;55:295–301. doi: 10.4269/ajtmh.1996.55.295. [DOI] [PubMed] [Google Scholar]
- 63.Mairiang E, et al. Ultrasound Screening for Opisthorchis viverrini-associated cholangiocarcinomas: experience in an endemic area. Asian Pac J Cancer Prev. 2006;7:431–433. [PubMed] [Google Scholar]
- 64.Mairiang E, et al. Relationship between intensity of Opisthorchis viverrini infection and hepatobiliary disease detected by ultrasonography. J Gastroenterol Hepatol. 1992;7:17–21. doi: 10.1111/j.1440-1746.1992.tb00928.x. [DOI] [PubMed] [Google Scholar]
- 65.Mairiang E, et al. Reversal of biliary tract abnormalities associated with Opisthorchis viverrini infection following praziquantel treatment. Trans R Soc Trop Med Hyg. 1993;87:194–197. doi: 10.1016/0035-9203(93)90489-d. [DOI] [PubMed] [Google Scholar]
- 66.Mairiang E, Mairiang P. Clinical manifestation of opisthorchiasis and treatment. Acta Trop. 2003;88:221–227. doi: 10.1016/j.actatropica.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 67.Mairiang E, et al. Ultrasonography assessment of hepatobiliary abnormalities in 3359 subjects with Opisthorchis viverrini infection in endemic areas of Thailand. Parasitol Int. 2012;61:208–211. doi: 10.1016/j.parint.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flavell DJ, et al. Opisthorchis viverrini: the relationship between egg production, worm size and intensity of infection in the hamster. Trans R Soc Trop Med Hyg. 1983;77:538–545. doi: 10.1016/0035-9203(83)90132-3. [DOI] [PubMed] [Google Scholar]
- 69.Loilome W, et al. Altered gene expression in Opisthorchis viverrini-associated cholangiocarcinoma in hamster model. Mol Carcinog. 2006;45:279–287. doi: 10.1002/mc.20094. [DOI] [PubMed] [Google Scholar]
- 70.Nithikathkul C, et al. Early stage biliary and intrahepatic migration of Opisthorchis viverrini in the golden hamster. J Helminthol. 2007;81:39–41. doi: 10.1017/S0022149X07212106. [DOI] [PubMed] [Google Scholar]
- 71.Sirisinha S, et al. Humoral immune responses in hamsters infected with Opisthorchis viverrini. SE Asian J Trop Med Pub Health. 1983;14:243–251. [PubMed] [Google Scholar]
- 72.Boonmars T, et al. Apoptosis-related gene expression in hamster opisthorchiasis post praziquantel treatment. Parasitol Res. 2008;102:447–455. doi: 10.1007/s00436-007-0783-5. [DOI] [PubMed] [Google Scholar]
- 73.Pinlaor S, et al. iNOS-dependent DNA damage via NF-kappaB expression in hamsters infected with Opisthorchis viverrini and its suppression by the antihelminthic drug praziquantel. Int J Cancer. 2006;119:1067–1072. doi: 10.1002/ijc.21893. [DOI] [PubMed] [Google Scholar]
- 74.Pinlaor S, et al. Oxidative and nitrative stress in Opisthorchis viverrini-infected hamsters: an indirect effect after praziquantel treatment. Am J Trop Med Hyg. 2008;78:564–573. [PubMed] [Google Scholar]
- 75.Pungpak S, et al. Opisthorchis viverrini infection in Thailand: studies on the morbidity of the infection and resolution following praziquantel treatment. Am J Trop Med Hyg. 1997;56:311–314. doi: 10.4269/ajtmh.1997.56.311. [DOI] [PubMed] [Google Scholar]
- 76.Goydos JS, et al. Marked elevation of serum interleukin-6 in patients with cholangiocarcinoma: validation of utility as a clinical marker. Ann Surg. 1998;227:398–404. doi: 10.1097/00000658-199803000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Balkwill F, et al. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 78.de Visser KE, et al. Paradoxical roles of the immune system during cancer development. Nature Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 79.Naugler WE, Karin M. The wolf in sheep's clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14:109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 80.Gatto M, et al. Cholangiocarcinoma: update and future perspectives. Dig Liver Dis. 2010;42:253–260. doi: 10.1016/j.dld.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 81.Alvaro D. Serum and bile biomarkers for cholangiocarcinoma. Curr Opin Gastroenterol. 2009;25:279–284. doi: 10.1097/mog.0b013e328325a894. [DOI] [PubMed] [Google Scholar]
- 82.Briggs CD, et al. Prognostic molecular markers in cholangiocarcinoma: a systematic review. Eur J Cancer. 2009;45:33–47. doi: 10.1016/j.ejca.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 83.Morris-Stiff G, et al. Cholangiocarcinoma complicating primary sclerosing cholangitis: a 24-year experience. Dig Surg. 2008;25:126–132. doi: 10.1159/000128169. [DOI] [PubMed] [Google Scholar]
- 84.Patel AH, et al. The utility of CA 19–9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:204–207. doi: 10.1111/j.1572-0241.2000.01685.x. [DOI] [PubMed] [Google Scholar]
- 85.Lempinen M, et al. Enhanced detection of cholangiocarcinoma with serum trypsinogen-2 in patients with severe bile duct strictures. J Hepatol. 2007;47:677–683. doi: 10.1016/j.jhep.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 86.Bamrungphon W, et al. A new mucin antibody/enzyme-linked lectin-sandwich assay of serum MUC5AC mucin for the diagnosis of cholangiocarcinoma. Cancer Lett. 2007;247:301–308. doi: 10.1016/j.canlet.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 87.Chapman MH, et al. Circulating CYFRA 21–1 is a Specific Diagnostic and Prognostic Biomarker in Biliary Tract Cancer. J Clin Exp Hepatol. 2011;1:6–12. doi: 10.1016/S0973-6883(11)60110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Uenishi T, et al. Serum cytokeratin 19 fragment (CYFRA21–1) as a prognostic factor in intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2008;15:583–589. doi: 10.1245/s10434-007-9650-y. [DOI] [PubMed] [Google Scholar]
- 89.Boonjaraspinyo S, et al. Overexpression of PDGFA and its receptor during carcinogenesis of Opisthorchis viverrini-associated cholangiocarcinoma. Parasitol Int. 2012;61:145–150. doi: 10.1016/j.parint.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 90.Techasen A, et al. Opisthorchis viverrini-antigen induces expression of MARCKS during inflammation-associated cholangiocarcinogenesis. Parasitol Int. 2012;61:140–144. doi: 10.1016/j.parint.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 91.Loilome W, et al. Expression of oxysterol binding protein isoforms in opisthorchiasis-associated cholangiocarcinoma: a potential molecular marker for tumor metastasis. Parasitol Int. 2012;61:136–139. doi: 10.1016/j.parint.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 92.Nair SS, et al. Inflammatory response to liver fluke Opisthorchis viverrini in mice depends on host master coregulator MTA1, a marker for parasite-induced cholangiocarcinoma in humans. Hepatol. 2011;54:1388–1397. doi: 10.1002/hep.24518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khoontawad J, et al. Proteomic identification of peroxiredoxin 6 for host defence against Opisthorchis viverrini infection. Parasite Immunol. 2010;32:314–323. doi: 10.1111/j.1365-3024.2009.01189.x. [DOI] [PubMed] [Google Scholar]
- 94.Thanan R, et al. Urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine in patients with parasite infection and effect of antiparasitic drug in relation to cholangiocarcinogenesis. Cancer Epidemiol Biomarkers Prev. 2008;17:518–524. doi: 10.1158/1055-9965.EPI-07-2717. [DOI] [PubMed] [Google Scholar]
- 95.Yonglitthipagon P, et al. Up-regulation of annexin A2 in cholangiocarcinoma caused by Opisthorchis viverrini and its implication as a prognostic marker. Int J Parasitol. 2010;40:1203–1212. doi: 10.1016/j.ijpara.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yonglitthipagon P, et al. Prognostic significance of peroxiredoxin 1 and ezrin-radixin-moesin-binding phosphoprotein 50 in cholangiocarcinoma. Human Path. 2012 Mar 23; doi: 10.1016/j.humpath.2011.11.021. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Latterich M, et al. Proteomics: new technologies and clinical applications. Eur J Cancer. 2008;44:2737–2741. doi: 10.1016/j.ejca.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 98.Fu Q, et al. Multiplex assays for biomarker research and clinical application: translational science coming of age. Proteomics Clin App. 2010;4:271–284. doi: 10.1002/prca.200900217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang X, et al. The draft genome of the carcinogenic human liver fluke Clonorchis sinensis. Genome Biol. 2011;12:R107. doi: 10.1186/gb-2011-12-10-r107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kaewkong W, et al. Genome size estimation of liver fluke Opisthorchis viverrini by real-time polymerase chain reaction based method. Parasitol Int. 2012;61:77–80. doi: 10.1016/j.parint.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 101.Kaewkong W, et al. Chromosomes and karyotype analysis of a liver fluke, Opisthorchis viverrini, by scanning electron microscopy. Parasitol Int. 2012;61:504–507. doi: 10.1016/j.parint.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 102.Rinaldi G, et al. Development of functional genomic tools in trematodes: RNA interference and luciferase reporter gene activity in Fasciola hepatica. PLoS Neglect Trop Dis. 2008;2:e260. doi: 10.1371/journal.pntd.0000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rinaldi G, et al. RNA interference targeting leucine aminopeptidase blocks hatching of Schistosoma mansoni eggs. Mol Biochem Parasitol. 2009;167:118–126. doi: 10.1016/j.molbiopara.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sripa J, et al. RNA interference targeting cathepsin B of the carcinogenic liver fluke, Opisthorchis viverrini. Parasitol Int. 2011;60:283–288. doi: 10.1016/j.parint.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Soukhathammavong P, et al. Efficacy and safety of mefloquine, artesunate, mefloquine-artesunate, tribendimidine, and praziquantel in patients with Opisthorchis viverrini: a randomised, exploratory, open-label, phase 2 trial. Lancet Infect Dis. 2011;11:110–118. doi: 10.1016/S1473-3099(10)70250-4. [DOI] [PubMed] [Google Scholar]
- 106.Frazer IH, et al. Prevention and treatment of papillomavirus-related cancers through immunization. Annu Rev Immunol. 2011;29:111–138. doi: 10.1146/annurev-immunol-031210-101308. [DOI] [PubMed] [Google Scholar]
- 107.Xiao S, et al. The evaluation of recombinant hookworm antigens as vaccines in hamsters (Mesocricetus auratus) challenged with human hookworm, Necator americanus. Exp Parasitol. 2008;118:32–40. doi: 10.1016/j.exppara.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 108.Quan FS, et al. Resistance to reinfection in rats induced by irradiated metacercariae of Clonorchis sinensis. Memorias do Instituto Oswaldo Cruz. 2005;100:549–554. doi: 10.1590/s0074-02762005000500016. [DOI] [PubMed] [Google Scholar]
- 109.Zhou Z, et al. Oral administration of a Bacillus subtilis spore-based vaccine expressing Clonorchis sinensis tegumental protein 22.3 kDa confers protection against Clonorchis sinensis. Vaccine. 2008;26:1817–1825. doi: 10.1016/j.vaccine.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 110.Lee JS, et al. Vaccination with DNA encoding cysteine proteinase confers protective immune response to rats infected with Clonorchis sinensis. Vaccine. 2006;24:2358–2366. doi: 10.1016/j.vaccine.2005.11.062. [DOI] [PubMed] [Google Scholar]
- 111.Kasny M, et al. Chapter 4. Peptidases of trematodes. Adv Parasitol. 2009;69:205–297. doi: 10.1016/S0065-308X(09)69004-7. [DOI] [PubMed] [Google Scholar]
- 112.Abdulla MH, et al. Schistosomiasis mansoni: novel chemotherapy using a cysteine protease inhibitor. PLoS Med. 2007;4:e14. doi: 10.1371/journal.pmed.0040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kenter GG, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 114.Hotez PJ, et al. Developing vaccines to combat hookworm infection and intestinal schistosomiasis. Nature Rev Microbiol. 2010;8:814–826. doi: 10.1038/nrmicro2438. [DOI] [PubMed] [Google Scholar]
- 115.Haswell-Elkins MR, et al. Immune responsiveness and parasite-specific antibody levels in human hepatobiliary disease associated with Opisthorchis viverrini infection. Clin Exp Immunol. 1991;84:213–218. doi: 10.1111/j.1365-2249.1991.tb08151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pinlaor S, et al. Mechanism of NO-mediated oxidative and nitrative DNA damage in hamsters infected with Opisthorchis viverrini: a model of inflammation-mediated carcinogenesis. Nitric Oxide. 2004;11:175–183. doi: 10.1016/j.niox.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 117.Pinlaor S, et al. Repeated infection with Opisthorchis viverrini induces accumulation of 8-nitroguanine and 8-oxo-7,8-dihydro-2'-deoxyguanine in the bile duct of hamsters via inducible nitric oxide synthase. Carcinogenesis. 2004;25:1535–1542. doi: 10.1093/carcin/bgh157. [DOI] [PubMed] [Google Scholar]
- 118.Thamavit W, et al. Repeated exposure to Opisthorchis viverrini and treatment with the antihelminthic Praziquantel lacks carcinogenic potential. Carcinogenesis. 1992;13:309–311. doi: 10.1093/carcin/13.2.309. [DOI] [PubMed] [Google Scholar]
- 119.Keiser J, Utzinger J. Chemotherapy for major food-borne trematodes: a review. Expert Opin Pharmacother. 2004;5:1711–1726. doi: 10.1517/14656566.5.8.1711. [DOI] [PubMed] [Google Scholar]
- 120.Loukas A, et al. Vaccinomics for the major blood feeding helminths of humans. OMICS. 2011;15:567–577. doi: 10.1089/omi.2010.0150. [DOI] [PubMed] [Google Scholar]
- 121.Smout MJ, et al. Infection with the carcinogenic human liver fluke, Opisthorchis viverrini. Mol Biosyst. 2011;7:1367–1375. doi: 10.1039/c0mb00295j. [DOI] [PMC free article] [PubMed] [Google Scholar]


