Abstract
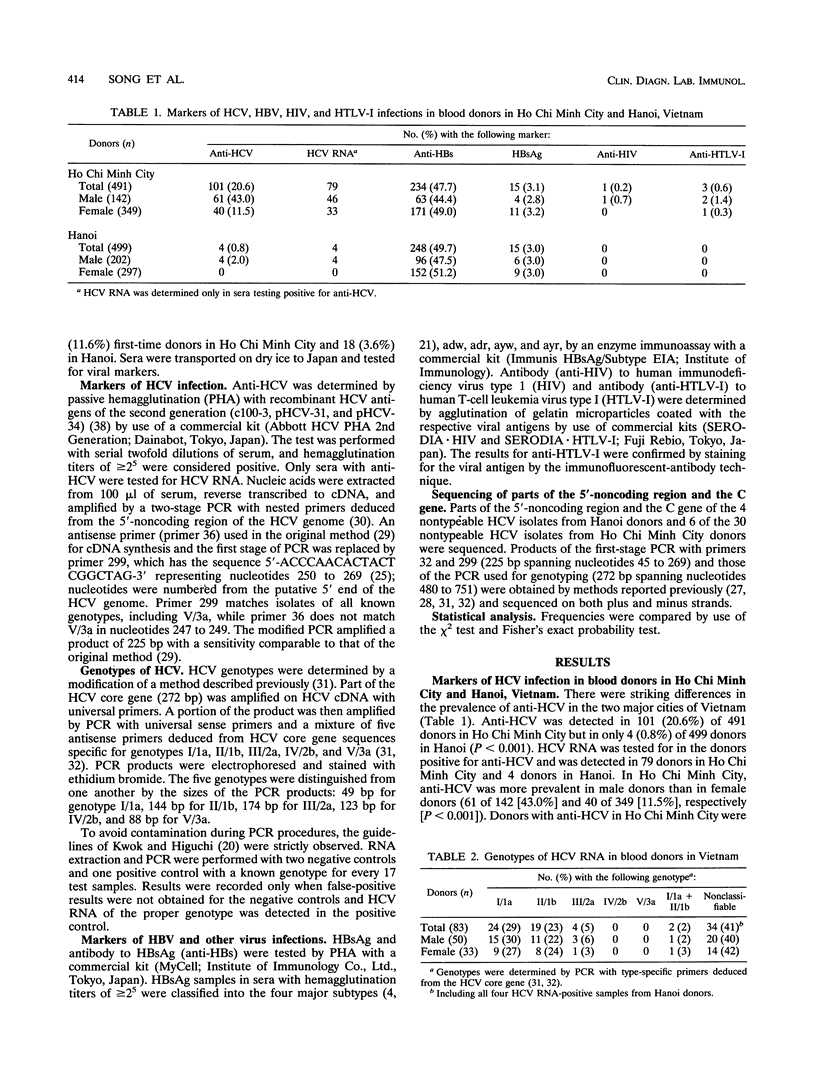
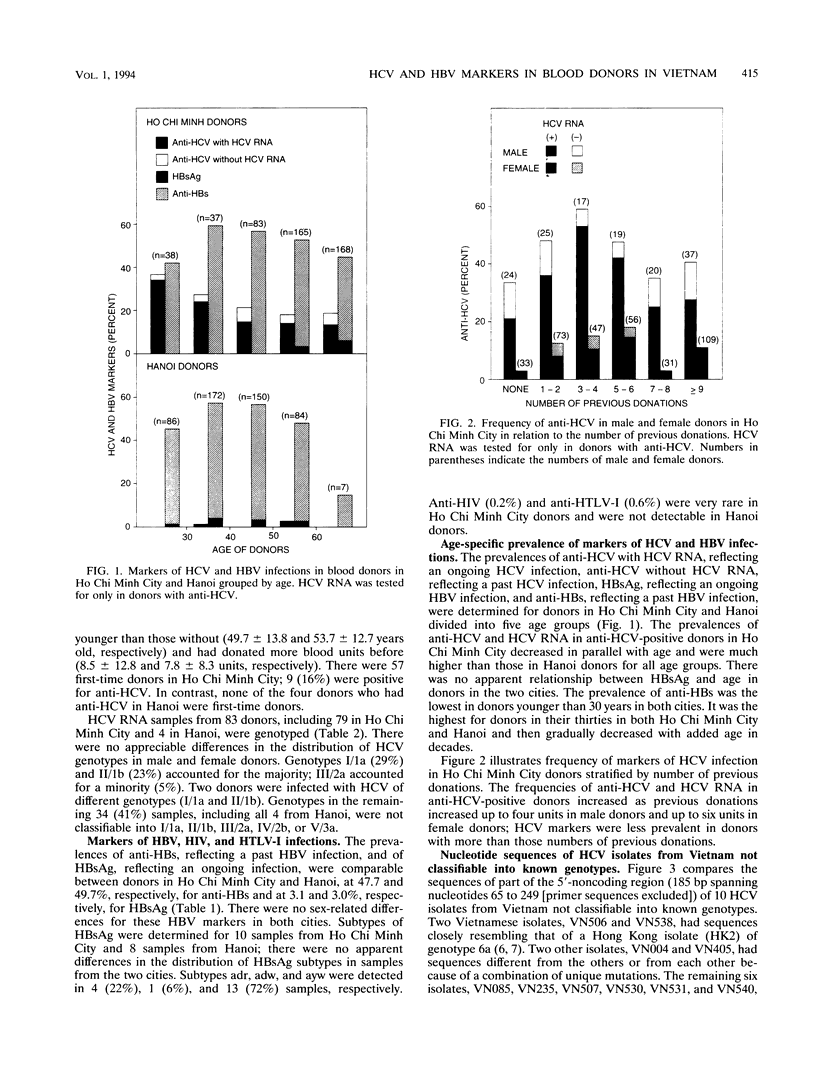
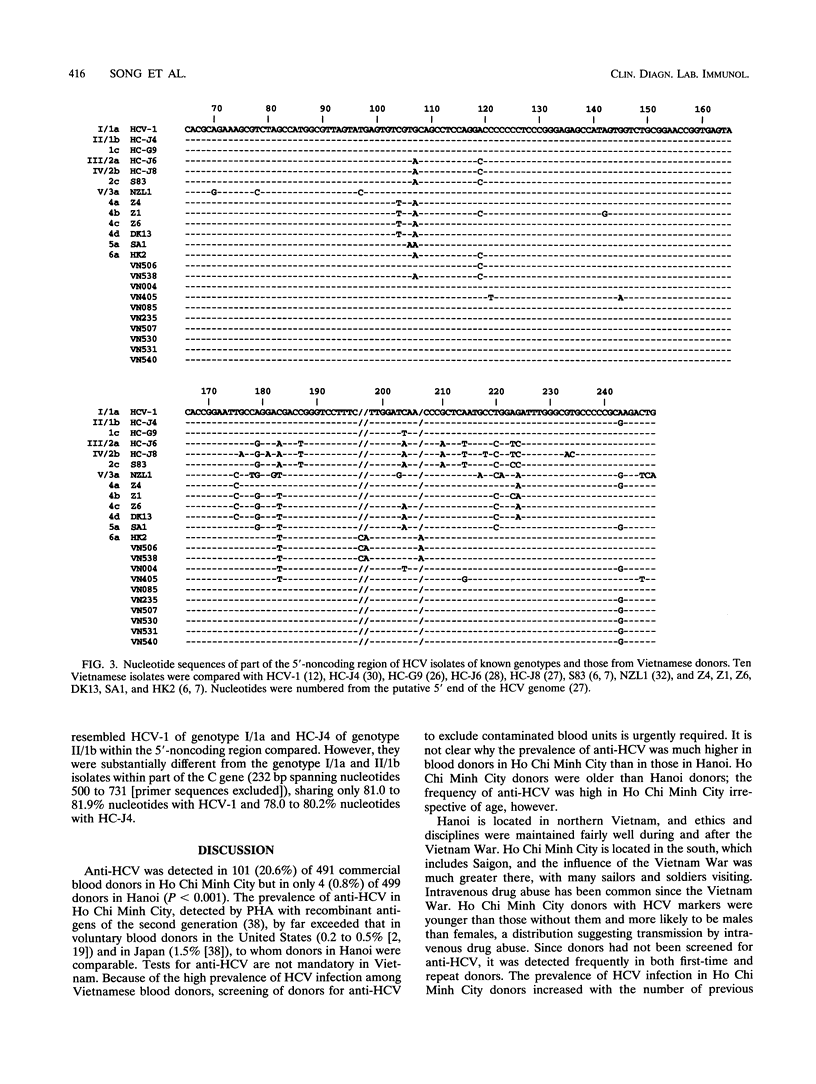
Blood donors in two cities in Vietnam were tested for markers of hepatitis C virus (HCV) and hepatitis B virus infections. Antibody to HCV was detected by passive hemagglutination with antigens of the second generation in 101 (20.6%) of 491 donors in Ho Chi Minh City; it was detected less frequently (P < 0.001) in donors in hanoi (4 [0.8%] of 499). HCV RNA was tested for in donors with antibody by PCR with nested primers from the 5'-noncoding region and detected in 79 donors in Ho Chi Minh City and 4 donors in Hanoi. HCV RNA was genotyped by PCR with type-specific primers from the core gene. Of 83 HCV carriers from Vietnam, 24 (29%) were infected with HCV of genotype I/1a 19 (23%) were infected with II/1b, 4 (5%) were infected with III/2a, and 2 (2%) were infected with mixed genotypes (I/1a and II/1b); HCV genotypes in the remaining 34 (41%) donors, including all 4 donors in Hanoi, were not classifiable into I/1a, II/2a, IV/2b, or V/3a. Of the 10 isolates with unclassifiable genotypes, 2 showed substantial sequence divergence within the 5'-noncoding region from reported isolates with known genotypes (I/1a to 6a). An analysis of part of the core gene sequence indicated that six of the remaining isolates most likely represented new HCV genotypes. Hepatitis B surface antigen and the corresponding antibody, respectively, were detected in 15 (3.1%) and 234 (47.7%) donors in Ho Chi Minh City as well as 15 (3.0%) and 248 (49.7%) donors in Hanoi. These results indicate an extensive spread of HCV among Ho Chi Minh City donors and HCV of novel genotypes in vietnam.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aach R. D., Stevens C. E., Hollinger F. B., Mosley J. W., Peterson D. A., Taylor P. E., Johnson R. G., Barbosa L. H., Nemo G. J. Hepatitis C virus infection in post-transfusion hepatitis. An analysis with first- and second-generation assays. N Engl J Med. 1991 Nov 7;325(19):1325–1329. doi: 10.1056/NEJM199111073251901. [DOI] [PubMed] [Google Scholar]
- Alter H. J. New kit on the block: evaluation of second-generation assays for detection of antibody to the hepatitis C virus. Hepatology. 1992 Feb;15(2):350–353. doi: 10.1002/hep.1840150228. [DOI] [PubMed] [Google Scholar]
- Alter H. J., Purcell R. H., Shih J. W., Melpolder J. C., Houghton M., Choo Q. L., Kuo G. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989 Nov 30;321(22):1494–1500. doi: 10.1056/NEJM198911303212202. [DOI] [PubMed] [Google Scholar]
- Bancroft W. H., Mundon F. K., Russell P. K. Detection of additional antigenic determinants of hepatitis B antigen. J Immunol. 1972 Oct;109(4):842–848. [PubMed] [Google Scholar]
- Bukh J., Purcell R. H., Miller R. H. At least 12 genotypes of hepatitis C virus predicted by sequence analysis of the putative E1 gene of isolates collected worldwide. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8234–8238. doi: 10.1073/pnas.90.17.8234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukh J., Purcell R. H., Miller R. H. Importance of primer selection for the detection of hepatitis C virus RNA with the polymerase chain reaction assay. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):187–191. doi: 10.1073/pnas.89.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukh J., Purcell R. H., Miller R. H. Sequence analysis of the 5' noncoding region of hepatitis C virus. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4942–4946. doi: 10.1073/pnas.89.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha T. A., Beall E., Irvine B., Kolberg J., Chien D., Kuo G., Urdea M. S. At least five related, but distinct, hepatitis C viral genotypes exist. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7144–7148. doi: 10.1073/pnas.89.15.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha T. A., Kolberg J., Irvine B., Stempien M., Beall E., Yano M., Choo Q. L., Houghton M., Kuo G., Han J. H. Use of a signature nucleotide sequence of hepatitis C virus for detection of viral RNA in human serum and plasma. J Clin Microbiol. 1991 Nov;29(11):2528–2534. doi: 10.1128/jcm.29.11.2528-2534.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. W., McOmish F., Holmes E. C., Dow B., Peutherer J. F., Follett E., Yap P. L., Simmonds P. Analysis of a new hepatitis C virus type and its phylogenetic relationship to existing variants. J Gen Virol. 1992 May;73(Pt 5):1131–1141. doi: 10.1099/0022-1317-73-5-1131. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Richman K. H., Han J. H., Berger K., Lee C., Dong C., Gallegos C., Coit D., Medina-Selby R., Barr P. J. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couroucé-Pauty A. M., Plançon A., Soulier J. P. Distribution of HBsAg subtypes in the world. Vox Sang. 1983;44(4):197–211. doi: 10.1111/j.1423-0410.1983.tb01885.x. [DOI] [PubMed] [Google Scholar]
- Hino K., Sainokami S., Shimoda K., Iino S., Wang Y., Okamoto H., Miyakawa Y., Mayumi M. Genotypes and titers of hepatitis C virus for predicting response to interferon in patients with chronic hepatitis C. J Med Virol. 1994 Mar;42(3):299–305. doi: 10.1002/jmv.1890420318. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Houghton M., Weiner A., Han J., Kuo G., Choo Q. L. Molecular biology of the hepatitis C viruses: implications for diagnosis, development and control of viral disease. Hepatology. 1991 Aug;14(2):381–388. [PubMed] [Google Scholar]
- Kanai K., Kako M., Okamoto H. HCV genotypes in chronic hepatitis C and response to interferon. Lancet. 1992 Jun 20;339(8808):1543–1543. doi: 10.1016/0140-6736(92)91311-u. [DOI] [PubMed] [Google Scholar]
- Kleinman S., Alter H., Busch M., Holland P., Tegtmeier G., Nelles M., Lee S., Page E., Wilber J., Polito A. Increased detection of hepatitis C virus (HCV)-infected blood donors by a multiple-antigen HCV enzyme immunoassay. Transfusion. 1992 Nov-Dec;32(9):805–813. doi: 10.1046/j.1537-2995.1992.32993110750.x. [DOI] [PubMed] [Google Scholar]
- Kuo G., Choo Q. L., Alter H. J., Gitnick G. L., Redeker A. G., Purcell R. H., Miyamura T., Dienstag J. L., Alter M. J., Stevens C. E. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989 Apr 21;244(4902):362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Le Bouvier G. L. The heterogeneity of Australia antigen. J Infect Dis. 1971 Jun;123(6):671–675. doi: 10.1093/infdis/123.6.671. [DOI] [PubMed] [Google Scholar]
- McHutchison J. G., Person J. L., Govindarajan S., Valinluck B., Gore T., Lee S. R., Nelles M., Polito A., Chien D., DiNello R. Improved detection of hepatitis C virus antibodies in high-risk populations. Hepatology. 1992 Jan;15(1):19–25. doi: 10.1002/hep.1840150105. [DOI] [PubMed] [Google Scholar]
- McOmish F., Chan S. W., Dow B. C., Gillon J., Frame W. D., Crawford R. J., Yap P. L., Follett E. A., Simmonds P. Detection of three types of hepatitis C virus in blood donors: investigation of type-specific differences in serologic reactivity and rate of alanine aminotransferase abnormalities. Transfusion. 1993 Jan;33(1):7–13. doi: 10.1046/j.1537-2995.1993.33193142314.x. [DOI] [PubMed] [Google Scholar]
- Mori S., Kato N., Yagyu A., Tanaka T., Ikeda Y., Petchclai B., Chiewsilp P., Kurimura T., Shimotohno K. A new type of hepatitis C virus in patients in Thailand. Biochem Biophys Res Commun. 1992 Feb 28;183(1):334–342. doi: 10.1016/0006-291x(92)91648-a. [DOI] [PubMed] [Google Scholar]
- Nagayama R., Tsuda F., Okamoto H., Wang Y., Mitsui T., Tanaka T., Miyakawa Y., Mayumi M. Genotype dependence of hepatitis C virus antibodies detectable by the first-generation enzyme-linked immunosorbent assay with C100-3 protein. J Clin Invest. 1993 Sep;92(3):1529–1533. doi: 10.1172/JCI116731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Kojima M., Sakamoto M., Iizuka H., Hadiwandowo S., Suwignyo S., Miyakawa Y., Mayumi M. The entire nucleotide sequence and classification of a hepatitis C virus isolate of a novel genotype from an Indonesian patient with chronic liver disease. J Gen Virol. 1994 Mar;75(Pt 3):629–635. doi: 10.1099/0022-1317-75-3-629. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Kurai K., Okada S., Yamamoto K., Lizuka H., Tanaka T., Fukuda S., Tsuda F., Mishiro S. Full-length sequence of a hepatitis C virus genome having poor homology to reported isolates: comparative study of four distinct genotypes. Virology. 1992 May;188(1):331–341. doi: 10.1016/0042-6822(92)90762-e. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Okada S., Sugiyama Y., Kurai K., Iizuka H., Machida A., Miyakawa Y., Mayumi M. Nucleotide sequence of the genomic RNA of hepatitis C virus isolated from a human carrier: comparison with reported isolates for conserved and divergent regions. J Gen Virol. 1991 Nov;72(Pt 11):2697–2704. doi: 10.1099/0022-1317-72-11-2697. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Okada S., Sugiyama Y., Tanaka T., Sugai Y., Akahane Y., Machida A., Mishiro S., Yoshizawa H., Miyakawa Y. Detection of hepatitis C virus RNA by a two-stage polymerase chain reaction with two pairs of primers deduced from the 5'-noncoding region. Jpn J Exp Med. 1990 Aug;60(4):215–222. [PubMed] [Google Scholar]
- Okamoto H., Okada S., Sugiyama Y., Yotsumoto S., Tanaka T., Yoshizawa H., Tsuda F., Miyakawa Y., Mayumi M. The 5'-terminal sequence of the hepatitis C virus genome. Jpn J Exp Med. 1990 Jun;60(3):167–177. [PubMed] [Google Scholar]
- Okamoto H., Sugiyama Y., Okada S., Kurai K., Akahane Y., Sugai Y., Tanaka T., Sato K., Tsuda F., Miyakawa Y. Typing hepatitis C virus by polymerase chain reaction with type-specific primers: application to clinical surveys and tracing infectious sources. J Gen Virol. 1992 Mar;73(Pt 3):673–679. doi: 10.1099/0022-1317-73-3-673. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Tokita H., Sakamoto M., Horikita M., Kojima M., Iizuka H., Mishiro S. Characterization of the genomic sequence of type V (or 3a) hepatitis C virus isolates and PCR primers for specific detection. J Gen Virol. 1993 Nov;74(Pt 11):2385–2390. doi: 10.1099/0022-1317-74-11-2385. [DOI] [PubMed] [Google Scholar]
- Pozzato G., Moretti M., Franzin F., Crocè L. S., Tiribelli C., Masayu T., Kaneko S., Unoura M., Kobayashi K. Severity of liver disease with different hepatitis C viral clones. Lancet. 1991 Aug 24;338(8765):509–509. doi: 10.1016/0140-6736(91)90578-d. [DOI] [PubMed] [Google Scholar]
- Simmonds P., McOmish F., Yap P. L., Chan S. W., Lin C. K., Dusheiko G., Saeed A. A., Holmes E. C. Sequence variability in the 5' non-coding region of hepatitis C virus: identification of a new virus type and restrictions on sequence diversity. J Gen Virol. 1993 Apr;74(Pt 4):661–668. doi: 10.1099/0022-1317-74-4-661. [DOI] [PubMed] [Google Scholar]
- Stuyver L., Rossau R., Wyseur A., Duhamel M., Vanderborght B., Van Heuverswyn H., Maertens G. Typing of hepatitis C virus isolates and characterization of new subtypes using a line probe assay. J Gen Virol. 1993 Jun;74(Pt 6):1093–1102. doi: 10.1099/0022-1317-74-6-1093. [DOI] [PubMed] [Google Scholar]
- Takada N., Takase S., Enomoto N., Takada A., Date T. Clinical backgrounds of the patients having different types of hepatitis C virus genomes. J Hepatol. 1992 Jan;14(1):35–40. doi: 10.1016/0168-8278(92)90128-c. [DOI] [PubMed] [Google Scholar]
- Takamizawa A., Mori C., Fuke I., Manabe S., Murakami S., Fujita J., Onishi E., Andoh T., Yoshida I., Okayama H. Structure and organization of the hepatitis C virus genome isolated from human carriers. J Virol. 1991 Mar;65(3):1105–1113. doi: 10.1128/jvi.65.3.1105-1113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe J., Matsumoto C., Fujimura K., Shimada T., Yoshizawa H., Okamoto H., Iizuka H., Tango T., Ikeda H., Endo N. Predictive value of screening tests for persistent hepatitis C virus infection evidenced by viraemia. Japanese experience. Vox Sang. 1993;65(3):199–203. doi: 10.1111/j.1423-0410.1993.tb02148.x. [DOI] [PubMed] [Google Scholar]
- Weiner A. J., Kuo G., Bradley D. W., Bonino F., Saracco G., Lee C., Rosenblatt J., Choo Q. L., Houghton M. Detection of hepatitis C viral sequences in non-A, non-B hepatitis. Lancet. 1990 Jan 6;335(8680):1–3. doi: 10.1016/0140-6736(90)90134-q. [DOI] [PubMed] [Google Scholar]
- Yoshioka K., Kakumu S., Wakita T., Ishikawa T., Itoh Y., Takayanagi M., Higashi Y., Shibata M., Morishima T. Detection of hepatitis C virus by polymerase chain reaction and response to interferon-alpha therapy: relationship to genotypes of hepatitis C virus. Hepatology. 1992 Aug;16(2):293–299. doi: 10.1002/hep.1840160203. [DOI] [PubMed] [Google Scholar]


