Abstract
This review of our research with rTMS to treat aphasia contains four parts: Part 1 reviews functional brain imaging studies related to recovery of language in aphasia with emphasis on nonfluent aphasia. Part 2 presents the rationale for using rTMS to treat nonfluent aphasia patients (based on results from functional imaging studies). Part 2 also reviews our current rTMS treatment protocol used with nonfluent aphasia patients, and our functional imaging results from overt naming fMRI scans, obtained pre- and post- a series of rTMS treatments. Part 3 presents results from a pilot study where rTMS treatments were followed immediately by constraint-induced language therapy (CILT). Part 4 reviews our diffusion tensor imaging (DTI) study that examined white matter connections between the horizontal, midportion of the arcuate fasciculus (hAF) to different parts within Broca’s area (pars triangularis, PTr; pars opercularis, POp), and the ventral premotor cortex (vPMC) in the RH and in the LH. Part 4 also addresses some of the possible mechanisms involved with improved naming and speech, following rTMS with nonfluent aphasia patients.
1. Brief Overview of TMS
Repetitive transcranial magnetic stimulation (rTMS) has been used in a growing number of research laboratories worldwide, since 1985 (Barker, Jalinous, and Freeston, 1985). It is investigated as a novel intervention to treat disorders associated with stroke including paralysis or hemispatial visual neglect; and to treat other disorders such as depression and epilepsy (Lefaucheur, 2006). Our ongoing research explores whether rTMS can be applied to improve naming and speech in stroke patients with chronic, nonfluent aphasia.
TMS is a noninvasive procedure that utilizes magnetic fields to create electric currents in discrete brain areas (Pascual-Leone et al., 2002; Wagner, Valero-Cabre and Pascual-Leone, 2007). TMS involves discharging a current through a coil of copper wire that is held over the subject’s scalp. The current pulse flowing through the coil generates a rapidly fluctuating magnetic field that penetrates the scalp and skull unimpeded, and induces a changing electrical field in the cerebral cortex below the coil. The physiologic response appears to be caused by current flow in the cortical tissue, which leads to neuronal depolarization (exciting or inhibiting the cortex) (Wagner, Valero-Cabre and Pascual-Leone, 2007; Wasserman et al., 2008). The participant feels a “light tap” on the scalp, may feel a twitch of the face muscles, and hears a brief, loud click as the current passing through the coil tightens the copper wire. Most participants find this not unpleasant. The stimulation of the brain itself is painless.
Multiple stimuli (called “trains”) of rTMS of appropriate frequency, intensity, and duration can lead to transient increases or decreases in excitability of the affected cortex that last beyond the duration of the train itself (Pascual-Leone et al., 1998). Slow rTMS, where one magnetic pulse is applied every second (1 Hz), delivered to the motor cortex can give rise to a lasting decrease in corticospinal excitability (Chen et al., 1997; Maeda et al., 2000; Pascual-Leone, Walsh, and Rothwell, 2000). Conversely, fast rTMS (5, 10, or 20 Hz) can induce a transient increase in cortical excitability (Pascual-Leone et al., 1994). The maximum output of a TMS device is usually in the range of 2 Tesla (e.g., Magstim Rapid Magnetic Stimulator Unit, Magstim Corporation, New York, NY).
2. Review of Research with TMS to Treat Aphasia
2.1. Part 1, Functional Imaging Studies with Aphasia Patients
Brain re-organization supporting recovery of language in stroke patients with aphasia is still unknown. Both the left hemisphere (LH) and the right hemisphere (RH) are thought to support language recovery after stroke (Crosson et al., 2007; Gold and Kertesz, 2000; Price and Crinion, 2005; Thompson, 2000). The relative contribution from each hemisphere is still debated. Factors including time poststroke when patients are studied (acute or chronic), lesion location and language tasks examined may affect the mechanisms involved in recovery (Price and Crinion, 2005; Thiel et al., 2006). The most rapid recovery occurs in the first 6 months poststroke onset (MPO) (Demeurisse and Capon, 1987); however, 50-60% of aphasia cases continue to have chronic, communicative impairment (Kertesz, 1984; Kertesz and McCabe, 1977). Approximately 20% have speech and language problems that include hesitant, poorly articulated, agrammatic speech with word-finding problems (nonfluent aphasia) (Pedersen, Vinter, and Olsen, 2004).
Since the 1870’s, some reports have suggested that the RH can support some recovery of language after LH stroke (Barlow, 1877; Basso et al., 1989; Gowers, 1886; Kinsbourne, 1971). In some recent studies that included a variety of patients with aphasia, RH activation was considered to be compensatory (Blasi et al., 2002; Musso et al., 1999; Peck et al., 2004; Thulborn, Carpenter, and Just, 1999; Weiller et al., 1995). When functional imaging studies have focused on patients with nonfluent aphasia, an increased activation (possible “over-activation”) in RH language homologues has often been observed (Belin et al., 1996; Martin et al., 2009; Naeser et al., 2004; Perani et al., 2003; Rosen et al., 2000). It is possible that unusually high RH activation is related to transcallosal disinhibition leading only to partial, or incomplete recovery. This increased RH activation may be ‘maladaptive’ and lead to a ‘dead-end’, inefficient strategy for recovery, particularly in nonfluent aphasia patients (Belin et al., 1996; Lefaucheur, 2006; Naeser et al., 2004; Price and Crinion, 2005; Rosen et al., 2000).
Heiss and Thiel (2006) have suggested that for long-term recovery, RH recruitment may be less efficient than restoring the LH network. For example, patients with better recovery have been observed to have higher activation in L superior temporal gyrus and L supplementary motor area (SMA) (Karbe et al., 1998; Saur et al., 2006). Recovery of naming has been associated with reperfusion of L Brodmann area (BA) 37 particularly, in acute stroke cases studied with perfusion weighted imaging (Hillis et al., 2006). Winhuisen et al. (2005) also observed as early as 2 weeks poststroke onset, that better performance on a verbal fluency test (and better recovery) was associated with the L inferior frontal gyrus (IFG).
After speech-language therapy with some chronic stroke patients, new LH activation has been associated with improvement in language (Breier et al., 2009; Cornelissen et al., 2003; Leger et al., 2002; Small, Flores, and Noll, 1998). Therapeutic success following treatment with constraint-induced language therapy (CILT) has been correlated with relative decrease of activation in RH areas, including the IFG/insular cortex (Richter, Miltner, and Straube, 2008) and with new activation in the L temporal lobe (Breier et al., 2009).
New RH activation has also been observed following speech-language therapy in some patients with aphasia (Cherney and Small, 2006; Crosson et al., 2005; Peck et al., 2004; Raboyeau et al., 2008). Fernandez et al. (2004) suggested that RH participation in the acute recovery stage of LH stroke may be followed later, by LH activation corresponding to further recovery, and that the RH may play a larger role in supporting recovery when there is greater damage to LH language areas.
Whether recovery in aphasia is mediated primarily from LH undamaged language or perilesional regions, or from RH language homologues (or both), the above-mentioned studies suggest there is potential for brain re-organization and improved language in chronic, post-stroke aphasia (Price and Crinion, 2005).
2.2. Part 2, Rationale for rTMS to Treat Nonfluent Aphasia
As reviewed in Part 1, several functional imaging studies with chronic, nonfluent aphasia patients have observed high activation, possibly “over-activation” during language tasks, in parts of R Broca’s area and other R perisylvian language homologues which may be ‘maladaptive’ (Belin et al., 1996; Martin et al., 2009; Naeser et al., 2004; Perani et al., 2003; Rosen et al., 2000). When applied to a specific ROI in the undamaged hemisphere, low-frequency (1 Hz) rTMS may suppress activity in that ROI. This may permit reactivation of some areas within the damaged hemisphere, promoting some functional recovery (Lefaucheur, 2006). This is similar to the phenomenon of ‘Paradoxical Functional Facilitation’ or PFF (Kapur, 1996). The phenomenon of PFF suggests that direct or indirect neural ‘damage’ to a specific area in the central nervous system may result in facilitation of behavior. Thus, suppressing a cortical ROI in the RH of a nonfluent aphasia patient using 1 Hz rTMS may result in a decrease of “over-activation” in that ROI, resulting in an overall modulation of the bilateral neural network for naming.
2.2.1 Review of rTMS Treatment Protocol with Nonfluent Aphasia Patients
Inclusion Criteria
Our studies include chronic aphasia patients who are at least 6 months post- single, unilateral LH stroke. They are R-handed, native English speakers, ranging in age from 40-80. If there is a history of seizures, the seizures are well controlled with medication, and the patient has not had a seizure for at least one year, prior to entry. [Note, slow, 1 Hz rTMS has been used to help reduce seizures (Tassinari et al., 2003) and has never been found to induce seizures when appropriate safety guidelines are followed (Rossi et al., 2009).] Patients have nonfluent speech, with a 1-4 word phrase length as measured with elicited propositional speech on the Cookie Theft Picture, Boston Diagnostic Aphasia Exam (BDAE) (Goodglass and Kaplan, 1983; Goodglass, Kaplan, and Barresi, 2001). Patients name at least 3 pictures from the first 20 pictures on the Boston Naming Test (BNT) (Kaplan, Goodglass, and Weintraub, 2001). Patients are requested not to receive any individualized speech therapy throughout their participation in our rTMS studies. Our primary language outcome measures are the BNT and selected subtests on the BDAE.
Our rTMS treatment protocol consists of first establishing an entry baseline naming ability for Snodgrass and Vanderwart pictures (Snodgrass and Vanderwart, 1980) for each patient. Then, each patient is entered into a Phase 1 of rTMS, followed by a Phase 2 of rTMS, as described below.
Baseline Snodgrass and Vanderwart Naming Ability
Prior to any rTMS sessions, a baseline naming ability for Snodgrass and Vanderwart (SandV) pictures is established. This baseline SandV naming score is later used during Phase 1 TMS sessions (explained below), to help establish the best RH cortical ROI to suppress with rTMS, in order to improve picture naming (e.g., the “best response” cortical ROI). During the baseline SandV naming testing, ten, 20-item SandV picture lists are administered across three separate testing sessions. Across the ten SandV lists, the baseline, mean number of SandV pictures named correctly is calculated, as well as the baseline, mean response time (RT).
Phase 1 of rTMS
During Phase 1 of rTMS, a “best-response” RH cortical ROI is located, which will later become the targeted location for rTMS during Phase 2. The effect of slow, 1 Hz rTMS for 10 min to suppress activity in each of at least four different RH frontal regions is examined, in separate rTMS treatment sessions. (Note, in our initial studies, the effect of rTMS suppression of the R Wernicke’s area was also studied.) See Fig. 1. These visits usually take place within a few weeks prior to the first Phase 2 rTMS treatment.
Fig. 1.
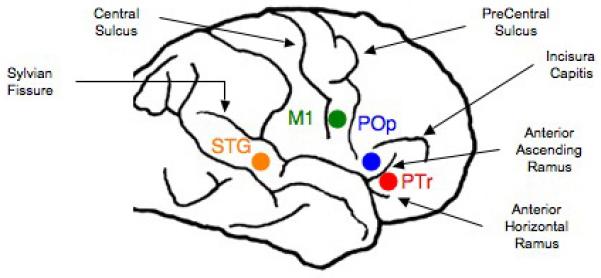
Schematic diagram showing the location of four RH cortical ROIs that were suppressed with 10 mins rTMS in separate sessions (Phase 1), to find the ‘best response’ cortical ROI for each patient. Abbreviations: rTMS = repetitive Transcranial Magnetic Stimulation; ROI = Region of Interest.
During Phase 1, a total of 600 magnetic pulses at 90% of motor threshold for the L first dorsal interosseus muscle (L FDI) are delivered to each RH ROI using the Super-Rapid High Frequency MagStim Magnetic Stimulator (MagStim, NY). The motor threshold (MT) is defined as the intensity of magnetic stimulation needed to elicit a muscle twitch in the thumb (L FDI), in 5 out of 10 trials when using single-pulse TMS applied to the primary motor cortex of the contralateral (R) hemisphere. Published guidelines for safety parameters of rTMS are based on stimulation intensities expressed as a percent of the individual’s MT (Wassermann, 1998). A figure-8 shaped rTMS coil with a 7 cm outside diameter on each wing is used. This allows direct stimulation of an area that is about one cubic centimeter. The RH cortical ROIs that are examined with slow rTMS, include the R M1, mouth (orbicularis oris muscle, as verified with MEP), and at least three subregions within R Broca’s area.
The subregions within the R Broca’s area homologue are labeled according to sulcal and gyral boundaries as defined by Amunts et al. (1999; 2004). Broca’s area is classically defined as the PTr and POp portions of the IFG. Although PTr and POp are sometimes considered to represent BA 45 and BA 44, respectively, only cytoarchitectonic studies from postmortem brain examination can determine the borders that define these Brodmann areas. The location of subregions within R Broca’s area are estimated from each patient’s 3D MPRAGE MRI scan. The three basic subregions within R Broca’s area (each about 1 cm apart) are defined as follows: 1) POp: gyrus that is rostral to the inferior precentral sulcus and caudal to the vertical ascending ramus; 2) PTr posterior: gyrus that is rostral to the vertical ascending ramus and caudal to the triangular sulcus; 3) PTr anterior: gyrus that is rostral to the triangular sulcus and caudal to the horizontal ramus. See Fig. 1.
In some cases, the anatomical landmarks used for delimiting subregions within the R Broca’s homologue are more complex - i.e., when a diagonal sulcus is present (Naeser et al., 2010). When present, the diagonal sulcus is located caudal to the vertical ascending ramus, within the IFG area (Amunts et al., 2004). These authors have observed at post mortem that a diagonal sulcus was present in only every second hemisphere studied, and they wrote that it can either mark the border between BA 44 and 45, or it can be inside BA 44. Thus, when a diagonal sulcus is present, without cytoarchitectonics, it is not possible to know whether the diagonal sulcus is actually a border between BA 44 and 45, or if it is within 44.
Taking this into consideration, when a diagonal sulcus is present in an aphasia patient who is participating our rTMS studies, we need to carefully examine at least four subregions within R Broca’s homologue (Naeser et al., 2010). These four subregions, each about 1 cm apart, are labeled (tentatively, without cytoarchitectonics) as follows: 1) POp: gyrus that is rostral to the inferior precentral sulcus and caudal to the diagonal sulcus; 2) PTr posterior: gyrus that is rostral to the diagonal sulcus and caudal to the vertical ascending ramus; 3) PTr middle: gyrus that is rostral to the vertical ascending ramus and caudal to the triangular sulcus; 4) PTr anterior: gyrus that is rostral to the triangular sulcus and caudal to the horizontal ramus.
The exact location of the “best response” RH ROI to suppress with 1 Hz rTMS can vary somewhat, from patient to patient, and it needs to be firmly established for each case. Surprisingly, the “best response” area is usually the gyrus that is located immediately rostral to the POp. In fact, the POp is usually a “poor response” area, where suppression of POp often transiently impairs naming (Naeser et al., 2002 and submitted). Suppression of the gyrus immediately rostral to this “poor response” area, e.g., suppression of a portion of the PTr, is usually the “best response” area. This differential/opposite effect is discussed further in Part 2.4.
During each rTMS session, the frameless stereotaxic system (Brainsight, Rogue Industries, Montreal) is used in combination with the patient’s 3D MPRAGE MRI scan, in order to guide the rTMS coil placement onto the targeted ROI. Before and after each 10-min rTMS application to a specific RH ROI, one of the SandV lists of pictures is presented for the patient to name. Each of the four or five RH ROIs is suppressed with ten min of rTMS, once, during separate rTMS sessions. If two ROIs are examined on one day, at least a 45 to 60 minute break is taken, before suppression of the second ROI, to establish a return to baseline SandV naming level.
The “best response” RH ROI is defined as that ROI which is associated with a SandV naming score that is at least 2 SD above the baseline, mean number of SandV pictures named correctly. This “best response” ROI will be suppressed for more minutes, and for more rTMS sessions during Phase 2.
Phase 2 of rTMS
During Phase 2, the “best response” RH ROI from Phase 1 is suppressed for 20 min, once a day, five days a week, for two weeks. On each day of treatment, the rTMS is applied at 1 Hz frequency (1200 pulses) at 90% of motor threshold (L FDI), using the same MagStim device as in Phase 1 (MagStim, NY). The frameless stereotaxic system (Brainsight, Rogue Industries, Montreal) is again used to guide the position of the rTMS coil on the patient’s scalp. On-line monitoring allows documentation of accurate targeting of the “best response” cortical ROI throughout the rTMS session, and from day-to-day. Coil orientation is held constant across sessions, at approximately 45 degrees. Our rTMS parameters are similar to those used in various studies where multiple rTMS treatments were given over time, to help treat depression (Kauffmann, Cheema, and Miller, 2004; Klein et al., 1999; Padberg et al., 1999). No negative side effects have been reported with these parameters.
Language testing is performed at approximately 2 and 6 months post-Phase 2 TMS.
Results, Language Outcome Measures
We have observed that for patients who have participated in both Phase 1 and Phase 2, at 2 months post-ten rTMS treatments to suppress the R PTr, significant improvement was present on three naming tests: 1) the BNT, first 20 items (p=.003); 2) the BDAE subtest, Animals (p=.02); and 3) the BDAE subtest, Tools/Implements (p=.04) (Naeser et al., 2005a). At 8 months post-TMS, all three naming test scores continued to improve relative to pre-TMS testing, but only Tools/Implements was significant (p=.003). BNT and naming Animals failed to reach significance because of one patient. See Fig. 2. Improvement was also observed in number of words per longest phrase length in elicited, propositional speech for two of the patients when tested with the BDAE at 2 months post-rTMS. A mild nonfluent patient increased from a three-word phrase length, to a five-word phrase length when describing the BDAE cookie theft picture; and a moderate nonfluent patient increased from a one-word phrase length, to a three-word phrase length.
Fig. 2.
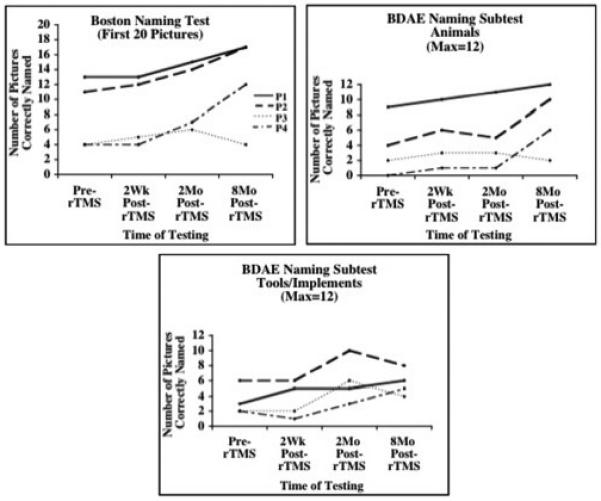
Graphs showing naming scores on three standardized naming tests administered pre- rTMS, and at 2 weeks, 2 months and 8 months after 10 rTMS treatments. Reprinted with author’s permission and Brain and Language (Naeser et al., 2005a).
Improvement in spontaneous speech has also been observed in a recent case report, following Phase 1 and Phase 2 rTMS (Hamilton et al., 2010). A 61 Yr., R-handed, chronic nonfluent aphasia patient had showed stable deficits in elicited propositional speech over the course of five years. At 7 years poststroke, he entered the study. Consistent with our prior rTMS studies, there was improvement on the BDAE naming subtest Tools/Implements, increasing from 7 pictures named, to 9. There was significant improvement in Action naming, increasing from 5, to 10/12 pictures named (p=.035). Significant improvement was also demonstrated on the BDAE cookie theft picture description at 2, 6, and 10 months after rTMS with respect to the number of narrative words (p=.045) and nouns (p=.027), mean sentence length over time (p=.017), and use of closed class words (p=.054). At 10 mo. after rTMS, compared to baseline performance, the patient showed significant improvement on the Western Aphasia Battery subscale for spontaneous speech (p=.032), with improvements on both the information content and fluency scales. These findings suggest that manipulation of the intact contralesional cortex in patients with nonfluent aphasia may result in language benefits that generalize beyond naming to include other aspects of language production.
Overt Naming Functional MRI Pre- and Post-rTMS in Two Nonfluent Aphasia Patients
We have used functional MRI to examine brain activation during overt naming, pre- and post- ten, 20-minute, 1 Hz rTMS treatments to suppress part of R PTr, in two chronic nonfluent aphasia patients (Martin et al., 2009). One patient was a ‘good responder’ (P1) with improved naming and phrase length in propositional speech, lasting out to almost 4 years post-TMS. The other patient was a ‘poor responder’ (P2) with no change in naming or propositional speech post-TMS.
Language Data (P1, Good Responder)
For P1, language was tested at 2, 6, 16, and 43 mo. post-Phase 2 rTMS. The BNT score increased from 11 pictures named (pre-TMS) to 14, 18, 15 and 15 (at the four testing times, post-TMS). The number of words for longest phrase (Cookie Theft picture description, BDAE) increased from 3 words (pre-TMS) to 5, 5, 5, and 6 words, respectively (post-TMS). Auditory comprehension was largely unchanged.
Language Data (P2, Poor Responder)
Pre-TMS, language testing was performed three times with P2 (1.5 yr. poststroke). At that time, his BNT scores were 1, 3, and 1 (mean=1.67; SD=1.15), and his longest phrase length was 1 word (Cookie Theft picture description, BDAE). His spontaneous speech consisted primarily of only stereotypies. Pre-TMS, his auditory comprehension for Commands was 8, 10, and 8 (mean=8.67; SD=1.15). At 2 and 6 mo. post-Phase 2 rTMS, his BNT score was only 1 picture named; his longest phrase length remained at 1 word, and spontaneous speech still consisted largely of stereotypies. Auditory comprehension for Commands improved by 2 SD, from a pre-TMS mean of 8.67, to 11, at 2 and 6 months post-rTMS. Auditory comprehension for Complex Ideational Material also improved by 2 SD, from pre-TMS mean, 2.33 (SD=0.58) to 4, at 6 mo. post-TMS.
Overt Naming fMRI Block Design Paradigm
Overt naming fMRIs were obtained in the same manner as Martin et al. (2005). A continuous sample, block design, overt naming fMRI paradigm was utilized that took advantage of the hemodynamic response delay where increased bloodflow remains for 4-8 seconds after the task (Friston, Jezzard, and Turner, 1994). Therefore, by obtaining the task-related information after the task, motion artifact was minimized (Barch et al., 1999; Birn, Cox, and Bandettini, 2004; Eden et al., 1999).
Images were modeled using a box-car reference function for the block design which consisted of two alternating conditions: silent viewing of black and white patterns (control condition) and overt picture naming. Sixty pictures were presented from the SandV database of black and white line drawings (Snodgrass and Vanderwart, 1980). Each pattern or picture was presented for 5 sec and was preceded by a 1-sec fixation dot for a total trial time of 6 sec. There were two runs (30 different pictures, each run) with 104 image volumes. Each run lasted 5 min 12 sec, with a short break between runs.
Functional Imaging Results for the ‘Good Responder’
We hypothesized that in chronic nonfluent aphasia, after rTMS treatment to suppress R PTr, a shift in activation from RH frontal areas to new activation in LH perilesional, perisylvian areas and L SMA would occur, if there was good response with improved naming. Pre-TMS, P1 who was a ‘good responder’, showed activation in R and L sensorimotor cortex (mouth area), R IFG, and in R and L SMA. He continued to show this general activation pattern at 3 months and at 16 months post-TMS.
At 16 mo. post-TMS, however, there was a significant change in SMA activation, where P1 showed significant increase in activation in the L SMA, compared to the pre-TMS, and to the 3 mo. post-TMS fMRI scan (p<.02; p<.05, respectively). See Fig. 3. There was also a trend towards significantly greater activation in L SMA versus R SMA at 16 mo. and at 46 mo. post-TMS (p<.08; p<.09, respectively). Pre-TMS there had been no difference between L and R SMA activation.
Fig. 3.
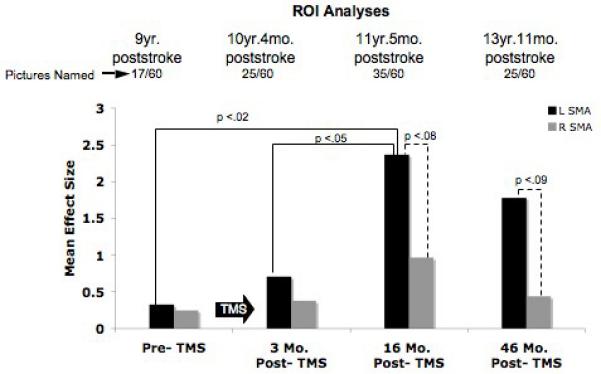
Overt naming fMRI results for ROI analyses, pre- and post- Phase 2 rTMS for P1, good responder. Bargraph displays the mean effect size for the L and R SMA activation for fMRI scans pre-TMS and 3, 16, and 46 mo. post-TMS. There was a significant shift to greater L SMA activation at 16 mo. post-TMS, compared to pre-TMS, and to 3 mo. post-TMS. Greater L SMA activation remained at 46 mo. post-TMS, relative to pre-TMS and 3 mo. post-TMS. Abbreviations: fMRI = functional Magnetic Resonance Imaging; rTMS = repetitive Transcranial Magnetic Stimulation; SMA = Supplementary Motor Area. Reprinted with authors’ and Brain and Language (Martin et al., 2009).
It is unknown exactly when, post-TMS, the shift to the stronger L SMA activation occurred for this patient during overt naming, however, it was first observed at 16 mo. post-TMS (his highest accuracy rate; 58% named). There were no intervening overt speech fMRI scans between 3 and 16 mo. post-TMS. The new LH activation remained present, even at 46 mo. post-TMS (nearly 4 years post-TMS) when the patient was 13 yr. 11 mo. poststroke. On the language outcome measures, P1 improved on the BNT from 11 pictures named pre-TMS, to scores ranging from 14-18 pictures, post-TMS (2 mo. to 43 mo. post-TMS). His longest phrase length improved from 3 words pre-TMS, to 5-6 words post-TMS.
Functional Imaging Results for the ‘Poor Responder’
Pre-TMS (1.5 yr. poststroke), P2 had significant activation in R IFG (3% pictures named). At 3 and 6 mo. post-TMS, there was no longer significant activation in R IFG, but significant activation was present in R sensorimotor cortex. Although P2 had significant activation in both the L and R SMA on all three fMRI scans (pre-TMS, and at 3 and 6 mo. post-TMS), ROI analyses showed no difference across sessions in the L or R SMA activation.
For P2, who was a ‘poor responder’, suppression of R PTr with rTMS resulted in no new, lasting perilesional LH activation across sessions. His naming remained only at 1-2 pictures during all three fMRI scans. His BNT score and longest phrase length remained at 1 word, post-TMS.
Lesion Location
Lesion location may play a role in each patient’s fMRI activation pattern and response to TMS treatment. P2 had an atypical frontal lesion in the L motor and premotor cortex that extended high, near brain vertex, with deep white matter lesion near L SMA. P2 also had frontal lesion in the posterior middle frontal gyrus at the junction of the premotor cortex, an area important for naming (Duffau et al., 2003). Additionally, P2 had lesion inferior and posterior to Wernicke’s area, in parts of BA 21 and 37. P1 had no lesion in these three areas.
P2 did not meet the inclusion criterion for entry regarding minimum number of pictures named on the BNT (3 pictures named on the first 20 items). In addition, his longest phrase length was 1 word (Cookie Theft picture) but consisted primarily of only stereotypies. Another severe nonfluent aphasia patient with a 1-word phrase length, who did not produce stereotypies, was, however, a good responder. She named 4 pictures on the BNT pre-TMS; 7 pictures, 2 mo. post-TMS; and 12 pictures, 8 mo. post-TMS (Naeser et al., 2005b).
Our results showing a significant increase in activation of the L SMA post-rTMS in the patient who improved in naming (P1), are compatible with previous fMRI studies that have also observed new L SMA activation to be present in aphasia patients with better outcome (Karbe et al., 1998; Saur et al., 2006). This improved LH activation is also compatible with previous studies that have observed better outcome following language therapy to be associated with new, LH activation (Breier et al., 2009; Cornelissen et al., 2003; Leger et al., 2002; Richter et al., 2008; Small et al., 1998).
2.3. Part 3, Pilot Study: TMS plus Constraint-Induced Language Therapy (CILT)
Our TMS studies with aphasia have shown that rTMS can induce long-term, language improvement in chronic stroke patients with aphasia (Naeser et al., 2005a,b; Martin et al., 2009; Naeser et al., 2010; Hamilton et al., 2010). These long-term rTMS effects are believed to engage mechanisms of neural plasticity, thus likely rendering this technique especially suitable for improving behavior when combined with appropriate behavioral intervention (Rossi and Rossini, 2004; Talelli and Rothwell, 2006). Along these lines, we have recently undertaken a pilot study to examine the effect of rTMS coupled with speech/language therapy in chronic aphasia patients. Our design, to combine rTMS plus speech/language therapy is similar to other recently published studies where improved behavior was associated with a combination of treatment modalities – e.g., pharmacological intervention plus speech therapy (Berthier et al., 2009; Kessler et al., 2010); or rTMS/tDCS plus physical therapy (Bolognini, Pascual-Leone and Fregni, 2009). Thus, our hypothesis is that a series of rTMS treatments to the appropriate cortical region, could prime a neural network that is appropriate for a specific task (e.g., naming pictures), and that neural network could then be reinforced immediately post-rTMS, by further practice of the task. It is expected that their mutual use can optimize the plastic changes induced by each, resulting in increased clinical gains, even in chronic stroke patients with stable language deficits.
Constraint-Induced Language Therapy
CILT has been observed to significantly improve naming, following a series of 10 CILT treatments (Maher et al., 2006; Meinzer et al., 2005; Pulvermuller et al., 2001). With CILT, a reinforcement strategy termed shaping is used (Taub, 2004; Taub et al., 1994). This strategy is based on operant training, and refers to the gradual successive approximation of behavior in small steps toward the desired goal. The response required from the patient is gradually increased from single words, up to phrases and even sentences. In our protocol, each CILT treatment lasts for 3 hours, and the patient is only allowed to respond with a spoken name for a stimulus picture (no gestures or writing or sound effects). An opaque screen is placed on a table where the Speech-Language Pathologist (SLP) is seated on one side, and the patient, on the other. There is eye contact above the screen.
Results from the Maher et al. (2006) study were striking in that although general improvement in language was observed immediately after the two weeks of CILT treatment, improvement in naming (BNT) was also apparent in 3 of the 4 patients, on the one-month follow-up testing. Maher et al. (2006) wrote that “…the impact of CILT may continue to be active beyond the direct treatment period.” This is also a pattern we have observed in our 2-month (and later) follow-up testing in our aphasia patients treated with rTMS (Naeser et al., 2005a,b; Martin et al., 2009; Naeser et al., 2010; Hamilton et al., 2010). Thus, rTMS plus CILT may promote maximum potential gains in naming for chronic aphasia patients.
rTMS plus CILT with Two Nonfluent Aphasia Patients
This is a pilot study to examine the feasibility of combining rTMS followed immediately by CILT. At this time, only patients who have completed Phase 1 and Phase 2 rTMS (including all follow-up testing at 2 and 6-8 months post-rTMS) are entered into our rTMS plus CILT study. The “best response” RH ROI that was suppressed with rTMS during Phase 2 (explained above, under 2.2.1) is also suppressed in the same manner in this pilot study - i.e., 1 Hz rTMS for 20 min, 90% MT for 10 sessions over a two-week period (weekdays only). A 3-hour CILT session immediately follows each 20-min rTMS session. Two nonfluent patients have completed our rTMS plus CILT protocol, a severe nonfluent patient and a mild nonfluent patient.
Severe Nonfluent Aphasia Patient
The severe nonfluent aphasia patient who had participated in our Phase 1 and Phase 2 rTMS protocol at 6.5 years poststroke also participated in the rTMS plus CILT protocol at 12.5 yr. poststroke. During the first series of rTMS treatments (Phase 1 and Phase 2 rTMS), she improved at 2 and 8 months post-Phase 2, on the BNT where her score increased from 4 pictures named pre-TMS, to 7 and 12 pictures, respectively, post- rTMS (Naeser et al., 2005b).
At 5 years, 10 months after the first rTMS series she participated in our rTMS plus CILT protocol (Naeser, Martin, Treglia et al., 2009). Prior to CILT, her object naming was tested on a set of 500 color pictures, 3 times, as a Baseline. During each CILT session, 6 pictures were presented at a time (2 pictures had been always named at entry Baseline testing; 2 pictures, sometimes; 2 pictures, never). A total of 18 pictures was presented each day (3 sets of 6 pictures each).
Naming Probe Testing
To examine changes that might occur during the CILT intervention, BDAE naming subtests (Actions, Animals, Tools/Implements), the BNT, and action naming pictures from the Object and Action Naming Battery (Druks and Masterson, 2000) were each administered 12 times pre-TMS; and daily, at the end of the 3-hour CILT treatment; and 10 times post-TMS. In addition, a R-hemisphere control-task, the Benton Line Orientation test was administered (Benton et al., 1994). The time-series data for each test were later analyzed using a double bootstrap method (McKnight, McKean, and Huitema, 2000; http://www.stat.wmich.edu/slab/Software/Timeseries.html).
The Language Outcome Measures utilized to examine long-term effects, included the following tests: subtests on the BDAE and the BNT (examined 3 times at entry Baseline); and at 1 and 6 months post- the 10th treatment. Significant improvement was defined as >2 SD above entry Baseline.
On the time-series analysis (Naming Probe Testing), there was significant improvement on BDAE Action Naming (p=.035) (Fig. 4); and Tools/Implements (p=.010). There was no significant change on the Benton Line Orientation (RH control task).
Fig. 4.
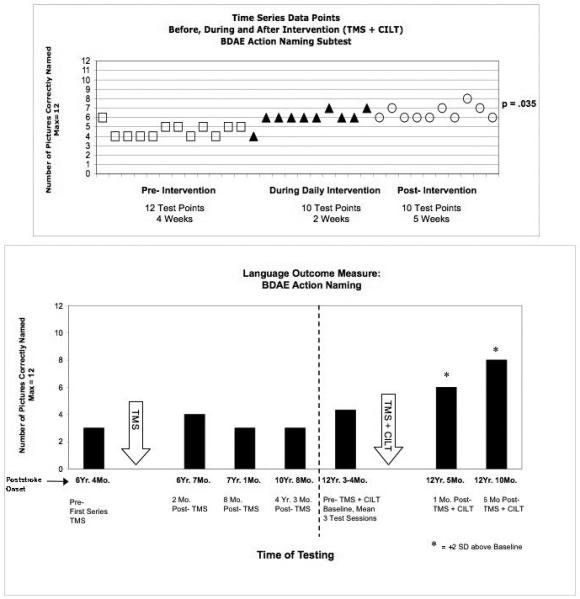
Significant improvement in Action naming test scores post- Intervention (TMS plus CILT) for a severe nonfluent, global aphasia patient. a) Time Series graph shows significant increase in pictures named over time (pre- Intervention, during Daily Intervention and post- Intervention; p=.035); b) Bargraph shows significant improvement (>2 SD) in BDAE Action Naming at 1 and 6 mo. post- Intervention, compared to Baseline testing pre- Intervention. Bargraph also displays scores pre- and post-TMS alone, without speech therapy. Abbreviations: TMS = transcranial magnetic stimulation; CILT = constraint-induced language therapy; BDAE = Boston Diagnostic Aphasia Exam.
On Language Outcome Measures, there was >2 SD improvement on BDAE verb Action Naming; Tools/Implements and Single Word Repetition. Her improvement in BDAE verb Action Naming was only observed following the rTMS plus CILT protocol, not during the first series of Phase 1 and Phase 2 rTMS treatments alone. See Fig. 4.
Mild Nonfluent Aphasia Patient
The second patient who has completed our rTMS plus CILT protocol was a mild nonfluent aphasia patient. He had suffered a L MCA embolic stroke at age 43. This patient received three types of treatments - e.g., 1) continuous positive airway pressure (CPAP) at night; 2) Phase 1 and Phase 2 rTMS; and 3) rTMS plus CILT. At 6 MPO, the patient had been diagnosed with obstructive sleep apnea (OSA). At 12.5 MPO, he began treatment for the OSA, using CPAP at night. He had received speech-language therapy only during the first year poststroke, and not during or after CPAP or rTMS.
Following 2-5 months of CPAP use, there was improvement in phrase length, auditory comprehension and some picture naming (BDAE) (Naeser et al., 2010). The patient used CPAP throughout the remainder of the study, and continues with CPAP. The best-response RH ROI in this case was the gyrus located rostral to the diagonal sulcus and caudal to the vertical ascending ramus - e.g., R PTr posterior.
Significant improvements at 3 and 6 mo. post- Phase 2 rTMS alone, were observed on phrase length for the BDAE cookie theft picture description, and on the BNT. Pre-TMS, the baseline mean for longest phrase length was 5 words; and at 3 and 6 months post-TMS, it was 11 and 7 words, respectively. The 11-word phrase length at 3 months post-TMS was, “his mother wash the dish up and the water fall down.” At 2.4 years post-TMS, the longest phrase length was 12 words: “she was getting her cookie jars and she started to fall back.”
At 3 and 6 months post- Phase 2 TMS, the BNT score improved from a mean of 8.67 pictures named at baseline, to 12 and 13. These gains in naming were stable at 2.4 years post- TMS, with scores of 15, 13 and 15.
At 4 yr. 8 months poststroke onset, this mild nonfluent aphasia patient entered the rTMS plus CILT protocol. At 2 months post- rTMS plus CILT, there was significant improvement (>2 SD) on the BNT where he named 17 pictures, compared to a mean of 14.33 (SD 1.15) pre-treatment. See Fig. 5. There was also significant improvement in naming Tools/Implements (pre-treatment, 5.67, SD 0.58; post-treatment, 8).
Fig. 5.
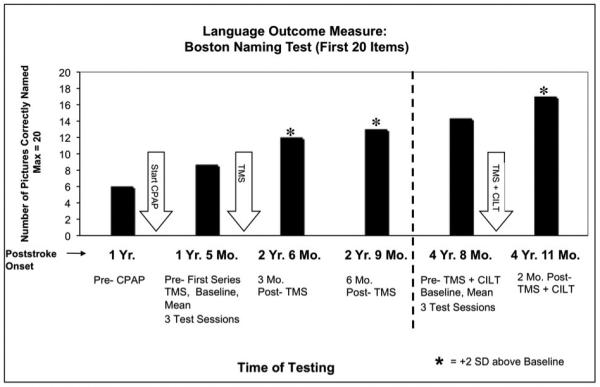
Significant improvement in Boston Naming Test (BNT) scores post- Intervention (TMS plus CILT) for a mild nonfluent aphasia patient. Bargraph shows significant improvement (>2 SD) at 2 mo. post- Intervention, compared to Baseline testing pre- Intervention. Bargraph also displays previous BNT scores pre- and post-TMS treatment alone (without speech therapy); and pre- and post- CPAP scores. Abbreviations: TMS = transcranial magnetic stimulation; CILT = constraint-induced language therapy; CPAP = Continuous Positive Airway Pressure.
On the BDAE cookie theft picture description, the pre-TMS plus CILT baseline mean for longest phrase length was 10.67 (SD 4.16), and at 2 mo. post-TMS plus CILT the longest phrase length was also 10. Further analyses, however, showed qualitative differences in those utterances. He had a significant increase (>2 SD) in number of narrative words (Baseline mean, 46 words and 2 mo. post-TMS plus CILT, 59 words). He also showed a significant increase (>2 SD) in the number of different nouns produced (Baseline mean, 8, SD 1.73 and 2 mo. post-TMS plus CILT, 12).
In summary, when rTMS was combined with CILT in these first two cases, significant improvement was observed in naming, as well as in aspects of propositional speech, beyond what was present post- rTMS alone. However, in the second patient (mild nonfluent aphasia), there were other medical factors that may have contributed to the observed effects (e.g., treatment of OSA with CPAP). A carefully controlled clinical trial is needed to assess these case study findings further.
2.4. Part 4, Diffusion Tensor Imaging (DTI) Study, and Possible Mechanisms Associated with Improvement post- rTMS
2.4.1. Diffusion Tensor Imaging Study
As reviewed above, our rTMS studies have observed long-term, improved naming in chronic nonfluent aphasia patients following a series of rTMS treatments to suppress the R PTr (Naeser et al., 2005a; Naeser et al., 2005b; Martin et al., 2009; Naeser et al., 2010; Hamilton et al., 2010). We have not observed the R POp to be a “best response” ROI to improve naming during Phase 1 in our nonfluent aphasia patients. In fact, suppression of the R POp was observed to impair naming (Naeser et al., 2002 and submitted). The differential/opposite effect observed on naming post- rTMS suppression of the R PTr versus the R POp prompted us, to conduct a DTI study where we could examine possible differences in white matter pathway connections of the POp and the PTr with the arcuate fasciculus (AF).
The AF is a white matter pathway traditionally considered to connect L Broca’s area with the posterior language zones (Catani and Mesulam, 2008, for review). We utilized DTI in eight healthy subjects (5M) to track the horizontal mid-portion of the AF (hAF) to parts of Broca’s area (PTr, POp), and the ventral premotor cortex (vPMC) in the RH and in the LH (Kaplan et al., under revision). White matter pathways from the specific parts of the R Broca’s homologue to the R AF have not previously been studied. Note, our designated AF is likely to have included some of the superior longitudinal fasciculus (SLF) III, because it is difficult to separate the two fiber bundles. The SLF III has been observed to connect the L supramarginal gyrus (SMG, BA 40) with POp (BA 44) and vPMC (ventral BA 6) (Croxson et al., 2005; Makris et al., 2005; Rushworth, Behrens, and Johansen-Berg, 2006).
Our DTI results showed that within R Broca’s homologue, only 1/8 subjects had direct R PTr connections with the hAF. However, 5/8 subjects had R POp connections with the hAF. Almost all subjects (7/8) had R vPMC connections with the hAF. Within L Broca’s area, our results also showed that only 1/8 subjects had L PTr connections with the hAF. However, all 8 subjects had L POp connections with the hAF. Also, all 8 subjects had L vPMC connections with the hAF. Thus, our DTI study showed robust connections between POp and/or vPMC with the hAF, in both the RH and in the LH, but almost no direct connections between PTr with the hAF in either hemisphere.
Our DTI results in the LH are similar to those reported by Frey et al. (2008) and Saur et al. (2008). Each of these DTI studies observed connections from L posterior Broca’s area (not L anterior Broca’s area) to the SMG via AF (and likely SLF III). Frey et al., 2008 observed L anterior Broca’s area connections to superior temporal gyrus to be via the extreme capsule, not via the AF. Thus, major connections from premotor cortices in the LH have been observed to follow a more dorsal route via the AF/SLF III to SMG (Crosxon et al., 2005; Frey et al., 2008; Saur et al., 2008); whereas major connections from ventrolateral prefrontal cortex (including PTr) follow a more ventral route via extreme capsule, to part of the superior temporal gyrus, or middle temporal gyrus (Frey et al., 2008; Saur et al., 2008). Other studies have also suggested separate dorsal and ventral pathways connecting parts of Broca’s area with posterior language zones in the LH (Parker et al., 2005; Rushworth et al., 2006).
The dorsal route in the LH, as recently summarized by Frey et al. (2008), is mainly restricted to sensory-motor mapping of sound to articulation and higher-order articulatory control of speech, where the POp is connected directly with premotor area 6 (involved with orofacial musculature) (Petrides, 2006; Petrides, Cadoret, and Mackey, 2005). This latter area, vPMC, was observed in our DTI study to have connections with the hAF in both the RH and the LH, similar to the POp. Thus, both the POp and vPMC are likely connecting with the SMG via the dorsal route in each hemisphere.
The ventral route in the LH, however, likely performs linguistic processing of sound to meaning, requiring temporo-frontal interaction and top-down regulation of linguistic processing such as that involved in verbal retrieval (Petrides, 2006), and lexical/semantic aspects of language processing (Devlin, Matthews, and Rushworth, 2003; Gold and Buckner, 2002; Nixon et al., 2004; Poldrack et al., 1999; Price et al., 1996; Saur et al., 2008). Heim et al. (2008) have suggested that the PTr may be more related to verbal fluency in general (including syntactic), and not restricted to semantic fluency. A dissociation between the roles of L PTr versus L POp in semantic versus phonological tasks has also been supported by TMS application to these two areas in normals, with differential/opposite effects observed (Gough, Nobre, and Devlin, 2005).
Our DTI results showed that fiber tracts from the R hAF connected primarily with R vPMC and R POp, but not R PTr. Thus, there may be a similar, dorsal route in the RH, which includes connections from the R POp and R vPMC to the R hAF (likely connecting with R SMG, although this posterior connection with SMG was not directly examined in our DTI study). A strong role for the R POp and R vPMC in promoting recovery of speech in nonfluent aphasia has been suggested since 1877 when Barlow reported his detailed anatomical study (Barlow, 1877). A 10 year-old boy lost speech for only 10 days following a first stroke restricted to L POp and L vPMC. One month later, however, a second stroke occurred, located in the same, RH homologous areas (R POp and R vPMC). Following the second stroke, he lost all speech again, and there was “loss of voluntary motor power over the muscles concerned in articulation and the first part of deglutition.” The boy died two months later, without any recovery of speech. Possible roles of R POp and vPMC in aphasia recovery are reviewed below.
Results from this DTI study suggest that pathways from the R POp to parieto-temporal areas likely follow a different route (via the hAF), from those for the R PTr. Thus, this difference may, in part, help to explain the differential/opposite effect following suppression of each of these two areas in chronic stroke patients with nonfluent aphasia.
2.4.2 Possible Mechanisms for Improved Naming and Speech, post- rTMS in Aphasia
The mechanisms associated with the long-term improvement in naming and narrative speech following rTMS suppression of the R PTr in chronic, nonfluent aphasia patients are unknown. Some possible mechanisms are reviewed here, including 1) release of remaining language areas in the LH, from overactivation of homologous areas in the RH (interhemispheric interactions); 2) role of R POp and vPMC in aphasia recovery; and 3) relationship between suppression of R PTr, and its effect on premotor and prefrontal areas associated with verb action naming.
Interhemispheric Interactions
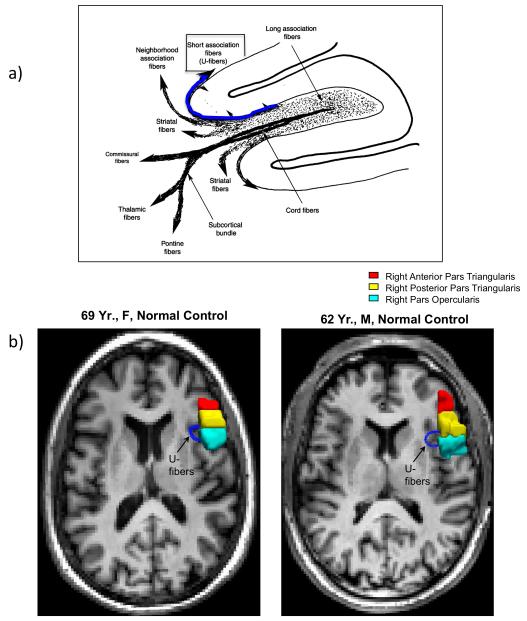
We have observed that suppression of the R PTr with rTMS improved naming in nonfluent aphasia. Although the mechanism for this beneficial effect is unknown, we would posit the following: In cases with nonfluent aphasia where LH lesion is present in L inferior frontal cortex, and/or subcortical white matter deep to it (adjacent to ventricle), hyperactivity of neurons in R PTr may be present, due to interhemispheric disinhibition from the damaged L frontal lobe. This R PTr hyperactivity could excessively suppress the R POp, via their shared U-fibers, which could possibly hinder recovery from aphasia. U-fibers are short association fibers that link adjacent gyri (Schmahmann and Pandya, 2006).
As part of our DTI study (reviewed above), we examined the possible presence of U-fibers in the POp and the PTr. We observed that when one seed point was placed in the R PTr, and a second seed point was placed in the R POp, direct U-fibers were mapped. See Fig. 6. Thus, it is possible that suppression of R PTr with rTMS may promote less inhibition of R POp from R PTr (via U-fibers), directly permitting better modulation of R POp. Modulation of R POp may also indirectly support better modulation of R vPMC. Both the R POp and vPMC connect with the hAF/SLF, likely connecting with the anterior SMG. Modulation of R POp and R vPMC could, in turn, promote better modulation of the overall, remaining bilateral temporo-parietal neural network for naming (Price et al., 2001; Gold and Buckner 2002; Damasio et al. 2004).
Fig. 6.
a) Schematic diagram showing U-fibers, short association fibers (blue arrow) (Schmahmann and Pandya, 2006). Reprinted with permission from Oxford University Press. b) DTI tractography showing U-fibers (dark blue) between R PTr and R POp in two normal controls.
The observation of improved function in chronic stroke patients following rTMS suppression of a target ROI in the undamaged hemisphere has now been reported across three domains of poststroke behavior - e.g., 1) motor deficits (Fregni et al., 2006); 2) visuospatial neglect following RH stroke (Brighina et al., 2003; Koch et al., 2008); and 3) aphasia as reviewed in this paper. A basic principle supporting these improvements poststroke is that rTMS may be used to modulate abnormal interhemispheric inhibition in chronic stroke where imbalanced excitability of the healthy hemisphere is present, due to release of inhibition from the damaged hemisphere.
All of these studies across the behavioral domains of motor impairment, visual neglect and language impairment suggest that following unilateral stroke, an imbalance in interhemispheric inhibition develops. Slow, 1 Hz rTMS may be used to suppress the disinhibition (hyperexcitability) of the undamaged hemisphere in these stroke patients, which in turn promotes better modulation of both hemispheres. Better modulation results in improved function and behavior, including language behavior, as reviewed here, following suppression of only one specific 1 cc target area in the RH - e.g., usually the R PTr. These studies support the notion of improvement for chronic stroke patients through neuromodulation based on noninvasive brain stimulation with rTMS.
Roles of R POp and R vPMC in Aphasia Recovery
The results from our rTMS studies in nonfluent aphasia patients support a role for the R POp in recovery of speech following L frontal lesion, because suppression of R POp with rTMS impaired naming (Naeser et al., 2002, and submitted). These rTMS results support data from a PET study by Blank et al. (2003), where it was observed that after lesion of the L POp, the R POp “contributed to processes involved in the assembly of the sound structure of speech.”
The POp (BA 44) and PMC (BA 6) in the human brain are thought to be analogous to the monkey’s F5 region, a locus of mirror neurons (Rizzolatti and Craighero, 2004). The mirror neuron system is bilateral, and these neurons fire during both production and perception of similar actions (Wilson et al., 2004; Iacoboni, 2008 for review). They are important in child language acquisition (Rizzolatti and Craighero, 2004). An rTMS study by Meister et al. (2007) has supported the role of vPMC as necessary for phonemic categorization in speech perception, where there is a functional interplay between vPMC and the L STG. The role of the R vPMC might be related more to the movement of the orofacial musculature (Petrides et al 2005; Petrides 2006); and the role of the R POp, related more to articulation.
The potential contribution of parts of the mirror neuron system to recovery in aphasia is unknown. The significant improvement we observed in relationship to “tools/implements” in all of our rTMS studies, including in our most recent pilot study with rTMS plus CILT, may be related to the mirror neuron system. The POp (BA 44, an area with mirror neurons) “mediates observation-execution matching for the goals of arm/hand actions” (Kemmerer & Gonzalez-Castille, 2010, for review); and the neural network for “tool use” is widely distributed, including temporal, parietal and frontal regions in the LH (Kemmerer, Gonzalez Castillo, Talavage, Patterson, Wiley, 2008). Thus, modulation of R POp and vPMC with rTMS may have permitted access to this uniquely, widespread neural network for tools/implements.
Our overall results suggest that both the R POp and the R vPMC may play a role in recovery of speech in nonfluent aphasia. This role was initially observed by Barlow (1877) in the childhood aphasia case, where there was no recovery of speech, following bilateral lesion in these two areas. The notion that the mirror neuron component within these two areas (R POp and R PTr) plays a role in aphasia recovery is hypothetical and remains to be established.
Premotor and prefrontal areas associated with verb action naming
Another area of language behavior where marked recovery has recently been observed in patients following rTMS suppression of the R PTr is in verb action naming (Hamilton et al., 2010; Naeser et al., 2009). See Fig. 4. For example, Hamilton and colleagues observed their chronic aphasia patient to have significant improvement in naming actions (but less so, for tools/implements or animals) at 6 months post-rTMS. The authors suggest that this preferential improvement in action naming following suppression of R PTr may be related to neural networks for naming in premotor/prefrontal areas. A previous lesion study has observed an association between action naming impairment, and lesions involving the L premotor and prefrontal areas (Tranel et al., 2001). In addition, rTMS to the L dorsolateral prefrontal cortex in nonaphasic individuals has been shown to affect both action naming (Cappa et al., 2002), and processing of verbs (Cappelletti et al., 2008). Thus, suppression of R PTr may have played a unique role in mediating action naming, a behavior associated with premotor/prefrontal areas of the LH (Hamilton et al., 2010; Naeser et al., 2009). Improvement of action naming following suppression of R PTr requires further study.
Other Transcranial Stimulation Studies
Another physiological intervention that holds promise for improvement in chronic aphasia patients includes transcranial direct current stimulation (tDCS) (Monti et al., 2008). Although the effect of only one session was examined (using cathodal tDCS to L Broca’s area), improved naming was observed with an increase of 33.6% (SEM 13.8%) immediately post-tDCS treatment, in eight chronic nonfluent aphasia patients.
3. Conclusion
Transcranial stimulation with either slow or fast rTMS, or tDCS, when placed on the proper cortical target area with specific treatment parameters, can induce changes in language behavior. Our studies with nonfluent aphasia demonstrate the critical importance of the focality of the rTMS target area in the undamaged RH. Future studies are needed to investigate the focality for rTMS target areas to promote language recovery in additional types of aphasia and lesion site patterns.
With the exception of our rTMS studies with nonfluent aphasia patients, studies with transcranial electrical stimulation (e.g., tDCS) have applied only a single session of treatment. It is suggested that more treatment sessions be applied over longer periods of time. Functional imaging studies pre- and post-TMS contribute information toward understanding of the plasticity of neural networks for language and recovery. The overt speech fMRI data with our nonfluent patient with good response to TMS support the hypothesis that restoration of parts of the LH language network is linked, at least in part, to better recovery of naming and phrase length in nonfluent aphasia. Additional fMRI studies pre- and post- rTMS are warranted. Also, the combination of rTMS or tDCS with speech therapy sessions provided immediately afterwards, might promote further language improvement in a variety of chronic aphasia patients.
Acknowledgments
Research supported by NIH grant RO1 DC05672 from the National Institute on Deafness and Other Communication Disorders (NIDCD), Bethesda, MD, and a grant from the Medical Research Service, Department of Veterans Affairs, Washington, D.C. (to M.A.N.); a K24 NIH award (RRO18875, to A.P.-L); BBVA Chair in Translational Medicine (to A.P.-L.); the Harvard-Thorndike General Clinical Research Center at BIDMC (NCRR MO1 RR01032) and Harvard Clinical and Translational Science Center (UL1 RR025758); and a P30 DC05207 NIDCD grant to the Harold Goodglass BU Aphasia Research Center.
References
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K. Broca’s region revisited: cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412(2):319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Amunts K, Weiss PH, Mohlberg H, Pieperhoff P, Eickhoff S, Gurd JM, Marshall JC, Shah NJ, Fink GR, Zilles K. Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space--the roles of Brodmann areas 44 and 45. Neuroimage. 2004;22(1):42–56. doi: 10.1016/j.neuroimage.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Barch DM, Sabb FW, Carter CS, Braver TS, Noll DC, Cohen JD. Overt verbal responding during fMRI scanning: empirical investigations of problems and potential solutions. Neuroimage. 1999;10(6):642–657. doi: 10.1006/nimg.1999.0500. [DOI] [PubMed] [Google Scholar]
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- Barlow T. Brit Med J. 1877:103. doi: 10.1136/bmj.2.865.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso A, Gardelli M, Grassi MP, Mariotti M. The Role of the Right Hemisphere in Recovery from Aphasia. Two Case Studies. Cortex. 1989;25(4):555–566. doi: 10.1016/s0010-9452(89)80017-6. [DOI] [PubMed] [Google Scholar]
- Belin P, Van Eeckhout P, Zilbovicius M, Remy P, Francois C, Guillaume S, Chain F, Rancurel G, Samson Y. Recovery from nonfluent aphasia after melodic intonation therapy: a PET study. Neurology. 1996;47(6):1504–1511. doi: 10.1212/wnl.47.6.1504. [DOI] [PubMed] [Google Scholar]
- Benton A, Sivan A, Hamsher K. d., Varney N, Spreen O. Contributions to Neuropsychological Assessment: A Clinical Manual. 2nd ed. Oxford University Press; New York: 1994. [Google Scholar]
- Berthier ML, Green C, Lara JP, Higueras C, Barbancho MA, Davila G, et al. Memantine and constraint-induced aphasia therapy in chronic poststroke aphasia. Ann Neurol. 2009;65(5):577–585. doi: 10.1002/ana.21597. [DOI] [PubMed] [Google Scholar]
- Birn RM, Cox RW, Bandettini PA. Experimental designs and processing strategies for fMRI studies involving overt verbal responses. Neuroimage. 2004;23(3):1046–1058. doi: 10.1016/j.neuroimage.2004.07.039. [DOI] [PubMed] [Google Scholar]
- Blank SC, Bird H, Turkheimer F, Wise RJ. Speech production after stroke: the role of the right pars opercularis. Ann Neurol. 2003;54(3):310–320. doi: 10.1002/ana.10656. [DOI] [PubMed] [Google Scholar]
- Blasi V, Young AC, Tansy AP, Petersen SE, Snyder AZ, Corbetta M. Word retrieval learning modulates right frontal cortex in patients with left frontal damage. Neuron. 2002;36(1):159–170. doi: 10.1016/s0896-6273(02)00936-4. [DOI] [PubMed] [Google Scholar]
- Bolognini N, Pascual-Leone A, Fregni F. Using non-invasive brain stimulation to augment motor training-induced plasticity. J Neuroeng Rehabil. 2009;6:8. doi: 10.1186/1743-0003-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier JI, Juranek J, Maher LM, Schmadeke S, Men D, Papanicolaou AC. Behavioral and neurophysiologic response to therapy for chronic aphasia. Arch Phys Med Rehabil. 2009;90(12):2026–2033. doi: 10.1016/j.apmr.2009.08.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighina F, Bisiach E, Oliveri M, Piazza A, La Bua V, Daniele O, Fierro B. 1 Hz repetitive transcranial magnetic stimulation of the unaffected hemisphere ameliorates contralesional visuospatial neglect in humans. Neurosci Lett. 2003;336(2):131–133. doi: 10.1016/s0304-3940(02)01283-1. [DOI] [PubMed] [Google Scholar]
- Cappa SF, Sandrini M, Rossini PM, Sosta K, Miniussi C. The role of the left frontal lobe in action naming: rTMS evidence. Neurology. 2002;59(5):720–723. doi: 10.1212/wnl.59.5.720. [DOI] [PubMed] [Google Scholar]
- Cappelletti M, Fregni F, Shapiro K, Pascual-Leone A, Caramazza A. Processing nouns and verbs in the left frontal cortex: a transcranial magnetic stimulation study. J Cogn Neurosci. 2008;20(4):707–720. doi: 10.1162/jocn.2008.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex. 2008;44(8):953–961. doi: 10.1016/j.cortex.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48(5):1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Cherney LR, Small SL. Task-dependent changes in brain activation following therapy for nonfluent aphasia: discussion of two individual cases. J Int Neuropsychol Soc. 2006;12(6):828–842. doi: 10.1017/S1355617706061017. [DOI] [PubMed] [Google Scholar]
- Cornelissen K, Laine M, Tarkiainen A, Jarvensivu T, Martin N, Salmelin R. Adult brain plasticity elicited by anomia treatment. J Cogn Neurosci. 2003;15(3):444–461. doi: 10.1162/089892903321593153. [DOI] [PubMed] [Google Scholar]
- Crosson B, McGregor K, Gopinath KS, Conway TW, Benjamin M, Chang YL, Moore AB, Raymer AM, Briggs RW, Sherod MG, Wierenga CE, White KD. Functional MRI of language in aphasia: a review of the literature and the methodological challenges. Neuropsychol Rev. 2007;17(2):157–177. doi: 10.1007/s11065-007-9024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson B, Moore AB, Gopinath K, White KD, Wierenga CE, Gaiefsky ME, Fabrizio KS, Peck KK, Soltysik D, Milsted C, Briggs RW, Conway TW, Gonzalez Rothi LJ. Role of the right and left hemispheres in recovery of function during treatment of intention in aphasia. J Cogn Neurosci. 2005;17(3):392–406. doi: 10.1162/0898929053279487. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Johansen-Berg H, Behrens TE, Robson MD, Pinsk MA, Gross CG, Richter W, Richter MC, Kastner S, Rushworth MF. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. J Neurosci. 2005;25(39):8854–8866. doi: 10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92(1-2):179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Demeurisse G, Capon A. Language Recovery in Aphasic Stroke Patients: Clinical, CT and CBF Studies. Aphasiology. 1987;1:301–315. [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MF. Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cogn Neurosci. 2003;15(1):71–84. doi: 10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- Druks J, Masterson J. An Object and Action Naming Battery. Psychology Press Ltd.; Hove, East Sussex, UK: 2000. [Google Scholar]
- Duffau H, Capelle L, Denvil D, Gatignol P, Sichez N, Lopes M, Sichez JP, Van Effenterre R. The role of dominant premotor cortex in language: a study using intraoperative functional mapping in awake patients. Neuroimage. 2003;20(4):1903–1914. doi: 10.1016/s1053-8119(03)00203-9. [DOI] [PubMed] [Google Scholar]
- Eden GF, Joseph JE, Brown HE, Brown CP, Zeffiro TA. Utilizing hemodynamic delay and dispersion to detect fMRI signal change without auditory interference: the behavior interleaved gradients technique. Magn Reson Med. 1999;41(1):13–20. doi: 10.1002/(sici)1522-2594(199901)41:1<13::aid-mrm4>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Fernandez B, Cardebat D, Demonet JF, Joseph PA, Mazaux JM, Barat M, Allard M. Functional MRI follow-up study of language processes in healthy subjects and during recovery in a case of aphasia. Stroke. 2004;35(9):2171–2176. doi: 10.1161/01.STR.0000139323.76769.b0. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Valle AC, Rocha RR, Duarte J, Ferreira MJ, Wagner T, Fecteau S, Rigonatti SP, Riberto M, Freedman SD, Pascual-Leone A. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37(8):2115–2122. doi: 10.1161/01.STR.0000231390.58967.6b. [DOI] [PubMed] [Google Scholar]
- Frey S, Campbell JS, Pike GB, Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J Neurosci. 2008;28(45):11435–11444. doi: 10.1523/JNEUROSCI.2388-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Jezzard P, Turner R. Analysis of functional MRI time-series. Hum Brain Mapp. 1994;1:153–177. [Google Scholar]
- Gold BT, Buckner RL. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;35(4):803–812. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Gold BT, Kertesz A. Right hemisphere semantic processing of visual words in an aphasic patient: an fMRI study. Brain Lang. 2000;73(3):456–465. doi: 10.1006/brln.2000.2317. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. Assessment of Aphasia and Related Disorders. Lea and Febiger; Philadelphia: 1983. [Google Scholar]
- Goodglass H, Kaplan E, Barresi B. The Assessment of Aphasia and Related Disorders. 3rd Edition Lippincott, Williams and Wilkins; Philadelphia, PA: 2001. [Google Scholar]
- Gough PM, Nobre AC, Devlin JT. Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. J Neurosci. 2005;25(35):8010–8016. doi: 10.1523/JNEUROSCI.2307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowers WR. A Manual of Diseases of the Nervous System. Churchill; London: 1886. [Google Scholar]
- Hamilton RH, Sanders L, Benson J, Faseyitan O, Norise C, Naeser M, et al. Stimulating conversation: enhancement of elicited propositional speech in a patient with chronic non-fluent aphasia following transcranial magnetic stimulation. Brain Lang. 2010;113(1):45–50. doi: 10.1016/j.bandl.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S, Eickhoff SB, Amunts K. Specialisation in Broca’s region for semantic, phonological, and syntactic fluency? Neuroimage. 2008;40(3):1362–1368. doi: 10.1016/j.neuroimage.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Thiel A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang. 2006;98(1):118–123. doi: 10.1016/j.bandl.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Kleinman JT, Newhart M, Heidler-Gary J, Gottesman R, Barker PB, Aldrich E, Llinas R, Wityk R, Chaudhry P. Restoring cerebral blood flow reveals neural regions critical for naming. J Neurosci. 2006;26(31):8069–8073. doi: 10.1523/JNEUROSCI.2088-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M. The role of premotor cortex in speech perception: evidence from fMRI and rTMS. J Physiol Paris. 2008;102(1-3):31–34. doi: 10.1016/j.jphysparis.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Lippincott, Williams and Wilkins; Philadelphia, PA: 2001. [Google Scholar]
- Kaplan E, Naeser MA, Martin PI, Ho M, Wang Y, Baker EH, Pascual-Leone A. Connections between Arcuate Fasciculus and Parts of Broca’s Area, and the Ventral Premotor Cortex in the Left and Right Hemispheres: A DTI Study. Under revision. Under revision. [Google Scholar]
- Kapur N. Paradoxical functional facilitation in brain-behaviour research. A critical review. Brain. 1996;119(Pt 5):1775–1790. doi: 10.1093/brain/119.5.1775. [DOI] [PubMed] [Google Scholar]
- Karbe H, Thiel A, Weber-Luxenburger G, Herholz K, Kessler J, Heiss WD. Brain plasticity in poststroke aphasia: what is the contribution of the right hemisphere? Brain Lang. 1998;64(2):215–230. doi: 10.1006/brln.1998.1961. [DOI] [PubMed] [Google Scholar]
- Kauffmann CD, Cheema MA, Miller BE. Slow right prefrontal transcranial magnetic stimulation as a treatment for medication-resistant depression: a double-blind, placebo-controlled study. Depress Anxiety. 2004;19(1):59–62. doi: 10.1002/da.10144. [DOI] [PubMed] [Google Scholar]
- Kemmerer D, Castillo JG, Talavage T, Patterson S, Wiley C. Neuroanatomical distribution of five semantic components of verbs: evidence from fMRI. Brain Lang. 2008;107(1):16–43. doi: 10.1016/j.bandl.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Kemmerer D, Gonzalez-Castillo J. The Two-Level Theory of verb meaning: An approach to integrating the semantics of action with the mirror neuron system. Brain Lang. 2010;112(1):54–76. doi: 10.1016/j.bandl.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A. Neurobiological aspects of recovery from aphasia in stroke. Int Rehabil Med. 1984;6(3):122–127. doi: 10.3109/03790798409165934. [DOI] [PubMed] [Google Scholar]
- Kertesz A, McCabe P. Recovery patterns and prognosis in aphasia. Brain. 1977;100(Pt 1):1–18. doi: 10.1093/brain/100.1.1. [DOI] [PubMed] [Google Scholar]
- Kessler J, Thiel A, Karbe H, Heiss WD. Piracetam improves activated blood flow and facilitates rehabilitation of poststroke aphasic patients. Stroke. 2000;31(9):2112–2116. doi: 10.1161/01.str.31.9.2112. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. The Minor Cerebral hemisphere as a Source of Aphasic Speech. Archives of Neurology. 1971;25(4):302–306. doi: 10.1001/archneur.1971.00490040028003. [DOI] [PubMed] [Google Scholar]
- Klein E, Kreinin I, Chistyakov A, Koren D, Mecz L, Marmur S, Ben-Shachar D, Feinsod M. Therapeutic efficacy of right prefrontal slow repetitive transcranial magnetic stimulation in major depression: a double-blind controlled study. Arch Gen Psychiatry. 1999;56(4):315–320. doi: 10.1001/archpsyc.56.4.315. [DOI] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Cheeran B, Ruge D, Lo Gerfo E, Salerno S, Torriero S, Marconi B, Mori F, Driver J, Rothwell JC, Caltagirone C. Hyperexcitability of parietal-motor functional connections in the intact left-hemisphere of patients with neglect. Brain. 2008;131(Pt 12):3147–3155. doi: 10.1093/brain/awn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur JP. Stroke recovery can be enhanced by using repetitive transcranial magnetic stimulation (rTMS) Neurophysiol Clin. 2006;36(3):105–115. doi: 10.1016/j.neucli.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Leger A, Demonet JF, Ruff S, Aithamon B, Touyeras B, Puel M, Boulanouar K, Cardebat D. Neural substrates of spoken language rehabilitation in an aphasic patient: an fMRI study. Neuroimage. 2002;17(1):174–183. doi: 10.1006/nimg.2002.1238. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111(5):800–805. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- Maher LM, Kendall D, Swearengin JA, Rodriguez A, Leon SA, Pingel K, Holland A, Rothi LJ. A pilot study of use-dependent learning in the context of Constraint Induced Language Therapy. J Int Neuropsychol Soc. 2006;12(6):843–852. doi: 10.1017/S1355617706061029. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr., Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15(6):854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Martin PI, Naeser MA, Doron KW, Bogdan A, Baker EH, Kurland J, Renshaw P, Yurgelun-Todd D. Overt naming in aphasia studied with a functional MRI hemodynamic delay design. Neuroimage. 2005;28(1):194–204. doi: 10.1016/j.neuroimage.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Martin PI, Naeser MA, Ho M, Doron KW, Kurland J, Kaplan J, Wang Y, Nicholas M, Baker EH, Fregni F, Pascual-Leone A. Overt naming fMRI pre- and post-TMS: Two nonfluent aphasia patients, with and without improved naming post- TMS. Brain Lang. 2009;111(1):20–35. doi: 10.1016/j.bandl.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight SD, McKean JW, Huitema BE. A double bootstrap method to analyze linear models with autoregressive error terms. Psychol Methods. 2000;5(1):87–101. doi: 10.1037/1082-989x.5.1.87. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Djundja D, Barthel G, Elbert T, Rockstroh B. Long-term stability of improved language functions in chronic aphasia after constraint-induced aphasia therapy. Stroke. 2005;36(7):1462–1466. doi: 10.1161/01.STR.0000169941.29831.2a. [DOI] [PubMed] [Google Scholar]
- Meister IG, Wilson SM, Deblieck C, Wu AD, Iacoboni M. The essential role of premotor cortex in speech perception. Curr Biol. 2007;17(19):1692–1696. doi: 10.1016/j.cub.2007.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti A, Cogiamanian F, Marceglia S, Ferrucci R, Mameli F, Mrakic-Sposta S, Vergari M, Zago S, Priori A. Improved naming after transcranial direct current stimulation in aphasia. J Neurol Neurosurg Psychiatry. 2008;79(4):451–453. doi: 10.1136/jnnp.2007.135277. [DOI] [PubMed] [Google Scholar]
- Musso M, Weiller C, Kiebel S, Muller SP, Bulau P, Rijntjes M. Training-induced brain plasticity in aphasia. Brain. 1999;122(Pt 9):1781–1790. doi: 10.1093/brain/122.9.1781. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin P, Fregni F, Theoret H, Kobayashi M, Nicholas M, Baker E, Maria-Tormos J, Steven M, Pascual-Leone A. Modulation of cortical areas with repetitive transcranial magnetic stimulation to improve naming in nonfluent aphasia [Abstract#133]; Proceedings of the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan: NeuroImage. 2002.Jun 2-6, [Google Scholar]
- Naeser MA, Martin P, Treglia E, Ho M, Baker E, Kaplan E, Bashir S, Pascual-Leone A. Improved Action Naming in A Severe, Nonfluent Aphasia Patient, following Transcranial Magnetic Stimulation plus Constraint-Induced Language Therapy [Abstract#72]; Poster presented at Academy of Aphasia 47th Annual Meeting; Boston, MA. 2009.Oct 18-20, [Google Scholar]
- Naeser MA, Martin PI, Baker EH, Hodge SM, Sczerzenie SE, Nicholas M, Palumbo CL, Goodglass H, Wingfield A, Samaraweera R, Harris G, Baird A, Renshaw P, Yurgelun-Todd D. Overt propositional speech in chronic nonfluent aphasia studied with the dynamic susceptibility contrast fMRI method. Neuroimage. 2004;22(1):29–41. doi: 10.1016/j.neuroimage.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Lundgren K, Klein R, Kaplan J, Treglia E, et al. Improved Language in a Chronic Nonfluent Aphasia Patient After Treatment With CPAP and TMS. Cogn Behav Neurol. 2010;23(1):29–38. doi: 10.1097/WNN.0b013e3181bf2d20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, Theoret H, Fregni F, Maria-Tormos J, Kurland J, Doron KW, Pascual-Leone A. Improved picture naming in chronic aphasia after TMS to part of right Broca’s area: an open-protocol study. Brain Lang. 2005;93(1):95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Helm-Estabrooks N, Cayer-Meade C, Kobayashi M, Theoret H, Fregni F, Tormos JM, Kurland J, Doron KW, Pascual-Leone A. Improved naming after TMS treatments in a chronic, global aphasia patient--case report. Neurocase. 2005;11(3):182–193. doi: 10.1080/13554790590944663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani N, Schurmann M, Amunts K, Hari R. Broca’s region: from action to language. Physiology (Bethesda) 2005;20:60–69. doi: 10.1152/physiol.00043.2004. [DOI] [PubMed] [Google Scholar]
- Nixon P, Lazarova J, Hodinott-Hill I, Gough P, Passingham R. The inferior frontal gyrus and phonological processing: an investigation using rTMS. J Cogn Neurosci. 2004;16(2):289–300. doi: 10.1162/089892904322984571. [DOI] [PubMed] [Google Scholar]
- Padberg F, Zwanzger P, Thoma H, Kathmann N, Haag C, Greenberg BD, Hampel H, Moller HJ. Repetitive transcranial magnetic stimulation (rTMS) in pharmacotherapy-refractory major depression: comparative study of fast, slow and sham rTMS. Psychiatry Res. 1999;88(3):163–171. doi: 10.1016/s0165-1781(99)00092-x. [DOI] [PubMed] [Google Scholar]
- Parker GJ, Luzzi S, Alexander DC, Wheeler-Kingshott CA, Ciccarelli O, Lambon Ralph MA. Lateralization of ventral and dorsal auditory-language pathways in the human brain. Neuroimage. 2005;24(3):656–666. doi: 10.1016/j.neuroimage.2004.08.047. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Davey N, Wasserman EM, Rothwell J, Puri B, editors. Handbook of Transcranial Magnetic Stimulation. Arnold Press; London, UK: 2002. [Google Scholar]
- Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15(4):333–343. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117(Pt 4):847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V, Rothwell J. Transcranial magnetic stimulation in cognitive neuroscience--virtual lesion, chronometry, and functional connectivity. Curr Opin Neurobiol. 2000;10(2):232–237. doi: 10.1016/s0959-4388(00)00081-7. [DOI] [PubMed] [Google Scholar]
- Peck KK, Moore AB, Crosson BA, Gaiefsky M, Gopinath KS, White K, Briggs RW. Functional magnetic resonance imaging before and after aphasia therapy: shifts in hemodynamic time to peak during an overt language task. Stroke. 2004;35(2):554–559. doi: 10.1161/01.STR.0000110983.50753.9D. [DOI] [PubMed] [Google Scholar]
- Pedersen PM, Vinter K, Olsen TS. Aphasia after stroke: type, severity and prognosis. The Copenhagen aphasia study. Cerebrovasc Dis. 2004;17(1):35–43. doi: 10.1159/000073896. [DOI] [PubMed] [Google Scholar]
- Perani D, Cappa SF, Tettamanti M, Rosa M, Scifo P, Miozzo A, Basso A, Fazio F. A fMRI study of word retrieval in aphasia. Brain Lang. 2003;85(3):357–368. doi: 10.1016/s0093-934x(02)00561-8. [DOI] [PubMed] [Google Scholar]
- Petrides M. Broca’s area in the human and the non-human primate brain. In: Amunts K, Grodzinsky Y, editors. Broca’s Region. Oxford, UP; Oxford: 2006. pp. 31–46. [Google Scholar]
- Petrides M, Cadoret G, Mackey S. Orofacial somatomotor responses in the macaque monkey homologue of Broca’s area. Nature. 2005;435(7046):1235–1238. doi: 10.1038/nature03628. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10(1):15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Price CJ, Crinion J. The latest on functional imaging studies of aphasic stroke. Curr Opin Neurol. 2005;18(4):429–434. doi: 10.1097/01.wco.0000168081.76859.c1. [DOI] [PubMed] [Google Scholar]
- Price CJ, Warburton EA, Moore CJ, Frackowiak RS, Friston KJ. Dynamic diaschisis: anatomically remote and context-sensitive human brain lesions. J Cogn Neurosci. 2001;13(4):419–429. doi: 10.1162/08989290152001853. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJ, Warburton EA, Moore CJ, Howard D, Patterson K, Frackowiak RS, Friston KJ. Hearing and saying. The functional neuro-anatomy of auditory word processing. Brain. 1996;119(Pt 3):919–931. doi: 10.1093/brain/119.3.919. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F, Neininger B, Elbert T, Mohr B, Rockstroh B, Koebbel P, Taub E. Constraint-induced therapy of chronic aphasia after stroke. Stroke. 2001;32(7):1621–1626. doi: 10.1161/01.str.32.7.1621. [DOI] [PubMed] [Google Scholar]
- Raboyeau G, De Boissezon X, Marie N, Balduyck S, Puel M, Bezy C, Demonet JF, Cardebat D. Right hemisphere activation in recovery from aphasia: lesion effect or function recruitment? Neurology. 2008;70(4):290–298. doi: 10.1212/01.wnl.0000287115.85956.87. [DOI] [PubMed] [Google Scholar]
- Richter M, Miltner WH, Straube T. Association between therapy outcome and right-hemispheric activation in chronic aphasia. Brain. 2008;131(Pt 5):1391–1401. doi: 10.1093/brain/awn043. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Petersen SE, Linenweber MR, Snyder AZ, White DA, Chapman L, Dromerick AW, Fiez JA, Corbetta MD. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000;55(12):1883–1894. doi: 10.1212/wnl.55.12.1883. [DOI] [PubMed] [Google Scholar]
- Rossi S, Rossini PM. TMS in cognitive plasticity and the potential for rehabilitation. Trends Cogn Sci. 2004;8(6):273–279. doi: 10.1016/j.tics.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Methods. 1997;74(2):113–122. doi: 10.1016/s0165-0270(97)02242-5. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, Johansen-Berg H. Connection patterns distinguish 3 regions of human parietal cortex. Cereb Cortex. 2006;16(10):1418–1430. doi: 10.1093/cercor/bhj079. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry MS, Umarova R, Musso M, Glauche V, Abel S, Huber W, Rijntjes M, Hennig J, Weiller C. Ventral and dorsal pathways for language. Proc Natl Acad Sci U S A. 2008;105(46):18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, Weiller C. Dynamics of language reorganization after stroke. Brain. 2006;129(Pt 6):1371–1384. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. Oxford University Press, Inc.; Oxford: 2006. [Google Scholar]
- Small SL, Flores DK, Noll DC. Different neural circuits subserve reading before and after therapy for acquired dyslexia. Brain Lang. 1998;62(2):298–308. doi: 10.1006/brln.1998.1951. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol [Hum Learn] 1980;6(2):174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Talelli P, Rothwell J. Does brain stimulation after stroke have a future? Curr Opin Neurol. 2006;19(6):543–550. doi: 10.1097/WCO.0b013e32801080d1. [DOI] [PubMed] [Google Scholar]
- Tassinari CA, Cincotta M, Zaccara G, Michelucci R. Transcranial magnetic stimulation and epilepsy. Clin Neurophysiol. 2003;114(5):777–798. doi: 10.1016/s1388-2457(03)00004-x. [DOI] [PubMed] [Google Scholar]
- Taub E. Harnessing brain plasticity through behavioral techniques to produce new treatments in neurorehabilitation. Am Psychol. 2004;59(8):692–704. doi: 10.1037/0003-066X.59.8.692. [DOI] [PubMed] [Google Scholar]
- Taub E, Crago JE, Burgio LD, Groomes TE, Cook EW, 3rd, DeLuca SC, Miller NE. An operant approach to rehabilitation medicine: overcoming learned nonuse by shaping. J Exp Anal Behav. 1994;61(2):281–293. doi: 10.1901/jeab.1994.61-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel A, Habedank B, Herholz K, Kessler J, Winhuisen L, Haupt WF, Heiss WD. From the left to the right: How the brain compensates progressive loss of language function. Brain Lang. 2006;98(1):57–65. doi: 10.1016/j.bandl.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Thompson CK. Neuroplasticity: evidence from aphasia. J Commun Disord. 2000;33(4):357–366. doi: 10.1016/s0021-9924(00)00031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulborn KR, Carpenter PA, Just MA. Plasticity of language-related brain function during recovery from stroke. Stroke. 1999;30(4):749–754. doi: 10.1161/01.str.30.4.749. [DOI] [PubMed] [Google Scholar]
- Tranel D, Adolphs R, Damasio H, Damasio AR. A Neural Basis for the Retrieval of Words for Actions. Cogn Neuropsych. 2001;18(7):655–670. doi: 10.1080/02643290126377. [DOI] [PubMed] [Google Scholar]
- Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- Wasserman EM, Epstein CM, Ziemann U, Walsh V, Paus T, Lisanby SH, editors. The Oxford Handbook of Transcranial Stimulation. Oxford Univeristy Press, Inc.; New York: 2008. [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5-7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108(1):1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Weiller C, Isensee C, Rijntjes M, Huber W, Muller S, Bier D, Dutschka K, Woods RP, Noth J, Diener HC. Recovery from Wernicke’s aphasia: a positron emission tomographic study. Ann Neurol. 1995;37(6):723–732. doi: 10.1002/ana.410370605. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Saygin AP, Sereno MI, Iacoboni M. Listening to speech activates motor areas involved in speech production. Nat Neurosci. 2004;7(7):701–702. doi: 10.1038/nn1263. [DOI] [PubMed] [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, Heiss WD. Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: a combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke. 2005;36(8):1759–1763. doi: 10.1161/01.STR.0000174487.81126.ef. [DOI] [PubMed] [Google Scholar]
Website
- McKnight S, McKean JW, Huitema B. [Accessed July 2009];Timeseries software. Available at http://www.stat.wmich.edu/slab/Software/Timeseries.html.