To the Editors
Interferon-gamma (IFN-γ) inducible protein 10 (IP-10), also known as CXCL10, is a chemokine involved in both innate and acquired immune responses that directs T cells to sites of inflammation1, 2. Plasma IP-10 has been shown to correlate closely with inflammation, liver fibrosis and Hepatitis C virus infection3, reflect HIV load in cerebral spinal fluid4 and be a useful marker for early HIV disease progression2, 5. In addition, genital tract levels of IP-10 may reflect vaginal HIV load6. Herein we report on plasma IP-10 concentrations correlating with plasma viral load that can be used as a predictive marker of viral replication in HIV infected adults.
Plasma concentrations of IP-10 were evaluated by Luminex assay using a Bio-Plex Cytokine reagent kit (BIO RAD Laboratories, Hercules, CA, USA) in 51 HIV uninfected and 55 HIV-infected individuals, including 7 participants on antiretroviral (ARV) treatment. The median plasma IP-10 concentration in HIV-uninfected controls was 340 ng/ml (IQR: 249–468) and in all HIV-infected individuals (including those on ARV) there was a significantly higher concentration (p<0.0001 using Mann-Whitney) of 1160 ng/ml (IQR: 779–2088). There was also a significantly higher concentration of IP-10 when comparing HIV-uninfected and HIV-infected individuals receiving ARV (median: 778 ng/ml, IQR: 534–924, p=0.0009), although all on ARV were below the threshold of 400 RNA copies/ml. Relative to ARV-naïve HIV-infected individuals, the lower IP-10 concentration in patients receiving ARV is compatible with reduced inflammation upon viral suppression7. However, the finding that IP-10 is significantly higher in ARV-treated individuals, relative to uninfected controls, also suggests that inflammatory signals are not completely dampened and may reflect viral activity below the 400 copies/ml threshold. When grouping all HIV-infected individuals, there was a significant positive correlation (p<0.0001) between IP-10 and viral load (r=0.71, 95% CI: 0.52–0.84; Figure 1A), compatible with the close association between this chemokine and viral burden. We also measured IP-10 longitudinally in 25 of the 55 HIV-infected individuals over 9 months, where a significant correlation was observed between changes in both IP-10 and viral loads over time (r=0.65; p=0.049), accentuating the parallel course of viral load and IP-10. We used Receiver-Operating-Characteristic (ROC) curves to identify the possible predictive nature of IP-10. A significant area-under-the-curve (AUC) was noted when selecting viral loads above or below 5000 RNA copies/ml (Figure 1B, AUC=0.88; 95% CI: 0.77–0.98). This was also reflected when a threshold of 2000 RNA copies/ml was chosen (AUC of 0.888; 95% CI: 0.78–0.996, p=0.0009, not shown). Together, these data show the robust ability of plasma IP-10 concentrations to predict different levels of viremia with good sensitivity and specificity. Using a cross-over plot to precisely identify the point where specificity and sensitivity intersect, an IP-10 concentration of 900ng/ml could predict viral loads above and below 5000 RNA copies/ml (Figure 1C). These data highlight that IP-10 is a suitable host marker for predicting viral load and may potentially be used instead of the latter assay. IP-10 has also been proposed as a useful biomarker for sputum clearance in TB patients [8] and there are reports showing that IP-10 is useful to track disease progression in HIV infected individuals5. Our data would concur with this and we extend the analysis to show a direct association with in vivo viral replication, as has been similarly shown in Hepatitis C infection9. When we extended the analysis to CD4 counts, we identified that there was no association between IP-10 and CD4 count changes over time, and ROC curve analysis showed an AUC of 0.58, with no significant predictive capability. Thus, although plasma IP-10 is an excellent proxy and predictor for viremia, it is unrelated to CD4 numbers.
Figure 1. Plasma IP-10 concentrations correlate and predict viral load in HIV-1 infected individuals.
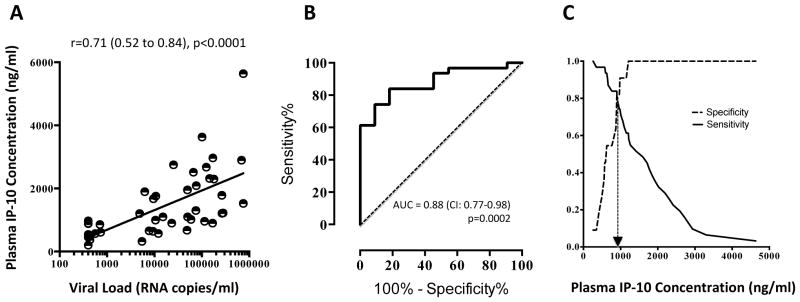
A. Spearman Rank correlation between IP-10 concentrations and Log10 RNA copies/ml; B. Receiver-Operating-Characteristic curve showing significant area under the curve for IP-10 above and below 5000 copies/ml; C. Specificity/sensitivity cross-over plots showing the intercept at which the concentration of IP-10 is predictive (denoted by hashed arrow line).
In summary, using ROC curve analysis, we have shown that IP-10 is a good predictor of viral load and is useful for tracking changes in viremia, including a response to ARV, and may be an alternative to the more costly viral load measurements. Whether there is a casual biological association between HIV and IP-10 is unclear, but we speculate that IP-10 is secreted as a direct effect of viral replication and activation of tumor necrosis factor alpha (TNF-α) and IFN-γ in host leukocytes1. As a result of this direct association, plasma IP-10 concentration can be used as an accurate proxy for viral load and could potentially be adapted to use as a point of care test. This is of great importance in a country such as South Africa, which has one of the largest ARV rollouts in the public health sector. Having a relatively quick colorimetric assay to measure this host-derived chemokine would obviate the need to send samples to regional or tertiary centres for viral load measurements.
Acknowledgments
We would like to thank Drs Landon Myer and Catherine Riou for useful discussions.
Footnotes
Financial Support & Conflicts of Interest:
HAH was a recipient of the National Research Foundation Scarce Skills Bursary, South Africa and a PHRI-Aurum Global Infectious Diseases Research Training Scholarship, (D43TW008264). This work was part-funded by the Poliomyelitis Research Foundation of South Africa, and in part by the following National Institute of Health (NIH) Fogarty Center Grant K01-TW00703-03 AI and the Canadian African Prevention Trials network grant to CMG.
References
- 1.Kaplan G, Luster AD, Hancock G, Cohn ZA. The expression of gamma interferon-induced protein (IP10) in delayed immune responses in human skin. JEM. 1987;166:1098–1108. doi: 10.1084/jem.166.4.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liovat AS, Rey-Cuille MA, Lecuroux C, Jacquelin B, Girault I, Petitjean G, et al. Acute plasma biomarkers of T cell activation set-point levels and of disease progression in HIV-1 infection. PLoS One. 2012;7:e46143. doi: 10.1371/journal.pone.0046143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grebely J, Feld JJ, Applegate T, Matthews GV, Hellard M, Sherker A, et al. Plasma interferon-gamma-inducible protein-10 (IP-10) levels during acute hepatitis C virus infection. Hepatology. 2013 Jan 16; doi: 10.1002/hep.26263. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cinque P, Bestetti A, Marenzi R, Sala S, Gisslen M, Hagberg L, et al. Cerebrospinal fluid interferon-gamma-inducible protein 10 (IP-10, CXCL10) in HIV-1 infection. J Neuroimmunol. 2005;168:154–163. doi: 10.1016/j.jneuroim.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Jiao Y, Zhang T, Wang R, Zhang H, Huang X, Yin J, et al. Plasma IP-10 is associated with rapid disease progression in early HIV-1 infection. Viral Immunol. 2012;25:333–337. doi: 10.1089/vim.2012.0011. [DOI] [PubMed] [Google Scholar]
- 6.Blish CA, McClelland RS, Richardson BA, Jaoko W, Mandaliya K, Baeten JM, et al. Genital Inflammation Predicts HIV-1 Shedding Independent of Plasma Viral Load and Systemic Inflammation. J Acquir Immune DeficSyndr. 2012;61:436–440. doi: 10.1097/QAI.0b013e31826c2edd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carsenti-Dellamonica H, Saidi H, Ticchioni M, Guillouet de Salvador F, DufayardCottalorda J, Garraffo R, et al. The suppression of immune activation during enfuvirtide-based salvage therapy is associated with reduced CCR5 expression and decreased concentrations of circulating interleukin-12 and IP-10 during 48 weeks of longitudinal follow-up. HIV Med. 2011;12:65–77. doi: 10.1111/j.1468-1293.2010.00848.x. [DOI] [PubMed] [Google Scholar]
- 8.Riou C, Perez Peixoto B, Roberts L, Ronacher K, Walzl G, Manca C, et al. Effect of standard tuberculosis treatment on plasma cytokine levels in patients with active pulmonary tuberculosis. PLoS One. 2012;7:e36886. doi: 10.1371/journal.pone.0036886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeremski M, Markatou M, Brown QB, Dorante G, Cunningham-Rundles S, Talal AH. Interferon gamma-inducible protein 10: a predictive marker of successful treatment response in hepatitis C virus/HIV-coinfected patients. J Acquir Immune Defic Syndr. 2007;45:262–268. doi: 10.1097/QAI.0b013e3180559219. [DOI] [PubMed] [Google Scholar]


