Abstract
Background
Patients with completely resected non-small cell lung cancer (NSCLC) have an excellent outcome; however tumor recurs in 30%–77% of patients. This study retrospectively analyzed the clinicopathologic features of patients with any operable stage of NSCLC to identify the prognostic factors that influence tumor recurrence, including intratumoral blood vessel invasion (IVI), tumor size, tumor necrosis, and intratumoral lymphatic invasion.
Methods
From January 2002 to December 2011, 227 consecutive patients were enrolled in this study. They were divided into two groups: the “no recurrence” group and the “recurrence” group. Recurrence-free survival was analyzed by multivariable Cox regression analysis, stratified by tumor staging, chemotherapy, and nodal involvement.
Results
IVI, tumor necrosis, tumor diameter more than 5 cm, and nodal involvement were identified as independent prognostic factors of tumor recurrence. The hazard ratio (HR) of patients with IVI was 2.1 times higher than that of patients without IVI (95% confident interval [CI]: 1.4–3.2) (P = 0.001).The HR of patients with tumor necrosis was 2.1 times higher than that of patients without tumor necrosis (95% CI: 1.3–3.4) (P = 0.001). Patients who had a maximum tumor diameter greater than 5 cm had significantly higher risk of recurrence than patients who had a maximum tumor diameter of less than 5 cm (HR 1.9, 95% CI: 1.0–3.5) (P = 0.033).
Conclusion
IVI, tumor diameter more than 5 cm, and tumor necrosis are prognostic factors of tumor recurrence in completely resected NSCLC. Therefore, NSCLC patients, with or without nodal involvement, who have one or more prognostic factors of tumor recurrence may benefit from adjuvant chemotherapy for prevention of tumor recurrence.
Keywords: intratumoral blood vessel invasion, recurrence, NSCLC
Introduction
Anatomical resection still remains the only method for curative treatment of non-small cell lung cancer (NSCLC) patients, not only in early stage (stage IA, IB and IIA, IIB), but also in a locally advanced stage (selected stage IIIA). Despite complete resection, recurrence occurs in the range of 30%–77%.1,2 The most important prognostic factor that may affect the tumor recurrence is nodal invasion; however previous studies have found other prognostic factors, such as intratumoral blood vessel invasion (IVI),3 intratumoral lymphatic invasion (ILI), visceral pleural invasion,4 or tumor size.5 Currently, there are no conclusions about prognostic factors for tumor recurrence in patients who have undergone a completely resected NSCLC. Also, the International Association for the Study of Lung Cancer (IASLC) does not include these factors in the TNM staging system.1 Patients who have one or more prognostic factors may benefit from adjuvant chemotherapy even when nodal involvement is negative. This study attempted to clarify the prognostic factors associated with tumor recurrence in completely resected NSCLC patients.
Patients and methods
Between January 2002 and December 2011, 227 patients underwent anatomical resection (lobectomy, sleeve lobectomy, bilobectomy, and pneumonectomy) with systematic mediastinal lymph node dissection at Chiang Mai University Hospital, Chiang Mai, Thailand. We retrospectively reviewed these 227 cases from the medical recording system with regard to patient characteristics, signs and symptoms, tumor pathology report, and follow-up status to examine the prognostic factors of tumor recurrence in all of the completely resected NSCLC patients. In the preoperative evaluation, standard laboratory tests, such as complete blood count (CBC), liver and renal function test, electrolytes, posteroanterior and lateral chest film, computed tomographic (CT) scan of the thorax and the upper abdomen, pulmonary function test, and bronchoscopy, were obtained for all of the patients. Bone scan and CT of the brain were obtained only in patients who had evidence of bone or brain metastasis. No positron emission tomography (PET) was done in the routine workup because this was not easily available. Patients who received neoadjuvant or adjuvant chemotherapy were included in this study. Patients were excluded from this study if they: had a single brain metastasis and underwent a craniectomy to remove their tumor before pulmonary resection (five patients); had evidence of residual tumor at the resection margin (five patients); or died within first 30 day of the surgery (postoperative mortality) (three patients). Standard anatomical resection (lobectomy, bilobectomy, or pneumonectomy) with systematic mediastinal lymph node dissection were performed in all cases, and all nodal stations were labeled according to the staging manual in Thoracic Oncology.6
All excised specimens were formalin-fixed and sliced at 10 mm intervals. Histopathologic examination was performed by the same pathologist. Pathologic staging was determined according to the IASLC TNM staging classification of NSCLC.7 Histologic subtypes of lung cancer were determined according to World Health Organization classification8 and IASLC/American Thoracic Society (ATS)/European Respiratory Society (ERS) International Multidisciplinary Classification of Lung Adenocarcinoma.1 Visceral pleural invasion was defined as evidence of penetration of thick outer elastic lamina by tumor during elastic tissue staining. The presence of IVI was defined by the identification of conspicuous findings of intravascular cancer clusters surrounded by an elastic layer at the maximum section of the primary lesion. The presence of ILI was defined by the identification of cancer cells within the lymphatic vessel lumen. Tumor necrosis was defined as coagulative necrosis identifiable in the tumor (macroscopic or microscopic finding). Tumor involvement of the epineurium was defined as perineural invasion.9
All patients were actively followed postoperatively at 2 weeks and at 3- to 6-month intervals for the first 2 years and yearly thereafter, with a CT scan of the chest and upper abdomen. Patients who had pathological nodal involvement (stages IIA, IIB, IIIA, and IIIB) received adjuvant chemotherapy. If patients developed signs or symptoms that correlated with tumor recurrence or metastasis, they would be worked up according to their signs or symptoms (ie, CT brain or bone scan). Tumor recurrence was defined as the evidence of tumor within the same lobe, the hilum, or the mediastinal lymph nodes (locoregional recurrence), or evidence of tumor in another lobe or elsewhere outside the hemithorax (distant recurrence). The interval to recurrence was defined as the interval between the time of the operation and the discovery of the recurrence by means of either imaging or cytopathalogic examination.
Patients were divided into two groups: the “no recurrence” group and the “recurrence” group. Categorical variables were expressed as count and percent and were analyzed by univariable analysis, using the Fisher exact test. Continuous variables were expressed as mean and standard deviation (SD) and were analyzed by univariable analysis, using the Student t-test. The recurrence-free survival curves were estimated using the Kaplan–Meier method. Tumor recurrence was expressed by using “time zero” as the date of surgery and recurrence as the end point. Comparison of recurrence-free survival between both groups was investigated using a Cox multivariable regression model stratified by stage of disease, chemotherapy, and nodal involvement. We stratified the model by the stage of disease and chemotherapy because we already knew that these factors influence tumor recurrence and we wanted to make comparisons between stages, different uses of chemotherapy, and between different patterns of nodal involvement. Univariable prognostic factors significant at the 0.10 level were considered for the multivariable models, and stepwise regression was used. All tests were two-tailed and performed with commercial statistical software (STATA 11.0; StataCorp LP, College Station, TX, USA).
This study was reviewed and approved by the Research Ethics Committee, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
Results
Table 1 summarizes the patient population for the groups with and without tumor recurrence. Tumor recurrence was identified in 120 patients, whereas 107 did not have tumor recurrence. There was no statistically significant difference in any of the patient characteristic variables. Nearly 50% in both groups of patients presented with chronic cough and nonmassive hemoptysis.
Table 1.
Patient characteristic between two groups
| Characteristics | Nonrecurrence (n = 107) | Recurrence (n = 120) | P-value |
|---|---|---|---|
| Age, mean ± SD | 61.4 ± 10.5 | 62.8 ± 10.5 | 0.322 |
| Male, n (%) | 64 (59.8) | 69 (57.5) | 0.788 |
| Smoking | 0.820 | ||
| Never smoked | 30 (28.0) | 30 (25.0) | |
| Stopped smoking | 71 (66.4) | 80 (66.7) | |
| Active smoker | 5 (4.7) | 7 (5.8) | |
| Passive smoker | 1 (0.9) | 3 (2.5) | |
| Packs per year, mean ± SD | 19.1 ± 16.9 | 19.1 ± 17.7 | 0.863 |
| Family history of malignancy | 7 (6.5) | 5 (4.2) | 0.555 |
| Underlying disease | |||
| Chronic lung disease | 14 (13.1) | 15 (12.5) | 1.000 |
| Diabetic mellitus | 12 (11.2) | 15 (12.5) | 0.839 |
| Essential hypertension | 34 (31.8) | 43 (35.8) | 0.575 |
| Dyslipidemia | 18 (16.8) | 15 (12.5) | 0.451 |
| Symptoms | |||
| Hemoptysis | 48 (44.9) | 48 (40.0) | 0.502 |
| Chronic cough | 48 (44.9) | 52 (43.3) | 0.894 |
| Poor appetite | 16 (15.0) | 15 (12.5) | 0.699 |
| Significant weight loss | 33 (30.8) | 30 (25.0) | 0.374 |
| Chest pain | 9 (8.4) | 11 (9.2) | 1.000 |
| Dyspnea | 17 (15.9) | 25 (20.8) | 0.393 |
| Asymptomatic | 38 (35.5) | 43 (35.8) | 1.000 |
Abbreviation: SD, standard deviation.
The surgical procedures consisted of 197 lobectomies (86.8%), 26 bilobectomies (11.5%), and four pneumonectomies (1.8%). A total of 104 patients (45.8%) received adjuvant chemotherapy because of diagnosed pathological nodal involvement (stage IIA, IIB, IIIA, or IIIB). Six patients (2.6%) received neoadjuvant chemotherapy because of N2 disease preoperatively diagnosed by mediastinoscopy. The summary of chemotherapy use between both groups was shown in Table 2. No patients were treated with postoperative radiotherapy. The histopathologic results are summarized in Table 3. There were no intraoperative deaths. There were no statistically significant differences in the surgical procedure, chemotherapy, histologic types, tumor grading, pathological stage, tumor diameter, visceral pleural invasion, and neural invasion. Univariable analysis showed that the stage of disease, nodal involvement, and IVI were associated with tumor recurrence (P = 0.041, P = 0.003, and P < 0.001, respectively). Median follow-up was 19 months (range, 0.7–144.9 months) in the nonrecurrent group and 24.5 months (range, 1.6–97.4 months) in the recurrent group. The overall mean interval to recurrence after the operation was 14.2 ± 13.9 months (range, 0.8–65.2 months). The sites of metastases are shown in Table 4. The lung was the most common metastatic site (49.1%), and the brain was the second most common (25.8%). The 2-year recurrence-free survival was 50.0% (95% confidence interval [CI]: 0.4%–0.6%), and the 5-year recurrence-free survival was 31.5% (95% CI: 0.2%–0.4%). The overall cumulative 2- and 5-year survival was 61.9% (95% CI: 0.5%–0.7%) and 31.5% (95% CI: 0.2%–0.4%), respectively. The mean survival was 32 months in the nonrecurrent group and was 24 months in the recurrent group.
Table 2.
Treatment modalities
| Procedure and chemotherapy | Nonrecurrence (n = 107 ) | Recurrence (n = 120) | P-value |
|---|---|---|---|
| Surgical procedures | 0.419 | ||
| Lobectomy | 89 (83.2) | 108 (90.0) | |
| Bilobectomy (RUL and RML) | 3 (2.8) | 3 (2.5) | |
| Bilobectomy (RLL and RML) | 12 (11.2) | 8 (6.7) | |
| Pneumonectomy | 3 (2.8) | 1 (0.8) | |
| Chemotherapy | 0.103 | ||
| No chemotherapy | 63 (58.9) | 54 (45.0) | |
| Adjuvant chemotherapy | 42 (39.3) | 62 (51.7) | |
| Neoadjuvant chemotherapy | 2 (1.9) | 4 (3.3) |
Abbreviations: RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe.
Table 3.
Histopathologic reports
| Covariates | Nonrecurrence (n = 107 ) | Recurrence (n = 120 ) | P-value |
|---|---|---|---|
| Histologic types | 0.848 | ||
| Adenocarcinoma | 61 (57.0) | 72 (60.0) | |
| Squamous cell carcinoma | 29 (27.1) | 32 (26.7) | |
| Others* | 17 (15.9) | 16 (13.3) | |
| Tumor grading | 0.644 | ||
| Well differentiated | 34 (31.8) | 48 (40.0) | |
| Moderately differentiated | 45 (42.1) | 44 (36.7) | |
| Poorly differentiated | 18 (16.8) | 22 (18.3) | |
| Undifferentiated | 4 (3.7) | 3 (2.5) | |
| Mucinous type of adenocarcinoma in situ | 3 (2.8) | 2 (1.7) | |
| Nonmucinous type of adenocarcinoma in situ | 3 (2.8) | 1 (0.8) | |
| Pathological staging | 0.041 | ||
| IA | 20 (18.7) | 13 (10.8) | |
| IB | 25 (23.4) | 21 (17.5) | |
| IIA | 17 (15.9) | 21 (17.5) | |
| IIB | 19 (17.8) | 12 (10.0) | |
| IIIA | 24 (23.4) | 51 (42.5) | |
| IIIB | 1 (0.9) | 2 (1.7) | |
| Tumor diameter (cm) | 0.325 | ||
| ≤5 | 75 (70.1) | 76 (63.3) | |
| >5 | 32 (29.9) | 44 (36.7) | |
| Nodal involvement | 0.003 | ||
| Nodal negative | 72 (67.3) | 57 (47.5) | |
| Nodal positive# | 35 (32.7) | 63 (52.5) | |
| Tumor necrosis | 36 (33.6) | 56 (46.7) | 0.058 |
| Visceral pleural invasion | 19 (17.8) | 29 (24.2) | 0.258 |
| Neural invasion | 3 (2.8) | 6 (5.0) | 0.506 |
| Intratumoral lymphatic invasion | 84 (78.5) | 104 (86.7) | 0.115 |
| Intratumoral blood vessel invasion | 32 (29.9) | 64 (53.3) | <0.001 |
Notes:
Other cell types include adenocarcinoma in situ,24 large cell carcinoma, neuroendocrine tumor, adenoid cystic carcinoma, metastasis, mucoepidermoid carcinoma, lymphoepithelioma-like carcinoma, adenosquamous cell carcinoma;
nodal positive refers to the presence of malignant cells, in any node level (1–14).
Table 4.
Sites of metastases
| Sites | Number of patients | % |
|---|---|---|
| Lung | 59 | 49.1 |
| Brain | 31 | 25.8 |
| Bone | 17 | 14.2 |
| Liver | 6 | 5.0 |
| Supraclavicular lymph node | 5 | 4.2 |
| Skin | 5 | 4.2 |
| Adrenal gland | 4 | 3.3 |
| Pleura | 4 | 3.3 |
| Mediastinal lymph node | 3 | 2.5 |
| Chest wall | 2 | 1.7 |
| Kidney | 1 | 0.8 |
| Cervical lymph node | 1 | 0.8 |
| Stomach | 1 | 0.8 |
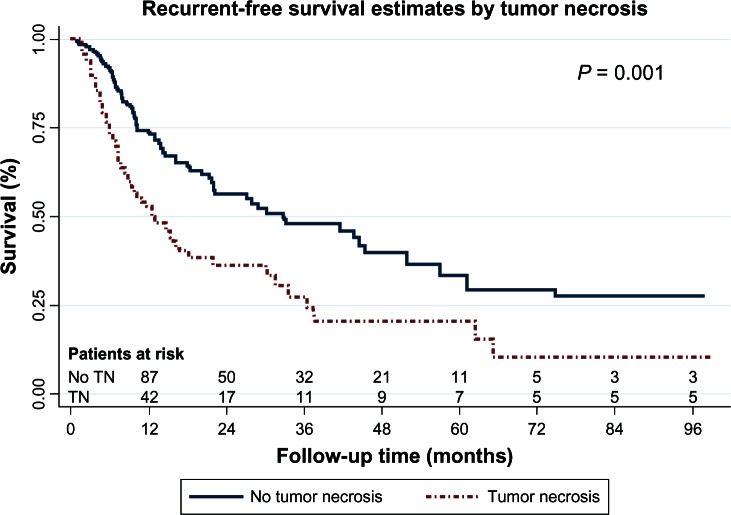
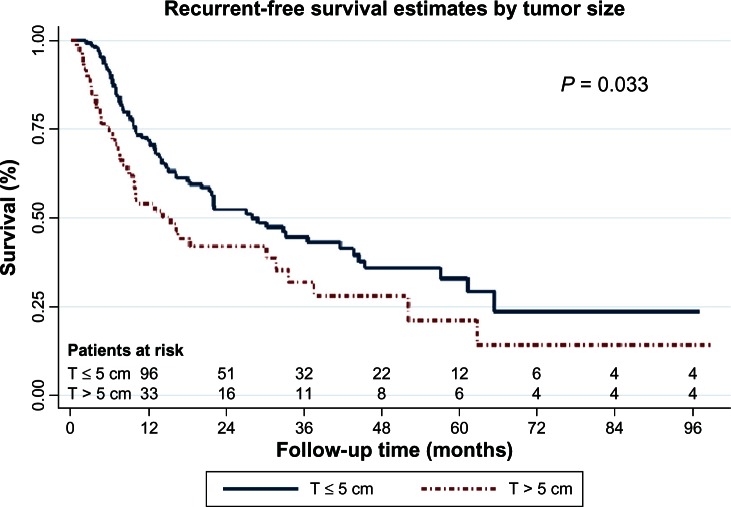
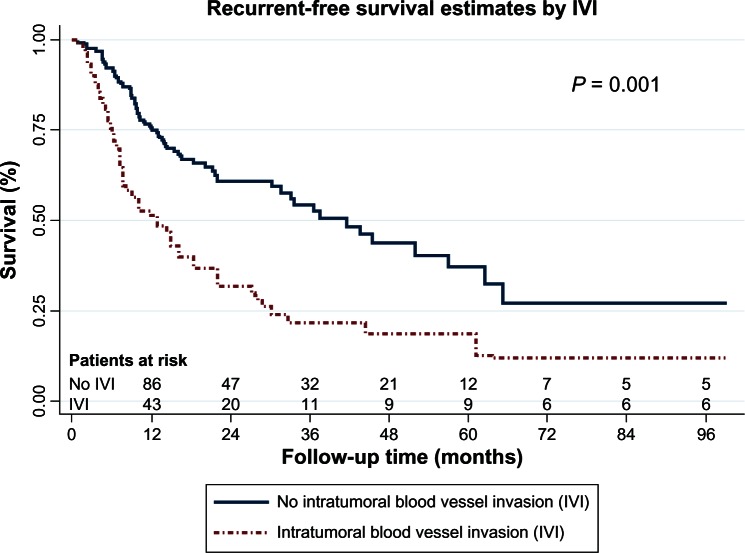
Multivariable analysis demonstrated that tumor necrosis, IVI, and tumor diameter greater than 5 cm were the only independent prognostic factors for tumor recurrence, as shown in Table 5. The recurrence-free survival curves stratified according to tumor necrosis, IVI, and tumor diameter are shown in Figures 1 to 3 respectively.
Table 5.
Multivariable Cox regression analysis stratified by tumor staging, chemotherapy treatment, and nodal involvement
| Covariates | Hazard ratio | 95% confidence interval | P-value |
|---|---|---|---|
| Age | 1.0 | 0.9–1.0 | 0.556 |
| Male | 1.0 | 0.7–1.5 | 0.837 |
| Tumor necrosis | 2.1 | 1.3–3.4 | 0.001 |
| Intratumoral blood vessel invasion | 2.1 | 1.4–3.2 | 0.001 |
| Intratumoral lymphatic invasion | 1.0 | 0.5–1.7 | 0.825 |
| Tumor diameter >5 cm | 1.9 | 1.0–3.5 | 0.033 |
Figure 1.
Recurrent-free survival curves by tumor necrosis.
Abbreviation: TN, tumor necrosis.
Figure 3.
Recurrent-free survival curves by tumor size.
Abbreviation: T, maximal tumor diameter (cm)
Discussion
The seventh edition of IASLC staging of lung cancer1 seems powerful in determining prognosis; however, many studies have tried to identify other prognostic factors of tumor recurrence in patients with completely resected NSCL patients. Histopathological examination may play an important role in this.10 There are several histopathological factors that affect tumor recurrence and survival, and which have been identified as prognostic factors, such as IVI,3,9,11,12 visceral pleural invasion,12–17 perineural invasion,10,18 mitotic index and nuclear atypia,19 and histologic grade.20
The results of this study found that IVI, tumor necrosis, tumor size greater than 5 cm, and nodal involvement affected tumor recurrence in any stage of completely resected NSCLC and that IVI was the only prognostic factor of tumor recurrence in completely resected stage I NSCLC. There have been some previously published studies on the topic of IVI. Yilmaz et al9 reported that lymphovascular invasion can show a higher risk of mortality; however, they did not separate lymphatic invasion from vascular invasion, therefore, we did not learn how either lymphatic invasion or vascular invasion exactly affects tumor recurrence. Pechet et al3 concluded that the presence of arterial invasion in stage I NSCLC patients was adversely associated with poor survival (hazard ratio [HR], 3.5) (P < 0.001). However, this study only studied and showed the survival rates of patients with stage I NSCLC. Miyoshi et al12 and Shoji et al21 concluded that IVI was independent prognostic factor in pathological stage I NSCLC patients. In the contrast, other work has not shown relevant prognostic factors.10 Currently, there are no firm conclusions about the role of IVI and tumor recurrence. In our study, we included all completely resected NSCLC and used a multivariable Cox proportional hazard model, as previously described, to identify the prognostic factor for tumor recurrence. The results showed that IVI is a strongly prognostic factor for tumor recurrence (HR, 2.1; 95% CI: 1.4–3.2) (P = 0.001), in any stage of completely resected NSCLC.
Among the tumor factors, the maximum tumor diameter is an available prognostic factor based on gross specimen. The seventh edition of the IASLC classification of lung cancer adopted a diameter of 50 mm as the threshold of stratification between T2a and T2b, for improvement of the ability to provide a differential prognosis. However, some previous studies have shown no significance of tumor size for prognosis in resected (p-)stage I NSCLC, by multivariable analysis.5,22,23 One explanation is that even in a large adenocarcinoma in situ (a subtype of adenocarcinoma according to the IASLC/ATS/ERS Classification of Lung Adenocarcinoma),24 growth is slow and there is a lack of involved stroma and vessels; thus even despite a large size, this subtype has been known to have a good clinical outcome.25 However, other work demonstrated that tumor size can predict survival in stage I and II NSCLC.26 Our study showed that a maximal tumor diameter of greater than 5 cm was associated with tumor recurrence (HR, 1.9; 95% CI: 1.0–3.5) (P = 0.033). This result was not contradicted by the characteristics of large adenocarcinoma in situ as described above, because we collected the data by separating adenocarcinoma in situ from adenocarcinoma. Our result supported the clinical relevance of tumor size as a prognostic factor for recurrence of completely resected NSCLC patients.
Tumor necrosis has not been mentioned as a prognostic factor of tumor recurrence before; however, in our study, this was shown to be a prognostic factor for tumor recurrence (HR, 2.1; 95% CI: 1.3–3.4) (P = 0.001). There was also a correlation between tumor size and tumor necrosis. Nearly 64% of the tumors with a size greater than 5 cm had the tumor necrosis, whereas 30% of the tumors with size less than 5 cm had tumor necrosis. The reason large tumors had more tumor necrosis was that there was a smaller vascular supply or blood vessels in the central part of the tumor; therefore, large tumors had a greater chances of presenting with tumor necrosis than did small ones.
Visceral pleural invasion has been recognized to be another strong prognostic factor associated with increased risk of recurrence and death;5,13,23,27–30 however, some researchers have found otherwise,4 and our study did not find any correlation with tumor recurrence.
ILI was previously reported to be a prognostic factor for tumor recurrence and survival, especially in completely resected stage I NSCLC patient;31 however our study did not show a significant correlation (HR, 1.0; 95% CI: 0.5–1.7) (P = 0.825).
Although the survival benefit of adjuvant therapy for resected NSCLC has been expected in some trials32,33 it is, so far, controversial with regard to stage I disease. Shoji et al21 reported that distant recurrence was observed in patients with stage IA or IB NSCLC with IVI more frequently than in those without IVI, and they suggested that patient in these groups seemed to be candidates for adjuvant chemotherapy. Bodendorf et al34 reported that the histologic evidence of IVI was more often followed by distant metastases than local recurrence and should be considered as an indication for adjuvant chemotherapy. The current series also supported the findings of their study.
Previously, five of the largest adjuvant trials35 to date were included in the Lung Adjuvant Cisplatin Evaluation (LACE) meta-analysis.36 Of the five, two trials – the National Cancer Institute of Canada (NCIC) JBR. 10 and the Adjuvant Navelbine® International Trialist Association (ANITA) – exclusively examined cisplatin-vinorelbine combinations, while the other three – the Big Lung Trial (BLT),39 the International Trialist Association Trial (IALT),32 and the Adjuvant Lung Project Italy (ALPI)40 – allowed investigator choice of cisplatin-based regimens. The adjuvant trial demonstrating the most striking benefit, NCIC JBR.10,37 included 482 patients with completely resected stage IB or II NSCLC, randomly assigned to observation or four cycles of weekly cisplatin 50 mg/m2 on days 1 and 8 plus vinorelbine 25 mg/m2 on days 1, 8, 15, 22, of a 28-day regimen. The ANITA trial38 evaluated adjuvant chemotherapy for 840 patients with completely resected stage IB–IIIA NSCLC, using cisplatin 100 mg/m2 day 1 (4 doses) plus vinorelbine at 30 mg/m2 on days 1, 8, 15, and 22 (16 doses), for four cycles, versus observation. Both the NCIC JBR.10 and the ANITA trials showed an overall survival benefit (HR 0.69 [P = 0.004] and HR 0.80 [P = 0.017], respectively); the survival benefit did not diminish over time and was 8.4% at the 7-year follow-up for ANITA trial.38 Alternately, the BLT,39 IALT,32 and ALPI39 trials evaluated an investigator-chosen cisplatin-based regimen. These trials demonstrated an overall survival benefit at 5 years, but not at 7 years. Why a long-term overall survival benefit was not achieved remains an interesting question.
Targeting pathways has shown benefit for patients who express particular gene mutation, for example, epidermal growth factor receptor (EGFR) gene mutation. The BR.19 trial40 randomized 503 patients with completely resected stage IB–IIIA NSCLC (only 21% of whom had EGFR mutations) to gefitinib versus placebo, but this trial was halted early because the interim analysis of the S0023 SWOG trial42 demonstrated that maintenance gefitinib was associated with a worse survival than placebo, after concurrent chemoradiation for stage III NSCLC. Recently, the Randomized Double-Blinded Trial in Adjuvant NSCLC with Tarceva® (RADIANT) trial was begun to evaluate the role of the EGFR-tyrosine kinase inhibitor (TKI) erlotinib in the adjuvant treatment of 945 patients with completely resected stage IB-IIIA NSCC whose tumors have overexpression of EGFR detected by immunohistochemistry or fluorescence in situ hybridization (FISH). In this 2-year trial, patients have been randomized in a 2:1 ratio to erlotinib or placebo and may have up to four cycles of chemotherapy after surgery. Patients randomized to erlotinib began 6 months from the day of surgery for patients who get chemotherapy and 3 months from the day of surgery for those who do not get chemotherapy. The RADIANT trial is ongoing, but no longer recruiting participants.
Clinical trials of adjuvant therapy in well-selected populations with completely resected early stage NSCLC presenting with poor prognostic factors are needed. IVI should be considered as one of the prognostic factors for tumor recurrence in completely resected early stage NSCLC and should be considered in future trials of adjuvant therapy. Furthermore, tumor size greater than 5 cm and tumor necrosis were also found to be prognostic for tumor recurrence; therefore, patients who have a maximal diameter geater than 5 cm or presenting with tumor necrosis or intratumoral blood vessel invasion without nodal involvement may also benefit from adjuvant chemotherapy.
One limitation of this study is its retrospective nature. Although there was missing data, we think that missing data randomly occurred in both comparison groups. We also controlled the quality of data by doing a quality audit, and we found that the data quality was respectable.
Conclusion
Our analyses indicated intratumoral blood vessel invasion, tumor diameter more than 5 cm, and tumor necrosis are prognostic factors of tumor recurrence, in any stage of completely resected NSCLC. Therefore, NSCLC patients with or without nodal involvement who have one of the prognostic factors of tumor recurrence may benefit from adjuvant chemotherapy for prevention of tumor recurrence. Further clinical trials are needed to support this hypothesis.
Figure 2.
Recurrent-free survival curves by intratumoral blood vessel invasion (IVI).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Goldstraw P, Crowley J, Chansky K, et al. International Association for the Study of Lung Cancer International Staging Committee. Participating Institutions The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2(8):706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 2.Subotic D, Mandaric D, Radosavljevic G, Stojsic J, Gajic M, Ercegovac M. Relapse in resected lung cancer revisited: does intensified follow up really matter? A prospective study. World J Surg Oncol. 2009;7:87. doi: 10.1186/1477-7819-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pechet TT, Carr SR, Collins JE, Cohn HE, Farber JL. Arterial invasion predicts early mortality in stage I non-small cell lung cancer. Ann Thorac Surg. 2004;78(5):1748–1753. doi: 10.1016/j.athoracsur.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 4.Schuchert MJ, Schumacher L, Kilic A, et al. Impact of angiolymphatic and pleural invasion on surgical outcomes for stage I non-small cell lung cancer. Ann Thorac Surg. 2011;91(4):1059–1065. doi: 10.1016/j.athoracsur.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 5.Ichinose Y, Yano T, Asoh H, Yokoyama H, Yoshino I, Katsuda Y. Prognostic factors obtained by a pathologic examination in completely resected non-small-cell lung cancer. An analysis in each pathologic stage. J Thorac Cardiovasc Surg. 1995;110(3):601–605. doi: 10.1016/S0022-5223(95)70090-0. [DOI] [PubMed] [Google Scholar]
- 6.Goldstraw P, editor. IASLC Staging Manual in Thoracic Oncology. Denver, CO: John Wiley & Sons Ltd; 2009. [Google Scholar]
- 7.Goldstraw P. The 7th edition of TNM in lung cancer: what now? J Thorac Oncol. 2009;4(6):671–673. doi: 10.1097/JTO.0b013e31819e7814. [DOI] [PubMed] [Google Scholar]
- 8.Travis WD BE, Brambilla E, Müller-Hermelink HK, Harris CC, editors. World Health Organization Classification of Tumors Pathology and Genetics: Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press; 2004. [Google Scholar]
- 9.Yilmaz A, Duyar SS, Cakir E, et al. Clinical impact of visceral pleural, lymphovascular and perineural invasion in completely resected non-small cell lung cancer. Eur J Cardiothorac Surg. 2011;40(3):664–670. doi: 10.1016/j.ejcts.2010.12.059. [DOI] [PubMed] [Google Scholar]
- 10.Sayar A, Turna A, Solak O, Kiliçgün A, Urer N, Gürses A. Nonanatomic prognostic factors in resected nonsmall cell lung carcinoma: the importance of perineural invasion as a new prognostic marker. Ann Thorac Surg. 2004;77(2):421–425. doi: 10.1016/S0003-4975(03)01645-X. [DOI] [PubMed] [Google Scholar]
- 11.Tsuchiya T, Hashizume S, Akamine S, et al. Upstaging by vessel invasion improves the pathology staging system of non-small cell lung cancer. Chest. 2007;132(1):170–177. doi: 10.1378/chest.06-1950. [DOI] [PubMed] [Google Scholar]
- 12.Miyoshi K, Moriyama S, Kunitomo T, Nawa S. Prognostic impact of intratumoral vessel invasion in completely resected pathologic stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137(2):429–434. doi: 10.1016/j.jtcvs.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu K, Yoshida J, Nagai K, et al. Visceral pleural invasion is an invasive and aggressive indicator of non-small cell lung cancer. J Thorac Cardiovasc Surg. 2005;130(1):160–165. doi: 10.1016/j.jtcvs.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 14.Ou SH, Zell JA, Ziogas A, Anton-Culver H. Prognostic significance of the non-size-based AJCC T2 descriptors: visceral pleura invasion, hilar atelectasis, or obstructive pneumonitis in stage IB non-small cell lung cancer is dependent on tumor size. Chest. 2008;133(3):662–669. doi: 10.1378/chest.07-1306. [DOI] [PubMed] [Google Scholar]
- 15.Osaki T, Nagashima A, Yoshimatsu T, Yamada S, Yasumoto K. Visceral pleural involvement in nonsmall cell lung cancer: prognostic significance. Ann Thorac Surg. 2004;77(5):1769–1773. doi: 10.1016/j.athoracsur.2003.10.058. [DOI] [PubMed] [Google Scholar]
- 16.Inoue M, Minami M, Shiono H, Sawabata N, Ideguchi K, Okumura M. Clinicopathologic study of resected, peripheral, small-sized, non-small cell lung cancer tumors of 2 cm or less in diameter: pleural invasion and increase of serum carcinoembryonic antigen level as predictors of nodal involvement. J Thorac Cardiovasc Surg. 2006;131(5):988–993. doi: 10.1016/j.jtcvs.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 17.Mulligan CR, Meram AD, Proctor CD, Wu H, Zhu K, Marrogi AJ. Lung cancer staging: a case for a new T definition. Ann Thorac Surg. 2006;82(1):220–226. doi: 10.1016/j.athoracsur.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 18.Poncelet AJ, Cornet J, Coulon C, Collard P, Noirhomme P, Weynand B, groupe d’oncologie thoraciques des Cliniques Saint Luc Intra-tumoral vascular or perineural invasion as prognostic factors for long-term survival in early stage non-small cell lung carcinoma. Eur J Cardiothorac Surg. 2008;33(5):799–804. doi: 10.1016/j.ejcts.2008.01.060. [DOI] [PubMed] [Google Scholar]
- 19.Takise A, Kodama T, Shimosato Y, Watanabe S, Suemasu K. Histopathologic prognostic factors in adenocarcinomas of the peripheral lung less than 2 cm in diameter. Cancer. 1988;61(10):2083–2088. doi: 10.1002/1097-0142(19880515)61:10<2083::aid-cncr2820611025>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Ou SH, Zell JA, Ziogas A, Anton-Culver H. Prognostic factors for survival of stage I nonsmall cell lung cancer patients : a population-based analysis of 19,702 stage I patients in the California Cancer Registry from 1989 to 2003. Cancer. 2007;110(7):1532–1541. doi: 10.1002/cncr.22938. [DOI] [PubMed] [Google Scholar]
- 21.Shoji F, Haro A, Yoshida T, et al. Prognostic significance of intratumoral blood vessel invasion in pathologic stage IA non-small cell lung cancer. Ann Thorac Surg. 2010;89(3):864–869. doi: 10.1016/j.athoracsur.2009.09.047. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki K, Nagai K, Yoshida J, et al. Conventional clinicopathologic prognostic factors in surgically resected nonsmall cell lung carcinoma. A comparison of prognostic factors for each pathologic TNM stage based on multivariate analyses. Cancer. 1999;86(10):1976–1984. doi: 10.1002/(sici)1097-0142(19991115)86:10<1976::aid-cncr14>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa T, Okumura N, Kokado Y, Miyoshi K, Matsuoka T, Kameyama K. Clinical relevance of intraoperative pleural lavage cytology in non-small cell lung cancer. Ann Thorac Surg. 2007;83(1):204–208. doi: 10.1016/j.athoracsur.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 24.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6(2):244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer. 1995;75(12):2844–2852. doi: 10.1002/1097-0142(19950615)75:12<2844::aid-cncr2820751209>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal M, Brahmanday G, Chmielewski GW, Welsh RJ, Ravikrishnan KP. Age, tumor size, type of surgery, and gender predict survival in early stage (stage I and II) non-small cell lung cancer after surgical resection. Lung Cancer. 2010;68(3):398–402. doi: 10.1016/j.lungcan.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki K, Nagai K, Yoshida J, Nishimura M, Nishiwaki Y. Predictors of lymph node and intrapulmonary metastasis in clinical stage IA non-small cell lung carcinoma. Ann Thorac Surg. 2001;72(2):352–356. doi: 10.1016/s0003-4975(01)02748-5. [DOI] [PubMed] [Google Scholar]
- 28.Harpole DH, Jr, Herndon JE, 2nd, Young WG, Jr, Wolfe WG, Sabiston DC., Jr Stage I nonsmall cell lung cancer. A multivariate analysis of treatment methods and patterns of recurrence. Cancer. 1995;76(5):787–796. doi: 10.1002/1097-0142(19950901)76:5<787::aid-cncr2820760512>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 29.Matsuguma H, Nakahara R, Igarashi S, et al. Pathologic stage I non-small cell lung cancer with high levels of preoperative serum carcinoembryonic antigen: clinicopathologic characteristics and prognosis. J Thorac Cardiovasc Surg. 2008;135(1):44–49. doi: 10.1016/j.jtcvs.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 30.Ohta Y, Waseda R, Minato H, et al. Surgical results in T2N0M0 nonsmall cell lung cancer patients with large tumors 5 cm or greater in diameter: what regulates outcome? Ann Thorac Surg. 2006;82(4):1180–1184. doi: 10.1016/j.athoracsur.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 31.Harada M, Hato T, Horio H. Intratumoral lymphatic vessel involvement is an invasive indicator of completely resected pathologic stage I non-small cell lung cancer. J Thorac Oncol. 2011;6(1):48–54. doi: 10.1097/JTO.0b013e3181f8a1f1. [DOI] [PubMed] [Google Scholar]
- 32.Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J, International Adjuvant Lung Cancer Trial Collaborative Group Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350(4):351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 33.Kato H, Ichinose Y, Ohta M, Japan Lung Cancer Research Group on Postsurgical Adjuvant Chemotherapy A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med. 2004;350(17):1713–1721. doi: 10.1056/NEJMoa032792. [DOI] [PubMed] [Google Scholar]
- 34.Bodendorf MO, Haas V, Laberke HG, Blumenstock G, Wex P, Graeter T. Prognostic value and therapeutic consequences of vascular invasion in non-small cell lung carcinoma. Lung Cancer. 2009;64(1):71–78. doi: 10.1016/j.lungcan.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 35.Patel MI, Wakelee HA. Adjuvant chemotherapy for early stage non-small cell lung cancer. Front Oncol. 2011;1:45. doi: 10.3389/fonc.2011.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008 Jul 20;26(21):3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 37.Winton T, Livingston R, Johnson D, et al. National Cancer Institute of Canada Clinical Trials Group. National Cancer Institute of the United States Intergroup JBR 10 Trial Investigators Vinorelbine plus cisplatin vs observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352(25:):2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 38.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB–IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7(9):719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 39.Waller D, Peake MD, Stephens RJ, et al. Chemotherapy for patients with non-small cell lung cancer: the surgical setting of the Big Lung Trial. Eur J Cardiothorac Surg. 2004;26(1):173–182. doi: 10.1016/j.ejcts.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 40.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 41.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366(9496):1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 42.Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol. 2008 May 20;26(15):2450–2456. doi: 10.1200/JCO.2007.14.4824. [DOI] [PubMed] [Google Scholar]