Abstract
A series of self-immolative boronic ester protected methyl salicylates and metal-binding groups with various linking strategies have been investigated for their use in the design of matrix metalloproteinase proinhibitors.
Prodrugs are a class of therapeutics that can be activated in vivo to generate an active drug, providing targeted inhibitory activity and thereby reducing side effects.1, 2 Approximately 6% of all drugs approved worldwide can be classified as prodrugs, including a handful of metalloprotein inhibitors.2–5 Recent efforts in prodrug strategies for metalloprotein inhibitors have focused largely on redox mechanisms for activation.6–10 However, general approaches for developing prodrugs that target metalloproteins has not been widely investigated.
In an effort to develop broadly applicable methods to metalloprotein prodrugs, we have focused on developing ‘proinhibitors’ of the zinc(II)-dependent matrix metalloproteinases (MMPs), a canonical metalloprotein target of medicinal interest.11, 12 MMP inhibitors (MMPi), like most metalloprotein inhibitors, generally employ a metal-binding group (MBG), which if blocked abolishes inhibitory activity. Prodrug matrix metalloproteinase inhibitors (‘proMMPi’) have been developed using enzymatic activation or activation by reactive oxygen species (ROS).13–15 The development of ROS-activated proMMPi proved particularly intriguing, because: a) these were the first ROS-activated prodrugs of any kind reported, and b) these proMMPi can simultaneously result in targeted delivery of an MMPi while scavenging tissue-damaging ROS.15 This ‘dual mode’ of action is particularly relevant to ischemia-reperfusion injury associated with stroke, where an increase in ROS (e.g. H2O2) and the concurrent activation of MMPs during the inflammatory response leads to the breakdown of the protective blood-brain barrier.16–18
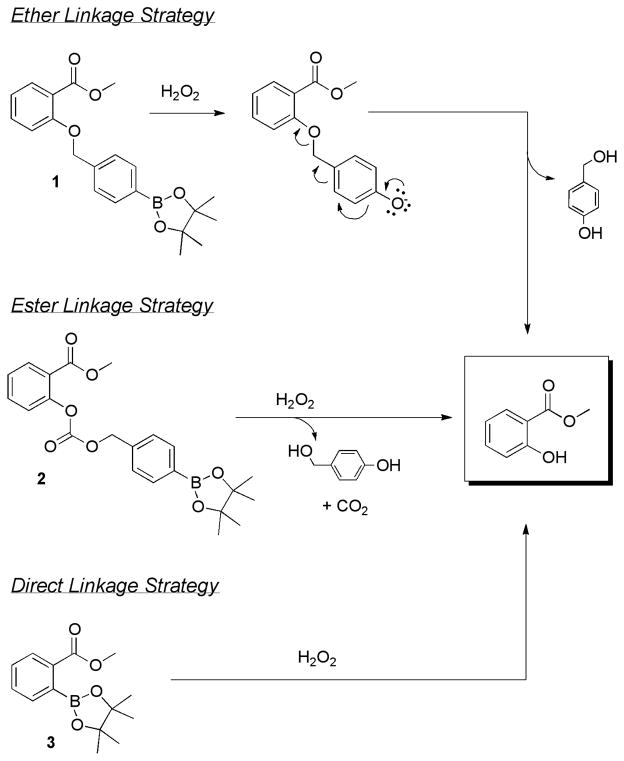
In the development of ROS-activated proMMPi, we employed a relatively underutilized self-immolative protecting group with several apparent advantages over previously described systems. The use of self-immolative linkers has become increasingly popular in drug development, molecular sensors, and polymeric delivery systems.1, 19–21 Linkers that undergo self-immolative elimination upon removal of the protecting group can release an active species through a 1,6-benzyl elimination (Fig. 1). This reaction is thermodynamically driven by the release of CO2 when a carbonate or carbamate ester linkage is employed.21–23 However, in the development of proMMPi, it was found that the use of an ether linkage between the activating group and the inhibitor was preferred over the more commonly used carbonate ester linkage (compare compound 1 vs. 2 in Fig. 1) due to better synthetic accessibility, superior hydrolytic stability, and comparably fast cleavage kinetics. Recently, this ether linkage was utilized in studies on ROS-sensitive luciferase probes24 and protease-sensitive fluorophores.22 Nonetheless, there are essentially no studies on the generality and utility of this promising linking strategy. This report investigates the scope of this ether-connected, self-immolative proinhibitor strategy with a variety of functional groups and MBGs.
Fig. 1.
Three approaches to the development of ROS-activated boronic ester proMMPi demonstrated with the methyl salicylate derivatives 1-3 using either an ether or ester linked self-immolative linker or through direct linkage of the protecting group.
Here we further investigate the behavior of different activation strategies using related, but distinct self-immolative linkers for coupling to the MBGs. All of the strategies studied here use boronic ester protecting groups that can be selectively removed by H2O2. A series of methyl salicylate derivatives containing phenol, thiophenol, aniline, and benzylamine leaving groups were investigated using either an ether linkage, a carbonate/carbamate ester linker, or no linker to the boronic ester protecting group (Fig. 1 and Fig. 2). In addition, we looked at a variety of MBGs protected with a boronic ester self-immolative leaving group to expand our inventory of MBGs for use in novel metalloprotein prodrugs.
Fig. 2.
Methyl salilcylate derivatives 4-9 investigated in this study with varying leaving groups and linkage strategies.
Compounds 1-3 were designed to release methyl salicylate in the presence of H2O2 using a self-immolative ether linkage (1), a carbonate ester linkage (2), or no self-immolative linker (3) to directly compare three possible designs of a prodrug scaffold. The syntheses of these compounds are described in the Supporting Information. Compounds 1-3 were first examined for activation in the presence of H2O2 using UV-Vis spectroscopy. To a solution of the boronic ester derivative in HEPES buffer (50 mM, pH 7.5) was added H2O2 and the change in absorbance was monitored over time. As shown in Fig. 3 for compound 1, the absorbance over time shows an increase at 302 nm indicative of the emergence of methyl salicylate with a clear isobestic point at 293 nm. Similar results were obtained with compounds 2 and 3. While compounds 1 and 2 achieved >90% cleavage within 45 min using an 18-fold excess of H2O2 (Fig. S1–S2), deprotection of compound 3 required a 180-fold excess of H2O2 to realize cleavage in a comparable time frame. Release of methyl salicylate for all three compounds was confirmed by HPLC (Fig. S3–S5).
Fig. 3.
Absorption spectra of 1 (50 μM in HEPES buffer, pH 7.5) in the presence of H2O2 (18 eq) monitored every 2 min over 60 min. The dashed line is the starting spectrum and the bold solid line is the final spectrum. A sample of methyl salicylate is shown as a dotted line. The arrow represents the change in absorption over time.
The rates of conversion to methyl salicylate were determined by monitoring the change in absorbance under pseudo first-order reaction conditions with an excess of H2O2. The calculated rate constants are presented in Table 1. Consistent with earlier reports, the carbonate ester derivative 2 displayed the fastest rate of conversion, but 2 also underwent spontaneous hydrolytic cleavage in buffer, whereas compound 1 was stable in buffer over a 4 h period (data not shown). Introduction of the carbonate group into the self-immolative linker of 2 leads to hydrolytic instability facilitated by nucleophilic attack of water at the carbonyl position which is not possible in compound 1.25 Interestingly, while the hydrolytic stability of 3 was comparable to the ether linkage used in 1, the rate of conversion for 3 was about two orders of magnitude slower than either 1 or 2, suggesting that use of self-immolative linker facilitates conversion to the desired active compound.
Table 1.
Pseudo first-order rate constants calculated with an excess of H2O2.
| Compound | k (M−1s−1) | Compound | k (M−1s−1) |
|---|---|---|---|
| 1 | 1.12 ± 0.04 | 11 | 5.9 ± 0.2 |
| 2 | 2.7 ± 0.1 | 12 | 3.5 ± 0.3 |
| 3 | 0.031 ± 0.002 | 13 | 2.9 ± 0.1 |
| 10 | 3.1 ± 0.5 | 14 | 4.1 ± 0.2 |
Based on the behavior of compounds 1-3, the most promising linking strategy is the benzyl ether linkage seen in compound 1. The benzyl ether linkage shows excellent stability in buffer while maintaining rapid cleavage kinetics upon activation. Therefore, we investigated the use of this motif with other leaving groups. Compounds 4-7 were synthesized to study the effects of using sulfur (4), aniline (5-6), or benzyl amine (7) leaving groups. Evaluation with UV-Vis absorption spectroscopy of 4-7 in the presence of H2O2 showed no cleavage of the protecting group suggesting that this strategy may only be relevant for oxygen-derived leaving groups (Fig. S6–S9). Further evaluation with LC-MS showed that the boronic ester of compound 6 was cleaved to the phenol group, but that the cascade reaction did not proceed as expected to release the aniline group (Fig. S10). This is reflective of the general robustness of these amine derivatives and their inability to become easily ionized.26 Compounds 8 and 9 were then evaluated to investigate the use of a carbamate ester linkage. Unlike compound 7, the carbamate self-immolative linkers in 8 and 9 showed that the desired benzyl amine is released in the presence of H2O2, thermodynamically driven by the release of CO2 (Fig. S11, S12). This suggests that for the release of nitrogen-derived leaving groups, the carbamate linkage may still be preferable for prodrug development.1
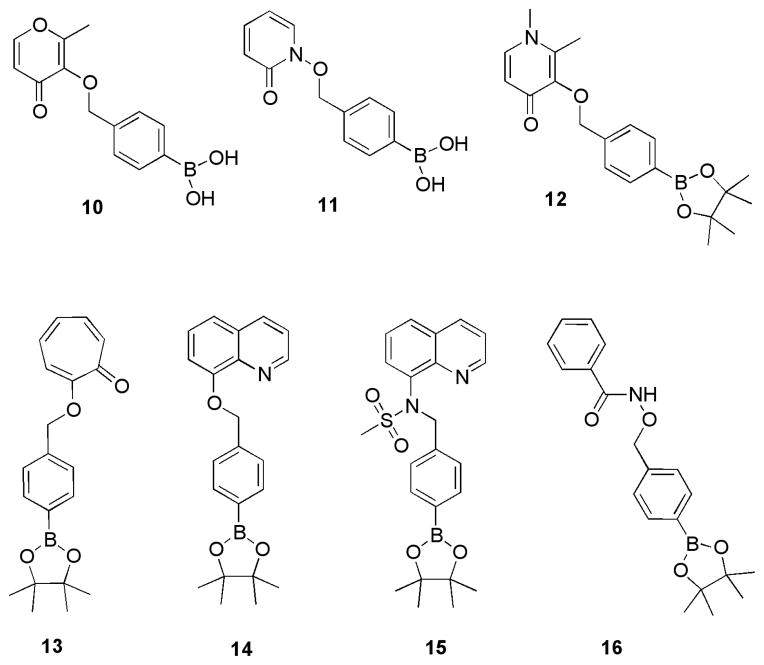
To validate our observations in the context of MBGs, a series of activatable MBGs (prochelators)27 were synthesized (compounds 10-14, Fig. 4) and evaluated. Compounds 10 and 11 were designed with a boronic acid protecting group to improve water solubility of the protected MBGs.15 Several other protected MBGs were examined including the oxygen-binding 3-hydroxy-1,2-dimethylpyridin-4(1H)-one (12), tropolone (13), and 8-hydroxyquinoline (14). Compounds 10-14 showed rapid cleavage to the desired MBG in the presence of H2O2, as determined by absorption spectroscopy (Table 1), thus confirming the broader utility of the benzyl ether self-immolative strategy for designing metalloprotein proinhibitors (Fig. S13–S17). Additionally, use of the boronic acid derivative in 10 and 11 shows both improved solubility and an increase in the rate of cleavage when compared to their boronic ester counterparts.15 The pinacol boronic ester analog of 10 had a rate constant of 2.9 M−1s−1, which is comparable to 10. However; compound 11 showed a notable improvement in rate, increasing from 4.0 M−1s−1 for the pinacol boronic ester to 5.9 M−1s−1 for 11. This rate approaches that of the reported rate of 6.7 M−1s−1 for the carbonate ester-linked boronic ester protected MBG.15 Compound 15 was synthesized and did not show cleavage in the presence of H2O2, confirming that this protection strategy is not effective with nitrogen-based MBGs.28 In the presence of H2O2, 15 shows similar deprotection of the boronic ester to the phenolic group as 6 and 7, but does not undergo release of the protecting group (Fig. S18). Overall, the results validate our findings that benzyl-ether linkages are best suited for oxygen-based MBGs.
Fig. 4.
Protected MBGs (prochelators) designed with a benzyl-ether self-immolative linker.
Hydroxamic acid MBGs are the most prevelant metal chelators in metalloprotein inhibitors, including MMPi, yet attempts to develop proMMPi using hydroxamic acid MBGs have not generally been successful.29 Therefore, compound 16, which is comprised of phenyl hydroxamic acid protected with the boronic-ester self-immolative linker, was synthesized and evaluated. In the presence of H2O2 compound 16 showed no release of the desired hydroxamic acid ligand. HPLC indicates exposure to H2O2 results in boronic ester cleavage to a phenol group, but no further cascade reaction occurs to release the hydroxamic acid (vide supra, Fig. S19).
A thorough investigation of boronic ester prochelators shows that the use of benzyl ether self-immolative linkers provides a superior platform for the development of metalloprotein proinhibitors with oxygen-based leaving groups. These compounds show excellent hydrolytic stability as well as fast rates of cleavage to the active compounds in the presence of H2O2. The use of boronic acids (instead of esters) results in even faster cleavage and better aqueous solubility with no loss in hydroylic stability. These findings are significant in the development of triggered metalloprotein proinhibitors, H2O2-activated prodrugs, and further examination of the benzyl-ether self-immolative strategy with other triggering groups is currently underway to obtain proinhibitors sensitive to a variety of chemical and biological stimuli.
Supplementary Material
Footnotes
Electronic Supplementary Information (ESI) available: Synthetic details, characterization of all compounds and details of hydrogen peroxide activation. Fig. S1–S19. See DOI: 10.1039/b000000x/
Notes and references
- 1.Kratz F, Muller IA, Ryppa C, Warnecke A. Chem Med Chem. 2008;3:20–53. doi: 10.1002/cmdc.200700159. [DOI] [PubMed] [Google Scholar]
- 2.Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Jarvinen T, Savolainen J. Nat Rev Drug Disc. 2008;7:255–270. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- 3.Tarasneko N, Nudelman A, Tarasneko I, Entin-Meer M, Hass-Kogan D, Inbal A, Rephaeli A. Clin Exp Metastasis. 2008;25:703–716. doi: 10.1007/s10585-008-9179-x. [DOI] [PubMed] [Google Scholar]
- 4.Rephaeli A, Zhuk R, Nudelman A. Drug Dev Res. 2000;50:379–391. [Google Scholar]
- 5.Reid T, Valone F, Lipera W, Irwin D, Paroly W, Natale R, Sreedharan S, Keer H, Lum B, Scappaticci F, Bhatnagar A. Lung Cancer. 2004;45:381–386. doi: 10.1016/j.lungcan.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Furumai R, Matsuyama A, Kobashi N, Lee KH, Nishiyama H, Tanaka A, Komatsu Y, Nishino N, Yoshida M, Horinouchi S. Cancer Res. 2002;62:4916–4921. [PubMed] [Google Scholar]
- 7.Bowers A, West N, Taunton J, Schreiber SL, Bradner JE, Williams RM. J Am Chem Soc. 2008;130:11219–11222. doi: 10.1021/ja8033763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Failes TW, Cullinane C, Diakos CI, Yamamoto N, Lyons JG, Hambley TW. Chemistry A European Journal. 2007;13:2974–2982. doi: 10.1002/chem.200601137. [DOI] [PubMed] [Google Scholar]
- 9.Failes TW, Hambley TW. Journal of Inorganic Biochemistry. 2007;101:396–403. doi: 10.1016/j.jinorgbio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 10.De Simone G, Vitale RM, Di Fiore A, Pedone C, Scozzafava A, Montero JL, Winum JY, Supuran CT. Journal of Medicinal Chemistry. 2006;49:5544–5551. doi: 10.1021/jm060531j. [DOI] [PubMed] [Google Scholar]
- 11.Whittaker M, Flyod CD, Brown P, Gearing AJH. Chem Rev. 1999;99:2735–2776. doi: 10.1021/cr0100345. [DOI] [PubMed] [Google Scholar]
- 12.Coussens LM, Fingleton B, Matrisian LM. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 13.Major Jourden JL, Cohen SM. Chem Commun. 2010;46:1241–1243. doi: 10.1039/b923302d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniel KB, Major Jourden JL, Negoescu KE, Cohen SM. J Biol Inorg Chem. 2011;16:313–323. doi: 10.1007/s00775-010-0727-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Major Jourden JL, Cohen SM. Angew Chem Int Ed. 2010;49:6795–6797. doi: 10.1002/anie.201003819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q, Tang XN, Yenari MA. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg GA, Cunningham LA, Wallace J, Alexander S, Estrada EY, Grossetete M, Razhagi A, Miller K, Gearing A. Brain Res. 2001;893:104–112. doi: 10.1016/s0006-8993(00)03294-7. [DOI] [PubMed] [Google Scholar]
- 18.Jin R, Yang G, Li G. Neurobio Disease. 2010;38:376–385. doi: 10.1016/j.nbd.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sella E, Shabat D. Chem Commun. 2008:5701–5703. doi: 10.1039/b814855d. [DOI] [PubMed] [Google Scholar]
- 20.Weinstain R, Baran PS, Shabat D. Bioconjugate Chem. 2009;20:1783–1791. doi: 10.1021/bc9002037. [DOI] [PubMed] [Google Scholar]
- 21.Blencowe CA, Russell AT, Greco F, Hayes W, Thornthwaite Polym Chem. 2011;2:773–790. [Google Scholar]
- 22.Meyer Y, Richard JA, Delest B, Noack P, Renard PY, Romieu A. Org Biomol Chem. 2010;8:1777–1780. doi: 10.1039/b926316k. [DOI] [PubMed] [Google Scholar]
- 23.Lee HY, Jiang X, Lee D. Org Lett. 2009;11:2065–2068. doi: 10.1021/ol900433g. [DOI] [PubMed] [Google Scholar]
- 24.Van de Bittner GC, Dubikovskaya EA, Bertozzi CR, Chang CJ. Proc Nat Acad Sci USA. 2010;107:21316–21321. doi: 10.1073/pnas.1012864107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostergaar J, Larsen C. Molecules. 2007;12:2396–2412. doi: 10.3390/12102396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simplicio AL, Clancy JM, Gilmer JF. Molecules. 2008;13:519–547. doi: 10.3390/molecules13030519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charkoudian LK, Pham DM, Franz KJ. J Am Chem Soc. 2006;128:12424–12425. doi: 10.1021/ja064806w. [DOI] [PubMed] [Google Scholar]
- 28.Rouffet M, de Oliveira CAF, Udi Y, Agrawal A, Sagi I, McCammon JA, Cohen SM. J Am Chem Soc. 2010;132:8232–8233. doi: 10.1021/ja101088j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell MB, Whitcombe IWA. Tet Lett. 2000;41:8829–8834. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.