Abstract
Recovery from end-stage organ failure presents a challenge for the medical community, considering the limitations of extracorporeal assist devices and the shortage of donors when organ replacement is needed. There is a need for new methods to promote recovery from organ failure and regenerative medicine is an option that should be considered. Recent progress in the field of tissue engineering has opened avenues for potential clinical applications, including the use of microfluidic devices for diagnostic purposes, and bioreactors or cell/tissue-based therapies for transplantation. Early attempts to engineer tissues produced thin, planar constructs; however, recent approaches using synthetic scaffolds and decellularized tissue have achieved a more complex level of tissue organization in organs such as the urinary bladder and trachea, with some success in clinical trials. In this context, the concept of decellularization technology has been applied to produce whole organ-derived scaffolds by removing cellular content while retaining all the necessary vascular and structural cues of the native organ. In this review, we focus on organ decellularization as a new regenerative medicine approach for whole organs, which may be applied in the field of digestive surgery.
Keywords: Organ transplantation alternatives, Tissue engineering, Organ engineering, Cell transplantation, Stem cells
Introduction
Organ transplantation is the definitive therapy for end-stage failure of digestive organs, including acute and chronic liver failure, end-stage diabetes, intestinal failure or short bowel syndrome. In fact, its success has now become one of its major obstacles, because of donor organ shortage [1-3]. The number of patients waiting for a liver transplant (>16000) or a pancreas-kidney transplant (>2000) far surpasses the supply of organs from standard criteria brain-dead donors, being 6000–7000 liver donors and 1000–1500 pancreas donors annually in the United States (OPTN/SRTR annual report); yet those who receive an organ transplant have far better 1-, 3-, and 5-year graft survival than patients who received a transplant just 20 years ago.
Tissue engineering is defined as an interdisciplinary field combining the technologies of biomaterials, bioengineering, and cell biology to produce tissues for therapeutic purposes as defined in 1972 by Fung [4] and popularized in 1993 by Langer and Vacanti [5]. It has been challenging to mimic the native 3D structure of human digestive organs to reproduce the functionality and metabolism necessary to make a clinical impact; for example, the peristaltic movement of mucous, acid secretion, and the complex 3D structure of the organ parenchyma. This requires the creation of micro-structures as well as large vessels or ducts for external secretions, including bile or pancreatic exocrine factors. The first attempts to isolate and decellularize organ-specific ECM were reported in the 1970s and 1980s [6-9]. More recent reports introduced this methodology into the field of digestive artificial organ construction using absorbable or non-absorbable scaffolds to accelerate the regeneration of epithelium, correct the defects of tubular organs [10-12], and construct bioreactors for the liver [13-15] and pancreas [16, 17], partly to support resident tissue regeneration. Although these new approaches yielded some successes in animal models and preclinical attempts, none has demonstrated long-term efficacy[18]. Thus, new methodologies for constructing organ substitutes are needed urgently.
There have been many recent advances in the field of stem cell biology. Stem cells can now be made to differentiate into many kinds of somatic cells, including endoderm-affiliated cells such as intestinal cells [19, 20], hepatocytes [21-26] or pancreatic β-cells [27-29]. These technologies opened the door for the application of regenerative therapy to the digestive organs, and yet acceptable therapeutic efficacy has not yet been achieved. Thus far, the experience has been that after implantation, cells gradually lose their function in vivo and have never shown long-term viability. Several studies have shown that the microenvironment plays a fundamental role, not only in cell maintenance or homeostasis but also for determining stem cell fate [30]. The extracellular matrix (ECM) and its 3D structure are both essential components of this microenvironment and have been exploited for the maintenance of somatic cells, cancer cells, or stem cells in vitro using tissue engineering approaches, demonstrating supportive activity in cell cultures [31, 32]. Therefore, ECM will be highly beneficial to combine stem cell biology with ECM technology for the further improvement of regenerative therapy.
Whole-organ decellularization has evolved as a tissue engineering approach based on a decade of basic tissue decellularization studies for thin, planar tissues, primarily dermis [33]. The first scientific report demonstrating perfusion decellularization technology in vital organs was demonstrated by Ott et al. [34]. Using this technology, whole organs including heart [34, 35], liver [36, 37] and lung [38, 39] have been decellularized following the perfusion over days of a detergent through a vascular access route. This preserves vessel structure, and more importantly, the ECM components such as collagen, laminin, or fibronectin as well as glycosaminoglycans (GAGs) [40]. This review summarizes the highlights of emerging strategies to develop organ substitutes. Whole-organ tissue engineering and cell therapy methodology are new technologies in various stages of development, with future implications for the surgical care and management of end-stage organ disease.
Tissue and organ decellularization
Decellularized organ scaffolds and the extracellular matrix
The extracellular matrix (ECM) plays a crucial role in the maintenance of tissue-specific function by controlling the cell microenvironment [41, 42]. The ECM represents the secreted products of resident cells dynamically responsive to different conditions of the local environment, and has been shown to provide fundamental signals for cell migration, proliferation, and differentiation [32, 43, 44]. Therefore, biological scaffolds composed of natural ECM have attracted much attention in relation to their therapeutic potential.
During the last decade, researchers have explored various methods for tissue decellularization, whereby different detergents are used to wash out all cellular contents from the resident tissue, with the aim of maintaining native ECM-contained cues essential for the cells [33]. These methods include a number of different agents such as acids and bases [45], hypotonic and hypertonic solutions [46], detergents [36, 38], alcohols [47], enzymes[48] and non-enzymatic agents [49] containing ethylenediaminetetraacetic acid (EDTA) or ethylene glycol tetraacetic acid (EGTA); as well as physical agents such as temperature [50], pressure, [51] and electroporation [52]. It is difficult to find the most effective agent for decellularization of each tissue and organ, since it depends on many factors, including the tissue’s cellularity (e.g., liver vs. tendon), density (e.g., dermis vs. adipose tissue), lipid content (e.g., brain vs. urinary bladder), and thickness (e.g., dermis vs. pericardium) [40].
Effects of the extracellular matrix on cellular fate
The fact that current regenerative medicine approaches, including cell transplantation and bioreactors, have limited clinical benefit [15, 53], suggests that even if the technology for promoting cell maturation and function from stem/progenitor cells is available, there are many hurdles to overcome to achieve clinical success [22, 27]. These disappointing results might be attributable to the lack of natural microenvironment for the cells during ex vivo culture. Therefore, native ECM scaffolds could offer a feasible shortcut from the stage of cell expansion to organ maturation by providing a microenvironment essential for cell function and tissue assembly. The cells in question, including stem/progenitor cells, interact directly with the ECM in its 3D form, as well as indirectly via cytokines and growth factors, which might be partially restored in the native ECM scaffold remaining after this tissue decellularization method is initiated. Indeed, hyaluronic acid, one of the major components of ECM, has been widely used to culture and propagate stem cells, since this component of ECM exists during early embryogenesis [54, 55]. Furthermore, the basement membrane, the first ECM produced by the developing embryo, was quickly identified as an important factor for modulating stem cell behavior, and has since been successfully employed in numerous methods as a substratum in vitro and as a bioactive support in vivo [56].
Decellularized scaffold as a platform for stem cell biology
The implementation of tissue engineering technologies for repairing or replacing digestive organs has been investigated extensively. Most approaches require a certain number of cells to naturally populate the scaffold and integrate into the surrounding tissue. The best cell source might be autologous material or cells from the native organ of an allogeneic donor because of their affinity for the resident tissue and low immunogenicity. However, the availability of any such cells would be extremely limited because too few autologous somatic/progenitor cells can be isolated and the opportunities to procure allogeneic cells from limited donor sources are rare. Recently, human ES cells [57], and even more recently, iPS cells [58], were isolated or generated and found to have a pluripotent ability to develop into a wide range of cell types [59]. This ability has drawn attention to ES/iPS cells as a novel source of cell populations for new therapeutic strategies in tissue engineering technology. There has been some success in inducing ES/iPS cells to differentiate into particular types of cells in endoderm lineages. Interestingly, it has been discovered that native matrix is essential to control stem cells [30, 31, 47] and guide their differentiation, maintenance [60], and maturation [61]. The first reports demonstrated that decellularized tissue matrices were valuable for answering basic questions of how stem cells contribute to homeostasis and repair. The unique approach of tissue decellularization combined with stem cell technology will represent a breakthrough in solving the current problems of organ and cell regenerative therapy such as donor shortage or life-long immunosuppression, especially in digestive surgery. Although this was reported by Macchiarini et al. [62] in a study using cadaveric donor trachea with recipient mesenchymal stem cells, there is no report describing the clinical application of a model for solid organ reconstruction, as it is still premature to generate functional organs requiring the seeding of multiple cell lineages. This contrasts with the relatively simple construction of skin, valves, and trachea. Another major caveat is that iPS long-term cell culture stability has yet to be investigated, although it is known that human ES cells grown in long-term culture show genetic instability. Indeed, subtle differences in the timing of onset and level of expression of different genes were found in these pluripotent cells [63]. Rapidly accumulating data suggests that the reprogramming process is often accompanied not only by genetic abnormalities, but also by epigenetic alterations, which are expected to increase tumorigenicity [64, 65]. Furthermore, the long-term clinical benefits of stem cell therapy have not been documented [66].
Immunogenicity of decellularized scaffolds
One of the earliest studies reporting a therapeutic benefit of ECM scaffolds manufactured by the tissue decellularization process concerned arterial grafts [67]. To obtain suitable long-term arterial replacements in vascular surgery, cellular components of the arterial wall were removed by detergent treatment, resulting in the production of a matrix tube that could be engrafted. Importantly, the findings of investigating the ECM in these arterial xenografts indicated that both the extracellular matrix and the cells contributed to graft immunogenicity [68]. A second study found that decellularized grafts elicited significantly lower levels of class I and class II HLA antibody formation than standard cryopreserved allografts [69]. Additional experiments revealed low immunogenicity of these matrices derived not only from animal tissues, but also cadaver veins used for the decellularization process. Such vein allografts thus retained little antigenic material. Subsequently, they were used for hemodialysis access in patients with renal failure and demonstrated no capacity for allo-sensitization [70]. The findings of these experiments suggested that the immunogenicity of these tissues was reduced, but not completely eliminated by the process of decellularization. This technique could, therefore, provide clinically relevant grafts with the necessary 3D structure and without cellular rejection. In fact, this methodology was applied early on in the clinical setting, especially for dermal repair. One of the first reports documented that decellularized porcine skin scaffolds significantly reduced wound contraction and improved the cosmetic outcome of full-thickness wounds in a porcine model [71]. After preclinical investigations in several animal models, LifeCell Corp., Branchburg, NJ, USA, developed AlloDerm, a human-derived decellularized allogeneic dermis, which has been used successfully to promote wound repair after burns [72], inguinal hernia surgery [73], and more adventurously in abdominal, pelvic and chest wall reconstruction [74, 75]. However, the imperfect decellularization of biologic scaffold materials can result in a macrophage response that can cause immuno-reactions in the host body. Therefore, effective decellularization remains an important component in the production of ECM-based scaffolds for therapeutic applications [76].
Using decellularized scaffolds as a platform for cell repopulation
The next step in developing therapeutic strategies of this nature is to recellularize the acellular ECM scaffold, to generate engineered cellular tissue ex vivo for further application in replacement therapy. An impressive example of this concept was carried out by Kaushal et al. [77], who seeded endothelial progenitor cells in decellularized blood vessels ex vivo and demonstrated long-term patency of the interposed graft. After this pioneering work, studies demonstrated that decellularized native matrix seeded with autologous cells was feasible for several different organs. Liu et al. [78] reported that decellularized bladder submucosa seeded with bladder cells could be maintained in cell culture, and that it promoted cell–matrix penetration in vitro and cell growth in vivo. More interestingly, Macchiarini et al. created a tissue-engineered airway which was recellularized with the patient’s autologous epithelial and progenitor cells. This was used clinically in a patient suffering from bronchial stenosis caused by tuberculosis, and allowed for normal functioning, free from the risks of rejection, for at least 1 year [62]. In juxtaposition to these encouraging results, there have also been reports on the difficulties and potential complications of this approach in clinical settings. When commercially available decellularized heart valves (SynerGraft, Cryolife Inc., Kennesaw, GA, USA) were implanted as xenografts, several patients died following rupture of the implanted grafts [79]. Another report described that after the initial implantation, the tissue-engineered heart valve had to be replaced with a homograft because of stenosis [80]. These reports suggest that the decellularized scaffold, when used without recellularization by cells to support its function, carried a risk of graft failure. Therefore, accumulating literature may indicate that in some cases the principle of tissue decellularization might require subsequent recellularization processes before becoming a feasible routine platform for regenerative therapy.
Organ engineering for digestive tract regeneration and replacement
The work reported by Isch et al. [81] is a great example of the application of this new technology, in the field of digestive surgery. Isch et al. [81] used decellularized human skin for patch esophagoplasty in large animals, and 3 months of follow-up revealed successful healing of epithelial injury of the esophagus and stimulated neovascularization. Subsequently, xenogeneic intestinal submucosa was successfully applied as a decellularized scaffold to replace pulmonary valves in a large animal model, resulting in symmetrical leaflet movement with good mobility [82]. Gabouev et al. [83] also used recellularized intestinal scaffolding to construct a tissue-engineered bladder wall. They reseeded syngeneic urothelial cells and bladder smooth muscle cells on decellularized porcine intestinal submucosa, maintained in culture for 6 weeks, and retained viable and functional cells in the scaffold. After these positive experimental results, Badylak et al. [84] used decellularized xenogeneic urinary bladder as a scaffold reseeded with autologous muscle cells, achieving functional reconstruction of the esophageal wall clinically. Moreover, Mertching et al. [85] investigated a transplantable intestine, which had been vascularized with human endothelial cells. These interesting reports document that using decellularized scaffolds reseeded with cells from an appropriate source to repair or replace the digestive tract is feasible. Based on these encouraging findings in hollow organs, investigators have also wondered how to develop tissue-engineered grafts for parenchymal organs. Lin et al. [86] reported the use of organ-specific ECM. Although they could maintain syngeneic primary hepatocytes for up to 45 days in a matrix derived from decellularized liver tissue from pigs, this was far from the generation of a clinically relevant engineered parenchymal organ. Subsequently, Linke et al. [87] reported the production of engineered liver-like tissue based on decellularized intestinal scaffolds by co-culturing porcine hepatocytes with endothelial cells. Interestingly, this reinforced the importance of endothelial cell support to preserve hepatic function in the engineered liver organoid, as reported in previous studies [88]. However, it seems difficult to mimic the liver-specific structures, such as liver sinusoid, using intestine-derived matrix. Indeed, the interactions between hepatocytes and other liver non-parenchymal cells lead to liver morphogenesis. Several studies have highlighted the importance of the function of hepatocytes when supported by non-parenchymal liver cells [89, 90]. The ability of liver non-parenchymal cells, including endothelial, stellate, and cholangiocytes, to support liver sinusoid formation to develop hepatic organoid in the matrix scaffold was demonstrated recently [91, 92].
The pancreas is a vital parenchymal organ strongly linked to diabetes causation. The methodology of tissue decellularization was similarly attempted by De Carlo et al. [93] for the maintenance of pancreatic islet cells. That study revealed that native matrix from the pancreas is important for the tissue-specific cell survival and function of the islet cells. However, it was also demonstrated that the ECM from native tissue plays a crucial role in the maintenance of islet cells by controlling the microenvironment.
Although previous investigators have documented the efficacy of ECM and constructs using decellularized tissue for cell maintenance in pre-clinical attempts, these techniques are not applicable for parenchymal organs that require vascular reconstruction, to provide nutrition and oxygen to the implanted cells. To preserve all the necessary cues for engineering a parenchymal organ graft that is clinically feasible for replacement therapy, the first excellent work by Ott et al. [34] used decellularized whole rodent heart, resulting in macroscopic ECM contractions and even pumping action following recellularization with primary cardiac cells. This work paved the way for the reconstruction of transplantable whole-organ scaffolds reseeded with functional cells. Subsequently, our group applied similar methodology to the liver and confirmed that it was feasible to create constructs for transplantation as partial liver grafts by vascular reconstruction in vivo [36, 94]. Similar work was done by Baptista et al. [95] using liver scaffolds, and recent reports by described a similar transplantable lung scaffold approach in rodents [38, 39]. It is known that local environmental factors induce hepatocyte homing, differentiation, and proliferation, and there is evidence that ES/iPS cells may differentiate toward mature hepatocytes when transferred into an injured liver [96]. It is reasonable to expect a similar beneficial response for engineering liver grafts. Thus, new techniques for decellularization of the liver matrix present great potential as the scaffold for hepatocyte maturation and transplantation. This process may be further manipulated by the sequential delivery of factors involved in the initiation and maturation of ES/iPS cells to liver cells, allowing temporal and spatial control over differentiation.
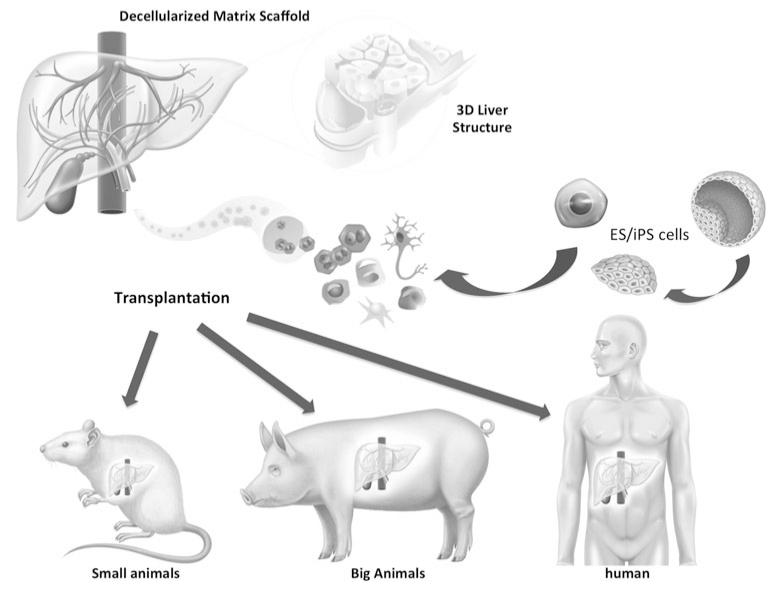
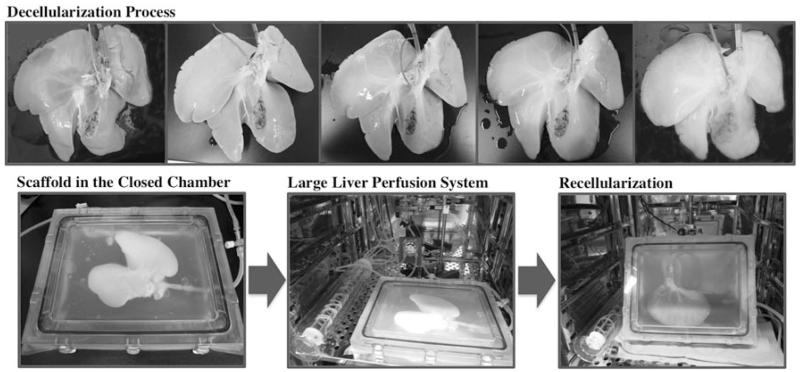
This technology of tissue decellularization might be applicable for any digestive organ. The first step to generate functional tissue which can be directly transplanted into humans requires the construction of perfusion-decellularized native ECM scaffolds that match human organs in size and structure (Fig. 1). Recently, our group demonstrated that this decellularization technology could be applied in a large animal model to generate a transplantable engineered liver graft (Fig. 2). However, it requires improvement and customization with regard to size, category, species, and cell sources. More importantly, the resulting scaffolds need to be reproducible, sterile, and preservable for future procedures.
Fig. 1.
Development of the engineering liver graft from small animals to human
Fig. 2.
Representative images of porcine livers during decellularization process and the closed perfusion system
Future prospects
We have reviewed the latest technology for whole-organ decellularization, developed in the field of digestive surgery, illustrating its necessity and efficacy in regenerative therapy applications. Compared with the successful clinical application of this technology in skin or vessels, which was established early on, many functional and structural issues need to be overcome before it can be used for the digestive organs. Thus far, this method has only achieved short-term functionality in vivo and then only in rodent models. Nevertheless, previous decades of work in the field of tissue engineering and current research in stem cells are producing encouraging results, which 1 day might solve the problem of organ assembly on demand. Scaling up this technology for clinical application is fundamentally feasible, and like many others, we are working toward this end.
Acknowledgments
We thank Keio University for a special Grant-in-Aid for Innovative Collaborative Research Projects, a grant from the Japan Society for the Promotion of Science KAKENHI (23689059), Takeda Science Foundation to H.Y. and the National Institutes of Health (DK083556) to A.S.-G.
Abbreviations
- ES
Embryonic stem
- iPS
Induced pluripotent stem
- ECM
Extracellular matrix
- GAGs
Glycosaminoglycans
- EDTA
Ethylenediaminetetraacetic acid
- EGTA
Ethylene glycol tetraacetic acid
- BME
Basement membrane extract
- HLA
Human leukocyte antigen
- OPTN
Organ procurement and transplantation network
- SRTR
Scientific registry of transplant recipients
Contributor Information
Hiroshi Yagi, Department of Surgery, School of Medicine, Keio University, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan.
Alejandro Soto-Gutierrez, Department of Pathology, Center for Innovative Regenerative Therapies, Department of Surgery, Transplantation Section, Children’s Hospital of Pittsburgh and the Starzl Transplantation Institute, University of Pittsburgh, Pittsburgh, PA 15261, USA.
Yuko Kitagawa, Department of Surgery, School of Medicine, Keio University, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan.
References
- 1.Lee SG, Hwang S, Kim KH, et al. Toward 300 liver transplants a year. Surg Today. 2009;39:367–73. doi: 10.1007/s00595-008-3917-1. [DOI] [PubMed] [Google Scholar]
- 2.Garg M, Jones RM, Vaughan RB, et al. Intestinal transplantation: current status and future directions. J Gastroenterol Hepatol. 2011;26:1221–8. doi: 10.1111/j.1440-1746.2011.06783.x. [DOI] [PubMed] [Google Scholar]
- 3.Gruessner AC, Sutherland DE, Gruessner RW. Long-term outcome after pancreas transplantation. Curr Opin Organ Transplant. 2012;17:100–5. doi: 10.1097/MOT.0b013e32834ee700. [DOI] [PubMed] [Google Scholar]
- 4.Fung YC, Sobin SS. Elasticity of the pulmonary alveolar sheet. Circ Res. 1972;30:451–69. doi: 10.1161/01.res.30.4.451. [DOI] [PubMed] [Google Scholar]
- 5.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 6.Meezan E, Hjelle JT, Brendel K, et al. A simple, versatile, nondisruptive method for the isolation of morphologically and chemically pure basement membranes from several tissues. Life Sci. 1975;17:1721–32. doi: 10.1016/0024-3205(75)90119-8. [DOI] [PubMed] [Google Scholar]
- 7.Rojkind M, Gatmaitan Z, Mackensen S, et al. Connective tissue biomatrix: its isolation and utilization for long-term cultures of normal rat hepatocytes. J Cell Biol. 1980;87:255–63. doi: 10.1083/jcb.87.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 9.Lwebuga-Mukasa JS, Ingbar DH, Madri JA. Repopulation of a human alveolar matrix by adult rat type II pneumocytes in vitro. A novel system for type II pneumocyte culture. Exp Cell Res. 1986;162:423–35. doi: 10.1016/0014-4827(86)90347-2. [DOI] [PubMed] [Google Scholar]
- 10.Kim SS, Kaihara S, Benvenuto MS, et al. Regenerative signals for intestinal epithelial organoid units transplanted on biodegradable polymer scaffolds for tissue engineering of small intestine. Transplantation. 1999;67:227–33. doi: 10.1097/00007890-199901270-00007. [DOI] [PubMed] [Google Scholar]
- 11.Nakase Y, Hagiwara A, Nakamura T, et al. Tissue engineering of small intestinal tissue using collagen sponge scaffolds seeded with smooth muscle cells. Tissue Eng. 2006;12:403–12. doi: 10.1089/ten.2006.12.403. [DOI] [PubMed] [Google Scholar]
- 12.Javaid Ur R, Waseem T. Intestinal tissue engineering: where do we stand? Surg Today. 2008;38:484–6. doi: 10.1007/s00595-007-3671-9. [DOI] [PubMed] [Google Scholar]
- 13.Demetriou AA, Whiting JF, Feldman D, et al. Replacement of liver function in rats by transplantation of microcarrier-attached hepatocytes. Science. 1986;233:1190–2. doi: 10.1126/science.2426782. [DOI] [PubMed] [Google Scholar]
- 14.Rozga J, Williams F, Ro MS, et al. Development of a bioartificial liver: properties and function of a hollow-fiber module inoculated with liver cells. Hepatology. 1993;17:258–65. [PubMed] [Google Scholar]
- 15.Stutchfield BM, Simpson K, Wigmore SJ. Systematic review and meta-analysis of survival following extracorporeal liver support. Br J Surg. 2011;98:623–31. doi: 10.1002/bjs.7418. [DOI] [PubMed] [Google Scholar]
- 16.Reach G, Jaffrin MY, Desjeux JF. A U-shaped bioartificial pancreas with rapid glucose-insulin kinetics. In vitro evaluation and kinetic modelling. Diabetes. 1984;33:752–61. doi: 10.2337/diab.33.8.752. [DOI] [PubMed] [Google Scholar]
- 17.Lombardi CP, Urso A, Catapano G, et al. Membrane bioreactors as hybrid artificial pancreas: experimental evaluation. Int J Artif Organs. 1992;15:126–30. [PubMed] [Google Scholar]
- 18.Park JK, Lee DH. Bioartificial liver systems: current status and future perspective. J Biosci Bioeng. 2005;99:311–9. doi: 10.1263/jbb.99.311. [DOI] [PubMed] [Google Scholar]
- 19.Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–5. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 20.Spence JR, Mayhew CN, Rankin SA, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–9. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hay DC, Fletcher J, Payne C, et al. Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proc Natl Acad Sci USA. 2008;105:12301–6. doi: 10.1073/pnas.0806522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basma H, Soto-Gutierrez A, Yannam GR, et al. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009;136:990–9. doi: 10.1053/j.gastro.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Si-Tayeb K, Noto FK, Nagaoka M, et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51:297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kajiwara M, Aoi T, Okita K, et al. Donor-dependent variations in hepatic differentiation from human-induced pluripotent stem cells. Proc Natl Acad Sci USA. 2012;109:12538–43. doi: 10.1073/pnas.1209979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woo DH, Kim SK, Lim HJ, et al. Direct and indirect contribution of human embryonic stem cell-derived hepatocyte-like cells to liver repair in mice. Gastroenterology. 2012;142:602–11. doi: 10.1053/j.gastro.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 26.Chen YF, Tseng CY, Wang HW, et al. Rapid generation of mature hepatocyte-like cells from human induced pluripotent stem cells by an efficient three-step protocol. Hepatology. 2012;55:1193–203. doi: 10.1002/hep.24790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonner-Weir S, Weir GC. New sources of pancreatic beta-cells. Nat Biotechnol. 2005;23:857–61. doi: 10.1038/nbt1115. [DOI] [PubMed] [Google Scholar]
- 28.D’Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 29.Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–52. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 30.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–7. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng HW, Tsui YK, Cheung KM, et al. Decellularization of chondrocyte-encapsulated collagen microspheres: a three-dimensional model to study the effects of acellular matrix on stem cell fate. Tissue Eng Part C Methods. 2009;15:697–706. doi: 10.1089/ten.TEC.2008.0635. [DOI] [PubMed] [Google Scholar]
- 32.Barkan D, Green JE, Chambers AF. Extracellular matrix: a gatekeeper in the transition from dormancy to metastatic growth. Eur J Cancer. 2010;46:1181–8. doi: 10.1016/j.ejca.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–93. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 34.Ott HC, Matthiesen TS, Goh SK, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 35.Wainwright JM, Czajka CA, Patel UB, et al. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng Part C Methods. 2010;16:525–32. doi: 10.1089/ten.tec.2009.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uygun BE, Soto-Gutierrez A, Yagi H, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–20. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baptista PM, Siddiqui MM, Lozier G, et al. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53:604–17. doi: 10.1002/hep.24067. [DOI] [PubMed] [Google Scholar]
- 38.Ott HC, Clippinger B, Conrad C, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–33. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 39.Price AP, England KA, Matson AM, et al. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng Part A. 2010;16:2581–91. doi: 10.1089/ten.tea.2009.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–43. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47:1394–400. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 42.Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341:126–40. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bissell MJ, Aggeler J. Dynamic reciprocity: how do extracellular matrix and hormones direct gene expression? Prog Clin Biol Res. 1987;249:251–62. [PubMed] [Google Scholar]
- 44.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cartmell JS, Dunn MG. Effect of chemical treatments on tendon cellularity and mechanical properties. J Biomed Mater Res. 2000;49:134–40. doi: 10.1002/(sici)1097-4636(200001)49:1<134::aid-jbm17>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 46.Yang B, Zhang Y, Zhou L, et al. Development of a porcine bladder acellular matrix with well-preserved extracellular bioactive factors for tissue engineering. Tissue Eng Part C Methods. 2010;16:1201–11. doi: 10.1089/ten.TEC.2009.0311. [DOI] [PubMed] [Google Scholar]
- 47.Flynn LE. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials. 2010;31:4715–24. doi: 10.1016/j.biomaterials.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 48.Cortiella J, Niles J, Cantu A, et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A. 2010;16:2565–80. doi: 10.1089/ten.tea.2009.0730. [DOI] [PubMed] [Google Scholar]
- 49.Gui L, Chan SA, Breuer CK, et al. Novel utilization of serum in tissue decellularization. Tissue Eng Part C Methods. 2010;16:173–84. doi: 10.1089/ten.tec.2009.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Remlinger NT, Czajka CA, Juhas ME, et al. Hydrated xenogeneic decellularized tracheal matrix as a scaffold for tracheal reconstruction. Biomaterials. 2010;31:3520–6. doi: 10.1016/j.biomaterials.2010.01.067. [DOI] [PubMed] [Google Scholar]
- 51.Hashimoto Y, Funamoto S, Sasaki S, et al. Preparation and characterization of decellularized cornea using high-hydrostatic pressurization for corneal tissue engineering. Biomaterials. 2010;31:3941–8. doi: 10.1016/j.biomaterials.2010.01.122. [DOI] [PubMed] [Google Scholar]
- 52.Phillips M, Maor E, Rubinsky B. Nonthermal irreversible electroporation for tissue decellularization. J Biomech Eng. 2010;132:091003. doi: 10.1115/1.4001882. [DOI] [PubMed] [Google Scholar]
- 53.Fox IJ, Chowdhury JR, Kaufman SS, et al. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338:1422–6. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- 54.Toole BP. Hyaluronan in morphogenesis. Semin Cell Dev Biol. 2001;12:79–87. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- 55.Matrosova VY, Orlovskaya IA, Serobyan N, et al. Hyaluronic acid facilitates the recovery of hematopoiesis following 5-fluorouracil administration. Stem cells. 2004;22:544–55. doi: 10.1634/stemcells.22-4-544. [DOI] [PubMed] [Google Scholar]
- 56.Arnaoutova I, George J, Kleinman HK, et al. Basement membrane matrix (BME) has multiple uses with stem cells. Stem cell Rev. 2012;8:163–9. doi: 10.1007/s12015-011-9278-y. [DOI] [PubMed] [Google Scholar]
- 57.Guhr A, Kurtz A, Friedgen K, et al. Current state of human embryonic stem cell research: an overview of cell lines and their use in experimental work. Stem cells. 2006;24:2187–91. doi: 10.1634/stemcells.2006-0053. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 59.Pournasr B, Khaloughi K, Salekdeh GH, et al. Concise review: alchemy of biology: generating desired cell types from abundant and accessible cells. Stem cells. 2011;29:1933–41. doi: 10.1002/stem.760. [DOI] [PubMed] [Google Scholar]
- 60.Sneddon JB, Borowiak M, Melton DA. Self-renewal of embryonic-stem-cell-derived progenitors by organ-matched mesenchyme. Nature. 2012 doi: 10.1038/nature11463. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coulombel L, Vuillet MH, Leroy C, et al. Lineage- and stage-specific adhesion of human hematopoietic progenitor cells to extracellular matrices from marrow fibroblasts. Blood. 1988;71:329–34. [PubMed] [Google Scholar]
- 62.Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–30. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 63.Adewumi O, Aflatoonian B, Ahrlund-Richter L, et al. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol. 2007;25:803–16. doi: 10.1038/nbt1318. [DOI] [PubMed] [Google Scholar]
- 64.Wu SM, Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol. 2011;13:497–505. doi: 10.1038/ncb0511-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11:268–77. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 66.Ilic D, Polak J. Stem cell based therapy-where are we going? Lancet. 2012;379:877–8. doi: 10.1016/S0140-6736(12)60155-X. [DOI] [PubMed] [Google Scholar]
- 67.Allaire E, Guettier C, Bruneval P, et al. Cell-free arterial grafts: morphologic characteristics of aortic isografts, allografts, and xenografts in rats. J Vasc Surg. 1994;19:446–56. doi: 10.1016/s0741-5214(94)70071-0. [DOI] [PubMed] [Google Scholar]
- 68.Allaire E, Bruneval P, Mandet C, et al. The immunogenicity of the extracellular matrix in arterial xenografts. Surgery. 1997;122:73–81. doi: 10.1016/s0039-6060(97)90267-1. [DOI] [PubMed] [Google Scholar]
- 69.Hawkins JA, Hillman ND, Lambert LM, et al. Immunogenicity of decellularized cryopreserved allografts in pediatric cardiac surgery: comparison with standard cryopreserved allografts. J Thorac Cardiovasc Surg. 2003;126:247–52. doi: 10.1016/s0022-5223(03)00116-8. discussion 252-3. [DOI] [PubMed] [Google Scholar]
- 70.Madden R, Lipkowitz G, Benedetto B, et al. Decellularized cadaver vein allografts used for hemodialysis access do not cause allosensitization or preclude kidney transplantation. Am J Kidney Dis Off J Natl Kidney Found. 2002;40:1240–3. doi: 10.1053/ajkd.2002.36892. [DOI] [PubMed] [Google Scholar]
- 71.Reagan BJ, Madden MR, Huo J, et al. Analysis of cellular and decellular allogeneic dermal grafts for the treatment of full-thickness wounds in a porcine model. J Trauma. 1997;43:458–66. doi: 10.1097/00005373-199709000-00012. [DOI] [PubMed] [Google Scholar]
- 72.Barret JP, Dziewulski P, McCauley RL, et al. Dural reconstruction of a class IV calvarial burn with decellularized human dermis. Burns J Int Soc Burn Inj. 1999;25:459–62. doi: 10.1016/s0305-4179(99)00018-2. [DOI] [PubMed] [Google Scholar]
- 73.Albo D, Awad SS, Berger DH, et al. Decellularized human cadaveric dermis provides a safe alternative for primary inguinal hernia repair in contaminated surgical fields. Am J Surg. 2006;192:e12–7. doi: 10.1016/j.amjsurg.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 74.Butler CE, Prieto VG. Reduction of adhesions with composite AlloDerm/polypropylene mesh implants for abdominal wall reconstruction. Plast Reconstr Surg. 2004;114:464–73. doi: 10.1097/01.prs.0000132670.81794.7e. [DOI] [PubMed] [Google Scholar]
- 75.Butler CE, Langstein HN, Kronowitz SJ. Pelvic, abdominal, and chest wall reconstruction with AlloDerm in patients at increased risk for mesh-related complications. Plastic Reconstr Surg. 2005;116:1263–75. doi: 10.1097/01.prs.0000181692.71901.bd. discussion 1276-7. [DOI] [PubMed] [Google Scholar]
- 76.Keane TJ, Londono R, Turner NJ, et al. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials. 2012;33:1771–81. doi: 10.1016/j.biomaterials.2011.10.054. [DOI] [PubMed] [Google Scholar]
- 77.Kaushal S, Amiel GE, Guleserian KJ, et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035–40. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Y, Bharadwaj S, Lee SJ, et al. Optimization of a natural collagen scaffold to aid cell-matrix penetration for urologic tissue engineering. Biomaterials. 2009;30:3865–73. doi: 10.1016/j.biomaterials.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 79.Simon P, Kasimir MT, Seebacher G, et al. Early failure of the tissue engineered porcine heart valve SYNERGRAFT in pediatric patients. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2003;23:1002–6. doi: 10.1016/s1010-7940(03)00094-0. discussion 1006. [DOI] [PubMed] [Google Scholar]
- 80.Schreiber C, Eicken A, Seidl S, et al. Unexpected early failure of a decellularized right ventricle to pulmonary artery graft. Ann Thorac Surg. 2008;86:2026–7. doi: 10.1016/j.athoracsur.2008.04.052. author reply 2027-8. [DOI] [PubMed] [Google Scholar]
- 81.Isch JA, Engum SA, Ruble CA, et al. Patch esophagoplasty using AlloDerm as a tissue scaffold. J Pediatr Surg. 2001;36:266–8. doi: 10.1053/jpsu.2001.20685. [DOI] [PubMed] [Google Scholar]
- 82.White JK, Agnihotri AK, Titus JS, et al. A stentless trileaflet valve from a sheet of decellularized porcine small intestinal submucosa. Ann Thorac Surg. 2005;80:704–7. doi: 10.1016/j.athoracsur.2004.08.063. [DOI] [PubMed] [Google Scholar]
- 83.Gabouev AI, Schultheiss D, Mertsching H, et al. In vitro construction of urinary bladder wall using porcine primary cells reseeded on acellularized bladder matrix and small intestinal submucosa. Int J Artif Organs. 2003;26:935–42. doi: 10.1177/039139880302601011. [DOI] [PubMed] [Google Scholar]
- 84.Badylak SF, Vorp DA, Spievack AR, et al. Esophageal reconstruction with ECM and muscle tissue in a dog model. J Surg Res. 2005;128:87–97. doi: 10.1016/j.jss.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 85.Mertsching H, Schanz J, Steger V, et al. Generation and transplantation of an autologous vascularized bioartificial human tissue. Transplantation. 2009;88:203–10. doi: 10.1097/TP.0b013e3181ac15e1. [DOI] [PubMed] [Google Scholar]
- 86.Lin P, Chan WC, Badylak SF, et al. Assessing porcine liver-derived biomatrix for hepatic tissue engineering. Tissue Eng. 2004;10:1046–53. doi: 10.1089/ten.2004.10.1046. [DOI] [PubMed] [Google Scholar]
- 87.Linke K, Schanz J, Hansmann J, et al. Engineered liver-like tissue on a capillarized matrix for applied research. Tissue Eng. 2007;13:2699–707. doi: 10.1089/ten.2006.0388. [DOI] [PubMed] [Google Scholar]
- 88.Nahmias Y, Schwartz RE, Hu WS, et al. Endothelium-mediated hepatocyte recruitment in the establishment of liver-like tissue in vitro. Tissue Eng. 2006;12:1627–38. doi: 10.1089/ten.2006.12.1627. [DOI] [PubMed] [Google Scholar]
- 89.Mitaka T, Sato F, Mizuguchi T, et al. Reconstruction of hepatic organoid by rat small hepatocytes and hepatic nonparenchymal cells. Hepatology. 1999;29:111–25. doi: 10.1002/hep.510290103. [DOI] [PubMed] [Google Scholar]
- 90.Michalopoulos GK, Bowen WC, Zajac VF, et al. Morphogenetic events in mixed cultures of rat hepatocytes and nonparenchymal cells maintained in biological matrices in the presence of hepatocyte growth factor and epidermal growth factor. Hepatology. 1999;29:90–100. doi: 10.1002/hep.510290149. [DOI] [PubMed] [Google Scholar]
- 91.Harada K, Mitaka T, Miyamoto S, et al. Rapid formation of hepatic organoid in collagen sponge by rat small hepatocytes and hepatic nonparenchymal cells. J Hepatol. 2003;39:716–23. doi: 10.1016/s0168-8278(03)00412-4. [DOI] [PubMed] [Google Scholar]
- 92.Soto-Gutierrez A, Navarro-Alvarez N, Yagi H, et al. Engineering of an hepatic organoid to develop liver assist devices. Cell Transplant. 2010;19:815–22. doi: 10.3727/096368910X508933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Floden EW, Malak SF, Basil-Jones MM, et al. Biophysical characterization of ovine forestomach extracellular matrix biomaterials. J Biomed Mater Res B Appl Biomater. 2011;96:67–75. doi: 10.1002/jbm.b.31740. [DOI] [PubMed] [Google Scholar]
- 94.Soto-Gutierrez A, Zhang L, Medberry C, et al. A whole-organ regenerative medicine approach for liver replacement. Tissue Eng Part C Methods. 2011;17:677–86. doi: 10.1089/ten.tec.2010.0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baptista PM, Siddiqui MM, Lozier G, et al. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53(2):604–17. doi: 10.1002/hep.24067. [DOI] [PubMed] [Google Scholar]
- 96.Sancho-Bru P, Najimi M, Caruso M, et al. Stem and progenitor cells for liver repopulation: can we standardise the process from bench to bedside? Gut. 2009;58:594–603. doi: 10.1136/gut.2008.171116. [DOI] [PubMed] [Google Scholar]