Abstract
Anti-B4-blocked ricin (anti-B4-bR) is a potent immunotoxin directed against the CD 19 antigen. Previous phase I and II studies suggested a possible role for anti-B4-bR as consolidation after high-dose chemotherapy and autologous stem cell transplant. Cancer and Leukemia Group B (CALGB) 9254 is a phase III study which randomized 157 patients with B-cell lymphoma in complete remission following autologous transplant to treatment with anti-B4-bR or observation. With a median follow-up time for patients of 5.8 years, the median event-free survival for protocol treatment and observation are 2.1 and 2.9 years, respectively (p = 0.275). The median overall survival for treatment and observation are 6.1 years and not reached, respectively (p = 0.063). Therefore, no differences were found in event-free survival and overall survival between protocol treatment and observation, although there was a trend toward improved survival with observation. These data fail to support a role for anti-B4-bR as consolidative therapy after bone marrow transplant in patients with B-cell lymphoma.
Keywords: Lymphoma, anti-B4-blocked ricin, autologous transplant, adjuvant therapy
Introduction
Diffuse large B-cell lymphoma is the most common sub-type of non-Hodgkin lymphoma (NHL) in the United States, representing approximately 30% of all patients [1]. Standard initial therapy for aggressive NHL is chemoimmunotherapy, which achieves complete response rates of 60–80% [2–4] and a 5-year progression-free survival of 50–75% [5–7]. For patients who subsequently relapse and respond to second-line therapy, high-dose chemotherapy followed by autologous stem cell transplant offers a potentially curative option. The Parma Trial demonstrated the superiority of myeloablative over non-myeloablative dosed chemotherapy for patients with aggressive NHL who were in chemosensitive relapse, with response rates of 84% versus 44%, but with an event-free survival at 5 years of only 46% [8]. Highdose chemotherapy followed by autologous stem cell transplant also has a role, though less clearly defined, in other recurrent B-cell lymphomas such as folli-cular lymphoma [9–12].
Despite the favorable outcomes for some patients, modifications in stem cell transplant-based approaches are necessary to further improve outcomes. Anti-B4-blocked ricin (anti-B4-bR) is a monoclonal antibody directed against the B4 antigen (CD19) present on B cells linked to the modified toxin blocked ricin, which inhibits protein synthesis [13,14]. Native ricin, found in castor beans, is a potent protein toxin that consists of two chains. The A-chain is an N-glycosidase that is responsible for the inhibition of ribosomal function. The B-chain serves to mediate: (1) binding to galactose-terminated oligosaccharides that are ubiquitous on the surfaces of eukaryotic cells and (2) translocation of the Achain into the cytoplasm. The non-specific binding of ricin is blocked by the covalent attachment of glycopeptides to the galactose binding sites of the Bchain, creating ‘blocked ricin’ [13–15]. Linking the toxin to an antibody directed against CD19 results in the specific targeting of the blocked ricin to CD19 positive cells. Once bound by the antibody, CD19 internalizes, mediating cell entry of the blocked ricin. Thus, anti-B4-bR replaces the loss of ubiquitous cell binding resulting from ‘blocking’ of the ricin with the specificity of a B-cell specific monoclonal antibody.
While initial in vitro and mouse models demonstrated anti-lymphoma effects of anti-B4-bR [13,16– 18], the drug failed to demonstrate significant single-agent activity in clinical trials [19,20]. Data suggested that a large tumor burden resulting in clearance of the immunotoxin and poor tissue penetration of the antibody represented potential key limitations to the efficacy of anti-B4-bR. In order to overcome these problems, anti-B4-bR was studied as an adjuvant after high-dose chemotherapy, when tumor burden would be minimal [21]. In phase I and II studies, anti-B4-bR demonstrated tolerability and encouraging disease-free survival when used as intensification after stem cell transplant [21,22]. In order to fully assess the safety and efficacy of anti-B4-bR, a randomized clinical trial was undertaken by the Cancer and Leukemia Group B (CALGB) and Eastern Cooperative Oncology Group (ECOG) in patients with relapsed B-cell NHL undergoing autologous bone marrow transplant.
Methods
Subjects
Eligible subjects for this study were between 18 and 65 years of age and were undergoing an autologous stem cell transplant for B-cell NHL that had relapsed after, or was refractory to, conventional chemotherapy or radiotherapy. All B-cell NHL histologies were eligible. All tumors demonstrated reactivity with anti-CD19 or anti-CD20 monoclonal antibodies prior to transplant. Subjects who demonstrated overt histologic bone marrow involvement by lymphoma at the time of marrow harvest or circulating lymphoma cells at the time of collection were required to have had their marrow or peripheral blood purged of residual tumor cells prior to reinfusion. Purging for subjects without overt marrow or peripheral blood involvement with lymphoma at harvest was at the discretion of the treating physician.
Each patient signed an institutional review boardapproved, study-specific informed consent and was registered to the study prior to stem cell transplant. Patients were subsequently eligible to receive treatment with anti-B4-bR between days 60 and 120 after marrow/stem cell reinfusion provided they were in a complete remission following the transplant. Complete remission was documented within 14 days for intermediate and aggressive lymphomas or 21 days for low-grade lymphomas prior to randomization via unilateral bone marrow biopsy (bilateral biopsies if marrow previously involved), gallium scan (if previously positive), and computed tomography (CT) scan. For randomization, subjects also had to demonstrate: stable engraftment (hematocrit > 25%, platelets > 50 000/µL, absolute neutrophil count > 500/µL), adequate kidney and liver function, negative human immunodeficiency virus (HIV) test, and an ECOG performance status of 0–2. Patients were excluded from receiving treatment with anti-B4-bR if they had a history of hemolytic uremic syndrome or veno-occlusive disease of the liver.
Randomization was either to observation or treatment with anti-B4-bR. In order to maintain a balanced randomization, patients were stratified based upon: (1) the institution’s transplant regimen; (2) disease sensitivity at the time of transplant (sensitive vs. resistant/unknown); (3) lymphoma grade (low vs. intermediate vs. high); and (4) time after transplant (6–90 days vs. 91–120 days).
Treatment
Subjects randomized to treatment received anti-B4-bR 30 µg/kg/day as a continuous intravenous infusion via a central catheter over 7 days on an outpatient basis. Patients were observed in the outpatient setting for the initial 3 h of the infusion for toxicities and variations in vital signs. Patients were eligible for a second cycle of treatment provided they did not develop HAMA (human anti-mouse antibody)/ HARA (human anti-ricin antibody) responses. The second cycle was to begin on day 15, but could be delayed up to 5 days to permit resolution of any toxicities that developed during the first infusion. Toxicities were evaluated utilizing the CALGB Expanded Toxicity Criteria.
Anti-B4-bR
Anti-B4-bR was manufactured and supplied by ImmunoGen, Inc. (Cambridge, MA) as previously described [13–15]. Anti-B4 is an immunoglobulin G1 (IgG1) murine monoclonal antibody produced using in vitro culture techniques, and is covalently linked to blocked ricin. Anti-B4-bR was administered intravenously at a concentration of 100 µg/mL in phosphate buffered saline using human serum albumin as a carrier.
Pharmacologic monitoring
Blood samples were obtained for determination of serum levels of anti-B4-bR immediately prior to initiation of the infusion, at day 4 ± 24 h, and on day 7 prior to discontinuation of the infusion. Repeat assessments were obtained at the same time points during the second cycle. Anti-B4-bR concentrations were performed by ImmunoGen, Inc. by enzyme-linked immunosorbent assay (ELISA) methods as described previously [23].
HAMA/HARA detection
Blood samples were assessed for the development of HAMA/HARA by ImmunoGen, Inc., utilizing standard ELISA techniques as previously described [15]. HAMA was considered positive if the value was > 0.468 µg/mL. HARA was considered positive if the value was > 20% above the pretreatment value. Samples were assessed prior to treatment with anti-B4-bR, on day 7, and on day 10–13 of course 1, and on days 0, 7, and 28 of course 2.
Response assessments
Responses were assessed starting 28 days after completion of treatment with anti-B4-bR, or 40–50 days after randomization for those on the observation arm, utilizing physical examination, blood work, chest X-ray, and CT scans. Subsequently, responses were assessed every 6 months by physical examination, chest X-ray, blood work, and abdominal/pelvic CT scanning. Chest CT or magnetic resonance imaging (MRI) was to be performed if the chest X-ray demonstrated adenopathy or if there was a history of adenopathy on chest X-ray.
Complete response (CR) required the disappearance of all signs and symptoms of measurable or evaluable disease by physical examination and laboratory and imaging studies persisting for more than 4 weeks, without the development of any new lesions. Complete response with residual abnormality was defined as a subject meeting all of the criteria for CR, but having a persistent mass that regressed by more than 50% following therapy and then stabilized with no further change over at least 2 additional months. If the mass demonstrated gallium avidity prior to treatment, it must be gallium negative on repeat imaging. Partial response was defined as a reduction greater than 50% in the sum of the products of the perpendicular diameters of all measurable lesions lasting more than 4 weeks, and the disappearance of any constitutional symptoms. Stable disease was defined as a less than 50% reduction and a less than 25% increase in the sum of the products of the perpendicular diameters of all measurable lesions, as well as the absence of any new lesions. Progressive disease was defined by an increase in the product of the perpendicular diameters of any lesion by greater than 25% or the appearance of any new lesion.
The primary end-point for this study is event-free survival. Event-free survival was defined as the time from randomization to recurrence of disease or treatment-related death. Recurrent disease was defined as the appearance on CT scan, gallium scan, or bone marrow biopsy of evidence of non-Hodgkin lymphoma. Survival represents the time from randomization to death.
Statistics
The study sample size was calculated based upon the primary end-point of the randomized portion of the study, event-free survival. Event-free survival at 2 years was estimated as 30% for the observation arm. Using a two-sided α = 0.05 level test with approximately 89% power, the sample size required to be randomized in order to detect a 70% increase in median event-free survival (from 30 to 49%) was 232 patients. Based upon the expectation that 60% of patients would be eligible for randomization, 388 patients were planned to be registered to the study. Survival curves were calculated according to the Kaplan–Meier method [24] and compared using two-sided log-rank test [25]. Formal interim analyses were planned to be performed after approximately 50%, 75%, and 100% of the expected study events (defined as recurrence or death) occurred in the pooled sample. The study was monitored at least twice annually by the CALGB Data Safety and Monitoring Board.
Patient registration and data collection were managed by the CALGB Statistical Center. Data quality was ensured by careful review of data by CALGB Statistical Center staff and by the study chairperson. Statistical analyses were performed by CALGB statisticians.
Auditing
As part of the quality assurance program of the CALGB, members of the Audit Committee visit all participating institutions at least once every 3 years to review source documents. The auditors verify compliance with federal regulations and protocol requirements, including those pertaining to eligibility, treatment, adverse events, tumor response, and outcome in a sample of protocols at each institution. Such on-site review of medical records was performed for a subgroup of 56 (11%) of the 511 patients under this study.
Results
Enrollment and treatment
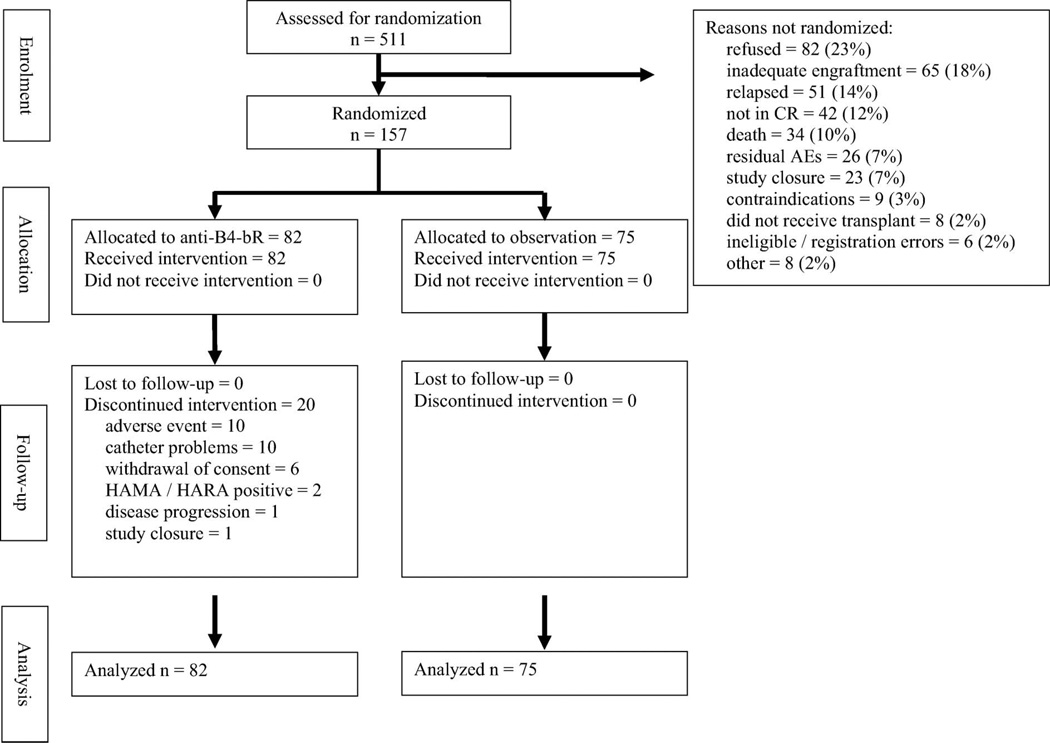
Between April 1993 and March 1997, 511 patients were registered onto the study, with 157 (30.7%) patients undergoing randomization (Figure 1 shows a Consolidated Standards of Reporting Trials [CON-SORT] flow chart depicting the outcomes of participants entered onto the study). The CALGB Data and Safety Monitoring Board recommended closure of the study after a planned interim analysis suggested it was highly unlikely that anti-B4-bR would be able to demonstrate any statistically significant benefit.
Figure 1.
CONSORT flow diagram depicting outcomes of patients enrolled onto study.
Characteristics of the patients enrolled onto the study are shown in Table I. The median patient age was 49 years, with 56.1% of patients having intermediate or high grade lymphoma by the International Working Formulation. Stage III or IV disease was noted in 72.7% of patients at relapse, and 91.7% of patients were chemotherapy-sensitive at the time of transplant. As stated, only 30.7% of the patients registered were randomized, compared to the 60% anticipated in the statistical design. There were no significant differences in characteristics between patients who were randomized and those who were not randomized except for histologic grade (data not shown). Patients with low-grade lymphomas were more likely to not be randomized compared with other histologies. The most common reasons for not being randomized to study treatment are listed in Figure 1
Table I.
Characteristics of the 511 patients registered on study.
| Total |
Randomized |
Not randomized |
||||
|---|---|---|---|---|---|---|
| Characteristic | n | % | n | % | n | % |
| Male | 286 | 56.0% | 78 | 49.7% | 208 | 58.8% |
| Female | 225 | 44.0% | 79 | 50.3% | 146 | 41.2% |
| B symptoms | 42 | 8.4% | 12 | 7.7% | 30 | 8.7% |
| Stage | ||||||
| I | 54 | 11.3% | 21 | 14.1% | 33 | 10.1% |
| II | 76 | 16.0% | 20 | 13.4% | 56 | 17.1% |
| III | 88 | 18.5% | 31 | 20.8% | 57 | 17.4% |
| V | 258 | 54.2% | 77 | 51.7% | 181 | 55.4% |
| Extranodal sites | ||||||
| 0 | 409 | 81.8% | 130 | 83.3% | 279 | 81.1% |
| 1 | 57 | 11.4% | 20 | 12.8% | 37 | 10.8% |
| 2 | 18 | 3.6% | 4 | 2.6% | 14 | 4.1% |
| 3+ | 16 | 3.2% | 2 | 1.3% | 14 | 4.1% |
| Performance status | ||||||
| 0 | 371 | 74.5% | 125 | 80.1% | 246 | 71.9% |
| 1 | 116 | 23.3% | 28 | 18.0% | 88 | 25.7% |
| 2 | 11 | 2.2% | 3 | 1.9% | 8 | 2.3% |
| IWF grade | ||||||
| Low | 191 | 38.8% | 73 | 47.4% | 118 | 34.9% |
| Intermediate | 239 | 48.6% | 68 | 44.2% | 171 | 50.6% |
| High | 37 | 7.5% | 6 | 3.9% | 31 | 9.2% |
| Miscellaneous | 25 | 5.1% | 7 | 4.6% | 18 | 5.3% |
| Disease status | ||||||
| Sensitive | 451 | 91.7% | 145 | 95.4% | 306 | 90.0% |
| Resistant | 41 | 8.3% | 7 | 4.6% | 34 | 10.0% |
| Age (years) | ||||||
| 20–29 | 20 | 3.9% | 3 | 1.9% | 17 | 4.8% |
| 30–39 | 83 | 16.2% | 30 | 10.1% | 53 | 15.0% |
| 40–49 | 159 | 31.1% | 50 | 31.9% | 109 | 30.8% |
| 50–59 | 175 | 34.2% | 57 | 36.3% | 118 | 33.3% |
| 60–69 | 73 | 14.3% | 17 | 10.8% | 56 | 15.8% |
| 70 + | 1 | 0.2% | 0 | 0% | 1 | 0.3% |
| Median/range | 49 | 20–70 | 48 | 26–69 | 49 | 20–70 |
| LDH, median/range | 252 | 65–9828 | 215 | 74–1267 | 278 | 65–9828 |
IWF, International Working Formulation; LDH, lactate dehydrogenase.
Of the 157 patients who underwent randomization, 82 were randomized to treatment with anti-B4-bR and 75 to observation. Of the 82 patients randomized to receive treatment with anti-B4-bR, only 52 (63.4%) completed all treatment. Table II lists the reasons for treatment discontinuation. The two most common reasons for not being able to complete anti-B4-bR treatment were adverse events and catheter problems (12% each). All patients were analyzed according to the treatment to which they were randomized, regardless of whether they received the treatment.
Table II.
Reasons for discontinuation of treatment.
| Reason treatment terminated | No. of patients | % |
|---|---|---|
| Completed treatment | 52 | 63.4% |
| Adverse event | 10 | 12.2% |
| Catheter problems | 10 | 12.2% |
| Patient withdrawal | 6 | 7.3% |
| HAMA/HARA positive | 2 | 2.4% |
| Disease progression | 1 | 1.2% |
| Study closure | 1 | 1.2% |
HAMA/HARA, human anti-mouse antibody/human anti-ricin antibody.
Adverse events
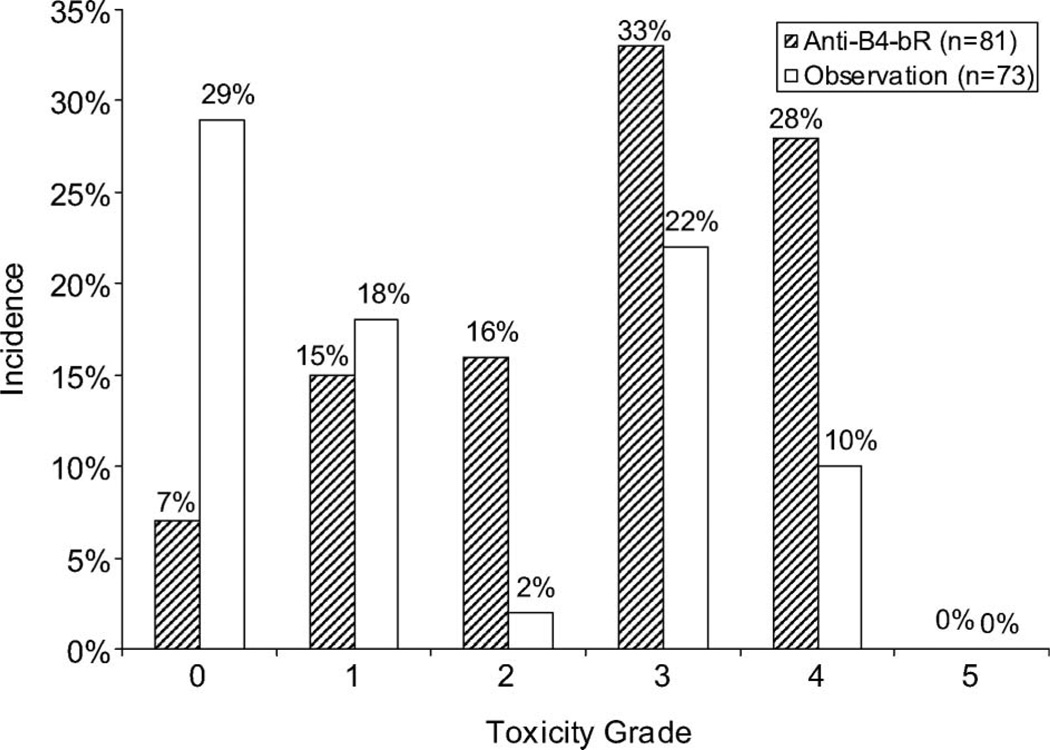
One hundred fifty-four patients were assessable for adverse events (AEs) (81 patients on anti-B4-bR and 73 patients on observation). The rates of higher grade hematologic, non-hematologic, and overall AEs were significantly greater in the anti-B4-bR arm compared with observation (p < 0.001) (Figure 2). Grade 3 or greater toxicities that were more likely to occur with anti-B4-bR treatment compared with observation were lymphopenia, phlebitis/thrombosis, peripheral edema, and malaise/fatigue. It is certainly possible that AEs were more likely to be reported for patients on the anti-B4-bR arm than for those patients on the observation arm, as this was not a blinded study. However, many of the AEs are those characteristically seen with the infusion of biological agents.
Figure 2.
Adverse events. Maximum hematologic toxicity as determined by Cochran–Armitage test for trend over all levels of toxicity for patients treated with anti-B4-bR versus observation.
Event-free and overall survival
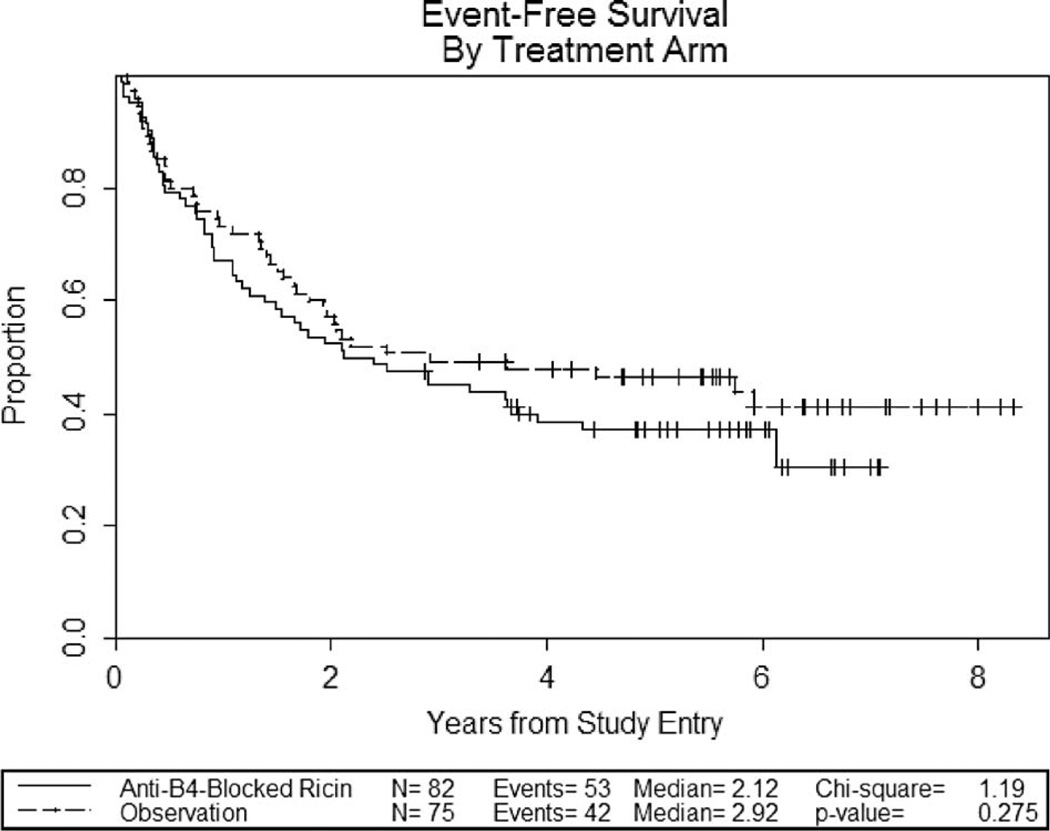
With a median follow-up time of 5.8 years (range 2.9–9.1 years), the median event-free survival is 2.1 for patients treated with anti-B4-bR versus 2.9 years for observation (p = 0.275) (Figure 3). The 2-year event-free survival was 52% for treatment with anti-B4-bR versus 57% for observation.
Figure 3.
Kaplan–Meier curves for event-free survival.
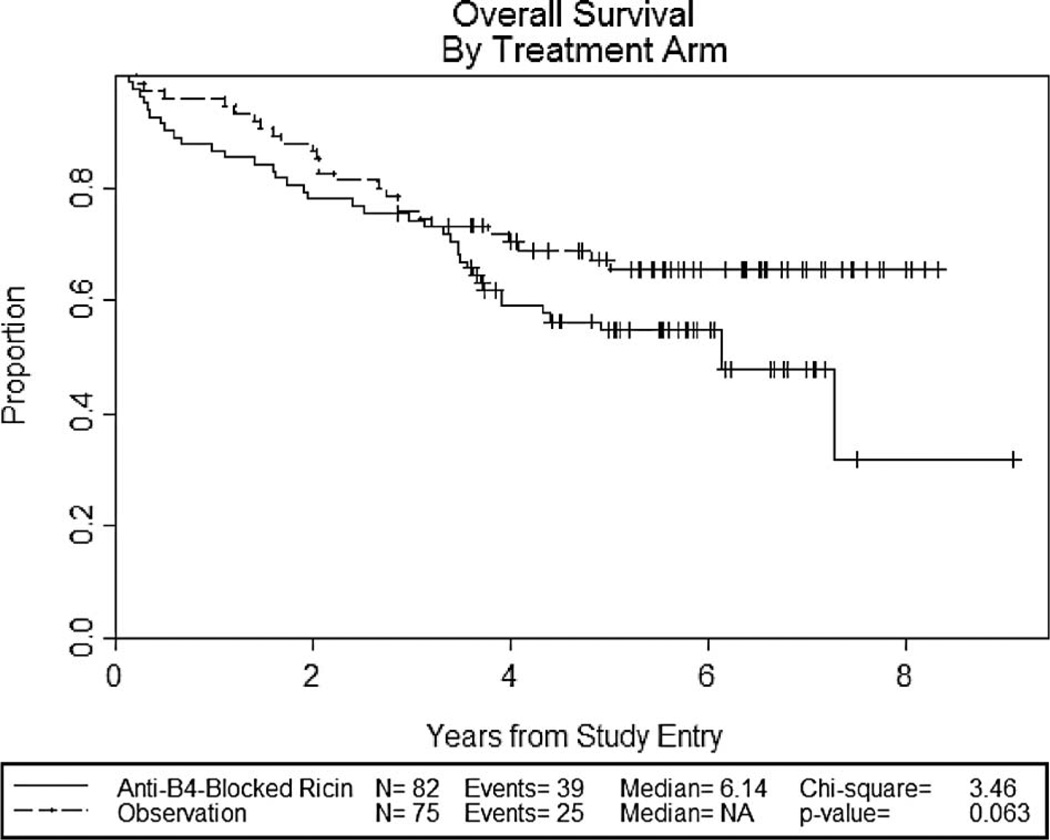
The median overall survival is 6.1 years for patients treated with anti-B4-bR versus not reached for observation (p = 0.063) (Figure 4). The 2-year overall survival is 78% for treatment with anti-B4-bR versus 88% for observation. While neither observation reached statistical significance, there was a trend toward decreased event-free survival and overall survival in patients treated with anti-B4-bR.
Figure 4.
Kaplan–Meier curves for overall survival.
Pharmacokinetics
The serum pharmacokinetics data for anti-B4-bR are shown in Table III. The mean value on day 4 of cycle 1 was 159 ng/mL and rose to 181 ng/mL by day 7. The mean difference in serum levels between day 4 and day 7 of cycle 1 for patients with samples available at both time points was 15 ng/mL, which was not statistically significant using the paired t-test, p = 0.219. During cycle 2, the mean serum levels of anti-B4-bR on days 0, 4, and 7 were 0, 118, and 105 ng/mL, respectively. The mean difference between day 4 and day 7 of cycle 2 was −16 ng/mL, which was statistically significant, p = 0.043. For the 36 patients with days 4 and 7 measurements from both cycles, the average course 1 mean value was 180 ng/ mL and the average course 2 mean value was 115 ng/ mL. The mean difference between the average course 1 and course 2 values was −64 ng/mL, which was statistically significant, p < 0.0001.
Table III.
Serum levels of anti-B4-bR.
| Time point | No. of patients | Mean (ng/mL) | 95% CI | Median (ng/mL) | Min. (ng/mL) | Max. (ng/mL) |
|---|---|---|---|---|---|---|
| Course 1, day 4 | 73 | 159 | 136–182 | 145 | 8 | 654 |
| Course 1, day 7 | 67 | 181 | 155–206 | 167 | 8 | 474 |
| Course 2, day 0 | 25 | 0 | NA | 0 | 0 | 0 |
| Course 2, day 4 | 49 | 118 | 96–140 | 120 | 5 | 422 |
| Course 2, day 7 | 43 | 105 | 79–132 | 84 | 8 | 319 |
anti-B4-bR, anti-B4-blocked ricin; CI, confidence interval; NA, not applicable.
HAMA/HARA
Although these patients were heavily immunosuppressed as a result of their lymphoma and prior therapies, antibody responses to the anti-B4-bR immunotoxin occurred in significant numbers of patients (Table IV). At baseline, five patients had detectable HAMA titers and none had HARA titers. While it is not possible to determine whether these positive HAMA titers resulted in more toxicity, none of the patients that were HAMA positive at baseline discontinued treatment due to adverse events. During the first cycle of anti-B4-bR treatment, two patients became HARA positive, and therefore did not receive a second cycle of treatment with anti-B4-bR.
Table IV.
Antibody responses to anti-B4-bR following treatment.
| Course 1 |
Course 2 |
|||||
|---|---|---|---|---|---|---|
| Pre-treatment | Day 7 | Days 10–13 | Pre-treatment | Day 7 | Day 28 | |
| No. of samples | 80 | 71 | 71 | 56 | 51 | 52 |
| HAMA(+) | 5 (6%) | 4 (6%) | 4 (6%) | 1 (2%) | 6 (12%) | 14 (27%) |
| HARA(+) | 0 (0%) | 0 (0%) | 1 (1%) | 0 (0%) | 19 (37%) | 37 (71%) |
anti-B4-bR, anti-B4-blocked ricin; HAMA, human anti-mouse antibody; HARA, human anti-ricin antibody.
Fifty-six patients continued onto course 2 of anti-B4-bR treatment. Only one patient, who was HAMA positive prior to course 1, was HAMA positive at the beginning of course 2 of treatment. By day 7 of cycle 2, significant numbers of patients converted to being HAMA (6; 12%) and/or HARA positive (19; 37%). The six subjects who were HAMA positive were also HARA positive. By day 28 of cycle 2, 14 (27%) patients were HAMA positive and 37 (71%) were HARA positive. Once again, all of the patients who were HAMA positive were HARA positive as well.
Discussion
New therapeutic modalities are necessary to eliminate chemotherapy-resistant lymphoma cells. Potent toxins, such as ricin, may potentially provide a means for killing lymphoma cells via pathways that remain operative in chemotherapy-resistant lymphoma cells. Anti-B4-bR has demonstrated considerable activity in vitro in multidrug-resistant lymphoma models [17,18]. Several problems potentially leading to the ineffectiveness of immunotherapy seen in earlier studies of anti-B4-bR include: achieving inadequate serum levels due to insufficient dosing or rapid clearance of the immunotherapy, large tumor burdens, or poor penetration into tissues. In this study utilizing high-dose chemotherapy and autologous stem cell transplant to help overcome some of these issues, anti-B4-bR was still unable to demonstrate any benefit.
More than half of the patients registered onto the study were not randomized, most commonly due to refusal or transplant failure (inadequate engraftment, relapse, did not achieve CR). The large number of patients not randomized would be unlikely to impact upon the results of the study. It is plausible that many of the patients who refused randomization did not want to go through additional therapy after enduring a transplant. Regarding the patients qualified as transplant failures, the design of the study, only randomizing patients in CR, was done to help maximize the possibility of seeing a benefit with the anti-B4-bR by ensuring a minimal amount of disease present at the time of transplant.
In vitro studies utilizing Namalwa cells predict that serum levels > 0.5 nM are sufficient to kill up to 3-log of cells after 24 h of exposure, and lower serum concentrations were able to achieve the same amount of cell killing with longer duration of exposure [15,23]. A previous phase I study in patients with relapsed B-cell lymphoma demonstrated that all patients treated with doses of 40 µg/kg/day or above as a 7-day continuous IV infusion achieved levels of anti-B4-bR greater than 1 nM [23]. When studied as an adjuvant to autologous bone marrow transplant, the maximum tolerated dose was found to be 40 µg/ kg/day for 7 days [21]. In order to improve the tolerability and to enable the administration of a second cycle of therapy prior to the development of HAMA/HARA responses, the treatment dose in our study was lowered to 30 µg/kg/day over 7 days and repeated 14 days later. A phase II study of this dosing regimen demonstrated that anti-B4-bR serum levels of 0.77 ± 0.41 nM were achieved on day 7 of course 1 [22]. The mean serum concentration of anti-B4-bR achieved in our study was 181 ng/mL (0.86 nM) after course 1 and 105 ng/mL (0.5 nM) after course 2. Based upon the in vitro data using Namalwa cells, a Burkitt lymphoma cell line, these levels would be predicted to be sufficient for therapeutic effect, but minimally. It is certainly possible that a Burkitt lymphoma cell line would be more sensitive to anti-B4-bR than lymphoma cells in vivo, and therefore underestimates the serum levels necessary for a therapeutic effect, leaving our levels sub-therapeutic.
A second possible reason for the lack of efficacy of anti-B4-bR was the development of neutralizing HAMA/HARA responses. In a study of indolent lymphoma patients, a median of only two cycles of anti-B4-bR was able to be administered out of a planned six, most often due to HAMA/HARA responses, which occurred in 27 of the 35 patients [26]. In the phase I study of anti-B4-bR as an adjuvant to myeloablative therapy, HAMA/HARA formation occurred in seven of 12 patients between days 14 and 38 [21].
In order to reduce an impact of HAMA/HARA formation on efficacy in our study, the second infusion of anti-B4-bR was moved to 14 days after the first. Only two patients (2.4%) were unable to receive the second cycle of therapy due to HAMA/ HARA. Significant rates of HAMA/HARA responses were still seen in our study, 27% and 71%, respectively, but not until completion of the second course. Additionally, while the development of these antibodies might neutralize subsequent cycles of treatment, they should not interfere with the efficacy of the initial cycle of immunotoxin. However, they might be responsible for the increased rate of clearance for the second cycle, resulting in the lower serum levels of anti-B4-bR seen in cycle 2. The ability of these patients, who were immunosuppressed from their lymphoma, the transplant, and the use of an agent (anti-B4-bR) that would deplete any residual normal B cells, to develop an antibody response suggests that perhaps the anti-B4-bR was having only a minimal impact.
For comparison, in the pivotal study of the immunotoxin denileukin diftitox in patients with relapsed cutaneous T-cell lymphoma, 19 out of 60 patients (32%) possessed neutralizing antibodies to denileukin diftitox at baseline, likely due to prior exposure to diphtheria toxin. After two cycles of denileukin diftitox, 59 out of 60 patients (98%) demonstrated neutralizing antibodies to denileukin diftitox. Importantly, the presence of neutralizing antibodies at baseline, their development, or the titer developed did not impair response [27]. For BL22 and LMB-2, two immunotoxins currently undergoing clinical trials for relapsed and refractory lymphomas, the presence of neutralizing antibodies has been reported in 4/16 (25%) and 13/35 (37%) of patients, respectively [28,29]. While these studies differ in tumor types and prior therapies, they do demonstrate and help support that the neutralizing antibodies are unlikely to explain the lack of efficacy for anti-B4-bR.
Earlier studies suggested poor tissue penetration as a possible explanation for the lack of effectiveness of anti-B4-bR [19,20]. Multani et al., using a daily bolus regimen of 50 µg/kg/day of anti-B4-bR, were able to achieve high serum levels of 2.7 ± 1.3 nM and detect immunotoxin in three out of three bone marrow biopsy specimens, but could only detect immunotoxin in two of seven lymph node specimens by immunohistochemistry [19]. This issue was avoided in our study by requiring patients to be in a complete response prior to being randomized.
Several other factors to explain a lack of efficacy include a lack of uptake of immunotoxin by the lymphoma cells or an inability for the ricin to be released into the cytoplasm and inhibit ribosomal function. As the trial utilized patients in complete response, obtaining tissue samples for analysis after treatment was not possible. Given earlier published reports of single agent activity of anti-B4-bR in patients with lymphomas, it is unlikely that one of these factors was likely to explain the lack of efficacy.
Finally, only 63% of patients were able to receive the two full courses of treatment. Of the patients unable to complete therapy, two-thirds were unable due to adverse events or catheter problems. While it is not possible to know whether this number of patients is sufficiently large to have had an impact upon the results of the study, it is only appropriate to analyze the data on an intent-to-treat basis. Should methods be identified that enable anti-B4-bR to be better tolerated or infused, reassessment of the efficacy of anti-B4-bR might be worthwhile.
In conclusion, anti-B4-bR is ineffective as a treatment for lymphomas when used at a dose of 30 µg/kg/day over 7 days as an adjuvant to myeloablative chemotherapy. The rationale and pre-clinical evidence for anti-B4-bR suggest that the immunotoxin could potentially be an effective clinical tool. While anti-B4-bR demonstrated no efficacy in this clinical study, it might provide further insights to shape any further trials involving anti-B4-bR. However, the future use of anti-B4-bR is uncertain given the more favorable activity and toxicity profiles of several agents that have been approved since this study was performed, including rituximab and radioimmunotherapy.
Acknowledgements
The following institutions participated in this study: Dana-Farber Cancer Institute, Boston, MA—Harold J. Burstein, MD, PhD, supported by CA32291. Dartmouth Medical School – Norris Cotton Cancer Center, Lebanon, NH—Konstantin Dragnev, MD, supported by CA04326.
Duke University Medical Center, Durham, NC—Jeffrey Crawford, MD, supported by CA47577. Massachusetts General Hospital, Boston, MA—Jeffrey W. Clark, MD, supported by CA32291. McGill University, Montreal, QC—Gerald Batist, MD.
Medical University of South Carolina, Charleston, SC—Mark Green, MD, supported by CA03927. Mount Sinai School of Medicine, New York, NY— Lewis R. Silverman, MD, supported by CA04457. North Shore University Health System CCOP, Evanston, IL—David L Grinblatt, MD, supported by CA35279.
Roswell Park Cancer Institute, Buffalo, NY—Ellis Levine, MD, supported by CA59518.
State University of New York Upstate Medical University, Syracuse, NY—Stephen L. Graziano, MD, supported by CA21060.
University of California at San Diego, San Diego, CA—Barbara A. Parker, MD, supported by CA11789.
University of California at San Francisco, San Francisco, CA—Charles J Ryan, MD, supported by CA60138.
University of Chicago, Chicago, IL—Hedy L Kindler, MD, supported by CA41287.
University of Iowa, Iowa City, IA—Daniel A. Vaena, MD, supported by CA47642.
University of Maryland Greenebaum Cancer Center, Baltimore, MD—Martin Edelman, MD, supported by CA31983.
University of Massachusetts Medical School, Worcester, MA—William V. Walsh, MD, supported by CA37135.
University of Missouri/Ellis Fischel Cancer Center, Columbia, MO—Michael C. Perry, MD, supported by CA12046.
University of North Carolina at Chapel Hill, Chapel Hill, NC—Thomas C. Shea, MD, supported by CA47559.
Wake Forest University School of Medicine, Winston-Salem, NC—David D. Hurd, MD, supported by CA03927.
Walter Reed Army Medical Center, Washington, DC—Brendan M. Weiss, MD, supported by CA26806.
Washington University School of Medicine, St. Louis, MO—Nancy Bartlett, MD, supported by CA77440.
Weill Medical College of Cornell University, New York, NY—John Leonard, MD, supported by CA07968.
Declaration of interest: The research for CALGB 9254 was supported, in part, by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Richard L. Schilsky, MD, Chairman) and to the CALGB Statistical Center (Stephen George, PhD, CA33601). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
References
- 1.Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1998;16:2780–2795. doi: 10.1200/JCO.1998.16.8.2780. [DOI] [PubMed] [Google Scholar]
- 2.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 3.Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 4.Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60) Lancet Oncol. 2008;9:105–116. doi: 10.1016/S1470-2045(08)70002-0. [DOI] [PubMed] [Google Scholar]
- 5.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 6.Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2005;23:4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 7.Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med. 1993;328:1002–1006. doi: 10.1056/NEJM199304083281404. [DOI] [PubMed] [Google Scholar]
- 8.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 9.Bierman PJ, Vose JM, Anderson JR, Bishop MR, Kessinger A, Armitage JO. High-dose therapy with autologous hematopoietic rescue for follicular low-grade non-Hodgkin’s lymphoma. J Clin Oncol. 1997;15:445–450. doi: 10.1200/JCO.1997.15.2.445. [DOI] [PubMed] [Google Scholar]
- 10.Schouten HC, Qian W, Kvaloy S, et al. High-dose therapy improves progression-free survival and survival in relapsed follicular non-Hodgkin’s lymphoma: results from the randomized European CUP trial. J Clin Oncol. 2003;21:3918–3927. doi: 10.1200/JCO.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Sebban C, Mounier N, Brousse N, et al. Standard chemotherapy with interferon compared with CHOP followed by high-dose therapy with autologous stem cell transplantation in untreated patients with advanced follicular lymphoma: the GELF-94 randomized study from the Groupe d’Etude des Lymphomes de l’Adulte (GELA) Blood. 2006;108:2540–2544. doi: 10.1182/blood-2006-03-013193. [DOI] [PubMed] [Google Scholar]
- 12.Gyan E, Foussard C, Bertrand P, et al. High-dose therapy followed by autologous purged stem cell transplantation and doxorubicin-based chemotherapy in patients with advanced follicular lymphoma: a randomized multicenter study by the GOELAMS with final results after a median follow-up of 9 years. Blood. 2009;113:995–1001. doi: 10.1182/blood-2008-05-160200. [DOI] [PubMed] [Google Scholar]
- 13.Lambert JM, Goldmacher VS, Collinson AR, Nadler LM, Blattler WA. An immunotoxin prepared with blocked ricin: a natural plant toxin adapted for therapeutic use. Cancer Res. 1991;51:6236–6242. [PubMed] [Google Scholar]
- 14.Lambert JM, McIntyre G, Gauthier MN, et al. The galactosebinding sites of the cytotoxic lectin ricin can be chemically blocked in high yield with reactive ligands prepared by chemical modification of glycopeptides containing triantennary N-linked oligosaccharides. Biochemistry. 1991;30:3234–3247. doi: 10.1021/bi00227a011. [DOI] [PubMed] [Google Scholar]
- 15.Grossbard ML, Freedman AS, Ritz J, et al. Serotherapy of Bcell neoplasms with anti-B4-blocked ricin: a phase I trial of daily bolus infusion. Blood. 1992;79:576–585. [PubMed] [Google Scholar]
- 16.Shah SA, Halloran PM, Ferris CA, et al. Anti-B4-blocked ricin immunotoxin shows therapeutic efficacy in four different SCID mouse tumor models. Cancer Res. 1993;53:1360–1367. [PubMed] [Google Scholar]
- 17.O’Connor R, Liu C, Ferris CA, et al. Anti-B4-blocked ricin synergizes with doxorubicin and etoposide on multidrug-resistant and drug-sensitive tumors. Blood. 1995;86:4286–4294. [PubMed] [Google Scholar]
- 18.Liu C, Lambert JM, Teicher BA, Blattler WA, O’Connor R. Cure of multidrug-resistant human B-cell lymphoma xenografts by combinations of anti-B4-blocked ricin and chemotherapeutic drugs. Blood. 1996;87:3892–3898. [PubMed] [Google Scholar]
- 19.Multani PS, O’Day S, Nadler LM, Grossbard ML. Phase II clinical trial of bolus infusion anti-B4 blocked ricin immunoconjugate in patients with relapsed B-cell non-Hodgkin’s lymphoma. Clin Cancer Res. 1998;4:2599–2604. [PubMed] [Google Scholar]
- 20.Tsimberidou AM, Giles FJ, Kantarjian HM, Keating MJ, O’Brien SM. Anti-B4 blocked ricin post chemotherapy in patients with chronic lymphocytic leukemia-long-term follow-up of a monoclonal antibody-based approach to residual disease. Leuk Lymphoma. 2003;44:1719–1725. doi: 10.1080/1042819031000116706. [DOI] [PubMed] [Google Scholar]
- 21.Grossbard ML, Gribben JG, Freedman AS, et al. Adjuvant immunotoxin therapy with anti-B4-blocked ricin after autologous bone marrow transplantation for patients with B-cell non-Hodgkin’s lymphoma. Blood. 1993;81:2263–2271. [PubMed] [Google Scholar]
- 22.Grossbard ML, Multani PS, Freedman AS, et al. A phase II study of adjuvant therapy with anti-B4-blocked ricin after autologous bone marrow transplantation for patients with relapsed B-cell non-Hodgkin’s lymphoma. Clin Cancer Res. 1999;5:2392–2398. [PubMed] [Google Scholar]
- 23.Grossbard ML, Lambert JM, Goldmacher VS, et al. Anti-B4-blocked ricin: a phase I trial of 7-day continuous infusion in patients with B-cell neoplasms. J Clin Oncol. 1993;11:726–737. doi: 10.1200/JCO.1993.11.4.726. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 25.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1996;50:163–170. [PubMed] [Google Scholar]
- 26.Longo DL, Duffey PL, Gribben JG, et al. Combination chemotherapy followed by an immunotoxin (anti-B4-blocked ricin) in patients with indolent lymphoma: results of a phase II study. Cancer J. 2000;6:146–150. [PubMed] [Google Scholar]
- 27.Olsen E, Duvic M, Frankel A, et al. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J Clin Oncol. 2001;19:376–388. doi: 10.1200/JCO.2001.19.2.376. [DOI] [PubMed] [Google Scholar]
- 28.Kreitman RJ, Wilson WH, Bergeron K, et al. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N Engl J Med. 2001;345:241–247. doi: 10.1056/NEJM200107263450402. [DOI] [PubMed] [Google Scholar]
- 29.Kreitman RJ, Wilson WH, White JD, et al. Phase I trial of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J Clin Oncol. 2000;18:1622–1636. doi: 10.1200/JCO.2000.18.8.1622. [DOI] [PubMed] [Google Scholar]