Abstract
Objective
To determine expressions of vascular endothelial growth factor (VEGF), pigment epithelial derived factor (PEDF) and respective receptors in retinas from a model of retinopathy of prematurity (ROP).
Method
Retinas isolated from the 50/10 oxygen-induced retinopathy model of ROP (50/10 OIR) and from room air raised pups (RA) at birth, age 14 days (time of persistent avascular retina in 50/10 OIR and complete retinal vascularization in RA), and age 18 days (time of intravitreous neovascularization in 50/10 OIR) were analyzed for mRNA of VEGF164, PEDF, VEGF receptor (R)-1 and -2, PEDF receptor, and neuropilin-1 and -2 by real-time polymerase chain reaction (PCR).
Results
Compared to RA, fold changes in expression of VEGF164, neuropilin-1 and -2 were significantly increased at day 14 while avascular retina persistent in 50/10 OIR and at 18 days during intravitreous neovascularization. There was also a trend for increased fold change in VEGFR2 at day 14 and a significant increase at day 18 in 50/10 OIR. PEDF receptor was significantly increased at day 14 in 50/10 OIR compared to RA.
Conclusions
Increased expression of VEGF164, and angiogenic receptors provides evidence that over-expressed VEGF may disorder developmental angiogenesis and contribute to peripheral avascular retina and intravitreous neovascularization.
Clinical Relevance
Disordered angiogenesis through increased VEGF signaling may contribute to avascular retina prior to the development of stage 3 ROP.
Introduction
Interactions between neural and vascular growth factors and receptors are recognized as important in retinal vascular development and in disease1. For example, the vascular endothelial growth factor (VEGF) family and signaling pathway is important in retinal vascular development2,3 and in neuronal survival4,5. The neuropilins, originally described as receptors for proteins involved in neuronal axon guidance, can form complexes with VEGF receptors and act as coreceptors for VEGF mediated angiogenic signaling1. Pigment epithelial derived factor (PEDF) is a known neurotrophic factor6 and is also involved in retinal vascular development7. One way PEDF was reported to inhibit pathologic angiogenesis is by downregulating VEGF expression and interfering with its binding to VEGF receptor 2 (VEGFR2)8, the receptor believed most involved with angiogenic processes9.
Since the VEGF signaling pathway and PEDF are important in physiologic and pathologic angiogenesis2,3,7,8, we wished to determine the expression of these factors and their respective receptors in a model that is relevant to ROP of modern-day. We previously studied the effects of oxygen stresses on the expression of VEGF isoforms and reported that VEGF164 was the most prevalent isoform and was increased by repeated fluctuations in oxygen10, a known risk factor for modern-day severe ROP11,12. Therefore, in this report, we used the same 50/10 oxygen induced retinopathy model of ROP (50/10 OIR) developed by John Penn13, which exposes rat pups to repeated fluctuations in oxygen. The model reproducibly and consistently develops peripheral avascular retina, similar to zone II ROP, followed by intravitreous neovascularization at the junction of vascular and avascular retina, similar to zone II, stage 3 ROP. Previous studies of ROP have used oxygen-induced retinopathy models that expose animals to extreme constant hyperoxia to create vaso-obliteration of the central retinal capillaries. The most know of these is the mouse model developed by Smith and D’Amore14. During the obliterative phase, VEGF mRNA expression was downregulated in conjunction with arrest and loss of developing retinal vasculature15. The newly formed capillaries can be protected from death by giving growth factors16,17,18 or nutrients19,20 prior to hyperoxia. Upon return to room air, a relative retinal hypoxia triggers signaling of VEGF and angiogenesis with capillary budding into the vitreous 21, and this proliferative phase can be prevented by administering agents to inhibit angiogenic factors, such as VEGF.22
We used a relevant model of zone II stage 3 ROP, the 50/10 OIR model to measure the expression of mRNAs of VEGF164, PEDF and their respective receptors. We proposed that the expression of angiogenic factors would be downregulated when avascular retina persisted in the model at day 14 compared to room air when the retina was fully vascularized and that angiogenic factors would be upregulated compared to room air during intravitreous neovascularization at day 18.
Methods
All animal studies complied with the University of North Carolina’s Institute for Laboratory Animal Research (Guide for the Care and Use of Laboratory Animals) and the ARVO Statement for the Use of Animals in Ophthalmic and Visual Research.
Rat 50/10 OIR Model
We used a model of zone II, stage 3 severe ROP, the rat 50/10 OIR model13. The model causes rat arterial oxygen levels13 that replicate transcutaneous oxygen extremes of modern-day human preterm infants who develop severe ROP12. Within 4 hours of birth at postnatal age 0 (p0), litters of 12–14 newborn Sprague-Dawley rat pups with their mothers (Charles River, Wilmington, MA) were placed into an Oxycycler incubator (Biospherix, New York, NY) to cycle inspired oxygen between 50% oxygen and 10% oxygen every 24 hours until p14. After 7 cycles of oxygen fluctuations, pups were then placed into room air for 4 days. Oxygen levels were monitored daily and recalibrated as needed. Carbon dioxide in the cage was also monitored daily and flushed from the system by maintaining sufficient gas-flow and by adding soda lime. The model reproducibly develops vascular tortuosity at p12, a peripheral avascular zone of retina at p14 (Figure 1) when retinal vascular development is complete in room air development, then intravitreous neovascularization at p18 (Figure 2)23.
Figure 1.
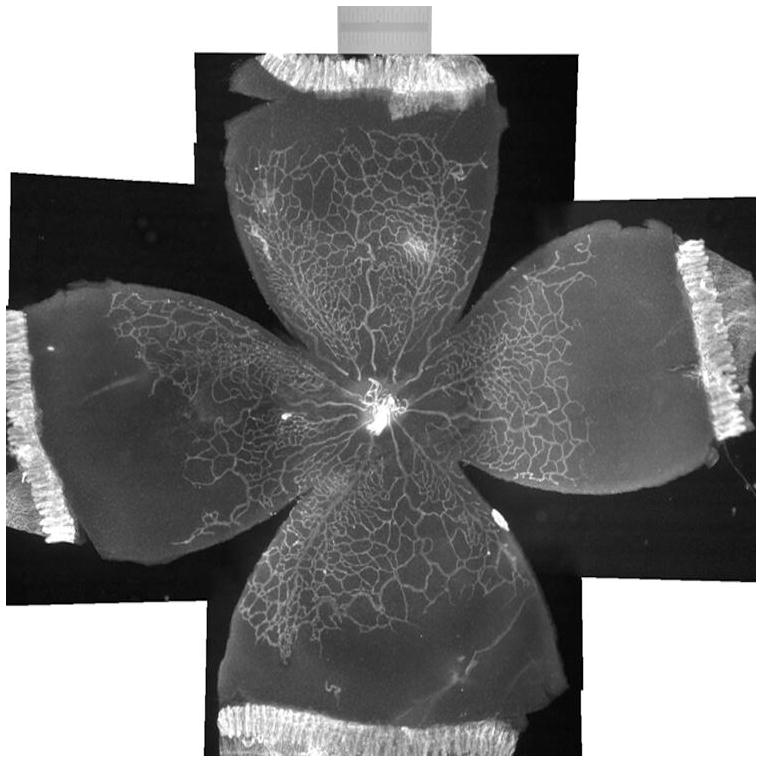
Lectin-stained retinal flatmount from postnatal day 14 rat pup in the 50/10 OIR model following 7 cycles of oxygen fluctuations every 24 hours between 50% and 10% oxygen. Peripheral avascular retina persists, whereas room air counterparts have fully vascularized retina.
Figure 2.
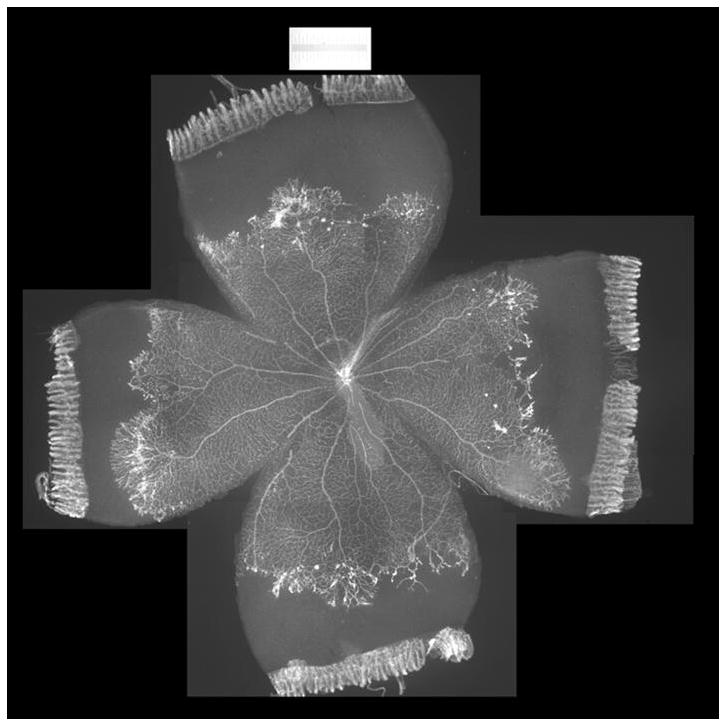
Lectin-stained retinal flatmount from postnatal day 18 rat pup in 50/10 OIR (7 cycles of oxygen fluctuations every 24 hours between 50% and 10%, followed by 4 days in room air). Intravitreous neovascularization exists at the border of vascular and avascular retina.
Previously, we showed that compared to rats raised in room air at the same time points after birth, those raised in an environment with oxygen cycling had increased areas of avascular retina10. At p14 after seven cycles of fluctuations in oxygen, the percent avascular to total retinal area was 33% in the 50/10 OIR model, whereas room air raised pups had fully vascularized retinas in which the inner capillary plexus covered the rat retina to the ora serrata.
Dissection of Retinal Tissue for mRNA Analyses
Pups were euthanized using pentobarbital (80 mg/kg intraperitoneal injection), and retinas were isolated using a modification of Chan-Ling24. Retinas were dissected, without ora serratas, from at least 5 separate pups for each time point in room air or the 50/10 OIR model. Separate litters were used for each time point and condition to assure adequate litter size and consistency of features in the 50/10 OIR model. Replicate experiments were performed for each condition and time point. Tissue was frozen in RNAlater (Applied Biosystems, CA) until analysis.
Real time PCR
Retinas were homogenized in lysis buffer and total RNA extracted by RNeasy Kit (Qiagen, CA). DNA-free Kit (Applied Biosystems, CA) was used to remove DNA contamination and RNA quantity was determined. 1μg of RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, CA). VEGF164 was chosen, because it is the most prevalent of VEGF isoforms associated with intravitreous neovascularization in the 50/10 OIR model, is upregulated by repeated oxygen fluctuations10, and it is also a ligand for neuropilin-1, which acts as a coreceptor with VEGFR21. Primers for: VEGF164: 5′GCACATAGGAGAGATGAGCT 3′, 5′GCTCACAGTGATTTT CTGGC3′, Fam TGCAG CATAGCAGATGTGAATGCAGACC Tamara; VEGFR1: 5′CCACCTCCATGTTTGAAGAC3′, 5′AGTCCAGGTGAATC GCTTCA3′, Fam TACCAGCAGTCTGCTGACCTCCCC Tamara; VEGFR2: 5′CTCCATCTTTTGGTGGGATG3′, 5′GCTGGTCTGGTTGGAGCCT3′, Fam AGGCCACAGACTCCCTGCTT TTACTG3 Tamara; PEDF: 5′CCAACTCTTTGCAGGACATG3′, 5′TCACAGGTTTGCCGGTAATC3′, Fam ACAGTCCTTGTTTGAGTCCCCTGAC Tamara; neuropilin-1 (NP-1): 5′GGAGCTACTGGGCTGTGAAG3′, 5′ATGTGGGGAAGTCTGA3′, Fam CACCCAATGGGAACCTA Tamara; neuropilin-2 (NP-2): 5′ACACAAGGAGCCATTTCCAG3′, 5′CGGATCCTGATGAAACGAGT3′, Fam CAAAGGGGGAAGATTGGATGTamara; PEDF receptor: 5′CCAACTCTTTGCAGGACATC3′, 5′TCACAGGTTTGCCGGTAATC3′, Fam ACAGTCCTTGTTTGAGTCCCCTGAC Tamara25 were made by UNC’s Oligonucleotide Core facility (http://www.med.unc.edu/olioli/index.htm). cDNA was mixed 1:1 with Taqman Universal Master Mix (Applied Biosystems, CA) and primers. Triplicate samples were cycled in the AB 7500 PCR System. The 7500 System Software calculates cycle threshold automatically for each well. Rat β-actin, which we found to be stably expressed in retinas under these oxygen stresses, was used as the control gene and was amplified with forward and reverse primers TGCCTGACGGTCAGGTCA and CAGGAAGGAAGGCTGGAAG, respectively. Duplicate reactions with a total volume of 16 μL were run for each sample and control using the AB 7500 PCR System. The 7500 System Software calculates cycle threshold automatically for each well. Cycle threshold values were normalized to β-actin and expressed as a fold increase over p0 value for each growth factor or receptor, thus permitting comparisons between room air and 50/10 OIR model for each growth factor or receptor.
Statistical Analysis
For real time PCR analysis, samples from room air and 50/10 OIR model were normalized to β-actin for each ligand or receptor group to allow comparisons of fold differences within each group. Student t-tests were used to analyze fold changes in expression of each ligand or receptor mRNA between room air and 50/10 OIR at postnatal day (p)14 (avascular retina) and p18 (intravitreous neovascularization).
Results
Expression of VEGF, VEGF receptors 1 and 2, neuropilins, PEDF and PEDF receptor in room air
In room air, the retinal extent is fully covered by retinal vessels at p14. VEGF164 mRNA expression increased 3-fold between p0 and p14 (Figure 3). The fold changes in expression differed between p0 and p14 for each of the 4 angiogenic receptors and coreceptors involved in VEGF signaling (VEGFR1, VEGFR2, neuropilin -1, neuropilin -2). Whereas VEGFR2 increased 5 fold (Figure 4), VEGFR1 increased approximately 42 fold (Figure 5). There was little change in expression of neuropilin -1 (Figure 6), and neuropilin -2 increased approximately 5-fold (Figure 7).
Figure 3.
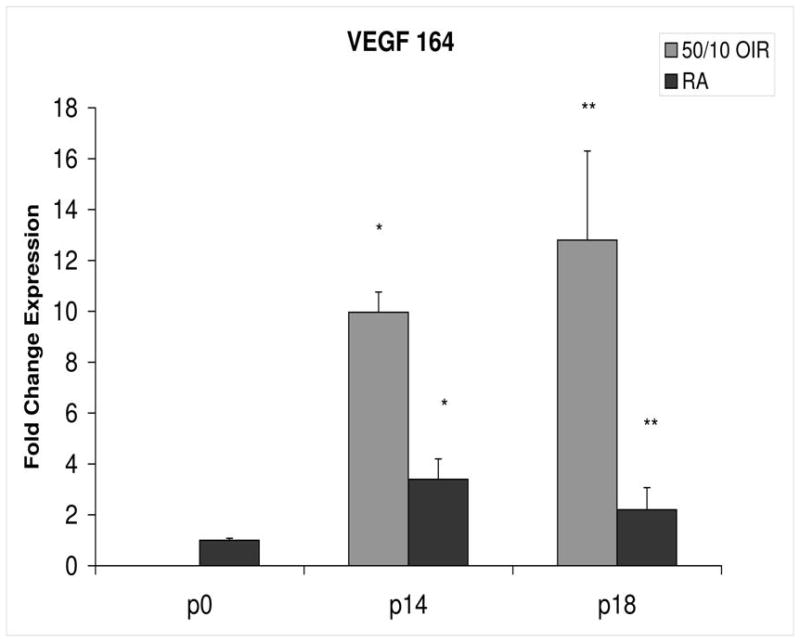
Fold change in VEGF164 mRNA expression, relative to β-actin, at p0, p14 and p18 room air (RA) and 50/10 OIR. Significantly increased fold expression in VEGF164 mRNA was present in 50/10 OIR compared to RA at p14 (*p=0.0004) and p18 (**p=0.02). Student t-tests.
Figure 4.
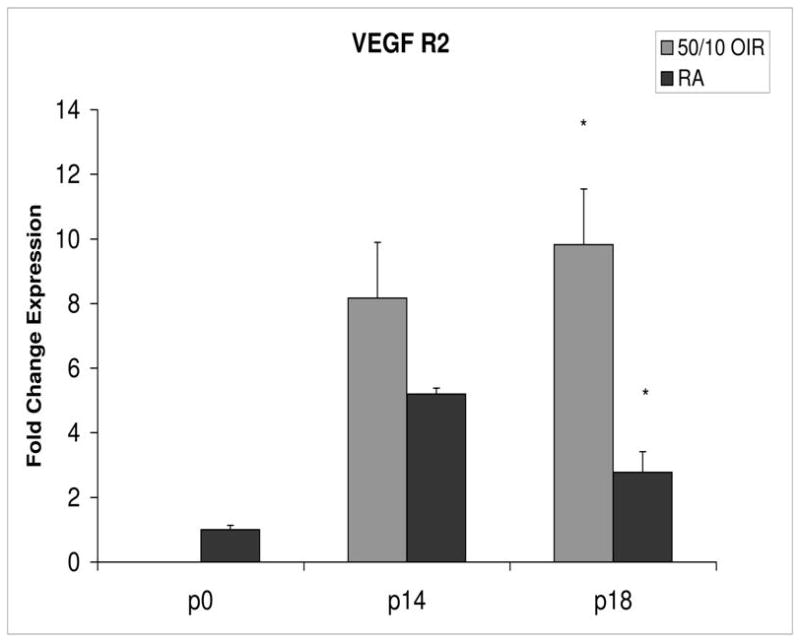
Fold change in expression of VEGFR2 mRNA, relative to β-actin, at p0, p14 and p18 room air (RA) and 50/10 OIR. Significantly increased fold expression in VEGFR2 mRNA was present in 50/10 OIR compared to RA at p18 (*p=0.004). A trend toward increased fold expression of VEGFR2 in 50/10 OIR was noted at p14 (p=0.06). Student t-tests.
Figure 5.
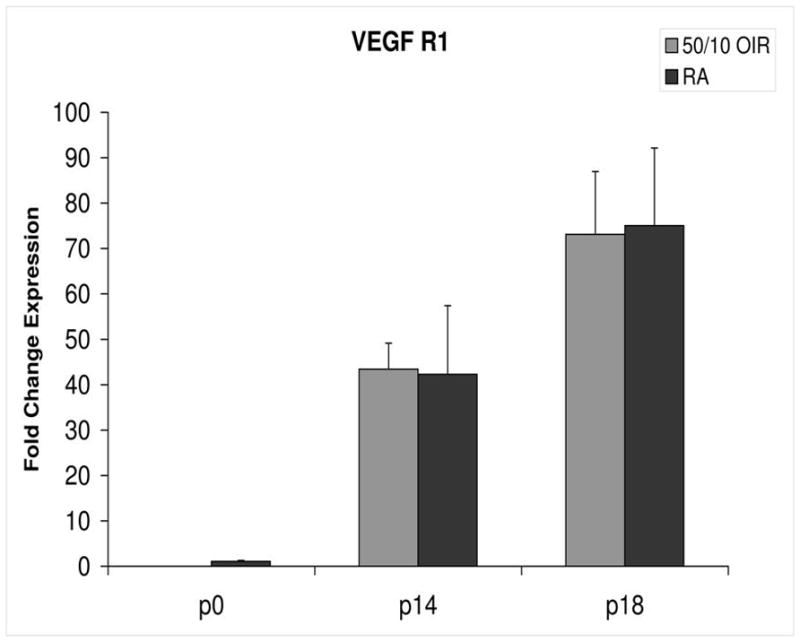
Fold change in expression of VEGFR1 mRNA, relative to β-actin, at p0, p14 and p18 room air (RA) and 50/10 OIR. No significant differences were found at p14 (p=0.47) or p18 (p=0.97). Student t-tests.
Figure 6.
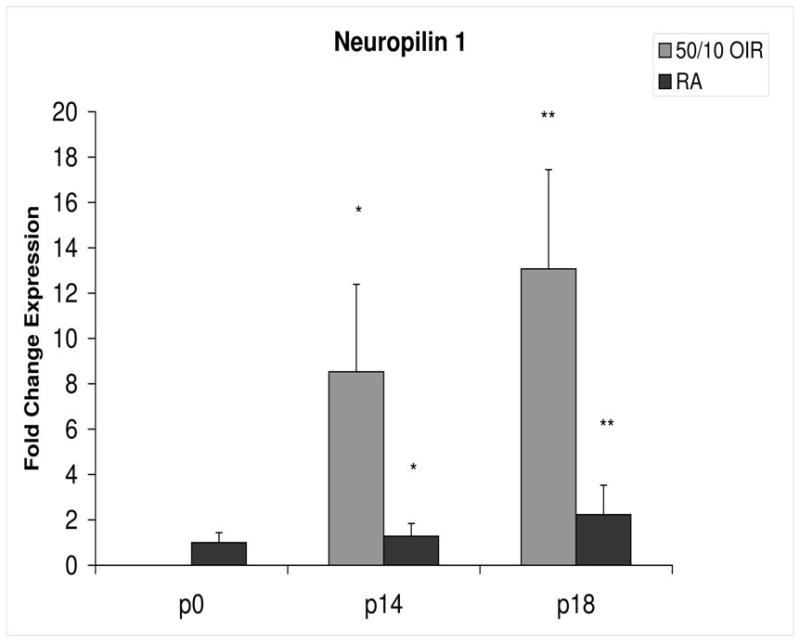
Fold change in expression of neuropilin-1 (NP-1) mRNA, relative to β-actin, at p0, p14 and p18 room air (RA) and 50/10 OIR. Significantly increased fold expression in NP-1 mRNA was present in 50/10 OIR compared to RA at p14 (*p=0.042) and p18 (**p=0.027). Student t-tests.
Figure 7.
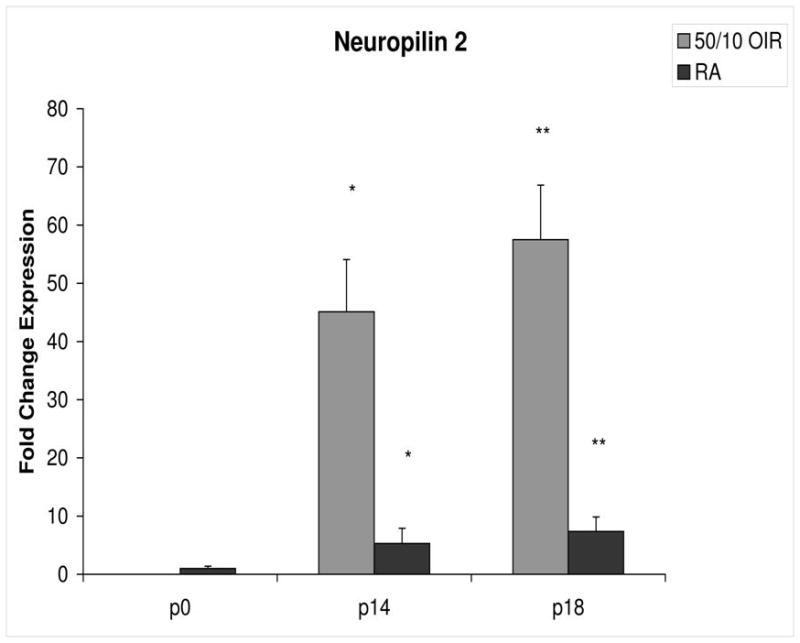
Fold change in expression of neuropilin-2 (NP-2) mRNA, relative to β-actin, at p0, p14 and p18 room air (RA) and 50/10 OIR. Significantly increased fold expression in NP-2 mRNA was present in 50/10 OIR compared to RA at p14 (*p=0.001) and p18 (**p=0.002). Student t-tests.
PEDF and PEDFR expression increased about 5- and 4-fold, respectively, between p0 and p14 (Figures 8 and 9).
Figure 8.
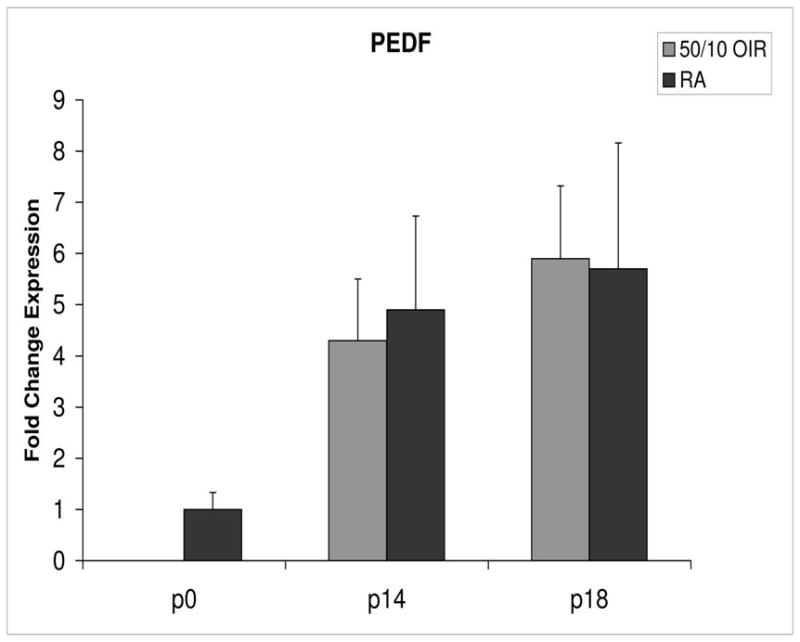
Fold change in expression of PEDF mRNA, relative to β-actin, at p0, p14 and p18 room air (RA) and 50/10 OIR. No significant difference was found at p14 (p=0.47) or p18 (p=0.39). Student t-tests.
Figure 9.
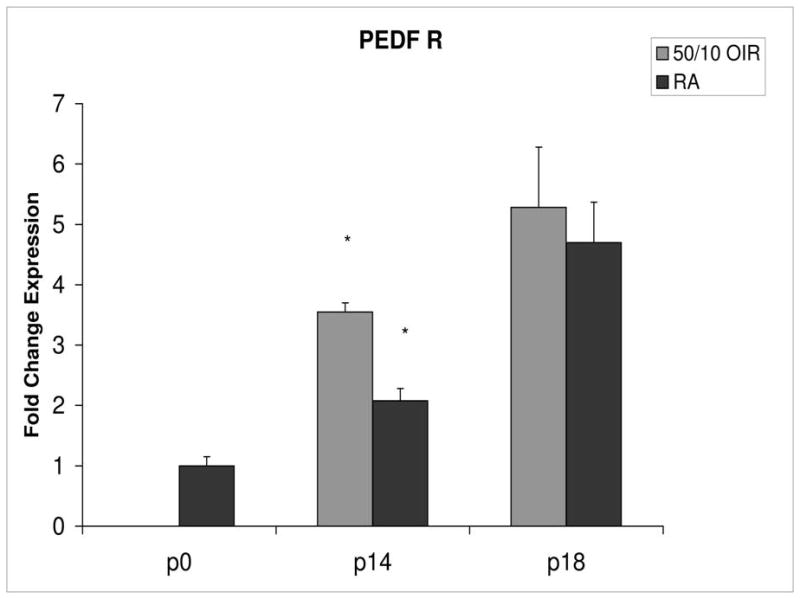
Fold change in expression of PEDFR mRNA, relative to β-actin, at p0, p14 and p18 room air (RA) and 50/10 OIR. Significantly increased fold expression in PEDFR mRNA was present in 50/10 OIR compared to RA at p14 (*p=0.0006). No significant difference was found at p18 (p=0.32). Student t-tests.
Expression of VEGF, VEGF receptors 1 and 2, neuropilins, PEDF and PEDF receptor at p14 during persistent avascular retina in the 50/10 OIR model
The 50/10 OIR model exposes animals to repeated fluctuations in hyperoxia and hypoxia and causes delayed retinal vascularization and retinal apoptosis26, both believed to contribute to peripheral avascular retina at p14, when room air raised pups have fully vascularized retinas. Since VEGF is important in developmental angiogenesis2,3, and the 50/10 OIR model had incomplete retinal vascularization compared to room air counterparts10, we anticipated that the expression of mRNAs of VEGF164, VEGF receptors and coreceptors would be lower at p14 in the 50/10 OIR model compared to those in room air. However, we found that VEGF164 expression increased with an almost 10-fold change between p0 and p14 in the 50/10 OIR model and this represented a 3-fold increase in expression compared to room air (Figure 3). This finding also contrasts with reports using the mouse oxygen-induced retinopathy model, in which VEGF expression was lower than room air during the phase of hyperoxia induced vaso-obliteration15.
Compared to p0, all receptors had increased expression at p14 in the 50/10 OIR model and all except for VEGFR1 had increased fold change in expression compared to room air values (Figures 4 through 7). The fold change in expression of neuropilin-1 and -2 were significantly increased in 50/10 OIR compared to room air, whereas there was a trend for increased fold expression of VEGFR2 in the 50/10 OIR model. The fold changes in PEDF and PEDFR mRNAs increased between p0 and p14, and there was a significant increase in fold expression of PEDFR mRNA in 50/10 OIR compared to room air (Figure 9). There was little fold difference in PEDF mRNA between room air and the 50/10 OIR model at p14 (Figure 8).
Expression of VEGF, VEGF receptors 1 and 2, neuropilins, PEDF and PEDF receptor at p18 during intravitreous neovascularization in 50/10 OIR model
In the 50/10 OIR model, pups are moved to a relatively hyperoxic environment (from 10% to 21% oxygen; i.e., room air) at day 14. At day 18, intravitreous neovascularization develops at the junctions of vascular and avascular retina. At p18, in association with the intravitreous neovascularization, we found greater fold changes in expression of VEGF164, VEGFR2, neuropilin -1, and neuropilin -2 compared to room air values (Figures 4–7). However, the fold change in expression of VEGFR1 was similar to that in room air (Figure 5).
Since PEDF is an angiogenic inhibitor, we anticipated that PEDF and PEDF receptor would be reduced at p18 compared to room air. At p18, however, the fold change in expression of PEDF and PEDF receptor were not significantly greater than those at room air (Figures 8 and 9).
Discussion
We found increased fold expression of mRNAs of VEGF164, VEGFR2, and the neuropilins at p18 in association with intravitreous neovascularization and compared to room air. This was anticipated because VEGF164, VEGFR2 and the neuropilins are involved in angiogenic processes. However, we also found that when avascular retina persisted in the 50/10 OIR model, the fold change in expression of mRNAs of VEGF164, both neuropilins, and a strong trend for VEGFR2 was greater than that in room air raised rats in which retinal vascularization was complete at p14. Furthermore, the fold expression of VEGFR1, believed to limit the amount of VEGF ligand present to signal through VEGFR2 during development, was not significantly elevated between the 50/10 OIR model and room air. These were unexpected findings based on data from the mouse model of oxygen-induced retinopathy in which VEGF was downregulated during vaso-obliteration15 and upregulated during angiogenesis22.
Both VEGF and PEDF are involved in pathologic angiogenesis and physiologic developmental retinal angiogenesis, and are regulated by oxygen tension2,7,27,28,3. VEGF expression is upregulated by hypoxia27, and PEDF by hyperoxia28. PEDF is a potent angiogenic inhibitor28, whereas VEGF is an agonist27. VEGFR2 is believed to be important in angiogenesis, whereas VEGFR1 is thought to bind and limit the amount of VEGF ligand that can signal through VEGFR29, thus permitting ordered angiogenesis. It is increasingly recognized that the neuropilins are also important in angiogenic processes1. Neuropilin-1 was initially described as a receptor for the semaphorins, and was involved in axonal guidance in the central nervous system. Akula et al. reported associations between VEGF, semaphorin 3A, vascular tortuosity, and electrophysiologic sensitivity parameters of rods and the post-receptor retina in the 50/10 OIR model suggesting that the post-receptor retina mediates vascular abnormalities in the model29. We now report that the neuropilins are upregulated in association with avascular retina and intravitreous neovascularization. Both neuropilins can function as receptors for a number of VEGF family members including VEGF isoforms, VEGF164/165 and VEGF145, placental growth factor-1, and VEGFB30. Less is known about PEDF receptor signaling25. However, counter to the anticipated role of PEDF as an angiogenic inhibitor, PEDF knockout mice were reported to develop larger areas of vaso-obliteration following hyperoxia than wild type littermates31. We found increased fold expression of PEDFR at p14 during persistent avascular retina in the 50/10 OIR compared to room air. This finding may have importance in the development of avascular retina, because PEDF is an inhibitor of angiogenesis28.
We used the 50/10 OIR model, which causes rat arterial oxygen levels13 to be similar to transcutaneous oxygen levels measured in preterm infants who develop severe ROP12 and uses repeated oxygen fluctuations, a risk for ROP12,11, rather than constant oxygen used in other models28,14,32,33. As in zone II Stage 3 ROP, in which fluorescein angiograms demonstrated reduced peripheral retinal vascularization and perfusion with minimal central nonperfusion (Lepore D., ARVO meeting, 2008, Abstract), the rat 50/10 OIR model also has peripheral avascular retina and minimal central obliteration13. The 50/10 OIR model reproducibly and consistently develops vessel tortuosity (analogous to plus disease)34,35, avascular retina at p14 (analogous to human zone II ROP)13,35, and subsequently intravitreous neovascularization at the junctions of vascular and avascular retina (analogous to stage 3 ROP)36. Other models of oxygen-induced retinopathy including the mouse model, use higher oxygen levels than that currently used in neonatal intensive care units in the US today and creates central rather than peripheral avascular retina.
There are several reasons we propose for our findings. At p14, VEGF expression may be increased as a result of previous exposure to 10% oxygen. We used real-time PCR to measure expression, because it is the most quantitative method available. The high mRNA is in alignment with later development of intravitreous neovascularization at p18, but it does not explain why blood vessel growth occurs into the vitreous rather than into the retina. Loss of the physiologic gradient of VEGF in the retina may be a reason for the growth of blood vessels into the vitreous. However, Geisen et al. measured vitreous VEGF and retinal VEGF in the 50/10 OIR model and found that the vitreous levels were 1/10 that of the retina at p1837, failing to provide support for a gradient of VEGF toward the vitreous and away from the retina. It is also possible that at p14, the model is moving toward a vasoproliferative phase. However, several papers have found that inhibition of VEGF bioactivity using either a neutralizing antibody37 or a VEGF receptor 2 kinase inhibitor38 reduced angiogenesis into the vitreous (intravitreous neovascularization), but did not interfere with angiogenesis into the retina (intraretinal vascularization). Excessive signaling through VEGFR2 has been shown to interfere with developmental angiogenesis in other models39. It is possible that increased signaling through VEGF receptors may disorder and interfere with developmental angiogenesis. Such a finding might delay normal vascularization and contribute to peripheral avascular retina. However, since VEGF is also a neuronal survival factor4,5, there is concern about possible systemic adverse effects of anti-VEGF agents that are absorbed into the blood stream of the developing preterm infant.
Although the measurement of mRNA with real-time PCR provides the most quantitative data of expression, it does not always correspond to the concentration of translated proteins or activated proteins involved in signaling pathways. Further study in these areas is warranted.
In summary, we found that during avascular retina, expression of VEGF164 and its receptors and coreceptors were increased in 50/10 OIR model compared to room air when retinal vascularization was complete. We propose that overexpression of VEGF may interfere with ongoing retinal vascular development and contribute to avascular retina. We also found that VEGF was increased during the period of intravitreous neovascularization. In addition, increased expression of PEDF receptor in 50/10 OIR model compared to room air may have importance in contributing to avascular retina.
Acknowledgments
Supported by NEI/NIH: EY015130, EY017011, March of Dimes, American Diabetes Association, Research to Prevent Blindness Physician Scientist Award (MEH).
Footnotes
MEH is a consultant for Ophthalmic Research Associates, Andover, MA for clinical trial development, not involved in this study.
MEH is the Principal Investigator and Corresponding Author and has had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer. 2008;8:632–645. doi: 10.1038/nrc2404. [DOI] [PubMed] [Google Scholar]
- 2.Chan-Ling T, Gock B, Stone J. The effect of oxygen on vasoformative cell division: Evidence that ‘physiological hypoxia’ is the stimulus for normal retinal vasculogenesis. Invest Ophthalmol Vis Sci. 1995;36:1201–1214. [PubMed] [Google Scholar]
- 3.Stone J, Itin A, Alon T, et al. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15:4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishijima K, Ng YS, Zhong L, et al. Vascular Endothelial Growth Factor-A Is a Survival Factor for Retinal Neurons and a Critical Neuroprotectant during the Adaptive Response to Ischemic Injury. Am J Path. 2007;171:53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oosthuyse B, Moons L, Storkebaum E, et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28:131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 6.Tombran-Tink J, Barnstable CJ. PEDF: A multifacted neurotrophic factor. Nature Reviews Neuroscience. 2003;4:628–636. doi: 10.1038/nrn1176. [DOI] [PubMed] [Google Scholar]
- 7.Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 8.Zhang SX, Wang JJ, Gao G, Parke K, Ma Jx. Pigment epithelium-derived factor downregulates vascular endothelial growth factor (VEGF) expression and inhibits VEGF-VEGF receptor 2 binding in diabetic retinopathy. J Mol Endocrinol. 2006;37:1–12. doi: 10.1677/jme.1.02008. [DOI] [PubMed] [Google Scholar]
- 9.Shibuya M. Differential Roles of Vascular Endothelial Growth Factor Receptor-1 and Receptor-2 in Angiogenesis. j Biochem Molecul Biol. 2006;39:469–478. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 10.McColm JR, Geisen P, Hartnett ME. VEGF isoforms and their expression after a single episode of hypoxia or repeated fluctuations between hyperoxia and hypoxia: Relevance to clinical ROP. Mol Vis. 2004;10:512–520. [PMC free article] [PubMed] [Google Scholar]
- 11.Tin W, Milligan DWA, Pennefather PM, Hey E. Pulse oximetry, severe retinopathy, and outcome at one year in babies of less than 28 weeks gestation. Arch Dis Child Fetal Neonat Ed. 2001;84:106–110. doi: 10.1136/fn.84.2.F106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham S, Fleck BW, Elton RA, Mclntosh N. Transcutaneous oxygen levels in retinopathy of prematurity. Lancet. 1995;346:1464–1465. doi: 10.1016/s0140-6736(95)92475-2. [DOI] [PubMed] [Google Scholar]
- 13.Penn JS, Henry MM, Tolman BL. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr Res. 1994;36:724–731. doi: 10.1203/00006450-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Smith LEH, Wesolowski E, McLellan A, et al. Oxygen induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 15.Pierce E, Foley E, Lois EH. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol. 1996;114:1219–1228. doi: 10.1001/archopht.1996.01100140419009. [DOI] [PubMed] [Google Scholar]
- 16.Shih SC, Ju M, Liu N, Smith LEH. Selective stimulation of VEGFR-1 prevents oxygen-induced retinal vascular degeneration in retinopathy of prematurity. J Clin Invest. 2003;112:50–57. doi: 10.1172/JCI17808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang KH, Chan-Ling T, McFarland EL, et al. IGF binding protein-3 regulates hematopoietic stem cell and endothelial precursor cell function during vascular development. PNAS. 2007;104:10595–10600. doi: 10.1073/pnas.0702072104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lofqvist C, Chen J, Connor KM, et al. From the Cover: IGFBP3 suppresses retinopathy through suppression of oxygen-induced vessel loss and promotion of vascular regrowth. PNAS. 2007;104:10589–10594. doi: 10.1073/pnas.0702031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of [omega]-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neu J, Afzal A, Pan H, Gallego E, et al. The Dipeptide Arg-Gln Inhibits Retinal Neovascularization in the Mouse Model of Oxygen-Induced Retinopathy. Investigative Ophthalmology Visual Science. 2006;47:3151–3155. doi: 10.1167/iovs.05-1473. [DOI] [PubMed] [Google Scholar]
- 21.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LEH. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson GS, Pierce EA, Rook SL, et al. Oligodeoxynucleotides inhibit retinal neovascularization in a murine model of proliferative retinopathy. Proc Natl Acad Sci USA. 1996;93:4851–4856. doi: 10.1073/pnas.93.10.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werdich XQ, Penn JS. Specific Involvement of Src Family Kinase Activation in the Pathogenesis of Retinal Neovascularization. Investigative Ophthalmology Visual Science. 2006;47:5047–5056. doi: 10.1167/iovs.05-1343. [DOI] [PubMed] [Google Scholar]
- 24.Chan-Ling T. Glial, vascular and neuronal cytogenesis in whole-mounted cat retina. Microsc Res Tech. 1997;36:1–16. doi: 10.1002/(SICI)1097-0029(19970101)36:1<1::AID-JEMT1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 25.Notari L, Baladron V, Aroca-Aguilar JD, et al. Identification of a Lipase-linked Cell Membrane Receptor for Pigment Epithelium-derived Factor. J Biol Chem. 2006;281:38022–38037. doi: 10.1074/jbc.M600353200. [DOI] [PubMed] [Google Scholar]
- 26.Saito Y, Geisen P, Uppal A, Hartnett ME. Inhibition of NAD(P)H oxidase reduces apoptosis and avascular retina in an animal model of retinopathy of prematurity. Mol Vis. 2007;13:840–853. [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrara N, Gerber HP, Lecouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 28.Gao G, Li Y, Zhang D, et al. Unbalanced expression of VEGF and PEDF in ischemia-induced retinal neovascularization. FEBS Lett. 2001;489:270–276. doi: 10.1016/s0014-5793(01)02110-x. [DOI] [PubMed] [Google Scholar]
- 29.Akula JD, Mocko JA, Benador IY, et al. The neurovascular relation in oxygen-induced retinopathy. Mol Vis. 2008;14:2499–2508. [PMC free article] [PubMed] [Google Scholar]
- 30.Bachelder RE, Lipscomb EA, Lin X, et al. Competing Autocrine Pathways Involving Alternative Neuropilin-1 Ligands Regulate Chemotaxis of Carcinoma Cells. Cancer Res. 2003;63:5230–5233. [PubMed] [Google Scholar]
- 31.Huang Q, Wang S, Sorenson CM, Sheibani N. PEDF-deficient mice exhibit an enhanced rate of retinal vascular expansion and are more sensitive to hyperoxia-mediated vessel obliteration. Exp Eye Res. 2008;87:226–241. doi: 10.1016/j.exer.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ernest JT, Goldstick TK. Retinal oxygen tension and oxygen reactivity in retinopathy of prematurity in kittens. Invest Ophthalmol Vis Sci. 1984;25:1129–1134. [PubMed] [Google Scholar]
- 33.McLeod DS, Crone SN, Lutty GA. Vasoproliferation in the neonatal dog model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 1996;37:1322–1333. [PubMed] [Google Scholar]
- 34.Liu K, Akula JD, Falk C, Hansen RM, Fulton AB. The Retinal Vasculature and Function of the Neural Retina in a Rat Model of Retinopathy of Prematurity. Investigative Ophthalmology Visual Science. 2006;47:2639–2647. doi: 10.1167/iovs.06-0016. [DOI] [PubMed] [Google Scholar]
- 35.Hartnett ME, Martiniuk DJ, Byfield GE, et al. Neutralizing VEGF decreases tortuosity and alters endothelial cell division orientation in arterioles and veins in rat model of ROP: Relevance to plus disease. Invest Ophthalmol Vis Sci. 2008;49(7):3107–14. doi: 10.1167/iovs.08-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Penn JS, Tolman BL, Henry MM. Oxygen-induced retinopathy in the rat: Relationship of retinal nonperfusion to subsequent neovascularization. Invest Ophthalmol Vis Sci. 1994;35:3429–3435. [PubMed] [Google Scholar]
- 37.Geisen P, Peterson L, Martiniuk D, et al. Neutralizing antibody to VEGF reduces intravitreous neovascularization and does not interfere with vascularization of avascular retina in an ROP model. Mol Vis. 2008;14:345–357. [PMC free article] [PubMed] [Google Scholar]
- 38.Budd S, Byfield G, Martiniuk D, Geisen P, Hartnett ME. Reduction in endothelial tip cell filopodia corresponds to reduced intravitreous but not intraretinal vascularization in a model of ROP. Exp Eye Res. 2009 doi: 10.1016/j.exer.2009.06.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng G, Taylor SM, McColm JR, et al. Orientation of endothelial cell division is regulated by VEGF signaling during blood vessel formation. Blood. 2007;109:1345–1352. doi: 10.1182/blood-2006-07-037952. [DOI] [PMC free article] [PubMed] [Google Scholar]


