Abstract
Events evoke seamlessly integrated stimulus evaluation and response preparation processing streams, guided by regulative functions that change behavior flexibly in accord with the internal goals and contextual demands. The neural basis of the effects of alcohol intoxication on these processing streams is poorly understood, despite the evidence of alcohol’s deleterious effects on both attention and motor control. In an attempt to separate and examine relative susceptibility of these two dimensions, we employed a color version of the Eriksen flanker task that manipulated compatibility at the stimulus- and response-processing levels. Functional magnetic resonance imaging (fMRI) was performed in healthy social drinkers as they participated in both alcohol (0.6 g/kg ethanol for men, 0.55 g/kg for women) and placebo conditions in a counterbalanced design. Alcohol increased reaction times to response-level incongruity and decreased accuracy overall. Relative to the no-conflict condition, the observed brain activity was predominantly evoked by response-related conflict in medial prefrontal and lateral prefrontal cortices under placebo, in agreement with extensive evidence of their role in conflict processing. Activity evoked by response incongruity in the medial frontal cortex and insula was insignificant under alcohol, indicating its interference with response inhibition and preparation. Conversely, activity in ventrolateral prefrontal and premotor areas was relatively greater under alcohol than placebo, suggesting their compensatory engagement. This finding is consistent with the compensatory prefrontal activity increase found in studies with chronic alcoholic individuals, indicating functional reorganization with a goal of optimizing response strategy. These results delineate functional differences and selective susceptibility of a prefrontal network subserving response-level conflict processing. Our findings are incompatible with notions that moderate alcohol primarily affects attentional or stimulus-related processing and argue instead that its primary influence is on response inhibition, selection, and execution, with ramifications for the models of behavioral self-control and the inability to refrain from drinking.
Keywords: Eriksen flanker, Anterior cingulated, Lateral prefrontal cortex, Compensatory activity
Introduction
Events that we encounter evoke two seamlessly integrated processing streams: one stream evaluates the stimulus (“input”), whereas the other prepares the response (“output”). These processing streams occur in parallel and are guided by regulative functions (“executive”). Defined as “those capacities that enable a person to engage successfully in independent, purposive, self-serving behavior” [1], executive functions (also termed “cognitive control”) commonly refer to a set of interdependent cognitive abilities that are needed to monitor and change behavior flexibly and in accord with the internal goals and with the contextual demands. Due to their fluidity, these terms are not well defined operationally but are usually evaluated with tasks that reflect constituent functions such as: selective attention, working memory, response inhibition, error-monitoring, etc. The prefrontal cortex plays an essential role in subserving these functions [2]. Notably, quite diverse tasks seem to regularly engage highly overlapping regions in the lateral and medial frontal cortex [3–5]. Recent theories have provided testable models of the neural basis of cognitive control by assigning evaluative and regulative roles to brain regions primarily in the lateral prefrontal cortex (PFC) presumed to apply the top-down, strategy-driven control, and the anterior cingulate cortex (ACC). The ACC is assumed to detect conflict as it is activated by tasks requiring novel strategies, or in which automatic, prepotent responses need to be inhibited in favor of a non-automatic response [6–11]. While many studies manipulating conflict observe activation in dorsal ACC [3,12–14], its neurofunctional role varies in rostro-caudal dimension depending on the complexity of task demands. According to some accounts, the anterior ACC appears to subsume more abstract, higher-order control while the posterior mediofrontal areas including the adjacent presupplementary and supplementary motor areas (pre-SMA and SMA) are primarily involved in motor planning and execution [15–17].
Executive deficits and the associated prefrontal damage have been well documented in chronic alcoholics [18–25]. Studies using acute intoxication challenge indicate that the “input” processing stream is adversely affected. For instance, attention is impaired by very low doses, indicating its high sensitivity to alcohol [26]. Electrophysiological studies indicate that attentional processes are affected both at the level of early sensory processing [27], and later stages of novelty detection [28–30]. There is also extensive evidence of alcohol’s deleterious effects on the “output” process. Acute alcohol intoxication increases commission errors in go/nogo and stop-signal behavioral tasks [31–34]. This deficit correlates with impulsivity and is reflected in premature motor preparation [35], indicating impairment of response inhibition and preparation. Given that alcohol interacts with most levels of the neuraxis [36–38], it is not surprising that its effects are evident during both stimulus evaluation and response preparation. Nevertheless, its effects are particularly detrimental in situations presenting unexpected events, in conflicting or ambiguous task demands [39], or when an automatic response has to be inhibited in favor of a new task-relevant response [40]. Our recent fMRI study using a modified Stroop paradigm shows that ACC activation to high-conflict and error responses is attenuated by moderate intoxication, indicating selective vulnerability of the regulative functions [41]. By disrupting strategic decision making, alcohol may interfere with goal-directed behavior, resulting in susceptibility to immediate cues and poor self-control. Indeed, a deficit in regulatory functions and the inability to maintain inhibitory control over drinking are considered fundamental to the development of alcohol abuse both as a dispositional risk factor and as a consequence of excessive drinking [42–45].
Neuroimaging data on alcohol effects on cognitive control are scant despite the crucial importance of understanding the neural basis of alcohol’s effects on behavioral self-regulation. Effects of alcohol on Stroop interference have been examined with event-related potential (ERP) [40] and fMRI methodology [41], suggesting that it primarily affects the regulative processes subserved by ACC. The Stroop task engages a series of complex processing stages including reading of the verbal stimuli, their evaluation on the perceptual dimension, rule activation, inhibiting the inappropriate and retrieving the appropriate stimulus-response (S-R) mapping, response selection, and execution. These processes rely on the complex interplay of the functional systems related to attention, working memory, response inhibition and motor control, and alcohol can interfere with these functions in complex ways depending on the intoxication level and task version difficulty. However, further research is needed to examine which aspects of incongruity processing are relatively more affected by intoxication. The Eriksen flanker task is suitable for contrasting the attentional and response control dimensions. It manipulates the compatibility between centrally presented targets and the task-irrelevant stimuli flanking the targets [46], resulting in performance cost [47]. Bartholow and colleagues [48] administered a high (0.8 g/kg ethanol) and a moderate (0.4 g/kg) alcohol dose to healthy subjects and recorded ERPs to Eriksen flanker incompatibility. They concluded that the higher level of intoxication primarily impairs response inhibition, whereas the lower dose affects allocation of attention. Low spatial resolution of the ERP method, however, did not permit assessment of the neural underpinnings of these effects.
In an attempt to parse out the “input” from the “output” processing dimensions and to examine their relative susceptibility to alcohol intoxication, we employed a modified version of the Eriksen flanker task in the current study, while measuring fMRI-BOLD (Blood Oxygenation Level Dependent) signal. In this version of the task [49], the stimuli are squares that are presented in one of four colors. Two flanker squares of the same color appear shortly before the central target, facilitating the response if they match in color (CO, congruent condition). A special feature of this task is pairing each response hand with two colors. When the target is of a different color but it maps on the same hand, the incongruity is only at the stimulus level (SI, stimulus incongruity). Conversely, when the S-R mapping of the flankers and the target is assigned to different hands, their incompatibility additionally includes response dimension (RI, response incongruity). Therefore, the aims of this study were to examine effects of moderate alcohol intoxication (0.6 g/kg) on the neural basis of conflict processing; to directly compare these effects at the stimulus encoding versus response preparation levels; to investigate alcohol effects on behavioral performance on the Eriksen flanker task and potential interactions of these effects with gender.
Methods
Participants
Twenty healthy volunteers (10 females; age (mean ± st. dev) = 24.8 ± 3.6 years, range = 21–35 years) participated in both alcohol and placebo sessions in a counterbalanced manner, serving as their own controls. All of the subjects were right-handed, non-smoking native English speakers who reported no medical problems and were medication-free at the time of the study. They were light social drinkers and reported drinking 1.9 ± 1 times per week, imbibing 2.4 ± 1 drinks per occasion on average (adapted Alcohol Use Questionnaire) [50]. Men and women did not differ significantly in the frequency or amount of drinking. Subjects did not report alcoholism-related symptoms on Short Michigan Alcoholism Screening Test (SMAST) [51] and were negative for family history of alcoholism or drug abuse. Their responses on personality questionnaires (see description below) were in the normal range. All participants gave written, informed consent approved by the human subject review board before participating in the study. Data from nineteen subjects (10 women) are reported here below, as one subject fell asleep in the scanner during one session.
Task
The Eriksen flanker task was developed to investigate how irrelevant information (flanker letters) influences decisions about targets (the central letter in a letter array) as a function of their compatibility [46]. Neuroimaging studies have commonly employed a task version with arrow signs that are compatible (≫≫>) or not (≫<≫) [52–54]. However, there are only two possible incongruous trials. In order to increase the number of incompatible combinations and to maintain visual complexity across trials, we used the color version of the task [49] with four colors and twelve different incongruent target-flanker arrangements. On each trial, two flanker squares were presented in green, red, blue, or yellow color, followed by a central target square 200 ms later, presented in one of these four colors for 200 ms (Figure 1). The next trial followed after 1300 ms of fixation, with a total onset-to-onset interval of 1.7 sec. Participants were asked to use their index fingers to respond to the color of a target square by pressing the left button to green or red and the right button to blue or yellow. This response contingency resulted in three stimulus categories: on Congruent (CO) trials the target and flankers were the same color. On Stimulus Incongruent (SI) trials the flankers and target differed in color, but their responses mapped on the same hand (e.g. red flankers and green target both require left-hand response). On Response Incongruent (RI) trials the flankers and target differed both in color and their respective response mapping. Task participation followed extensive practice of color-response mapping. Response speed and accuracy were analyzed with a mixed model ANOVA with gender as a between-group factor and beverage and trial type as within-subject factors (Figure 2) [55]. The stimuli were shown in the center of a rear-projection screen with the Presentation software package (Neurobehavioral Systems) in a manner synchronized with the scanner via transistor-transistor (TTL) pulses. Each subject was presented with four runs that comprised the total of 200 Congruous, 120 Stimulus-Incongruous, and 120 Response Incongruous trials. In addition, 152 fixation trials were randomly interspersed in an event-related sequence providing temporal jitter for optimal deconvolution of the BOLD signal [56]. Optimized randomization of the event-related sequence was achieved with the Optseq program within the FS-FAST software (http://surfer.nmr.mgh.harvard.edu/optseq).
Figure 1.
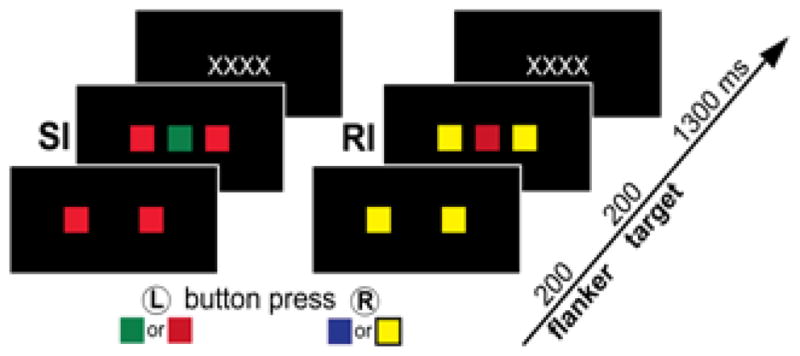
Stimulus sequence of the color version of Eriksen flanker task proceeds as follows: Two flankers of the same color are presented for 200 ms on the black background. A centrally presented target completes the set which is presented for 200ms and is then replaced by a fixation string for 1300ms with a total trial length of 1700ms. Using their index fingers, participants are instructed to press the left button to green or red targets and the right button to blue or yellow targets. Besides the Congruent (CO) condition in which flankers are the same color as the target, the stimulus sets can be incongruent in two ways: on Stimulus Incongruent (SI) trials, flankers and the target are incongruent at the color level, but are mapped to the same response hand. Response Incongruent (RI) trials are incongruent in both color and response mapping.
Figure 2.
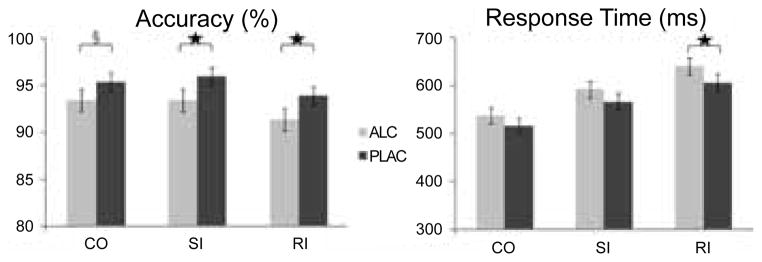
Response-level incompatibility on RI trials, on which the responses to flankers and targets were mapped onto the opposite hands, resulted in the lowest accuracy and slowest RTs. Alcohol intoxication decreased accuracy overall and increased RTs especially on RI trials. Alcohol vs. placebo comparisons: * p < 0.05, § p < 0.06.
Experimental design and procedure
All subjects served as their own controls and participated in both alcohol and placebo sessions in a counterbalanced manner. Prior to the experimental sessions, they took part in an introductory session during which they were familiarized with the laboratory setting and experimental procedure, abating potential effects of situation-induced arousal. At this time, the participants provided detailed information about their medical status, family history of alcoholism, level of response to alcohol [57], quantity and frequency of alcohol use [50,58], severity of their alcoholism-related symptoms (SMAST) [59], and handedness [60]. A battery of questionnaires was used in order to obtain a comprehensive dispositional profile for each subject, particularly with respect to disinhibitory, novelty seeking, and socialization traits. The battery contained the following questionnaires: Childhood Hyperactivity Questionnaire (HK/ MBD) [61]; Eysenck Personality Questionnaire (EPQ) [62]; Eysenck Impulsiveness and Venturesomeness Scale [63]; Socialization Scale of the California Psychological Inventory [64]; Zuckerman Sensation Seeking Scale [65]. All subjects gave written informed consent approved by the Human Research Committee at Massachusetts General Hospital and the Partners Healthcare Network.
During the two experimental sessions that were scheduled 30 days apart on average, participants were given either alcohol or placebo in a counterbalanced order. Half of the subjects were given alcohol beverage in the first session and vice versa. Upon their arrival to the laboratory, participants were asked about their last meal and about the last time they drank alcohol to verify their compliance with the requirement to abstain from food for 3 hours and from alcohol at least 48 hours before each experimental session. Prior to each scanning session female subjects were given a pregnancy test to ascertain that they were not pregnant. Breath alcohol concentration (BAC) was measured with a breathalyzer (Draeger, Inc.) upon arrival and on several occasions when the subjects were outside the scanner. Since no electronic device can be used in the scanner room, Q.E.D. Saliva Alcohol Test (OraSure Techn, Inc.) was used to estimate the BAC during the actual scans. Participants rated their moods and feelings with the adapted Biphasic Alcohol Effects Scale [BAES, 66] three times during each session: prior to drinking, immediately before and after the scan, corresponding to the ascending and descending BAC limbs respectively. The participants rated their momentary feelings on the “stimulating” (e.g. vigorous) and “sedating” (e.g. sluggish) subscales, in addition to rating how tired, worn-out, high, euphoric, or sexy they felt (Figure 3). Beverage was administered as a cocktail containing vodka (Grey Goose, Bacardi), 20% v/v and orange juice [67]. Alcohol beverage contained 0.60 g/kg of ethanol for men and 0.55 g/kg for women and placebo beverage contained the same volume of orange juice. The task was administered 77 ± 11 min after the subjects were presented with the beverage and lasted 18 minutes. It followed another task, the results of which have been presented elsewhere [41]. The average BAC measured before the task was 0.052% ± 0.01 and 0.047% ± 0.01 following the task, indicating that it was performed close to the peak, on the descending BAC limb. Upon completion of each experimental session participants filled out a detailed questionnaire querying them about perceived task difficulty, type and content of the beverage they imbibed, and about how intoxicated, nauseous or dizzy they felt.
Figure 3.
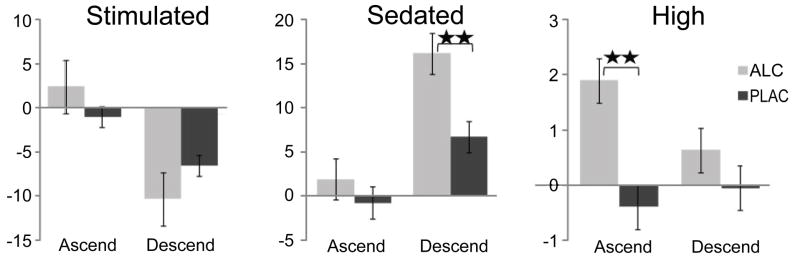
Group average momentary ratings of moods and feelings on the modified BAES subscales of “stimulation” and “sedation”, and the “high” adjective. The ratings obtained on the ascending (before the experiment) and on the descending BAC limbs (after the experiment) are expressed relative to baseline. Overall, participants reported feeling less stimulated and more sedated at the end of the experiment. When intoxicated, the participants felt more “high” on the ascending and more sedated on the descending BAC limbs. ** p < 0.001.
Image acquisition and analysis
Functional and structural brain images were acquired with a 3 Tesla Siemens Trio whole-body scanner (Siemens, Erlangen, Germany) at the Massachusetts General Hospital Martinos Center in Charlestown, MA. Exposure to scanner noise was reduced with 29dB earplugs and customized pillow padding. Special care was taken to minimize head movements with additional foam padding and head “clamps” which assured stable position as well as participants’ comfort. As they lay on the scanner bed, subjects viewed stimuli on a mirror fitted onto the head coil. Two high-resolution 3D MPRAGE (magnetization-prepared rapid gradient echo) T1-weighted sequences that optimize contrast for a range of tissue properties were obtained with the following parameters: TR = 2.53 sec, TE = 3.25 msec, flip angle = 7°, FOV = 256, 128 sagittal slices, 1.33 mm thickness, in-plane resolution 1 × 1 mm were obtained for each subject. These two high-resolution structural images were used for spatial normalization and cortical surface reconstruction. A series of functional whole-brain BOLD images was collected using a T2*-weighted EPI sequence of 28 interleaved 5mm thick slices in axial-oblique AC-PC orientation with TR = 1.7 sec, TE = 30 msec, flip angle = 90°, FOV = 200mm, matrix = 64 × 64, with 3.125 × 3.125 in-plane resolution.
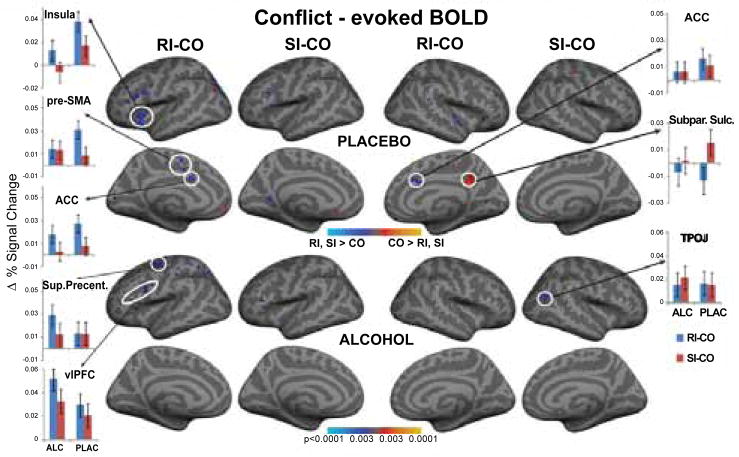
Brain images were analyzed with FreeSurfer and FS-FAST (Free-Surfer - Functional Analysis Stream) package [68–70]. Each subject’s cortical surface was reconstructed using an automatic gray/white segmentation, tessellation and inflation of the folded surface tessellation patterns (http://surfer.nmr.mgh.harvard.edu/). Each surface was registered with a canonical brain surface [71] permitting high-resolution group averaging based on surface alignment. Motion correction of the functional data was performed with AFNI software [72]. The data were spatially smoothed with a 3D 5-mm Gaussian full-width half-maximum filter, corrected for temporal drift and normalized to correct for signal intensity changes. The data were carefully checked for motion or other artifacts. Finite impulse-response model (FIR) was used to estimate event-related hemodynamic responses (HDR) for each TR within a time window of 18.7 sec. (prestimulus 5.1 sec). This model makes no a priori assumptions about the shape of the HDR and provides unbiased estimates of the average signal intensity at each time point for each trial type [68]. Motion parameters derived from realignment correction were entered into the model as regressors. Averaged responses for each contrast were obtained for trials with correct responses, avoiding potential bias due to error-related processing. The resulting F-distributed statistical activation maps were resampled onto the common cortical surface space and projected onto an inflated brain with average curvature [71]. A random-effects statistical model was used to calculate group averages. This approach takes into account the inter-subject variance and allows for inferences to the population [73]. In order to mitigate possible vasoactive effects of alcohol, potential baseline shifts were removed by subtracting the average hemodynamic response prior to stimulus onset from the hemodynamic response waveform for both placebo and alcohol conditions respectively, thus equating the two conditions at the baseline. Voxel-wise group-average activity (N = 19) of the two types of conflict effects (i.e. RI vs. CO and SI vs. CO contrasts) was calculated for placebo and alcohol conditions. These contrast images based on the RI-CO and SI-CO contrasts are shown in Fig 4. In order to explore possible effects and interactions of the factors of gender, beverage, and conflict, region-of-interest (ROI) analyses were carried out in addition to voxel-wise group maps. The ROIs were defined based on the overall group average for the conflict contrast summed across both beverage conditions at the 5.1 – 6.8 sec latency. The ROIs were identical across all subjects as they were automatically transferred from the average cortical surface onto each individual surface by means of a spherical morphing procedure [71] in a manner that was blind to each subject’s activation pattern. Percent signal change from baseline was computed for each ROI and each subject for all task and beverage conditions (Figure 4). Mixed design ANOVAs were carried out with gender as a between group and condition (CO, SI, RI) and beverage (alcohol, placebo), as within-subject factors for each ROI [55]. Results of these statistical comparisons are presented in Table 1, along with the Talairach coordinates of the ROIs.
Figure 4.
Random effects group average activity to both RI-CO and SI-CO conflict contrasts are displayed on the lateral and medial views of the inflated cortical surfaces of both hemispheres. Bar graphs along the sides of the activity maps show baseline-normalized conflict-related difference in percent signal change for the ROIs in both hemispheres for alcohol and placebo. The response-level conflict elicited activity in predominantly left prefrontal areas. Alcohol intoxication attenuated activity in the medial prefrontal cortex and insula. Conversely, increase in compensatory activity can be observed in the vlPFC and premotor areas. Pre-SMA: presupplementary motor area; ACC: anterior cingulate cortex; Sup. Precent.: superior precentral area; vlPFC: ventrolateral prefrontal cortex; Subpar. sulc: subparietal sulcus; TPOJ: temporo-parieto-occipital junction.
Table 1.
Summary of statistical comparisons for ROIs including their Talairach coordinates and significance of the overall RI-CO and SI-CO differences as well as for each beverage separately and beverage x conflict interactions, expressed as F1,17 and the associated p values.
| RI - CO contrast (F1,17) | SI - CO contrast (F1,17) | RI-CO (F1,17) | SI-CO (F1,17) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Area | Tal.coord. | Placebo | Alcohol | Plac-Alc | Placebo | Alcohol | Plac-Alc | overall | overall |
| L. insula | −28 21 1 | 17.1 < 0.001** | 1.2 > 0.2 | 5.8 < 0.05* | 3.3 < 0.1+ | 0.2 > 0.5 | 2.6 > 0.1 | 8.3 < 0.01** | 0.4 > 0.5 |
| L. pre-SMA | −9 1 48 | 11.2 < 0.005** | 1.8 > 0.2 | 1.9 < 0.2 | 0.4 > 0.5 | 2.2 > 0.1 | 0.1 > 0.5 | 10.03 < 0.01** | 2.0 > 0.1 |
| L. ACC | −6 11 26 | 13.0 < 0.005** | 1.1 > 0.3 | 1.3 > 0.2 | 0.7 > 0.4 | 0.0 > 0.5 | 0.1 > 0.5 | 5.8 < 0.05* | 0.2 > 0.5 |
| L. Sup. Precent. | −28 −3 41 | 1.7 > 0.2 | 4.0 < 0.06+ | 1.0 > 0.3 | 1.1 > 0.3 | 0.7 > 0.4 | 0.0 > 0.5 | 4.8 < 0.05* | 2.5 > 0.1 |
| L. vlPFC | −39 16 20 | 7.0 < 0.05* | 8.5 < 0.01** | 1.5 > 0.2 | 3.5 < 0.1+ | 4.4 = 0.05* | 0.6 > 0.4 | 12.1 < 0.005** | 6.7 < 0.05* |
| R. ACC | 7 17 29 | 3.9 < 0.07+ | 0.2 > 0.5 | 0.5 > 0.5 | 1.0 > 0.3 | 0.5 > 0.5 | 0.0 > 0.5 | 1.9 > 0.1 | 1.6 > 0.2 |
| R. Subpar. Sulc. | 11 −45 30 | 1.0 > 0.3 | 0.3 > 0.5 | 0.1 > 0.5 | 1.3 > 0.2 | 0.0 > 0.5 | 0.6 > 0.4 | 1.2 > 0.2 | 1.0 > 0.3 |
| R. TPOJ | 38 −58 20 | 1.8 > 0.2 | 0.6 > 0.4 | 0.1 > 0.5 | 1.1 > 0.3 | 2.0 > 0.1 | 0.1 > 0.5 | 1.7 > 0.2 | 2.3 > 0.1 |
p < 0.01,
p < 0.05,
p < 0.1.
Pre-SMA: pre-supplementary motor area; ACC: anterior cingulate cortex; Sup. Precent.: superior precentral area; vlPFC: ventrolateral prefrontal cortex; Subpar. sulc: subparietal sulcus; TPOJ: temporo-parieto-occipital junction.
Results
Behavioral Measures
Performance
Participants performed more accurately when given placebo (mean ± s.d., 95.1% ± 4.3) compared to alcohol (92.7% ± 5.1), as indicated by the main effect of beverage (F1,17 = 9.3, p < 0.01) (Figure 2). The effect of beverage was significant for the two incongruous conditions, RI, (F1,17 = 4.7, p < 0.05) and SI, (F1,17 = 5.9, p < 0.05) and only marginally so for the congruous condition, (F1,17 = 4.2, p < 0.06). Overall, the accuracy was higher on the CO, as compared to the RI trials (F1,17 = 9.0, p < 0.01), whereas the SI accuracy did not differ from CO or RI. Reaction times (RTs) were significantly longer only on the RI trials under alcohol (F1,17 = 5.8, p < 0.05), with the overall tendency of alcohol to induce longer RTs, (F1,17 = 3.14, p < 0.1). RTs were progressively longer across the three conditions, (F2,34 = 119.3, p < 0.0001), with the shortest RTs on CO trials (527 ± 63), followed by the SI (579ms ± 70), and RI trials (623ms ± 63). Women and men did not differ in performance speed or accuracy.
Mood ratings
Participants rated their momentary moods and feelings on three occasions during each session with the modified BAES scale: upon arrival to the laboratory (the baseline rating), prior to entering the scanner (ascending BAC limb), and after the experiment (descending BAC limb). Effects of gender, beverage, and the time of rating were examined with a mixed ANOVA. Since no differences between groups or sessions were observed at the baseline, the subsequent ratings were expressed as the relative change from baseline (Figure 3). Overall, participants reported being less stimulated (F1,17 = 68.5, p < 0.001) and more sedated (F1,17 = 19.4, p < 0.001) at the end, as compared to the beginning of the experiment. Alcohol intoxication rendered them significantly more sedated on the descending BAC limb, F1,17 = 17.2, p < 0.001). Conversely, they reported being more “high” on the ascending BAC limb when intoxicated, F1,17 = 17.2, p < 0.001. The feeling of being “high” dissipated by the end of the experiment, F1,17 = 13.1, p < 0.01, giving way to sedation. No gender differences were observed on any of the mood reports.
Post-experimental questionnaire
After each experimental session participants used Likert scales (1–5) to rate the perceived task difficulty, beverage content and perceived level of intoxication, feeling of dizziness, and nausea. Effects of gender and beverage were analyzed for each scale with a mixed model ANOVA. Overall, the task was rated as being fairly easy (2.3 ± 0.96), but when intoxicated, men tended to rate it as being easier than women, with ratings of 1.8 ± 0.8 (men) and 2.6 ± 0.9 (women), (F1,17 = 3.9, p < 0.06). When asked to rate the contents of the beverage ranging from 1 (“definitely contains no alcohol”) to 5 (“definitely contains alcohol”), participants correctly perceived the beverage contents under both alcohol (4.5 ± 1.0) and placebo conditions (1.4 ± 1.0), F1,17 = 50.3, p < 0.0001. When asked how intoxicated they felt ranging from 1 (“not at all”) to 5 (“very much”), women reported being more intoxicated then men (3.2 ± 0.8 and 2.3 ± 0.8 respectively), F1,17 = 5.2, p < 0.05. While alcohol did not increase nausea (overall mean = 1.1 ± 0.4), participants reported feeling more dizzy when intoxicated (1.9 ± 1.1) as compared to placebo condition (1.2 ± 0.4), F1,17 = 9.8, p < 0.01.
Neuroimaging results
Results of the voxel-wise random effects analysis for both beverages are shown in Figure 4, expressed as the statistical maps of the RI-CO and SI-CO contrasts. These group averages included only trials with correct responses, to avoid any bias due to error-related processing. Relative to the no-conflict CO condition, the conflict evoked stronger responses especially in left prefrontal areas. The observed activity was due predominantly to the response-related conflict with much weaker contributions from the stimulus-related conflict. Bar graphs flanking the activity maps in Figure 4 show baseline-normalized percent signal change for the ROIs in the left and right hemispheres. Further details of the statistical comparisons are given in Table 1. Inspection of the figure indicates that distinct frontal areas were differentially sensitive to the effects of alcohol and conflict. Activity to the RI-related conflict was pronounced under placebo in the medial prefrontal areas including left ACC and pre-SMA and right ACC, suggesting that those areas participate in response inhibition, selection and execution. Activity to RI-CO contrast was much weaker in those areas, indicating that the effects of alcohol are particularly detrimental to response-related conflict. The left insula was sensitive to both levels of conflict as the activity to the stimulus-related conflict was marginally greater than the activity to the CO condition under placebo (Table 1). Alcohol blunted the activity in insula to RI-CO condition, (RI-CO x beverage interaction, F1,17 = 5.8, p < 0.05) implicating insula’s role in subserving response conflict and the vulnerability of this function to acute intoxication. The only two areas that were relatively more activated under alcohol intoxication than placebo were the left ventrolateral prefrontal and superior precentral regions. This activity pattern indicates that performance accuracy may be maintained by relying on partially different frontal areas, compensating for alcohol-induced impairments. ROI-based analysis confirmed that the right hemisphere contributions to conflict processing were minimal. With the exception of the marginally significant RI-CO activity in the right ACC under placebo, no other significant right hemisphere contributions were observed, indicating the left lateralized activity dominance during conflict processing. No gender effects were observed on any of these effects with one exception. Stronger RI-CO activity in the left ACC under placebo was particularly prominent in men, F1,17 = 16.9, p < 0.001, but not women, F1,17 = 0.9, p < 0.38.
Discussion
Results of this study indicate that moderate alcohol intoxication primarily affects response inhibition, preparation and control under the conditions of response conflict. The version of the Eriksen flanker task employed in this study manipulates the level of S-R compatibility between targets and task-irrelevant flankers, allowing a comparison between the stimulus-level and response-level incongruity. Presentation of the flankers triggers stimulus evaluation and primes a particular S-R mapping. Prevalent models of S-R compatibility suggest that automatic and intentional activation of the response mapping are simultaneously initiated [74,75]. Response speed is facilitated by flanker presentation on congruent trials. On SI trials, the perceptual difference between the targets and flankers needs to be evaluated, but the same S-R rule applies. However, when the flankers activate an incorrect S-R mapping, the automatically activated response needs to be inhibited and the correct response planned and executed, resulting in slower RTs and increased error rate. Our study examined the behavioral and neural characteristics of this interference. At the behavioral level, the RTs were progressively longer to SI and RI as the conflict conditions incurred cost, reflecting increased processing demands due to response inhibition and selection. This finding is consistent with other observations that flanker interference primarily affects response selection, and not perceptual feature extraction [47]. Alcohol affected accuracy in both SI and RI conflict conditions, but the RTs were significantly longer only on RI trials. Overall, accuracy was more sensitive to alcohol intoxication than RTs, in agreement with other studies using the Eriksen flanker task [48], or other tasks probing response inhibition [76]. Furthermore, the fMRI-BOLD signal was elicited predominantly by the RI conflict in the left frontal areas and it was particularly sensitive to alcohol effects. These findings lend support to the response inhibition accounts of alcohol’s effect on conflict processing [77].
In a companion study using the Stroop task [41], moderate alcohol intoxication selectively attenuated ACC activation during high-conflict and error trials, indicating vulnerability of the regulative functions [39]. The present study confirmed that the ACC activity to RI conflict was sensitive to alcohol intoxication. Indeed, extensive evidence highlights the ACC as a central node in a predominantly frontal cortical network subserving cognitive control [6–10]. Our placebo results are in agreement with previous fMRI studies showing that the ACC is activated by the RI, but not SI conflict [78,79]. In the present study, the medial frontal activation to RI conflict also encompassed the left pre-SMA area, which had a very similar activity pattern to the ACC activity. This finding is consistent with suggestions that the medial frontal cortex exhibits an activation gradient as a function of the task complexity, with the anterior ACC implicated in higher-order control, and more posterior areas, inclusive of the pre-SMA and SMA, subserving motor control [15–17]. The ACC activation in the current study is more posterior than the one observed to Stroop interference [41], likely a result of the emphasis on motor control by the RI manipulation. It corresponds to the posterior rostral cingulate zone that is primarily involved in response selection [80,81]. Evidence obtained with a variety of methods converges on the pre-SMA as an important part of the circuitry involved in both motor planning and response inhibition [17,82,83]. Furthermore, pre-SMA lesions impair the ability to inhibit ongoing movement [84], suggesting its critical role in planning and inhibiting actions under response conflict conditions. The medial prefrontal cortex inclusive of the ACC and pre-SMA has widespread anatomical connections with lateral prefrontal cortex, motor cortex, spinal cord, and limbic structures, making it suitable for its multifaceted role in self-regulation and motor planning [3,85–87]. In agreement with van Veen et al., [78], our results indicate that the medial frontal cortex, inclusive of ACC and the left pre-SMA, is not involved in detecting conflict in general. Instead, it plays an important role in inhibiting the response primed by distracting flankers and in selecting the correct response. Whereas the activity in those areas was significantly greater to RI than CO under placebo, the difference was nonsignificant under alcohol intoxication, indicating that alcohol interferes with overriding primed responses and executing a correct response.
The activity in the left anterior insula was greater to RI than CO under placebo. This difference was significantly attenuated by alcohol (Table 1, Figure 4). Left anterior insula was also marginally activated by the SI, indicating its contributions to the task performance. Aside from its association with contextual influences on rewards [88], insula is considered to be a part of a “core” system controlling task set [89]. Activity in the left anterior insula is correlated with stopping efficiency [90], implicating it in response inhibition. However, it is commonly activated in tasks of cognitive control, suggesting that its function may be at the level of maintaining task rules and coordinating response in accordance with those rules as a function of their salience [91,92]. Evidence of the synchronous activity changes between the ACC and the anterior insula indicates that they may be closely functionally related [93]. In the current study, the pattern of alcohol-induced attenuation in the left insula is very similar to the one observed in the left ACC, in support of the conjecture of their joint activity. Thus, alcohol may exert its deleterious effects at the level of a functional system that subserves conflict processing related to response selection and control.
The only area that showed relatively greater activity under alcohol than placebo to both RI and SI was left ventrolateral prefrontal cortex (vlPFC). This region has been strongly implicated in subserving the top-down influence on behavior [94]. It is selectively activated by cognitive conflict in a verbal flanker task [95] and it plays an essential role in making a selection from an array of possible responses [96,97]. It is the only area that was significantly more activated by the SI as compared to the CO condition, in agreement with its role in the ventral attentional system [98] and with suggestions that it subserves the retrieval of response rules [99,100]. The conflict monitoring account [52] proposes that the vlPFC is functionally related to the ACC. It is suggested that the ACC engages the lateral PFC to actually implement top-down control by carrying out the necessary behavioral adjustments. On this view, conflict detection and response selection are regulated by their functional interplay. Indeed, our observation of the attenuated ACC activity and concomitantly increased vlPFC activity under alcohol are consistent with that proposal. Alcohol-induced increase in vlPFC activity may have compensated for the blunted ACC response by maintaining rule identification and retrieval. Additional engagement of the superior precentral motor area to RI under alcohol may be a part of this compensatory circuitry during response inhibition and selection as lesion studies indicate that it is involved in response inhibition [101]. While the vlPFC assists with S-R rule retrieval, the premotor area carries out the response selection and execution in the RI condition under alcohol. The top-down regulation that is normally carried out by the medial frontal cortex is blunted by alcohol intoxication. The increased lateral prefrontal activity under alcohol compensates for this deficit albeit at a cost in speed and accuracy. Indeed, a compensatory hypothesis has been advanced based on the studies in chronic alcoholics [18,23,102,103]. These studies have reported increased activity in the left vlPFC and parieto-cerebellar network in chronic alcoholic individuals as compared to healthy controls on working memory tasks. Similarly, increased activity of the right vlPFC was observed in compensation for the blunted amygdala activity during emotional processing in chronic alcoholics [104]. Taken together, it appears that the impairments of limbic structures may be offset by lateral prefrontal activity in an effort to maintain performance. Compensatory increase in ERP amplitudes has also been observed on Eriksen flanker task in younger alcoholic individuals [105].
Consistent with the Stroop-based interference effects [41], the activity observed on this non-verbal task was strongly left-lateralized. Contributions of the right hemisphere were minimal and none were significant, with the exception of the marginally larger RI-CO activity in the ACC under placebo. Nevertheless, while these effects did not quite reach our stringent criteria, they may be potentially informative. The subparietal sulcus is a part of the “default” network and its deactivation during task performance is consistent with its role in performance optimization [106,107]. The only area that was relatively (though not significantly) more activated to SI-CO was the right temporo-parieto-occipital junction (TPOJ). Given that it is a part of visually responsive association cortex which is activated by perceptual conflict [98,108], its potential contribution to perceptual attention is quite likely.
A possible caveat to consider is that alcohol exerts vasoactive effects on resting cerebral blood flow [109] as a function of gender and cortical region, and may bias fMRI-BOLD signal. In an effort to mitigate such potential effects, all analyses were carried out with baseline-normalized activity differences, thus equating the two beverage conditions at the baseline. Furthermore, the conflict-related effects were calculated relative to the no-conflict condition, further equating such possible bias. Nonetheless, caution is warranted when interpreting fMRI BOLD magnitude changes.
In sum, our results are incompatible with notions that moderate alcohol primarily affects attentional or stimulus-related processing and argue instead that its primary influence is on the response inhibition, selection, and execution. In a task that manipulated flanker-target compatibility, the observed brain activity was predominantly evoked by response-related conflict in prefrontal areas. Alcohol intoxication preferentially attenuated regulative input from the medial prefrontal cortex and the anterior insula, resulting in behavioral deficits. This impairment was partially offset by the compensatory increase in the lateral frontal involvement with a goal of optimizing response strategy. These results suggest that a network of frontal areas is engaged during response-level conflict and alcohol intoxication underscores their functional differences and their selective susceptibility. Prefrontal cortex plays an important role in guiding behavior in accordance with intents and goals. The finding that alcohol intoxication primarily impairs response-level regulation has ramifications for the models of behavioral self-control and the inability to refrain from drinking.
Acknowledgments
This work was supported by funds from the National Institutes of Health (R01-AA016624 and P41RR14075); Beverage Medical Research Foundation, and Medical Investigation of Neurodevelopmental Disorders (MIND) Institute. The study was carried out at Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA. We thank Elinor Artsy and Nevena Padovan for assistance.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Lezak MD. Neuropsychological Assessment. New York: Oxford University Press, USA; 1995. [Google Scholar]
- 2.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 3.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 4.Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 5.Posner MI, Raichle ME. Images of Mind (Revised) Scientific American Books; Washington, DC: 1996. [Google Scholar]
- 6.Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci. 2007;7:356–366. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- 7.Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- 8.Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- 9.Posner MI, Rothbart MK, Sheese BE, Tang Y. The anterior cingulate gyrus and the mechanism of self-regulation. Cogn Affect Behav Neurosci. 2007;7:391–395. doi: 10.3758/cabn.7.4.391. [DOI] [PubMed] [Google Scholar]
- 10.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Ulbert I, Schomer DL, Marinkovic K, Halgren E. Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. J Neurosci. 2005;25:604–613. doi: 10.1523/JNEUROSCI.4151-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 13.Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, et al. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 14.Procyk E, Tanaka YL, Joseph JP. Anterior cingulate activity during routine and non-routine sequential behaviors in macaques. Nat Neurosci. 2000;3:502–508. doi: 10.1038/74880. [DOI] [PubMed] [Google Scholar]
- 15.Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn Sci. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Rushworth MF, Buckley MJ, Behrens TE, Walton ME, Bannerman DM. Functional organization of the medial frontal cortex. Curr Opin Neurobiol. 2007;17:220–227. doi: 10.1016/j.conb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Nachev P, Rees G, Parton A, Kennard C, Husain M. Volition and conflict in human medial frontal cortex. Curr Biol. 2005;15:122–128. doi: 10.1016/j.cub.2005.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oscar-Berman M, Marinković K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, et al. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- 20.Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, et al. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol. 2001;36:357–368. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- 22.Adams KM, Gilman S, Koeppe RA, Kluin KJ, Brunberg JA, et al. Neuropsychological deficits are correlated with frontal hypometabolism in positron emission tomography studies of older alcoholic patients. Alcohol Clin Exp Res. 1993;17:205–210. doi: 10.1111/j.1530-0277.1993.tb00750.x. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- 24.Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- 25.Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, et al. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcohol Clin Exp Res. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- 26.Koelega HS. Alcohol and vigilance performance: a review. Psychopharmacology (Berl) 1995;118:233–249. doi: 10.1007/BF02245951. [DOI] [PubMed] [Google Scholar]
- 27.Kenemans JL, Hebly W, van den Heuvel EH, Grent-’T-Jong T. Moderate alcohol disrupts a mechanism for detection of rare events in human visual cortex. J Psychopharmacol. 2010;24:839–845. doi: 10.1177/0269881108098868. [DOI] [PubMed] [Google Scholar]
- 28.Grillon C, Sinha R, O’Malley SS. Effects of ethanol on the processing of low probability stimuli: an ERP study. Psychopharmacology (Berl) 1995;119:455–465. doi: 10.1007/BF02245862. [DOI] [PubMed] [Google Scholar]
- 29.Jääskeläinen IP, Schröger E, Näätänen R. Electrophysiological indices of acute effects of ethanol on involuntary attention shifting. Psychopharmacology (Berl) 1999;141:16–21. doi: 10.1007/s002130050801. [DOI] [PubMed] [Google Scholar]
- 30.Marinkovic K, Halgren E, Maltzman I. Arousal-related P3a to novel auditory stimuli is abolished by moderately low alcohol dose. Alcohol and Alcoholism. 2001;36:529–539. doi: 10.1093/alcalc/36.6.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marczinski CA, Fillmore MT. Preresponse cues reduce the impairing effects of alcohol on the execution and suppression of responses. Exp Clin Psychopharmacol. 2003;11:110–117. doi: 10.1037//1064-1297.11.1.110. [DOI] [PubMed] [Google Scholar]
- 32.Abroms BD, Fillmore MT, Marczinski CA. Alcohol-induced impairment of behavioral control: effects on the alteration and suppression of prepotent responses. J Stud Alcohol. 2003;64:687–695. doi: 10.15288/jsa.2003.64.687. [DOI] [PubMed] [Google Scholar]
- 33.de Wit H, Crean J, Richards JB. Effects of d-amphetamine and ethanol on a measure of behavioral inhibition in humans. Behav Neurosci. 2000;114:830–837. doi: 10.1037//0735-7044.114.4.830. [DOI] [PubMed] [Google Scholar]
- 34.Dougherty DM, Moeller FG, Steinberg JL, Marsh DM, Hines SE, et al. Alcohol increases commission error rates for a continuous performance test. Alcohol Clin Exp Res. 1999;23:1342–1351. [PubMed] [Google Scholar]
- 35.Marinkovic K, Halgren E, Klopp J, Maltzman I. Alcohol effects on movement-related potentials: a measure of impulsivity? J Stud Alcohol. 2000;61:24–31. doi: 10.15288/jsa.2000.61.24. [DOI] [PubMed] [Google Scholar]
- 36.Kalant H. In: Effects of ethanol on the nervous system, in Alcohol and derivatives: International Encyclopedia of Pharmacology and Therapeutics. Tremolieres J, editor. Pergamon Press; Oxford: 1970. [Google Scholar]
- 37.Begleiter H, Kissin B. The pharmacology of alcohol and alcohol dependence. Oxford University Press; New York: 1996. [Google Scholar]
- 38.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ridderinkhof KR, de Vlugt Y, Bramlage A, Spaan M, Elton M, et al. Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Science. 2002;298:2209–2211. doi: 10.1126/science.1076929. [DOI] [PubMed] [Google Scholar]
- 40.Curtin JJ, Fairchild BA. Alcohol and cognitive control: implications for regulation of behavior during response conflict. J Abnorm Psychol. 2003;112:424–436. doi: 10.1037/0021-843x.112.3.424. [DOI] [PubMed] [Google Scholar]
- 41.Marinkovic K, Rickenbacher E, Azma S, Artsy E. Acute alcohol intoxication impairs top-down regulation of stroop incongruity as revealed by blood oxygen level-dependent functional magnetic resonance imaging. Hum Brain Mapp. 2011;33:319–333. doi: 10.1002/hbm.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Field M, Wiers RW, Christiansen P, Fillmore MT, Verster JC. Acute alcohol effects on inhibitory control and implicit cognition: implications for loss of control over drinking. Alcohol Clin Exp Res. 2010;34:1346–1352. doi: 10.1111/j.1530-0277.2010.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyvers M. “Loss of control” in alcoholism and drug addiction: a neuroscientific interpretation. Exp Clin Psychopharmacol. 2000;8:225–249. doi: 10.1037//1064-1297.8.2.225. [DOI] [PubMed] [Google Scholar]
- 44.Finn P. In: Acute effects of alcohol on cognition and impulsive-disinhibited behavior, in Review of NIAAA’s neuroscience and behavioral research portfolio. Noronha A, Eckardt M, Warren K, editors. US Department of health and human services; Bethesda, MD: 2000. pp. 337–356. [Google Scholar]
- 45.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of target letters in a non-search task. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- 47.Sanders AF, Lamers JM. The Eriksen flanker effect revisited. Acta Psychol (Amst) 2002;109:41–56. doi: 10.1016/s0001-6918(01)00048-8. [DOI] [PubMed] [Google Scholar]
- 48.Bartholow BD, Pearson M, Sher KJ, Wieman LC, Fabiani M, et al. Effects of alcohol consumption and alcohol susceptibility on cognition: a psychophysiological examination. Biol Psychol. 2003;64:167–190. doi: 10.1016/s0301-0511(03)00108-x. [DOI] [PubMed] [Google Scholar]
- 49.Hazeltine E, Poldrack R, Gabrieli JD. Neural activation during response competition. J Cogn Neurosci. 2000;12:118–129. doi: 10.1162/089892900563984. [DOI] [PubMed] [Google Scholar]
- 50.Cahalan D, Cisin IH, Crossley HM. Monograph #6. Rutgers Center of Alcohol Studies; New Brunswick, NJ: 1969. American drinking practices: A national study of drinking behavior and attitudes. [Google Scholar]
- 51.Selzer ML, Vinokur A, van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) J Stud Alcohol. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- 52.Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- 53.Casey BJ, Thomas KM, Welsh TF, Badgaiyan RD, Eccard CH, Jennings JR, et al. Dissociation of response conflict, attentional selection, and expectancy with functional magnetic resonance imaging. Proc Natl Acad Sci U S A. 2000;97:8728–8733. doi: 10.1073/pnas.97.15.8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fan J, Hof PR, Guise KG, Fossella JA, Posner MI. The functional integration of the anterior cingulate cortex during conflict processing. Cereb Cortex. 2008;18:796–805. doi: 10.1093/cercor/bhm125. [DOI] [PubMed] [Google Scholar]
- 55.Woodward JA, Bonett DG, Brecht ML. Introduction to linear models and experimental design. San Diego: Harcourt Brace Jovanovich; 1990. [Google Scholar]
- 56.Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. Neuroreport. 1998;9:3735–3739. doi: 10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- 57.Schuckit MA, Smith TL, Tipp JE. The Self-Rating of the Effects of alcohol (SRE) form as a retrospective measure of the risk for alcoholism. Addiction. 1997;92:979–988. [PubMed] [Google Scholar]
- 58.Mills Kenneth C, Neal Edward Max, Neal lola Peed. Handbook for alcohol education: The community approach. Cambridge, MA: Ballinger; 1983. [Google Scholar]
- 59.Selzer ML. The Michigan alcoholism screening test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- 60.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 61.Tarter RE, McBride H, Buonpane N, Schneider DU. Differentiation of alcoholics. Childhood history of minimal brain dysfunction, family history, and drinking pattern. Arch Gen Psychiatry. 1977;34:761–768. doi: 10.1001/archpsyc.1977.01770190023002. [DOI] [PubMed] [Google Scholar]
- 62.Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Questionnaire. London: Hodder & Staughton; 1975. [Google Scholar]
- 63.Eysenck SB, Eysenck HJ. Impulsiveness and venturesomeness: their position in a dimensional system of personality description. Psychol Rep. 1978;43:1247–1255. doi: 10.2466/pr0.1978.43.3f.1247. [DOI] [PubMed] [Google Scholar]
- 64.Gough HG. Theory, development, and interpretation of the CPI socialization scale. Psychol Rep. 1994;75:651–700. doi: 10.2466/pr0.1994.75.1.651. [DOI] [PubMed] [Google Scholar]
- 65.Zuckerman M. Dimensions of sensation seeking. Journal of Consulting and Clinical Psychology. 1971;36:45–52. doi: 10.1037/h0026047. [DOI] [PubMed] [Google Scholar]
- 66.Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. 1993 doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 67.Marinkovic K, Halgren E, Maltzman I. Effects of alcohol on verbal processing: An event- related potential study. Alcohol Clin Exp Res. 2004;28:415–423. doi: 10.1097/01.alc.0000117828.88597.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burock MA, Dale AM. Estimation and detection of event-related fMRI signals with temporally correlated noise: a statistically efficient and unbiased approach. Hum Brain Mapp. 2000;11:249–260. doi: 10.1002/1097-0193(200012)11:4<249::AID-HBM20>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 70.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 71.Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 73.Friston KJ, Holmes AP, Price CJ, Büchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. Neuroimage. 1999;10:385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- 74.Kornblum S, Hasbroucq T, Osman A. Dimensional overlap: cognitive basis for stimulus-response compatibility--a model and taxonomy. Psychol Re. 1990;97:253–270. doi: 10.1037/0033-295x.97.2.253. [DOI] [PubMed] [Google Scholar]
- 75.Lien MC, Proctor RW. Stimulus-response compatibility and psychological refractory period effects: implications for response selection. Psychon Bull Rev. 2002;9:212–238. doi: 10.3758/bf03196277. [DOI] [PubMed] [Google Scholar]
- 76.Mulvihill LE, Skilling TA, Vogel-Sprott M. Alcohol and the ability to inhibit behavior in men and women. J Stud Alcohol. 1997;58:600–605. doi: 10.15288/jsa.1997.58.600. [DOI] [PubMed] [Google Scholar]
- 77.Fillmoroe MT, Vogel-Sprott M. Response inhibition under alcohol: effects of cognitive and motivational conflict. J Stud Alcohol. 2000;61:239–246. doi: 10.15288/jsa.2000.61.239. [DOI] [PubMed] [Google Scholar]
- 78.van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage. 2001;14:1302–8. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- 79.Lau H, Rogers RD, Passingham RE. Dissociating response selection and conflict in the medial frontal surface. Neuroimage. 2006;29:446–451. doi: 10.1016/j.neuroimage.2005.07.050. [DOI] [PubMed] [Google Scholar]
- 80.Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- 81.Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- 82.Mostofsky SH, Simmonds DJ. Response inhibition and response selection: two sides of the same coin. J Cogn Neurosci. 2008;20:751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- 83.Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 84.Nachev Parashkev, Wydell Henrietta, O’Neill Kevin, Husain Masud, Kennard Christopher. The role of the pre-supplementary motor area in the control of action. Neuroimage. 2007;36:155–163. doi: 10.1016/j.neuroimage.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res Bull. 2000;52:319–330. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- 86.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 87.Wise SP. The primate premotor cortex: past, present, and preparatory. Annu Rev Neurosci. 1985;8:1–19. doi: 10.1146/annurev.ne.08.030185.000245. [DOI] [PubMed] [Google Scholar]
- 88.Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. J Neurosci. 2000;20:6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boehler CN, Appelbaum LG, Krebs RM, Hopf JM, Woldorff MG. Pinning down response inhibition in the brain--conjunction analyses of the Stop-signal task. Neuroimage. 2010;52:1621–1632. doi: 10.1016/j.neuroimage.2010.04.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Menon V, Uddin LQ. Saliency, switchingattention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56:1655–65. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- 93.Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Struct Funct. 2010;214:535–549. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tomita H, Ohbayashi M, Nakahara K, Hasegawa I, Miyashita Y. Top-down signal from prefrontal cortex in executive control of memory retrieval. Nature. 1999;40:699–703. doi: 10.1038/44372. [DOI] [PubMed] [Google Scholar]
- 95.Ochsner KN, Hughes B, Robertson ER, Cooper JC, Gabrieli JD. Neural systems supporting the control of affective and cognitive conflicts. J Cogn Neurosci. 2009;21:1842–1855. doi: 10.1162/jocn.2009.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci U S A. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 98.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 99.Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cereb Cortex. 2006;16:1679–1689. doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- 100.Brass M, von Cramon DY. The role of the frontal cortex in task preparation. Cereb Cortex. 2002;12:908–914. doi: 10.1093/cercor/12.9.908. [DOI] [PubMed] [Google Scholar]
- 101.Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, et al. Effects of focal frontal lesions on response inhibition. Cereb Cortex. 2007;17:826–838. doi: 10.1093/cercor/bhk031. [DOI] [PubMed] [Google Scholar]
- 102.Desmond JE, Chen SH, DeRosa E, Pryor MR, Pfefferbaum A, et al. Increased frontocerebellar activation in alcoholics during verbal working memory: an fMRI study. Neuroimage. 2003;19:1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- 103.Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, et al. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. Neuroimage. 2001;14:7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- 104.Marinkovic K, Oscar-Berman M, Urban T, O’Reilly CE, Howard JA, et al. Alcoholism and dampened temporal limbic activation to emotional faces. Alcohol Clin Exp Res. 2009;33:1880–1892. doi: 10.1111/j.1530-0277.2009.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Padilla ML, Colrain IM, Sullivan EV, Mayer BZ, Turlington SR, et al. Electrophysiological evidence of enhanced performance monitoring in recently abstinent alcoholic men. Psychopharmacology (Berl) 2011;213:81–91. doi: 10.1007/s00213-010-2018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 107.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 108.Papeo L, Longo MR, Feurra M, Haggard P. The role of the right temporoparietal junction in intersensory conflict: detection or resolution? Exp Brain Res. 2010;206:129–139. doi: 10.1007/s00221-010-2198-2. [DOI] [PubMed] [Google Scholar]
- 109.Rickenbacher E, Greve DN, Azma S, Pfeuffer J, Marinkovic K. Effects of alcohol intoxication and gender on cerebral perfusion: An Arterial Spin Labeling study. Alcohol. 2011;45:725–737. doi: 10.1016/j.alcohol.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]