Abstract
Normal function of the placenta is pivotal for optimal fetal growth and development. Fetal programming commonly is associated with placental dysfunction that predisposes to obstetric complications and .suboptimal fetal outcomes. We consider several clinical phenotypes for placental dysfunction that likely predispose to fetal programming. Some of these reflect abnormal development of the chorioallantoic placenta in size, shape, or histopathology. Others result when exogenous stressors in the maternal environment combine with maladaptation of the placental response to yield small placentas with limited reserve, as typical of early-onset intrauterine growth restriction and preeclampsia. Still others reflect epigenetic changes, including altered expression of imprinted genes, altered enzymatic activity, or altered efficiencies in nutrient transport. Although the human placenta is a transient organ that persists only 9 months, the effects of this organ on the offspring remain for a lifetime.
Keywords: Placenta, trophoblast, developmental origins of human adult disease, epigenetics, fetal programming
The phenomenon of changing the pattern or content of developmental signals in utero to result in cardiovascular or metabolic disease of the adult is termed fetal programming. The developmental origins of health and disease, abbreviated as the mnemonic DOHaD, are based on the concept of fetal programming. Much attention is focused on the molecular mechanisms for the programming effects, with hopes of targeted therapy in the population at risk. Several observations regarding placental function and outcomes of pregnancy suggests this organ is pivotal, either directly or indirectly, in DOHaD.1,2 First, placental dysfunction is a common feature of pregnancies that yields programming effects on the fetus, most commonly recognized in offspring with intrauterine growth restriction (IUGR). Second, fetal programming occurs in both small for gestational age and large for gestational age offspring and, because placental weight correlates with fetal weight, programming occurs when placentas are maladapted, either too small or excessively large.3,4 Third, programming effects are not unique to a single clinical phenotype, but instead they can be detected after a variety of clinical maladies, including pregnancies complicated by IUGR, preterm birth, preeclampsia, or uncontrolled gestational diabetes, among others. Finally, the etiology of the placental dysfunction that predisposes to fetal programming is not singular but instead is multifactorial with abundant gene and environment interactions. Etiologies include maldevelopment of the placenta in the first trimester, injury to villous tissues from underperfusion, mechanical damage, or reoxygenation in the third trimester, or from maladaptive changes that evolve in the placenta throughout pregnancy in response to the dynamic maternal environment. Imprinting disorders and epigenetic modulations provide the background for interaction with these placental phenotypes to yield fetal programming.
Intuitively, the placenta interfaces the fetus and mother and thus creates, or at least transmits, the environment in which fetal programming occurs in the offspring. The fetal environment is the result of multiple placental functions, including directional transport of nutrients and oxygen, secretion of cytokines, hormones, and growth factors, and adaptive responses to environmental cues (Fig. 1). We first describe some aspects of early human placental development that previously were infrequently considered in the context of fetal programming. We then highlight how modifications in the gross structure of the placenta and exposure to exogenous insults relate to placental dysfunction and placental effects on fetal programming. We next overview the roles of epigenetic changes, imprinted genes, activity of placental 11 (β hydroxysteroid dehydrogenase (11 (β HSD), and nutrient sensors. Finally, we consider therapeutic interventions that might influence the processes we discuss. We hope that further research will provide clinicians with more options to improve placental function in pregnancies predisposed to fetal programming, thereby improving both short-term and long-term outcomes.
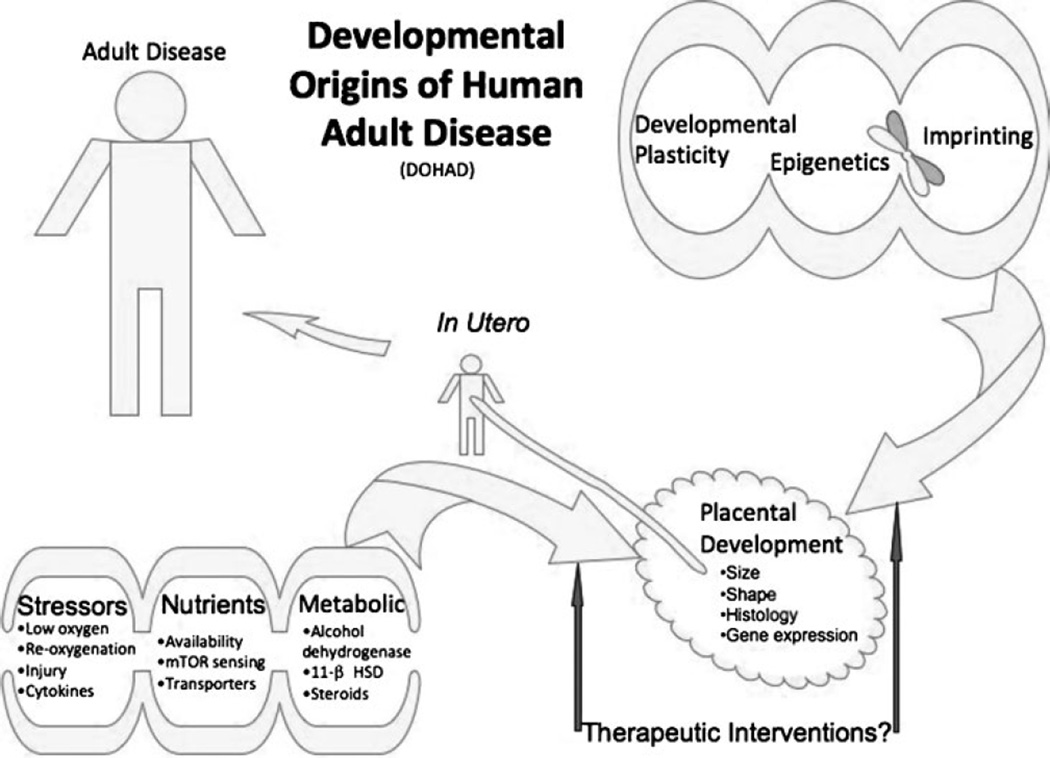
Figure 1.
Opportunities for fetal programming mediated by the placenta. Emerging possibilities, discussed in the text, indicate the placenta is an important mediator of the in utero environment predisposing to adult disease. Placental structure and function are affected by developmental plasticity, epigenetic effects, as well as by exogenous or endogenous stressors, nutrient limitation, or metabolic imbalances. The resulting placental dysfunction can affect fetal development in utero and predispose the adult to a variety of diseases. The placenta may be a possible target for clinical interventions in the future. 11 β HSD, 11 β hydroxysteroid dehydrogenase; mTOR, mammalian target of rapamycin.
COULD EMBRYONIC PROGRAMMING OCCUR IN TANDEM WITH EARLY PLACENTAL DEVELOPMENT?
All would accept the premise that placental development is pivotal for survival of Homo sapiens. However, we posit that fetal programming or, more specifically, embryonic programming may occur as a result of exogenous stress created in the intrauterine environment even before the definitive placenta evolves. We briefly outline the premise on which this hypothesis is based as an introduction to placental development.
Zygote-associated changes in a gene expression profile, determined by the nucleotide sequences, are modulated by epigenetic effects. These combine to orchestrate a highly regulated sequence of developmental events that produce the embryo and chorioallantoic placenta. For example, the progression from zygote to morula to blastocyst culminates in differentiation of cells into the trophectoderm, which will form the placenta, and inner cell mass, which will form the fetus. Trophectoderm of human blastocysts are highly invasive and penetrate the uterine epithelium to establish interstitial implantation. Multiple trophoblast phenotypes along with mesenchymal angiogenesis and vasculogenesis then contribute to the development of chorioallantoic placenta with trees of highly branched villi that are involved in all aspects of the maternal–fetal exchange. Importantly, two recent discoveries have changed our perception of the early environment in which both the conceptus and placenta develop. First, the villous structures only passively contribute to conceptus nutrition in the first 2 months of gestation, with organogenesis occurring in an environment where nutrition is not directly supplied by maternal blood but instead by uterine secretions called histiotroph.4–7 Second, there is an absence of perfusion of the intervillous space with maternal blood in normal early pregnancy. Invasive endovascular trophoblasts from the placenta migrate into the maternal basal plate where they penetrate and plug the openings in maternal spiral arterioles. This restricts the flow of oxygenated blood into the intervillous space until the end of the first trimester. Thus embryogenesis and early placental development occur in an environment with an oxygen tension < 20 mm Hg, perhaps as a mechanism to limit oxidative stress. Removal of the blockage of the spiral arteries and perfusion of the intervillous space ensues at 10 to 12 weeks of gestation, with the placental villi directly bathed with oxygen- and nutrient-rich maternal blood until delivery. Notably, premature flow of maternal blood into the intervillous lacunae in the first 2 months of gestation, as demonstrated by ultrasound assessment, associates with a higher rate of spontaneous pregnancy loss.8,10 The pregnancy loss is attributed to damaging reactive oxygen species and inadequate placental antioxidant defenses, which normally do not appear until 10 to 12 weeks of gestation.
The findings just outlined suggest opportunities for the occurrence of fetal programming earlier in gestation than commonly described. For example, through an altered content of the maternal supply of histiotroph, the conceptus may modulate gene expression and epigenetic profiles in the embryo to predispose to permanent programming changes in the fetus and, ultimately, to the adult. Moreover, exposure of the conceptus to excessive reactive oxygen species, as would occur with premature perfusion of placental villi with oxygenated blood, could affect the developmental potential of the chorioallantoic placenta, the developing embryo, or both, to affect permanent programming that manifests as a predisposition to disease later in life. Experimental exploration of these hypotheses clearly will require study in animal models because such studies are ethically unlikely in humans.
CHORIOALLANTOIC PLACENTAL DEVELOPMENT
Although the events just described evolve during early embryogenesis, the chorioallantoic placenta is forming trees of villi, some free floating and others anchored to the maternal basal plate decidua. In the presence of the invasive endovascular extravillous trophoblasts, the decidual spiral arterioles lose their smooth muscle coat and their vessel walls are replaced by fibrinoid. This anatomical change in these maternal vessels allows adequate, nonvasoreactive perfusion of the placental intervillous space and associates with oxygen delivery to the rapidly developing conceptus. Free-floating villi are exposed to an oxygen tension of 40 to 80 mm Hg from the end of the first trimester through term.11 Importantly, oxygen and nutrient delivery to the fetus from this point forward is primarily the responsibility of the villous trophoblast bilayer.
Injury to the villous trophoblast bilayer offers a possible mechanism for fetal programming that normally does not influence early fetal embryogenesis. The outer syncytiotrophoblast component of the trophoblast bilayer is a terminally differentiated, nonmitotic epithelium derived through differentiation and fusion of the subjacent mitotically active cytotrophoblast stem cells, to form a unique multinucleated, and apparently continuous, syncytium on the villous surface. Homeostasis of the trophoblast bilayer in normal development and in response to injury requires modulation of the proliferation, differentiation, and fusion of villous cytotrophoblasts to replenish the syncytiotrophoblast layer. Turnover of syncytiotrophoblast occurs, in part, through shedding of fragments from the syncytium into the intervillous space, yielding denudations on the villous surface. Deposits of fibrin on the basement membrane of the trophoblast identify these denudations, and the fibrin provides a matrix for reepithelialization by trophoblast at the site of the injury.12 Clusters of nuclei with condensed chromatin typical of apoptosis, so-called syncytial knots, are characteristic of the histopathology in the syncytiotrophoblast of the villous tree of complicated pregnancies and are especially apparent when preeclampsia and IUGR occur together.13 Whether the syncytial knots are released into the maternal circulation during normal troph-oblast turnover is a debated point,14–16 but the presence of increased knots in complicated pregnancies likely reflects a response to villous injury because knots also form in vitro when term villi are cultured in low oxygen tensions.17
Discontinuities in the syncytiotrophoblast affect the normal regulation of the passage of molecules through the syncytium. At sites of epithelial denudation, the barrier to passage of molecules from the maternal circulation to the fetal circulation becomes only the basement membrane that delimits the villous core connective tissue and the vessels within the core that merge into the fetal umbilical circulation. In vitro perfusion studies suggest that breaks in the trophoblast layer provide an unregulated paracellular route for molecules to cross the placenta based on their coefficient of diffusion in water.4,18 There is evidence of increased villous injury reflected by an increased number of fibrin deposits at breaks in the trophoblast layer in preeclampsia and in placentas from pregnancies with IUGR.19 It is possible, although not shown, that the fetus can be programmed, in part, by exposure to abnormal concentrations of ions, glucose, or other small molecules that pass from the maternal to fetal circulations through these unregulated regions of denuded villi.
Programming changes in the fetus are especially associated with placental histopathology where inadequate modification of the maternal spiral arterioles is present and so–called placental insufficiency ensues. There is a complex relationship between gene interactions and the environment to establish the spiral arteriole modifications, and dysregulation of this process commonly leads to a suboptimal pregnancy outcome and offspring with long-term health issues.20 Placental dysfunction is especially prominent when suboptimal pregnancy outcomes manifest as early onset IUGR, preeclampsia, or both, but basal plate histopathology may also manifest as other clinical phenotypes, such as preterm birth or premature preterm rupture of membranes.21 Interestingly, fetal growth in the latter phenotypes is commonly suboptimal even though not classified as IUGR.22 All of these suboptimal clinical outcomes associate with increased risks for fetal programming with long-term effects on the afflicted offspring.
Inadequate remodeling of the maternal spiral arterioles by placental trophoblasts results in no, or only limited, widening of the lumen and retention of the muscle in the vessel walls, so that the arterioles remain responsive to endogenous vasoactive agents. These maladaptive changes result in an increase in exposure of the placental villi to injury from reactive oxygen and nitrative species, to underperfusion induced hypoxia-reoxygenation, or to a combination of these stressors.23 Maladaptation of the spiral arterioles also increases the pressure of blood entering the intervillous space, likely inducing mechanical damage to the exposed villi.24 These injuries may yield a range of downstream responses manifested as placental insufficiency. Key signaling pathways may be differentially affected by these injuries, including dysregulation of the nuclear factor κB–mediated inflammatory pathways to yield preterm birth in some cases, and nitrative induced reductions of p38 mitogen-activated protein kinase (MAPK) activity to yield diminished placental growth and development of the villous trophoblast in cases of preeclampsia.23,25 Notably, the p38 MAPK pathway has been shown to regulate trophoblast differentiation.26,27
The histopathology of placentas from infants delivered with the diagnosis of IUGR with absent-end diastolic flow includes elongated terminal villi, with reduced capillary branching angiogenesis.28 Histopathological changes also occur in pregnancies at high altitude or with severe maternal anemia, with increased capillary branching, likely reflecting placental hypoxia.29 In all these situations, the alterations in placental villous structure provide opportunities for placental dysfunction and fetal programming.
Placental Size, Shape, and Weight Matter
We know that reduced placental growth, reflected by weight and volume, generally precedes diminished fetal growth.30,31 As importantly, placental weight can be modified by maternal cues because placental weight at delivery is increased when mothers receive high carbohydrate loads in the first trimester and high protein loads in later pregnancy.32 How might the gross appearances of shape and placental weight influence the outcomes of pregnancy and fetal programming?
A simple observation indicates that on a population basis the weight of the placenta at birth correlates with the weight of the infant.22,33 The placenta is typically described as either round or oval, but other shapes such as irregular, bilobate, or circumvallate, among others, are not infrequent.34,35 Irregular placental shapes have been associated with lower birthweight,36,37 suggesting they are associated with altered placental function. The relationship between placental weight and birthweight is sometimes expressed as the fetoplacental ratio, a proportion with fetal weight in the numerator and placental weight in the denominator. This ratio is influenced by exogenous signals in the fetus, the mother, or both. For example, maternal anemia, high body mass index, and maternal smoking all increase the placental ratio, even though smoking during pregnancy diminishes the absolute weight of the placenta compared with nonsmoking mothers.38 Women with preeclampsia have placentas with reduced surface area, with a shape that is more likely oval than round, and with offspring that have an increased risk for hypertension as adults.39 Indeed, several animal models and data on birthweight and placental weights in humans have correlated offspring vulnerability to cardiovascular disease and placental insufficiency, as recently reviewed.40
Salafia and Yampolsky conducted mathematical modeling studies to show that placental proportions, such as deviations in placental shape from round and the relative thickness, modify placental functional efficiency above and beyond shape and weight relationships alone. The effects of these factors explain some of the range of birthweights possible in a normal population delivered from placental weights in the same range and account for some of the variance in birthweight that otherwise is attributed to effects of maternal factors, such as age, parity, race, ethnicity, and socioeconomic status.22,42
Insertions of the umbilical cord into the placenta margin or into the fetal membranes (velamentous insertion) rather than into the main placental mass are well known to pathologists to associate with small placentas and small infants.34 Concomitant alterations of the umbilical blood supply to the fetus, perhaps by torsion of the umbilical cord, is a risk factor for fetal programming that has not been studied. However, Salafia and colleagues hypothesized that the association of cord insertions to suboptimal outcomes is not limited only to these extreme insertions. They recently applied mathematical analyses to the gross parameters of the placenta and showed that nonmarginal, but markedly eccentric, cord insertion associates with a sparser chorionic vascular distribution, a reduced transport efficiency of the placental vasculature, and a reduced birthweight for a given placental weight.35,43 The more eccentric the umbilical cord insertion, independent of the placental shape, the less efficient the placenta is in terms of the birthweight per gram of placental weight. Indeed, placentas with a noncentrally inserted umbilical cord and with finite diameters on the surface tended to be heavier because of increased thickness, a response that reflects the developmental plasticity of the placenta to supply fetal demands for nutrients. These data suggest that infants with eccentric insertions of the umbilical cord may be predisposed to effects of fetal programming that are not directly related to maternal nutrient deficiency or reductions in the maternal supply line to the placenta. They also indicate that the development of the gross appearance of the placenta can influence fetal outcomes and, in the process, predispose the fetus to programming.
Epigenetic Modulation of Placental Function
Epigenetics likely plays a prominent role in fetal programming, either by modulating placental gene expression and placental function or by directly affecting gene expression in the fetus during its orchestrated development and growth. Simply stated, epigenetics is changes in phenotype or gene expression caused by mechanisms other than alteration in DNA sequence. Five-methyl-cytosine methylation occurs by specific DNA methylases, typically in C/G-rich regions of the genome. The DNA methyltransferase I pathway involves folic acid, offering a potential clinical approach to modification of the methylation status of cellular DNA, as described later. Moreover, acetylation and methylation modification of histones regulates the transcriptional activity of associated genes. Epigenetic modifications can result in a change of information content even for future cell and organismal generations. Epigenetic phenomena offer a mechanism of developmental plasticity of the fetus and the placenta that allows each to respond to environmental stressors, perhaps promoting offspring survival, albeit at a potential long-term cost. A case in point is the small fetus associated with placental dysfunction that is consequently programmed through epigenetic effects to later develop postnatal diabetes and impaired insulin action.3
The presence of epigenetic modulations of human placental development remains to be identified, but animal models have provided evidence for proof of principle that epigenetic changes occur in response to environmental factors, as reviewed.44 For example, the Agouti mouse is yellow as a result of a single gene locus that is normally hypomethylated. Supplementation of mothers with methyl donors, including folic acid and vitamin B12, increases methylation of the coat gene and yields more offspring with a brown coat color compared with offspring of unsupplemented mothers.45 A recent functional analysis of gene expression in placentas of mice exposed to stress found increased expression of genes involved in DNA methylation and histone modification compared with placentas of unstressed mice.46 Similar studies in human placentas exposed to in utero stress are likely to identify key pathways that are susceptible to epigenetic regulation during placental development.
Imprinting and the Placenta
Over 100 protein-coding genes in humans are differentially expressed depending on parent-of-origin, a process called imprinting.47 Most imprinted genes are present in clusters of two or more genes that contain a differentially methylated region of relatively high CpG content. A surprising number of imprinted protein encoded genes are expressed in the placenta, indicating the importance of epigenetic gene expression in this organ.48–50 Interestingly, the paternal genome is normally demethylated within hours after fertilization of the zygote, whereas the maternal genome is demethylated during the initial cleavages that yield the morula and precede implantation, a phenomenon that varies among species. Imprinted genes are spared this phenomenon of demethylation, or erasure, because they are critical to ongoing development.51,52 Genomic imprinting is con- served among primates, rodents, and mammals, highlighting the importance of this phenomenon to placental development.53,54 In addition to protein coding genes, mammals express > 700 microRNAs (miRNAs), which are short RNAs that regulate gene expression at the post-transcriptional level. Many miRNAs are subject to epigenetic regulation and can themselves affect epige- netic pathways, providing feedback control.55–57
Studies on protein encoding imprinted RNAs provide the general insight that maternally imprinted genes tend to result in reduced placental growth whereas paternally expressed genes tend to enhance placental and fetal growth,49 supporting the conflict hypothesis58 that proposes paternally expressed genes enhance fetal growth and maternally expressed genes suppress fetal growth. An example is the insulinlike growth factor 2 system. The insulinlike growth factor 2 (Igf2) gene is maternally imprinted (paternally expressed), whereas the insulinlike growth factor 2 receptor (Igf2r) gene is paternally imprinted (maternally expressed). These genes have opposite effects on fetal and placental growth: Mice null for igf2 show reduced growth of the placenta and fetus;59,60 mice null for igf2r show increased placental and fetal growth.61,62 Alterations of epigenetic expression of these and other imprinted genes provide a mechanism by which nutrient and other stress in placental growth and development can ultimately alter the programming of the fetus.
Several miRNAs are expressed in the placenta and cultured human trophoblasts.63,66 Two groups found differential up- and downregulation of miRNAs in preeclamptic placentas compared with normal placen- tas,67,68 with, interestingly, several miRNA clusters identified, including one on 14q32.31, a chromosomal region subject to imprinting. Notably, miRNAs are present extracellularly in the maternal circulation in 50- to 100-nm diameter secreted vesicles termed exo-somes, and at least some of these miRNAs appear to be produced by the placenta.64,69 Hypoxia regulates miRNA expression in cultured human trophoblasts,65 although expression of this miRNA subset was not significantly altered in women with growth-restricted fetuses.69 Together, these data suggest the strong possibility that changes in miRNA expression, perhaps through epigenetic mechanisms, could alter the fetal environment and lead to fetal programming.
Placental 11 β Hydroxysteroid Dehydrogenase
The placenta produces and metabolizes steroids, as reviewed.44 A key step in steroid metabolism is the action of 11 β HSD type 2 to convert glucocorticoids to inactive metabolites.70 The placenta in this capacity protects the fetus from excess active glucocorticoids because the maternal concentration of active glucocorticoids is 1000-fold higher than the concentration in the fetal circulation. Notably, glucocorticoids impair the expression and function of Glut glucose transporters,71 providing a direct connection between fetal nutrient supply and the activity of 11 β HSD2. Expression of this enzyme normally increases with gestational age,72 and 11 β HSD2 activity changes in response to hypo- xia.73 Importantly, there is a reduction in 11 β HSD2 enzyme expression and activity in placentas from pre- eclamptic women,74 and the reduced activity may contribute to the abnormally high steroid levels in the fetus of preeclamptic women. Such higher than normal steroid levels could result in in utero programming changes of the hypothalamic-pituitary-adrenal (HPA) axis that last a lifetime because the HPA axis in the fetus normally develops independently of the effects of maternal stress steroid hormones.
The fetal HPA axis is especially influenced by alcohol exposure during pregnancy, with a higher HPA tone apparent in offspring exposed in utero to maternal alcohol abuse.75 Alcohol crosses the placenta with impunity, as the lipophilic nature of this small molecule is amenable to rapid transit. Notably, alcohol dehydrogenase is present at low concentrations in the placenta but may be inducible in some patients.76 The passage of alcohol to the fetus can modulate methyl group supply in cells77 and can alter methionine metabolism to alter protein synthesis.75 Alcohol thereby may yield long-lasting epigenetic changes in the fetus that manifest in adult life, through modulation of the HPA axis.
Glucocorticoids enhance differentiation in a variety of tissues, and the presence of higher than normal levels of these potent inducers of organ maturation may contribute to the long-term effects of in utero stress, even into the offspring’s adult life. Suboptimal maternal dietary intake reduces placental 11 β HSD2 activity. This result can also lead not only to abnormally high fetal levels of glucocorticoids, which modulate the fetal HPA axis long term, but also to effects on differentiation of other fetal organs, if exposed at critical stages of development. A case in point is kidney development in a sheep animal model,78 where transient exposure to exogenous steroids reduces nephron numbers in the fetus and predisposes to kidney disorders and hypertension as adults.
The short- and long-term effects of reduced placental 11 β HSD2 activity, excess maternal stress hormones, or a combination of these clearly contributes to fetal programming and subsequent adult disease, and placental dysfunction is often central to the effects induced.
NUTRIENT SUPPLY EFFECTS ON FETAL PROGRAMMING
The fetus depends on nutrients for growth, and the placenta mediates the transfer of nutrients from ~10 weeks of gestation until delivery, as previously dis- cussed and reviewed in Jansson and Powell.79 Placental supplied nutrient availability for fetal growth relates to multiple parameters, including the metabolism of the placenta itself, the mass and surface area of placental villi, blood flow into and out of the placenta, the concentration gradient between maternal and fetal circulations, and the expression and activity of rate-limiting transporters and binding proteins in the syncytiotrophoblast layer.4 Function of the syncytiotrophoblasts in hormone production and substrate metabolism is also crucial. Placental metabolism of substrates otherwise destined for the fetus may be regulated in response to demands.80 Importantly, villous trophoblasts in placentas exposed to environmental insults express altered hormone production 81 and mal-adaptive expression of nutrient transporters.82 Saudi Arabian babies who were in their second or third trimesters of gestation during Ramadan, a month-long period of daytime fasting for Muslims, had decreased placental weight and reduced ratio of placental to birthweight, although with unchanged birthweight, suggesting compensatory changes in placental efficiency.83 Although not yet investigated, it will be important to see if there are any long-term consequences of this in utero nutritional stress.
Doppler studies of the maternal arcuate arteries and the fetal umbilical arteries show that blood flow into and out of the placenta is reduced in IUGR, compared with pregnancies with average for gestational age (AGA) growth. Whether this reduced flow, and thus nutrient delivery, accounts for the reduced growth of the fetus is debated, but ultimately it is unlikely because most substrates are not flow limited. However, it is noteworthy that glucose is a primary energy source, and glucose transport in villous explants from 6 to 8 weeks of gestation, but not in term villi, is regulated by insulin.84 Thus the hyperinsulinemic state that characterizes the second half of pregnancy is unable to compensate and enhance glucose transport to the fetus if the placental or villous surface area(s) or Glut transporter function is inadequate for optimal fetal growth.
There is a concentration gradient for most amino acids between the maternal and fetal circulations that generally provides abundant substrates for protein synthesis in the fetus. However, the fetal circulation in IUGR has a reduced concentration of some amino acids compared with AGA fetuses.85,86 Concordantly, there is reduced activity in IUGR, compared with AGA, fetuses for system A, taurine, leucine, and cationic amino acid transporters.79
Jansson and Powell87 have proposed the intriguing model that the mammalian target of rapamycin (mTOR) signaling pathway is a nutrient sensor within the placenta. mTOR signaling influences the use of substrates for cell growth in a variety of systems, and this kinase integrates signals from growth factors, extracellular matrix, and nutritional substrates to optimize fetal growth. Importantly, extreme nutrient deficiency signals autophagy in cells through the mTOR pathway. mTOR also regulates system L amino acid transport in placental villi,88 and we recently found that mTOR is involved with the response to hypoxia-induced autophagy in cultures of human trophoblast.89 Localized trophoblast autophagy in vivo in response to exogenous stressors such as hypoxia, nutrient deprivation, or reactive oxygen species would temporarily benefit the fetus by providing critical substrates for ongoing growth in the presence of insufficient nutrient supply. However, this adaptive placental response may concurrently set the stage for fetal programming effects, as excessive autophagy would eventually yield placental dysfunction that would impact fetal development. Clearly, the roles of the mTOR pathway in sensing and responding to nutrient levels and stress, and in fetal programming, deserve further study.
FUTURE THERAPEUTIC POSSIBILITIES
The preceding review underscores the observation that fetal programming commonly associates with placental dysfunction. We have considered several clinical pheno-types for placental dysfunction that likely predispose to fetal programming. Some of these reflect abnormal development of the chorioallantoic placenta in size, shape, or histopathology. Others result when exogenous stressors in the maternal environment combine with maladaptation of the placental response to yield small placentas with limited reserve, as typical of early-onset IUGR and preeclampsia. Still others reflect epigenetic changes, imprinted gene expression, altered enzymatic activity, or nutrient transport deficiencies.
Although targeted modification of the placental development program is unlikely to be a clinical option in the foreseeable future, there may be some interventions that prove of benefit to reduce the effects of placental dysfunction and thus the predisposition to in utero programming of the fetus. A case in point is the fact that epigenetic changes are, by definition, reversible. Future therapeutic options for modulation of placental development may take the form of targeting individuals at high risk for IUGR or preeclampsia by increasing the administration of folate, B12, or choline to influence methylation of DNA, histone proteins, or both. Further studies of animal models may also direct us to more targeted therapies that involve the insulinlike growth factors administered to enhance both placental and fetal growth. We clearly need more research into normal placental development and function, and into placental dysfunction that predisposes to in utero programming of adult diseases, before any therapy will become a clinical reality.3
ACKNOWLEDGMENTS
This work was supported by NIH R01-HD29190. We thank Baosheng Chen and Fred Kraus for helpful discussions.
REFERENCES
- 1.Clifton VL. Review: Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31(Suppl):S33–S39. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Graves JA. Review: Sex chromosome evolution and the expression of sex-specific genes in the placenta. Placenta. 2010;31(Suppl):S27–S32. doi: 10.1016/j.placenta.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 3.Gatford KL, Simmons RA, De Blasio MJ, Robinson JS, Owens JA. Review: Placental programming of postnatal diabetes and impaired insulin action after IUGR. Placenta. 2010;31(Suppl):S60–S65. doi: 10.1016/j.placenta.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sibley CP, Brownbill P, Dilworth M, Glazier JD. Review: Adaptation in placental nutrient supply to meet fetal growth demand: implications for programming. Placenta. 2010;31(Suppl):S70–S74. doi: 10.1016/j.placenta.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 5.Burton GJ, Jauniaux E, Charnock-Jones DS. The influence of the intrauterine environment on human placental development. Int J Dev Biol. 2010;54(2–3):303–312. doi: 10.1387/ijdb.082764gb. [DOI] [PubMed] [Google Scholar]
- 6.Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metab. 2002;87(6):2954–2959. doi: 10.1210/jcem.87.6.8563. [DOI] [PubMed] [Google Scholar]
- 7.Jones CJ, Aplin JD, Burton GJ. First trimester histiotrophe shows altered sialylation compared with secretory phase glycoconjugates in human endometrium. Placenta. 2010;31(7):576–580. doi: 10.1016/j.placenta.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Jauniaux E, Hempstock J, Greenwold N, Burton GJ. Trophoblastic oxidative stress in relation to temporal and regional differences in maternal placental blood flow in normal and abnormal early pregnancies. Am J Pathol. 2003;162(1):115–125. doi: 10.1016/S0002-9440(10)63803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jauniaux E, Watson A, Burton G. Evaluation of respiratory gases and acid-base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks’ gestation. Am J Obstet Gynecol. 2001;184(5):998–1003. doi: 10.1067/mob.2001.111935. [DOI] [PubMed] [Google Scholar]
- 10.Jauniaux E, Johns J, Burton GJ. The role of ultrasound imaging in diagnosing and investigating early pregnancy failure. Ultrasound Obstet Gynecol. 2005;25(6):613–624. doi: 10.1002/uog.1892. [DOI] [PubMed] [Google Scholar]
- 11.Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum Reprod Update. 2006;12(6):747–755. doi: 10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson DM, Crouch EC, Curran EM, Farmer DR. Trophoblast interaction with fibrin matrix. Epithelialization of perivillous fibrin deposits as a mechanism for villous repair in the human placenta. Am J Pathol. 1990;136(4):855–865. [PMC free article] [PubMed] [Google Scholar]
- 13.Levy R, Smith SD, Yusuf K, et al. Trophoblast apoptosis from pregnancies complicated by fetal growth restriction is associated with enhanced p53 expression. Am J Obstet Gynecol. 2002;186(5):1056–1061. doi: 10.1067/mob.2002.122250. [DOI] [PubMed] [Google Scholar]
- 14.Burton GJ, Jones CJ. Syncytial knots, sprouts, apoptosis, and trophoblast deportation from the human placenta. Taiwan J Obstet Gynecol. 2009;48(1):28–37. doi: 10.1016/S1028-4559(09)60032-2. [DOI] [PubMed] [Google Scholar]
- 15.Huppertz B. The anatomy of the normal placenta. J Clin Pathol. 2008;61(12):1296–1302. doi: 10.1136/jcp.2008.055277. [DOI] [PubMed] [Google Scholar]
- 16.Huppertz B. IFPA Award in Placentology Lecture: Biology of the placental syncytiotrophoblast—myths and facts. Placenta. 2010;31(Suppl):S75–S81. doi: 10.1016/j.placenta.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Heazell AE, Moll SJ, Jones CJ, Baker PN, Crocker IP. Formation of syncytial knots is increased by hyperoxia, hypoxia and reactive oxygen species. Placenta. 2007;28(Suppl A):S33–S40. doi: 10.1016/j.placenta.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Brownbill P, Mahendran D, Owen D, et al. Denudations as paracellular routes for alphafetoprotein and creatinine across the human syncytiotrophoblast. Am J Physiol Regul Integr Comp Physiol. 2000;278(3):R677–R683. doi: 10.1152/ajpregu.2000.278.3.R677. [DOI] [PubMed] [Google Scholar]
- 19.Rampersad R, Barton A, Sadovsky Y, Nelson DM. The C5b-9 membrane attack complex of complement activation localizes to villous trophoblast injury in vivo and modulates human trophoblast function in vitro. Placenta. 2008;29(10):855–861. doi: 10.1016/j.placenta.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts CT. IFPA Award in Placentology Lecture: Complicated interactions between genes and the environment in placentation, pregnancy outcome and long term health. Placenta. 2010;31(Suppl):S47–S53. doi: 10.1016/j.placenta.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Sherer DM, Salafia CM, Minior VK, Sanders M, Ernst L, Vintzileos AM. Placental basal plate myometrial fibers: clinical correlations of abnormally deep trophoblast invasion. Obstet Gynecol. 1996;87(3):444–449. doi: 10.1016/0029-7844(95)00426-2. [DOI] [PubMed] [Google Scholar]
- 22.Salafia CM, Zhang J, Charles AK, et al. Placental characteristics and birthweight. Paediatr Perinat Epidemiol. 2008;22(3):229–239. doi: 10.1111/j.1365-3016.2008.00935.x. [DOI] [PubMed] [Google Scholar]
- 23.Myatt L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta. 2010;31(Suppl):S66–S69. doi: 10.1016/j.placenta.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30(6):473–482. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindstrom TM, Bennett PR. The role of nuclear factor kappa B in human labour. Reproduction. 2005;130(5):569–581. doi: 10.1530/rep.1.00197. [DOI] [PubMed] [Google Scholar]
- 26.Daoud G, Amyot M, Rassart E, Masse A, Simoneau L, Lafond J. ERK1/2 and p38 regulate trophoblasts differentiation in human term placenta. J Physiol. 2005;566(Pt 2):409–423. doi: 10.1113/jphysiol.2005.089326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnstone ED, Sibley CP, Lowen B, Guilbert LJ. Epidermal growth factor stimulation of trophoblast differentiation requires MAPK11/14 (p38 MAP kinase) activation. Biol Reprod. 2005;73(6):1282–1288. doi: 10.1095/biolreprod.105.044206. [DOI] [PubMed] [Google Scholar]
- 28.Krebs C, Macara LM, Leiser R, Bowman AW, Greer IA, Kingdom JC. Intrauterine growth restriction with absent end-diastolic flow velocity in the umbilical artery is associated with maldevelopment of the placental terminal villous tree. Am J Obstet Gynecol. 1996;175(6):1534–1542. doi: 10.1016/s0002-9378(96)70103-5. [DOI] [PubMed] [Google Scholar]
- 29.Kingdom JC, Kaufmann P. Oxygen and placental villous development: origins of fetal hypoxia. Placenta. 1997;18(8):613–621. doi: 10.1016/s0143-4004(97)90000-x. discussion 623–626. [DOI] [PubMed] [Google Scholar]
- 30.Hafner E, Metzenbauer M, Hofinger D, et al. Placental growth from the first to the second trimester of pregnancy in SGA-foetuses and pre-eclamptic pregnancies compared to normal foetuses. Placenta. 2003;24(4):336–342. doi: 10.1053/plac.2002.0918. [DOI] [PubMed] [Google Scholar]
- 31.Jansson T, Thordstein M, Kjellmer I. Placental blood flow and fetal weight following uterine artery ligation. Temporal aspects of intrauterine growth retardation in the guinea pig. Biol Neonate. 1986;49(3):172–180. doi: 10.1159/000242528. [DOI] [PubMed] [Google Scholar]
- 32.Godfrey K, Robinson S, Barker DJ, Osmond C, Cox V. Maternal nutrition in early and late pregnancy in relation to placental and fetal growth. BMJ. 1996;312(7028):410–414. doi: 10.1136/bmj.312.7028.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baptiste-Roberts K, Salafia CM, Nicholson WK, Duggan A, Wang NY, Brancati FL. Gross placental measures and childhood growth. J Matern Fetal Neonatal Med. 2009;22(1):13–23. doi: 10.1080/14767050802415728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benirschke K, Kaufmann P, Baergen R. Pathology of the Human Placenta. 5th ed. New York, NY: Springer; 2006. [Google Scholar]
- 35.Yampolsky M, Salafia CM, Shlakhter O, Haas D, Eucker B, Thorp J. Modeling the variability of shapes of a human placenta. Placenta. 2008;29(9):790–797. doi: 10.1016/j.placenta.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salafia CM, Maas E, Thorp JM, Eucker B, Pezzullo JC, Savitz DA. Measures of placental growth in relation to birth weight and gestational age. Am J Epidemiol. 2005;162(10):991–998. doi: 10.1093/aje/kwi305. [DOI] [PubMed] [Google Scholar]
- 37.Salafia CM, Misra DP, Yampolsky M, Charles AK, Miller RK. Allometric metabolic scaling and fetal and placental weight. Placenta. 2009;30(4):355–360. doi: 10.1016/j.placenta.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Godfrey KM, Redman CW, Barker DJ, Osmond C. The effect of maternal anaemia and iron deficiency on the ratio of fetal weight to placental weight. Br J Obstet Gynaecol. 1991;98(9):886–891. doi: 10.1111/j.1471-0528.1991.tb13510.x. [DOI] [PubMed] [Google Scholar]
- 39.Kajantie E, Thornburg KL, Eriksson JG, Osmond C, Barker DJ. In preeclampsia, the placenta grows slowly along its minor axis. Int J Dev Biol. 2010;54(2–3):469–473. doi: 10.1387/ijdb.082833ek. [DOI] [PubMed] [Google Scholar]
- 40.Thornburg KL, O’Tierney PF, Louey S. Review: The placenta is a programming agent for cardiovascular disease. Placenta. 2010;31(Suppl):S54–S59. doi: 10.1016/j.placenta.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salafia CM, Yampolsky M. Metabolic scaling law for fetus and placenta. Placenta. 2009;30(5):468–471. doi: 10.1016/j.placenta.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salafia CM, Zhang J, Miller RK, Charles AK, Shrout P, Sun W. Placental growth patterns affect birth weight for given placental weight. Birth Defects Res A Clin Mol Teratol. 2007;79(4):281–288. doi: 10.1002/bdra.20345. [DOI] [PubMed] [Google Scholar]
- 43.Yampolsky M, Salafia CM, Shlakhter O, Haas D, Eucker B, Thorp J. Centrality of the umbilical cord insertion in a human placenta influences the placental efficiency. Placenta. 2009;30(12):1058–1064. doi: 10.1016/j.placenta.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langley-Evans SC. Nutritional programming of disease: unravelling the mechanism. J Anat. 2009;215(1):36–51. doi: 10.1111/j.1469-7580.2008.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23(15):5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gheorghe CP, Goyal R, Mittal A, Longo LD. Gene expression in the placenta: maternal stress and epigenetic responses. Int J Dev Biol. 2010;54(2–3):507–523. doi: 10.1387/ijdb.082770cg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sha K. A mechanistic view of genomic imprinting. Annu Rev Genomics Hum Genet. 2008;9:197–216. doi: 10.1146/annurev.genom.122007.110031. [DOI] [PubMed] [Google Scholar]
- 48.Bressan FF, De Bem TH, Perecin F, et al. Unearthing the roles of imprinted genes in the placenta. Placenta. 2009;30(10):823–834. doi: 10.1016/j.placenta.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 49.Coan PM, Burton GJ, Ferguson-Smith AC. Imprinted genes in the placenta—a review. Placenta. 2005;26(Suppl A):S10–S20. doi: 10.1016/j.placenta.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 50.Wood AJ, Oakey RJ. Genomic imprinting in mammals: emerging themes and established theories. PLoS Genet. 2006;2(11):e147. doi: 10.1371/journal.pgen.0020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mayer W, Niveleau A, Walter J, Fundele R, Haaf T. Demethylation of the zygotic paternal genome. Nature. 2000;403(6769):501–502. doi: 10.1038/35000656. [DOI] [PubMed] [Google Scholar]
- 52.Tremblay KD, Saam JR, Ingram RS, Tilghman SM, Bartolomei MS. A paternal-specific methylation imprint marks the alleles of the mouse H19 gene. Nat Genet. 1995;9(4):407–413. doi: 10.1038/ng0495-407. [DOI] [PubMed] [Google Scholar]
- 53.Dean W, Ferguson-Smith A. Genomic imprinting: mother maintains methylation marks. Curr Biol. 2001;11(13):R527–R530. doi: 10.1016/s0960-9822(01)00311-6. [DOI] [PubMed] [Google Scholar]
- 54.Wagschal A, Feil R. Genomic imprinting in the placenta. Cytogenet Genome Res. 2006;113(1–4):90–98. doi: 10.1159/000090819. [DOI] [PubMed] [Google Scholar]
- 55.Chuang JC, Jones PA. Epigenetics and microRNAs. Pediatr Res. 2007;61(5 Pt 2):24R–29R. doi: 10.1203/pdr.0b013e3180457684. [DOI] [PubMed] [Google Scholar]
- 56.Iorio MV, Piovan C, Croce CM. Interplay between micro-RNAs and the epigenetic machinery: an intricate network. Biochim Biophys Acta. 2010;1799(10–12):694–701. doi: 10.1016/j.bbagrm.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 57.Maccani MA, Marsit CJ. Epigenetics in the placenta. Am J Reprod Immunol. 2009;62(2):78–89. doi: 10.1111/j.1600-0897.2009.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haig D, Graham C. Genomic imprinting and the strange case of the insulin-like growth factor II receptor. Cell. 1991;64(6):1045–1046. doi: 10.1016/0092-8674(91)90256-x. [DOI] [PubMed] [Google Scholar]
- 59.Constancia M, Hemberger M, Hughes J, et al. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature. 2002;417(6892):945–948. doi: 10.1038/nature00819. [DOI] [PubMed] [Google Scholar]
- 60.Coan PM, Fowden AL, Constancia M, Ferguson-Smith AC, Burton GJ, Sibley CP. Disproportional effects of Igf2 knockout on placental morphology and diffusional exchange characteristics in the mouse. J Physiol. 2008;586(Pt 20):5023–5032. doi: 10.1113/jphysiol.2008.157313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lau MM, Stewart CE, Liu Z, Bhatt H, Rotwein P, Stewart CL. Loss of the imprinted IGF2/cation-independent man-nose 6-phosphate receptor results in fetal overgrowth and perinatal lethality. Genes Dev. 1994;8(24):2953–2963. doi: 10.1101/gad.8.24.2953. [DOI] [PubMed] [Google Scholar]
- 62.Ludwig T, Eggenschwiler J, Fisher P, D’Ercole AJ, Davenport ML, Efstratiadis A. Mouse mutants lacking the type 2 IGF receptor (IGF2R) are rescued from perinatal lethality in Igf2 and Igf1r null backgrounds. Dev Biol. 1996;177(2):517–535. doi: 10.1006/dbio.1996.0182. [DOI] [PubMed] [Google Scholar]
- 63.Barad O, Meiri E, Avniel A, et al. MicroRNA expression detected by oligonucleotide microarrays: system establishment and expression profiling in human tissues. Genome Res. 2004;14(12):2486–2494. doi: 10.1101/gr.2845604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luo SS, Ishibashi O, Ishikawa G, et al. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biol Reprod. 2009;81(4):717–729. doi: 10.1095/biolreprod.108.075481. [DOI] [PubMed] [Google Scholar]
- 65.Mouillet JF, Chu T, Nelson DM, Mishima T, Sadovsky Y. MiR-205 silences MED1 in hypoxic primary human trophoblasts. FASEB J. 2010;24(6):2030–2039. doi: 10.1096/fj.09-149724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Su L, Zhao S, Zhu M, Yu M. Differential expression of microRNAs in porcine placentas on days 30 and 90 of gestation. Reprod Fertil Dev. 2010;22(8):1175–1182. doi: 10.1071/RD10046. [DOI] [PubMed] [Google Scholar]
- 67.Pineles BL, Romero R, Montenegro D, et al. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am J Obstet Gynecol. 2007;196(3):261. doi: 10.1016/j.ajog.2007.01.008. e1–e6. [DOI] [PubMed] [Google Scholar]
- 68.Zhu XM, Han T, Sargent IL, Yin GW, Yao YQ. Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies vs normal pregnancies. Am J Obstet Gynecol. 2009;200(6):661. doi: 10.1016/j.ajog.2008.12.045. e1–e7. [DOI] [PubMed] [Google Scholar]
- 69.Mouillet JF, Chu T, Hubel CA, Nelson DM, Parks WT, Sadovsky Y. The levels of hypoxia-regulated microRNAs in plasma of pregnant women with fetal growth restriction. Placenta. 2010;31(9):781–784. doi: 10.1016/j.placenta.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Edwards CR, Benediktsson R, Lindsay RS, Seckl JR. 11 β-Hydroxysteroid dehydrogenases: key enzymes in determining tissue-specific glucocorticoid effects. Steroids. 1996;61(4):263–269. doi: 10.1016/0039-128x(96)00033-5. [DOI] [PubMed] [Google Scholar]
- 71.Hahn T, Barth S, Graf R, et al. Placental glucose transporter expression is regulated by glucocorticoids. J Clin Endocrinol Metab. 1999;84(4):1445–1452. doi: 10.1210/jcem.84.4.5607. [DOI] [PubMed] [Google Scholar]
- 72.Murphy VE, Clifton VL. Alterations in human placental 11p-hydroxysteroid dehydrogenase type 1 and 2 with gestational age and labour. Placenta. 2003;24(7):739–744. doi: 10.1016/s0143-4004(03)00103-6. [DOI] [PubMed] [Google Scholar]
- 73.Alfaidy N, Gupta S, DeMarco C, Caniggia I, Challis JR. Oxygen regulation of placental 11 P-hydroxysteroid dehydrogenase 2: physiological and pathological implications. J Clin Endocrinol Metab. 2002;87(10):4797–4805. doi: 10.1210/jc.2002-020310. [DOI] [PubMed] [Google Scholar]
- 74.Challis JR, Sloboda DM, Alfaidy N, et al. Prostaglandins and mechanisms of preterm birth. Reproduction. 2002;124(1):1–17. doi: 10.1530/rep.0.1240001. [DOI] [PubMed] [Google Scholar]
- 75.Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinol. 2008;20(4):470–488. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rasheed A, Hines RN, McCarver-May DG. Variation in induction of human placental CYP2E1: possible role in susceptibility to fetal alcohol syndrome? Toxicol Appl Pharmacol. 1997;144(2):396–400. doi: 10.1006/taap.1997.8152. [DOI] [PubMed] [Google Scholar]
- 77.Schalinske KL, Nieman KM. Disruption of methyl group metabolism by ethanol. Nutr Rev. 2005;63(11):387–391. doi: 10.1111/j.1753-4887.2005.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 78.Dodic M, Abouantoun T, O’Connor A, Wintour EM, Moritz KM. Programming effects of short prenatal exposure to dexamethasone in sheep. Hypertension. 2002;40(5):729–734. doi: 10.1161/01.hyp.0000036455.62159.7e. [DOI] [PubMed] [Google Scholar]
- 79.Jansson T, Powell TL. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci (Lond) 2007;113(1):1–13. doi: 10.1042/CS20060339. [DOI] [PubMed] [Google Scholar]
- 80.Illsley NP, Caniggia I, Zamudio S. Placental metabolic reprogramming: do changes in the mix of energy-generating substrates modulate fetal growth? Int J Dev Biol. 2010;54(2–3):409–419. doi: 10.1387/ijdb.082798ni. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McMullen S, Osgerby JC, Thurston LM, et al. Alterations in placental 11 P-hydroxysteroid dehydrogenase (11 betaHSD) activities and fetal cortisol:cortisone ratios induced by nutritional restriction prior to conception and at defined stages of gestation in ewes. Reproduction. 2004;127(6):717–725. doi: 10.1530/rep.1.00070. [DOI] [PubMed] [Google Scholar]
- 82.Jansson T, Ekstrand Y, Bjorn C, Wennergren M, Powell TL. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes. 2002;51(7):2214–2219. doi: 10.2337/diabetes.51.7.2214. [DOI] [PubMed] [Google Scholar]
- 83.Alwasel SH, Abotalib Z, Aljarallah JS, et al. Changes in placental size during Ramadan. Placenta. 2010;31(7):607–610. doi: 10.1016/j.placenta.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 84.Ericsson A, Hamark B, Powell TL, Jansson T. Glucose transporter isoform 4 is expressed in the syncytiotrophoblast of first trimester human placenta. Hum Reprod. 2005;20(2):521–530. doi: 10.1093/humrep/deh596. [DOI] [PubMed] [Google Scholar]
- 85.Cetin I, Ronzoni S, Marconi AM, et al. Maternal concentrations and fetal-maternal concentration differences of plasma amino acids in normal and intrauterine growth-restricted pregnancies. Am J Obstet Gynecol. 1996;174(5):1575–1583. doi: 10.1016/s0002-9378(96)70609-9. [DOI] [PubMed] [Google Scholar]
- 86.Cetin I, Alvino G. Intrauterine growth restriction: implications for placental metabolism and transport. A review. Placenta. 2009;30(Suppl A):S77–S82. doi: 10.1016/j.placenta.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 87.Jansson T, Powell TL. IFPA 2005 Award in Placentology Lecture. Human placental transport in altered fetal growth: does the placenta function as a nutrient sensor?—a review. Placenta. 2006;27(Suppl A):S91–S97. doi: 10.1016/j.placenta.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 88.Roos S, Kanai Y, Prasad PD, Powell TL, Jansson T. Regulation of placental amino acid transporter activity by mammalian target of rapamycin. Am J Physiol Cell Physiol. 2009;296(1):C142–C150. doi: 10.1152/ajpcell.00330.2008. [DOI] [PubMed] [Google Scholar]
- 89.Longtine M, Chen B, Nelson D. Hypoxia induces autophagy in cultured human placental trophoblasts; Presented at: The 57th meeting for the Society for Gynecological Investigation; March 16–19 2011; Orlando, FL. 2011. [Google Scholar]